Abstract
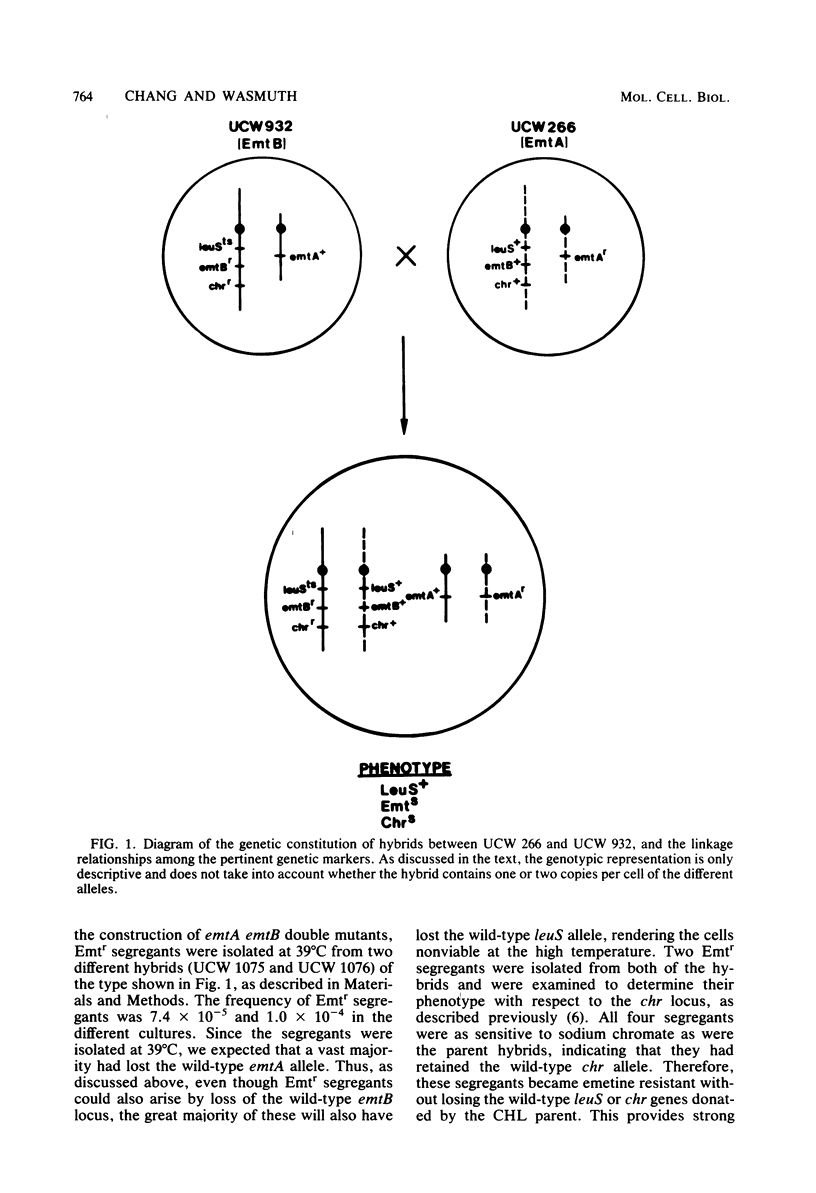
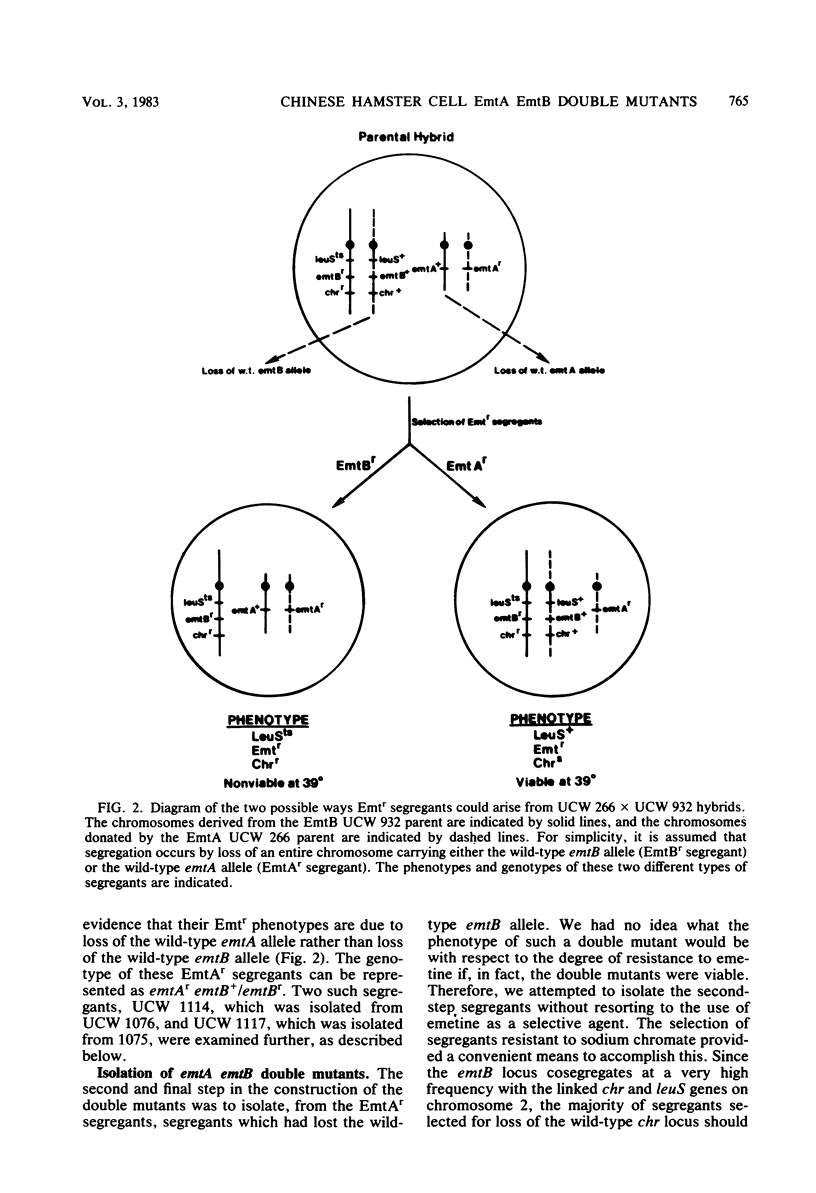
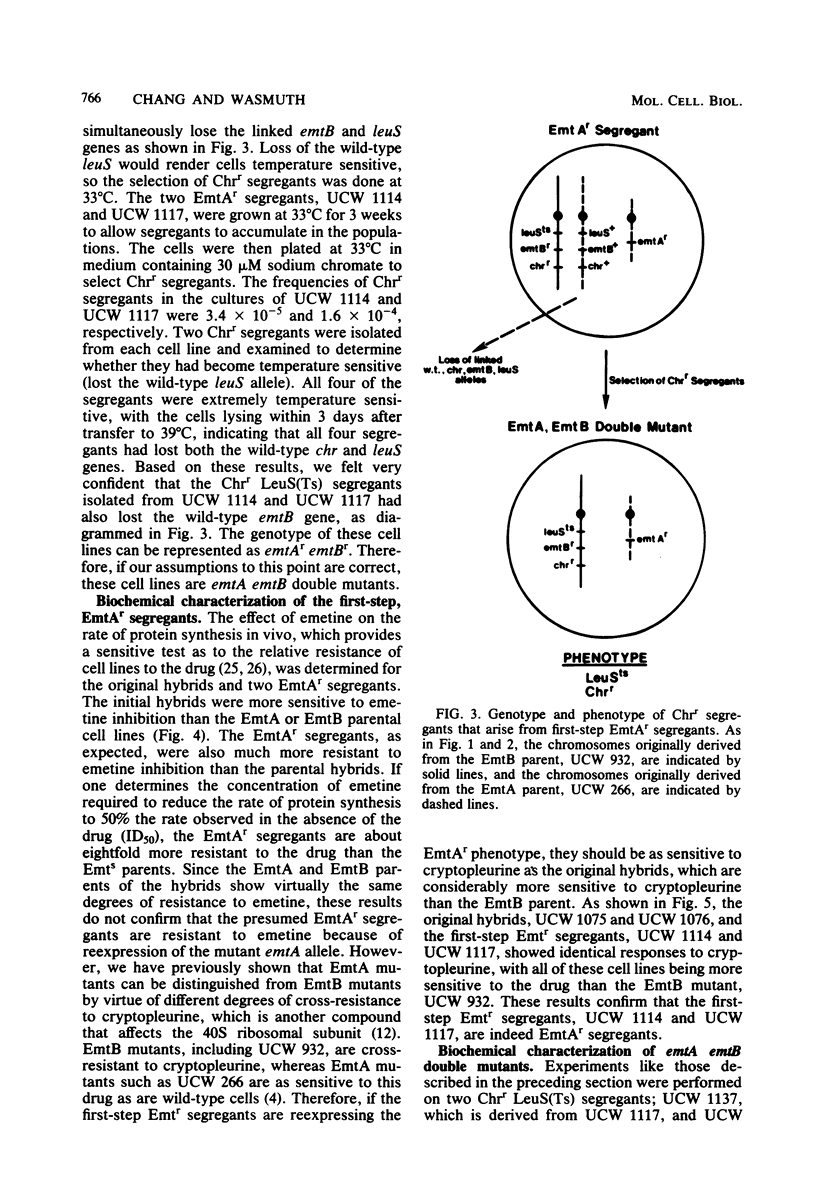
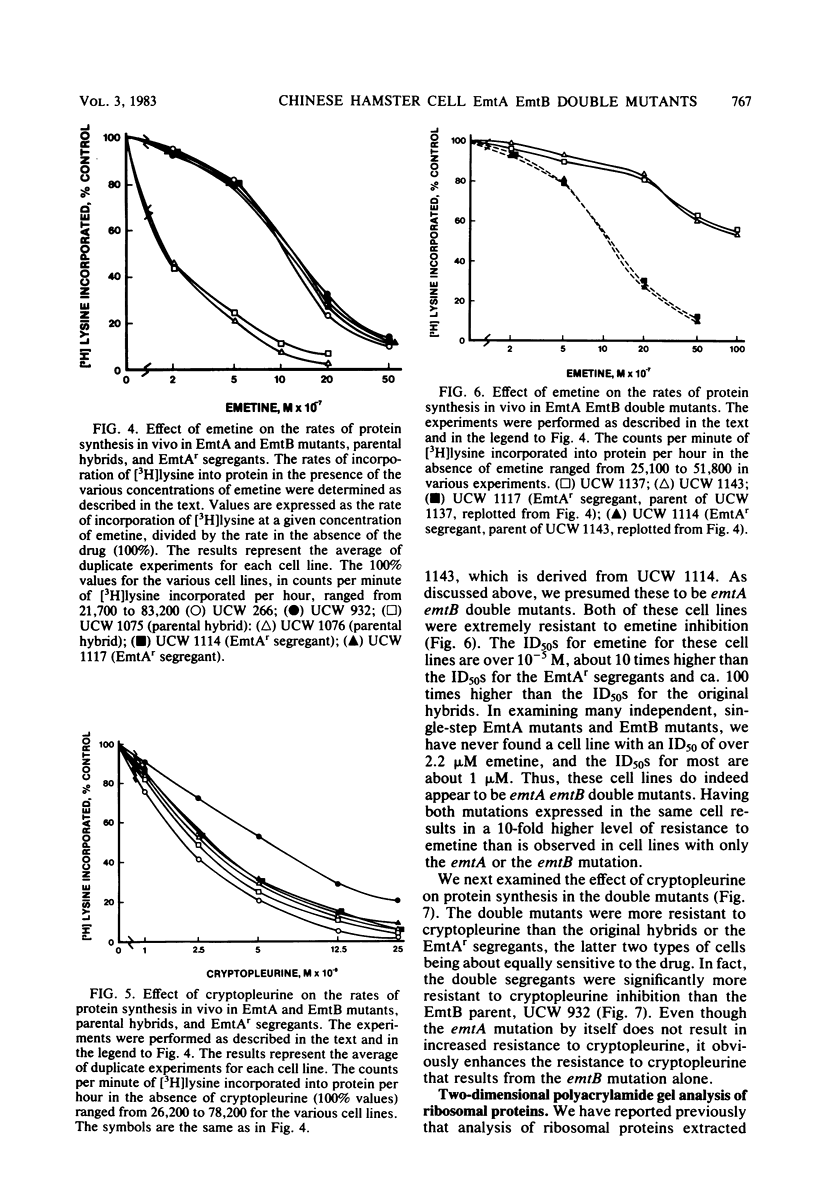
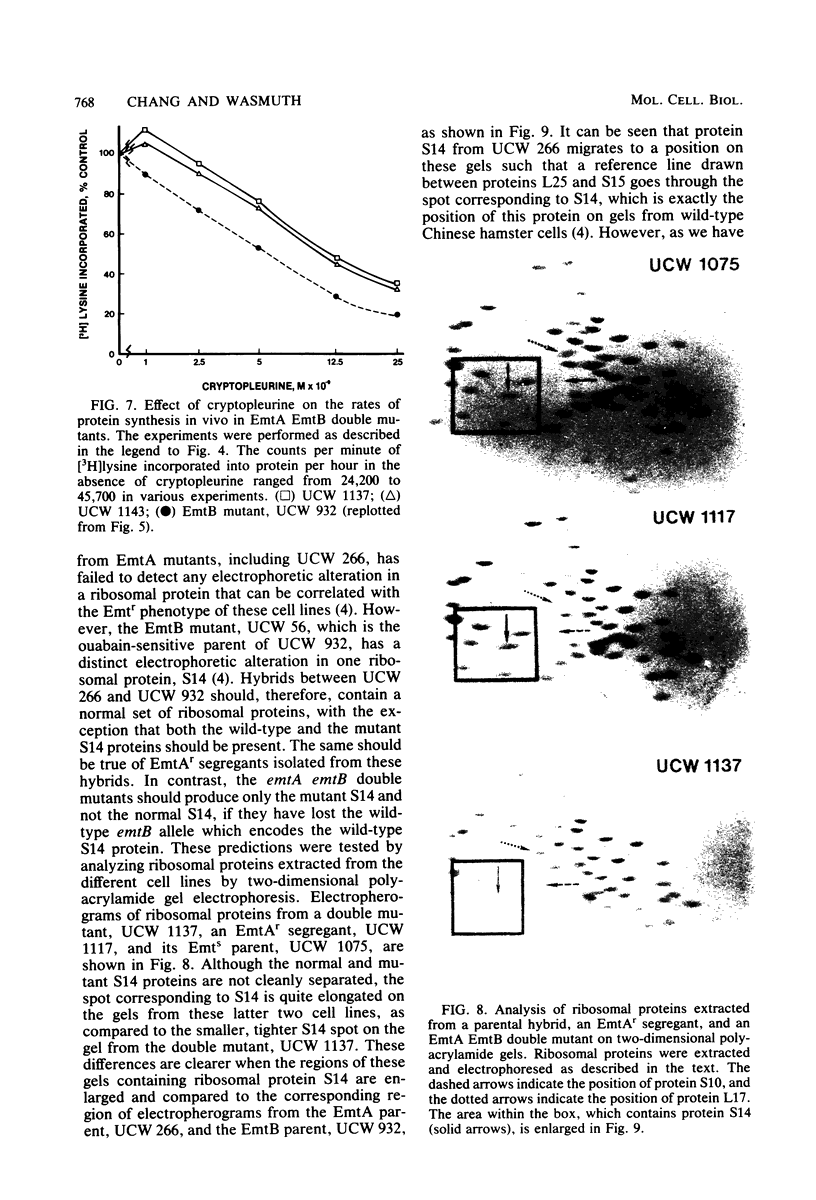
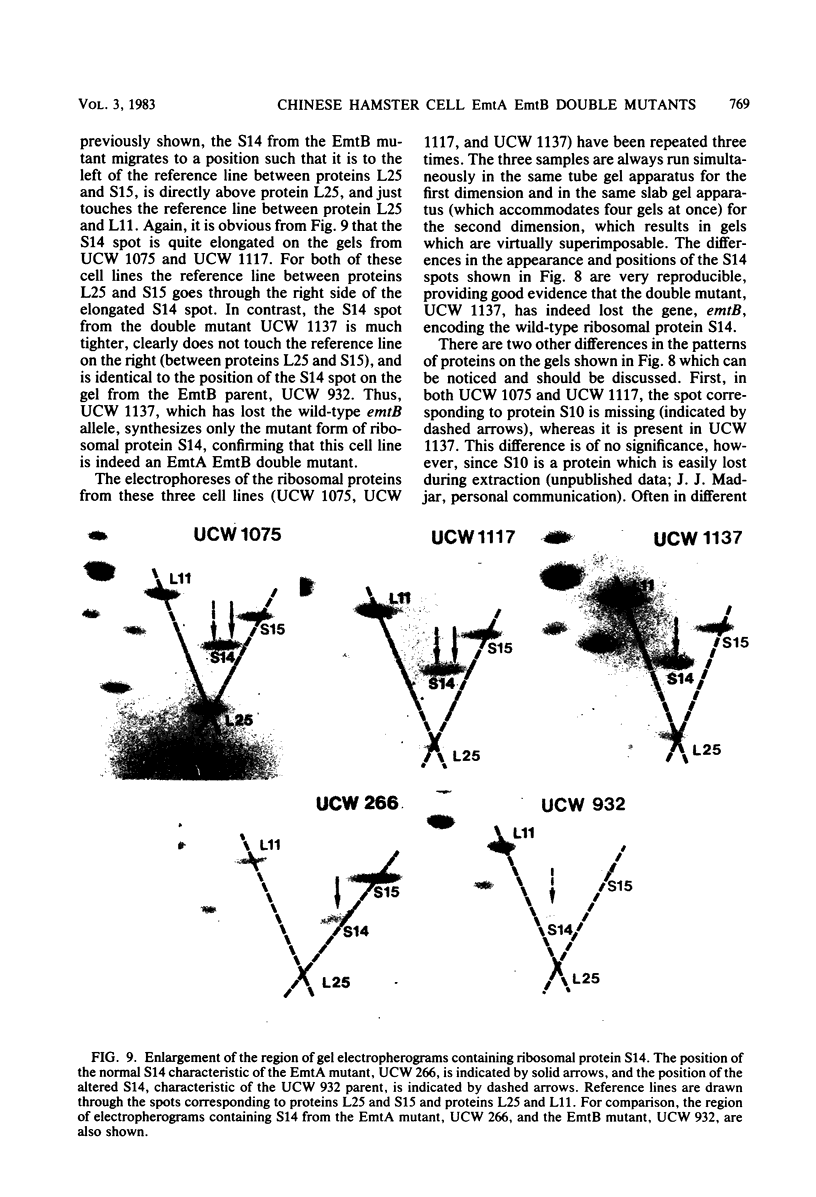
Starting with hybrid cell lines between a Chinese hamster cell EmtA mutant and a Chinese hamster cell EmtB mutant, we have constructed cell lines that are homozygous for mutant alleles at both the emtA locus and the emtB locus, by using a two-step segregation protocol. The EmtA EmtB double mutants are approximately 10-fold more resistant to emetine inhibition than either of the parental mutants. Having both the EmtA mutation and the EmtB mutation expressed in the same cell also results in a level of resistance to cryptopleurine that is significantly higher than a simple additive effect of the two mutations alone. Analysis of ribosomal proteins by two-dimensional polyacrylamide gel electrophoresis demonstrated that a parental hybrid and a first-step segregant, which has lost the wild-type emtA allele, synthesize both a normal and an altered form of ribosomal protein S14, whereas an EmtA EmtB double mutant synthesizes only the altered form of this ribosomal protein. This result confirms that the emtB locus is the structural gene for ribosomal protein S14. Our results also suggest that the products of the emtA and emtB loci interact directly, indicating that the emtA locus, like the emtB locus, encodes a component of the ribosome.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boersma D., McGill S. M., Mollenkamp J. W., Roufa D. J. Emetine resistance in Chinese hamster cells is linked genetically with an altered 40S ribosomal subunit protein, S20. Proc Natl Acad Sci U S A. 1979 Jan;76(1):415–419. doi: 10.1073/pnas.76.1.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C. E., Worton R. G. Linkage of genetic markers emt and chr in Chinese hamster cells. Somatic Cell Genet. 1980 Mar;6(2):215–224. doi: 10.1007/BF01538797. [DOI] [PubMed] [Google Scholar]
- Campbell C. E., Worton R. G. Segregation of recessive phenotypes in somatic cell hybrids: role of mitotic recombination, gene inactivation, and chromosome nondisjunction. Mol Cell Biol. 1981 Apr;1(4):336–346. doi: 10.1128/mcb.1.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Wasmuth J. J. Genetic and biochemical distinction among Chinese hamster cell emtA, emtB, and emtC mutants. Mol Cell Biol. 1983 Mar;3(3):429–438. doi: 10.1128/mcb.3.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzet M., Bégueret J. Cold-sensitivity of a double mutant strain combining two ribosomal mutations in the ascomycete Podospora anserina. Mol Gen Genet. 1978 Oct 24;165(3):283–288. doi: 10.1007/BF00332528. [DOI] [PubMed] [Google Scholar]
- D'Eustachio P., Meyuhas O., Ruddle F., Perry R. P. Chromosomal distribution of ribosomal protein genes in the mouse. Cell. 1981 May;24(2):307–312. doi: 10.1016/0092-8674(81)90320-2. [DOI] [PubMed] [Google Scholar]
- Dana S., Wasmuth J. J. Linkage of the leuS, emtB, and chr genes on chromosome 5 in humans and expression of human genes encoding protein synthetic components in human--Chinese hamster hybrids. Somatic Cell Genet. 1982 Mar;8(2):245–264. doi: 10.1007/BF01538680. [DOI] [PubMed] [Google Scholar]
- Deaven L. L., Petersen D. F. The chromosomes of CHO, an aneuploid Chinese hamster cell line: G-band, C-band, and autoradiographic analyses. Chromosoma. 1973;41(2):129–144. doi: 10.1007/BF00319690. [DOI] [PubMed] [Google Scholar]
- Dequard M., Couderc J. L., Legrain P., Belcour L., Picard-Bennoun M. Search for ribosomal mutants in Podospora anserina: genetic analysis of mutants resistant to paromomycin. Biochem Genet. 1980 Apr;18(3-4):263–280. doi: 10.1007/BF00484241. [DOI] [PubMed] [Google Scholar]
- Farrell S. A., Worton R. G. Chromosome loss is responsible for segregation at the HPRT locus in Chinese hamster cell hybrids. Somatic Cell Genet. 1977 Sep;3(5):539–551. doi: 10.1007/BF01539124. [DOI] [PubMed] [Google Scholar]
- Fried H. M., Warner J. R. Cloning of yeast gene for trichodermin resistance and ribosomal protein L3. Proc Natl Acad Sci U S A. 1981 Jan;78(1):238–242. doi: 10.1073/pnas.78.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P., Sánchez L., Jiménez A. Cryptopleurine resistance: genetic locus for a 40S ribosomal component in Saccharomyces cerevisiae. J Bacteriol. 1974 Dec;120(3):1308–1314. doi: 10.1128/jb.120.3.1308-1314.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. Genetic and biochemical characterization of mutants of CHO cells resistant to the protein synthesis inhibitor trichodermin. Somatic Cell Genet. 1978 May;4(3):355–374. doi: 10.1007/BF01542848. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. Mutants of CHO cells resistant to the protein synthesis inhibitor emetine: genetic and biochemical characterization of second-step mutants. Somatic Cell Genet. 1978 Jan;4(1):77–94. doi: 10.1007/BF01546494. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. The isolation and preliminary characterization of somatic cell mutants resistant to the protein synthesis inhibitor-emetine. Cell. 1976 Oct;9(2):213–219. doi: 10.1016/0092-8674(76)90112-4. [DOI] [PubMed] [Google Scholar]
- Haralson M. A., Roufa D. J. A temperature-sensitive mutation affecting the mammalian 60 S ribosome. J Biol Chem. 1975 Nov 25;250(22):8618–8623. [PubMed] [Google Scholar]
- Madjar J. J., Arpin M., Buisson M., Reboud J. P. Spot position of rat liver ribosomal proteins by four different two-dimensional electrophoreses in polyacrylamide gel. Mol Gen Genet. 1979 Mar 20;171(2):121–134. doi: 10.1007/BF00269998. [DOI] [PubMed] [Google Scholar]
- Madjar J. J., Michel S., Cozzone A. J., Reboud J. P. A method to identify individual proteins in four different two-dimensional gel electrophoresis systems: application to Escherichia coli ribosomal proteins. Anal Biochem. 1979 Jan 1;92(1):174–182. doi: 10.1016/0003-2697(79)90641-9. [DOI] [PubMed] [Google Scholar]
- Madjar J. J., Nielsen-Smith K., Frahm M., Roufa D. J. Emetine resistance in chinese hamster ovary cells is associated with an altered ribosomal protein S14 mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1003–1007. doi: 10.1073/pnas.79.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madjar J. J., Traut R. R. Differences in electrophoretic behaviour of eight ribosomal proteins from rat and rabbit tissues and evidence for proteolytic action on liver proteins. Mol Gen Genet. 1980;179(1):89–101. doi: 10.1007/BF00268450. [DOI] [PubMed] [Google Scholar]
- McConkey E. H., Bielka H., Gordon J., Lastick S. M., Lin A., Ogata K., Reboud J. P., Traugh J. A., Traut R. R., Warner J. R. Proposed uniform nomenclature for mammalian ribosomal proteins. Mol Gen Genet. 1979 Jan 16;169(1):1–6. doi: 10.1007/BF00267538. [DOI] [PubMed] [Google Scholar]
- Monk R. J., Meyuhas O., Perry R. P. Mammals have multiple genes for individual ribosomal proteins. Cell. 1981 May;24(2):301–306. doi: 10.1016/0092-8674(81)90319-6. [DOI] [PubMed] [Google Scholar]
- Picard-Bennoun M. Mutations affecting translational fidelity in the eucaryote Podospora anserina: characterization of two ribosomal restrictive mutations. Mol Gen Genet. 1981;183(1):175–180. doi: 10.1007/BF00270158. [DOI] [PubMed] [Google Scholar]
- Wasmuth J. J., Chu L. Y. Linkage in cultured Chinese hamster cells of two genes, emtB and leuS, involved in protein synthesis and isolation of cell lines with mutations in three linked genes. J Cell Biol. 1980 Dec;87(3 Pt 1):697–702. doi: 10.1083/jcb.87.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmuth J. J., Hill J. M., Vock L. S. Biochemical and genetic evidence for a new class of emetine-resistant Chinese hamster cells with alterations in the protein biosynthetic machinery. Somatic Cell Genet. 1980 Jul;6(4):495–516. doi: 10.1007/BF01539152. [DOI] [PubMed] [Google Scholar]
- Wasmuth J. J., Hill J. M., Vock L. S. Identification and characterization of a third complementation group of emetine-resistant Chinese hamster cell mutants. Mol Cell Biol. 1981 Jan;1(1):58–65. doi: 10.1128/mcb.1.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worton R. G., Ho C. C., Duff C. Chromosome stability in CHO cells. Somatic Cell Genet. 1977 Jan;3(1):27–45. doi: 10.1007/BF01550985. [DOI] [PubMed] [Google Scholar]