Abstract
Dissemination of cancer cells to distant organ sites is the leading cause of death due to treatment failure in different types of cancer. Mehlen and Puisieux have reviewed the importance of the development of inappropriate cell survival signalling for various steps in the metastatic process and have noted the particular importance of aberrant cell survival to successful colonization at the metastatic site. Therefore, the understanding of mechanisms that govern cell survival fate of these metastatic cells could lead to the understanding of a new paradigm for control of metastatic potential and could provide the basis for developing novel strategies for the treatment of metastases. Numerous studies have documented the widespread role of Akt in cell survival and metastasis in colorectal cancer, as well as many other types of cancer. Akt acts as a key signalling node that bridges the link between oncogenic receptors to many essential pro-survival cellular functions, and is perhaps the most commonly activated signalling pathway in human cancer. In recent years, Akt2 and Akt3 have emerged as significant contributors to malignancy alongside the well-characterized Akt1 isoform, with distinct non-overlapping functions. This review is aimed at gaining a better understanding of the Akt-driven cell survival mechanisms that contribute to cancer progression and metastasis and the pharmacological inhibitors in clinical trials designed to counter the Akt-driven cell survival responses in cancer.
Keywords: Akt isoforms, PI3K, Colorectal cancer, Metastasis, Cell survival, TGFβ/PKA signalling, XIAP and survivin, Akt inhibitors
1. Introduction
Colorectal cancer (CRC) is one of the most common malignancies with high incidence rates globally [1]. It develops due to various genetic, epigenetic and environmental factors. The stage I CRC is localized to the mucosal and submucosal layers of the colon wall. Surgery and multimodal treatments are the preferred strategies following early stage diagnosis of CRC with a 5-year survival rate of approximately 100%. This rate in 5-year survival is significantly decreased during stage III CRC which is characterized by lymph node metastasis [2]. However, metastasis to distant organ sites (stage IV) is the most frequent cause of cancer-related deaths with a 5-year survival rate of <5% [3, 4]. Although CRCs progress slowly to an invasive stage but the progression from invasive carcinoma to metastatic phase occurs rapidly [5]. The disseminating CRC cells acquire enhanced cell survival capabilities to counteract apoptosis initiated by the multi-step metastatic cascade including anoikis [6], migration through the basement membrane and intravasation into the blood and/or lymphatic vessels. The CRC cells develop the ability to proliferate and colonize secondary organ sites with unrelated microenvironments [6]. There has been limited success in the early identification of patients at high risk for developing metastasis through the tissue-based biomarkers currently available for such purposes [7, 8]. The mechanisms involved in regulating the early stages of the metastatic cascade, that are crucial for diagnosis are currently not fully understood [9–12]. In recent years, several aberrant cell survival mechanisms has been linked to successful metastatic colonization [13] including the deregulation of pro-survival Bcl-2 family proteins [14], inhibitor-of-apoptosis (IAP) family proteins [15] and the PI3K/AKT signalling. The Bcl-2 family members are either pro-apoptotic or anti-apoptotic in nature [16]. The pro-apoptotic proteins such as BID, BAX and BAK translocate in the mitochondria leading to caspase activation thus leading to cell death. The Bcl-2 family members have a BH3 domain that inhibits the anti-apoptotic members of the Bcl-2 family thus leading to apoptosis. Bcl-XL protein inhibits the activity of BAX and BAK by binding to them and inhibiting apoptosis [17]. BAX and BAK are responsible for caspase activation thus downregulating cell survival. Several lines of evidence have implicated the role of Bcl-2 overexpression in promoting cell survival and metastasis [18, 19]. However, contrasting reports from Subhawong et al.(2010) concluded that Bcl2 is infrequently up-regulated in metastatic breast carcinoma and hormone therapy resistance may lead to downregulation of Bcl-2 [20]. IAPs are also responsible for regulating apoptosis by inhibiting caspases. Some IAPs have been shown to inhibit caspases by inhibiting their enzyme activity and/or by targeting them for proteasomal degradation [21]. Caspases 3,7 and 9 bind to the BIR (Baculoviral IAP repeat) domains of IAPs leading to its inhibition and increased cell survival [22]. Recently, Altieri laboratory has shown that IAP family members survivin and XIAP are playing an essential role in metastasis [23]. Overexpression of survivin has been observed in colorectal tumorigenesis. Survivin plays a role in the progression of adenomas from the mild dysplasia to the more advanced highly dysplastic lesions [24]. Several survivin inhibitors are currently in the phase I or II clinical trials in solid tumors and non-Hodgkins lymphoma [25]. YM155 (1-(2-Methoxyethyl)-2-methyl-4,9-dioxo-3-(pyrazin-2-ylmethyl)-4,9-ihydro-H-naphtho[2,3]-dimidazolium bromide) (Astellas Pharma Inc) is a transcriptional inhibitor of survivin. It has been demonstrated to have a potent anti-tumor activity and has been used as a radio-sensitizing agent to potentiate the anti-cancer functions of various chemotherapeutic agents [25–27]. The phosphoinositide 3-kinase (PI3K) – Akt signalling pathway, which transmits anti-apoptotic signals, is involved in a significant fraction of human tumors promoting cancer cell growth, metabolism, survival and has been implicated in EMT, angiogenesis and metastasis (see reference [28–32] for extensive reviews on PI3K/Akt signalling in cancer). A better understanding of the molecular signalling pathways involved in the process of metastasis will help in effectively targeting these aggressive cancer cells using novel therapeutic strategies.
In this review, we have focused on the emerging roles of Akt isoforms in various types of cancer including colorectal, breast, lung and pancreatic cancer and have discussed the TGFβ/PKA metastatic suppressor pathway that negatively regulates Akt-driven aberrant cell survival mechanisms that contribute to metastatic progression in CRC. Additionally, we have provided updates on various Akt inhibitors that are in clinical trials.
2. PI3K – Akt signalling in cancer
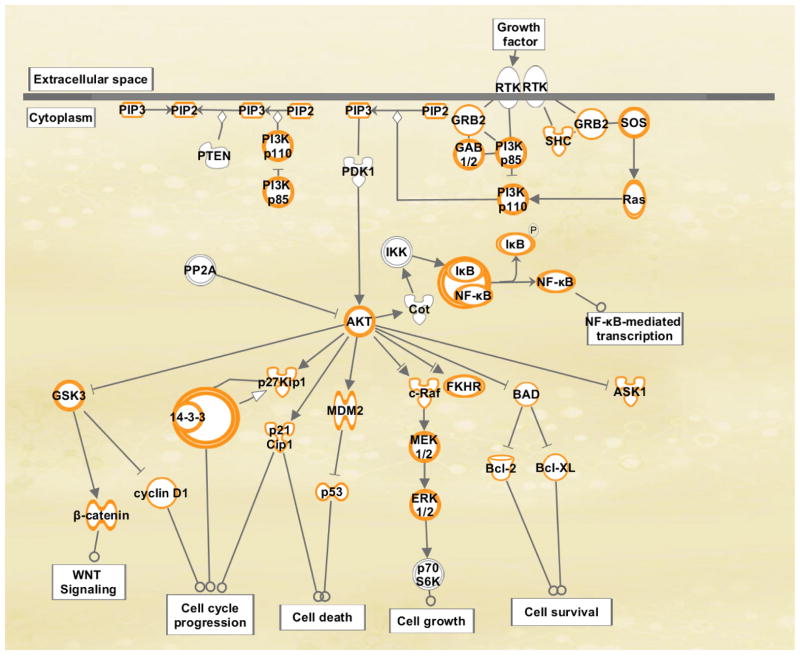
The PI3K signalling pathway is a major link between oncogenic receptors to downstream pro-survival molecules and is one of the most frequently activated signalling pathway in human cancers [33–35]. It is known that multiple small GTPase that belong to Ras and Rho-kinase family of GTPases activate PI3K [36]. It has also been shown that Rho GTPases are downstream activators of PI3K. These Rho GTPases amplify the PI3K activity by feedback mechanisms [37]. The stimulation by growth factors leads to the activation of receptor tyrosine kinases (RTKs), that in turn recruits the class IA PI3Ks, consisting of p110α–p85 subunits to the membrane due to the direct protein-protein interaction between p85 and activated RTKs (for example, IGFR and EGFR). Alternatively, p85 subunit can also interact with specific adaptor proteins, such as Insulin Receptor Substrate 1/2 (IRS1/2) associated with IGFR (Figure 1). The activated p110α catalytic subunit converts phosphatidylinositol-4,5-bis phosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3) at the membrane. The conversion of PIP2 to PIP3 provides docking sites for pleckstrin homology (PH) domain-containing proteins, including 3-phosphoinositide-dependent protein kinase 1 (PDPK1) and serine–threonine protein kinase Akt (also known as protein kinase B). PDPK1 phosphorylates Akt at Ser473 and PDK2 phosphorylates Akt at Thr308. The dual activation of Akt at Ser473 and Thr308 phosphorylation sites elicits a broad range of downstream signalling events as shown in Figure 2. The tumor suppressor phosphatase and tensin homologue deleted on chromosome 10 (PTEN) inhibits PI3K activation and downstream signalling by dephosphorylating PIP3 [38]. Deletion, mutation and hypermethylation of PTEN are observed in various cancer conditions thus resulting in elevated Akt activity [39]. A study in rhabdomyosarcoma cells has shown that PTEN is responsible for dephosphorylation specifically at the Ser473 site of Akt [40]. PHLPP (PH domain leucine-rich repeat protein phosphatase) specifically dephosphorylates Akt at Ser473 thus attenuating Akt signalling [41]. It has two isoforms PHLPP1 and PHLPP2, which are very specific in their mode of action. PHLPP1 and PHLPP2 specifically dephosphorylate Akt2 and Akt1 respectively [42]. The expression of these phosphatases decrease in many cancer types including colon cancer and glioblastoma, thus resulting in increased Akt activation and its downstream oncogenic signalling pathways [43]. PP2A (protein phosphatase 2A) is a serine threonine phosphatase, which is ubiquitously expressed in the tissues. It regulates Akt by inhibiting its phosphorylation at Thr308 [44]. It is a tumor suppressor gene since its inhibition by okadaic acid results in increase in cell survival thus increasing tumor growth [45]. It has been shown that there is an increase in the expression of PP2A inhibitors in various cancer conditions [46]. We have recently demonstrated a novel pathway of cell death in colon cancer, mediated by PP2A where it inhibits the phosphorylation of Akt, thus disrupting the XIAP/Survivin complex resulting in increased cell death [47]. The overall mechanism of cell death mediated by this pathway has been explained in detail in the later part of review.
Figure 1.
PI3K/Akt signalling in cancer
Figure 2.
Akt isoform functions in cancer
Genomic analyses have revealed that many components of the PI3K/Akt pathway are frequently mutated or altered in common human cancers underscoring the importance of this pathway in cancer [48]. The genes encoding the PI3K catalytic subunits PI3KCA, PI3KCB, regulatory subunit of PI3K, p85, PDK1, PTEN undergo loss of function or overexpression in various tumor conditions thus leading to cancer.
3. Distinct roles for Akt isoforms in cancer progression and metastasis
Akt is an evolutionarily conserved serine/threonine kinase consisting of 3 members, i.e., Akt1 (PKBα), Akt2 (PKBβ) and Akt3 (PKBγ). The Akt isoforms have distinct non-redundant functional characteristics despite sharing high level of structural homology and similar mechanisms of activation [49, 50]. The Akt isoforms are ubiquitously expressed in all cell types and tissues. However, the pattern of Akt3 expression is restricted [51]. Akt1 is widely expressed in brain, heart and lungs; however, Akt2 is mostly expressed in skeletal muscles [45, 52]. Akt3 is expressed in brain, kidney and embryonic heart [53]. These Akt isoforms also show difference in their cellular localization. Akt1 is known to localize in the cytoplasm;Akt2 localizes in the nucleus, cytoplasm and mitochondria [49, 54–57]. However, Akt3 is known to localizein the nuclear membrane [57, 58]. Knockdown of individual Akt isoforms does not affect the localization pattern of other Akt isoforms and also has been shown to have no compensatory effects on the level of other isoforms [59]. Numerous studies have been done in genetically engineered mice deficient for Akt1, Akt2 or Akt3 to confirm that these isoforms have different physiological functions [60]. Variable phenotypic differences are observed following the genetic inactivation and/or removal of the Akt isoforms. Akt1 knockout mice exhibit retardation in growth and an increase in perinatal lethality [58, 61]. Removal of Akt2 in mice results in insulin resistance and diabetes mellitus [62] whereas Akt3 knockout results in reduction in brain size and development [63]. Double knockout mice harboring deficiency of both Akt1 and Akt2 isoforms die immediately after birth [64]. However there are no studies available related to double knockdown of Akt3 in combination with Akt1 or Akt2.
Although Akt isoforms are aberrantly expressed in tumor condition but these isoforms have tumor specific expression. Akt1 amplification is commonly observed in gastric cancer cells and knockdown of Akt1 increases the sensitivity of gastric cancer cells to chemotherapy as determined by treatment with cisplatin. Akt1 knockdown in gastric cancer cells increases the expression of Bax and reduces the expression of Bcl2 thus increasing cell death in vitro and in vivo [65]. Akt2 is abnormally expressed in breast, ovarian and colon cancers [45, 63, 66]. Akt3 undergoes amplification in breast and prostate cancer [53, 65, 67]. In the following section, we have discussed the role of Akt isoforms in colorectal, breast, lung and pancreatic cancer. Numerous cross talk and feedback mechanisms regulate PI3K/Akt signalling. Recently, Heron-Milhanet et al. has extensively reviewed the differential action of Akt1 and Akt2 in cancer and pointed towards the significance of targeting isoform-specific downstream events for the development of effective anti-cancer therapies involving Akt kinases [68].
3.1 Colorectal cancer
Several studies have documented the inhibition of apoptosis as a critical event in the development of colorectal malignancies. The overexpression of anti-apoptotic proteins or the inactivation of pro-apoptotic proteins is a common event in colorectal carcinogenesis that is usually dependent on the genetic background of the tumor [69]. Sporadic colon cancers are associated with inhibition of apoptosis in association with the loss of function of tumor suppressor adenomatous polyposis coli (APC), along with overexpression of anti-apoptotic Bcl-2 proteins [70]. However, CRC that originates due to DNA mismatch repair deficiency resulting in microsatellite instability (MSI) are usually associated with inactivating mutations of the pro-apoptotic BAX protein. About 6–15% of sporadic (non-hereditary) colon cancers have MSI positive, while majority of tumors from patients with hereditary non-polyposis colorectal cancer (HNPCC) are MSI positive [71]. Roy et al. demonstrated for the first time that Akt overexpression is an early event during sporadic colon carcinogenesis [69]. This increased Akt expression was specific to sporadic colon carcinogenesis as opposed to the human MSI-high tumors represented by a series of HNPCC tumors. It was shown that 57% of all colorectal cancer specimens tested were positively immnuostained for Akt. However, normal colonic mucosa and hyperplastic polyps expressed negligible levels of Akt. Roy et al. also reported for the first time that 57% of all adenomas overexpressed Akt indicating that overexpression of Akt is an early event in colon carcinogenesis. Interestingly, Akt2 isoform was indicated as a predominant player in colon carcinogenesis. Additionally, Akt phosphorylation at Ser473 was detected in colon carcinomas but not in normal epithelium. This study concluded that Akt overexpression (especially Akt2) may be important in the early inhibition of apoptosis during colon carcinogenesis [69].
The independent roles for Akt isoforms in regulating malignant progression in colorectal and breast cancer was recently reported by Yoeli-Lerner et al. [51, 72] demonstrating that Akt1 overexpression inhibits the transcriptional activity of NFAT (nuclear factor of activated T cells) thus resulting in the blockade of invasion and migration. It is postulated that NFAT is targeted through Akt1-HDM2 (Human analogue of MDM2) pathway. HDM2 is an E3 ubiquitin ligase, that ubiquitinates its target proteins, such as NFAT for proteasomal degradation. It was observed that NFAT degradation was rescued in the presence of HDM2 siRNA [73]. Feng et al. has shown that Akt1 stabilizes MDM2 by phosphorylating Ser166 and Ser188 residues, thus inhibiting its self-ubiquitination and increasing its stability. [74]. In contrast, Akt2 overexpression up-regulates β1-integrin(a component of collagen IV- binding receptor; referred to here as β1) expression both in vivo and in vitro [75]. Increased β1 expression is responsible for increased metastasis [76, 77]. This capability of Akt2 has been attributed to the fact that Akt2, in addition to regulating cell migration and invasion, also inhibits apoptosis. Thus Akt1 inhibits metastasis and invasion by degrading NFAT however, Akt2 up-regulates β1-integrin expression resulting in increased cell migration and invasion depicting their contradictory roles in cancer. Amplification and overexpression of Akt2 has been shown to play a critical role in CRC metastatic colonization [78]. Akt2 is a proto-oncogene, and is highly expressed in metastatic colon carcinoma as compared to primary colon cancer [79]. Genetic inactivation of Akt2 has been shown to results in reduced ability of colon carcinoma cells to metastasize thus confirming that Akt2 is required for the establishment of colon cancer metastasis [80]. However, the exact mechanism of Akt2-driven metastasis is poorly understood. Genetic inactivation of Akt1 and Akt2 results in reduction in clonal growth of colon cancer cells in vitro, but this reduction was much more significant when the cells were cultured in media lacking growth factors [80]. This led to the conclusion that tumor microenvironment plays a significant role in regulating the effects of gene inactivation [12]. Inactivation of Akt1 and Akt2 also results in reduced metastasis to liver and reduced tumor burden [80].
Activated Akt regulates the expression of Bcl-2 and FAK (focal adhesion kinase) proteins mediating CRC metastasis [81, 82]. In response to stress, Akt1 binds directly to FAK thus phosphorylating it at three serine residues (Ser 517, 601, 695) [83]. Phosphorylation of the 3 serine residues in turn phosphorylate the tyrosine residue (Tyr397) thus activating it. This in turn induces cell adhesion by increasing the binding of integrins to matrix thus leading to increased metastasis [84].
In addition to the overexpression of Akt isoforms in different cancer types, Akt phosphorylation in human CRCs has been shown to correlates with cell proliferation and apoptosis inhibition, and has also been demonstrated to increase with advancement of CRC [85, 86]. It is well documented that mTOR (mammalian target of rapamycin), which promotes growth, protein translation and metabolism is regulated by Akt kinase. Sabatini laboratory have made the seminal discovery that mTOR is a direct substrate of Akt and identified Ser2448 residue as the Akt phosphorylation site on mTOR [87]. Evers laboratory has recently demonstrated that mTOR1/mTOR2 proteins, Raptor and Rictor are highly expressed in CRC tissues [88]. Importantly, Rictor expression was correlated with pAKT Ser473 expression in CRCs derived from same patient. Johnson et al. showed that p85α, Akt1, Akt2 and pmTOR Ser2448 were all overexpression in CRC compared to control and additionally reported that the expression of Akt1 and Akt2 was more pronounced in the left sided CRCs compared to the right sided CRC [89].
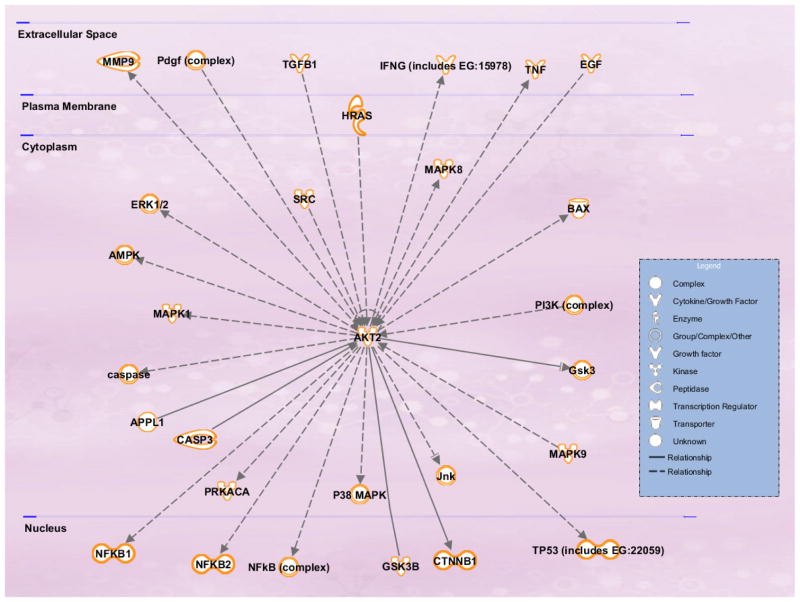
The molecular networks and pathways affected by the predicted mRNA targets of Akt2 in context of CRC metastasis signalling generated using the Ingenuity Pathway Analysis (IPA) tool has been shown in Figure 3. The IPA analysis of Akt2 revealed that Akt2 targets multiple genes frequently deregulated in cancer. Some of these target genes have been identified previously as directs targets for Akt2 and have been implicated in invasion and metastasis (including the MMP-9, EGF and SRC). Additionally, the predicted mRNA targets of Akt2 also included apoptosis regulators such as Bax, JNK/p38, TP53, NF-κB and Caspase3. Yuan et al [90] demonstrated that Akt2 altered the cisplatin-induced apoptosis in ovarian cancer cells by regulating the ASK1/JNK/p38 pathway. ASK1 (apoptosis signal-regulating kinase 1) is a member of the mitogen-activated protein kinase kinase kinase family that mediates cell death in response to various stress including chemotherapeutic drugs such as cisplatin and paclitaxel [90]. Stimulation of JNK/p38 activity has also been shown to be critical for cisplatin-induced apoptosis in certain cancer cell types. It was shown that AKT2 interacts with and phosphorylates ASK1 (apoptosis signal-regulating kinase 1) at Ser-83 residue. This leads to the inhibition of ASK1 kinase activity, blockade of JNK/p38 activation and activation of Bax. This work provided a new highlight on the role of Akt2 in regulating the ASK1/JNK/p38/Bax pathway and provided a new mechanistic insight into the anti-apoptotic effects of Akt2 [90].
Figure 3.
Predicted mRNA targets of Akt2 in context of colorectal cancer metastasis signalling
3.2 Breast cancer
In breast cancer, Akt upregulates matrix metalloproteases (MMPs) mediated matrix degradation resulting in increased metastasis [91]. In estrogen receptor-positive (ER+) breast cancer cells, Akt2 activation is triggered by ER-α. Akt2 expression is higher in Her2/neu breast cancer compared to Her2 negative tumors with increased resistance to stress mediated cell death and less susceptibility to chemotherapeutics [67]. Due to the intrinsic tyrosine kinase activity of Her2/neu, the receptor undergoes dimerization and activation, even in the absence of ligand, which in turn activates PI3K [92, 93]. Akt1 is upregulated in breast cancer samples although there is no significant correlation between Her2/neu and Akt1 expression [67]. In contrast, ER-β causes reduction in Akt signalling in ERα positive breast cancer cells, thus affecting cell proliferation and survival along with increasing the sensitivity of cells for tamoxifen (ER antagonist) treatment [94]. Increased expression of Akt3 has been observed in hormone unresponsive breast tumors thus contributing to their aggressive and metastatic phenotype. Chung et al, [95] showed that N-cadherin specifically suppressed Akt3 activity and promoted tumor cell migration in breast cancer cells, however, N-cadherin had no effects on Akt1 and Akt2 isoforms, thereby associating Akt3 with epithelial-to-mesenchymal transition (EMT), a crucial step in metastasis. These studies were further confirmed by knockdown of Akt3, which resulted in increased cell motility with no effect on cell proliferation [95]. The mechanism of reduction in Akt3 activity by N-cadherin is yet to be determined however, it was observed that the expression of Akt3 mRNA was affected by N-cadherin but the protein expression remains unchanged, thus the transcription of Akt3 is reduced by N-cadherin.
3.3 Lung cancer
Grabinski et al. reported a high activity of Akt3 isoform in lung cancer cells that regulates the expression of cyclin D3, thus promoting G1-S transition of cell cycle [96]. Akt3 siRNA knockdown reduced proliferation, survival and migration of lung cancer cells. In contrast, Akt1 siRNA knockdown resulted in a reduction in MEK/ERK1/2 activity [97] and IkB protein expression leading to cell death [98]. However, reduction in Akt2 expression induces MCL-1 cleavage cell death through mitochondrial membrane potential loss and release of cytochrome c in the cytosol [81]. Akt1 undergoes a mutation 17th position (Glutamic acid is mutated to LysineE17K)this mutation is generally observed in breast, colon and ovarian cancers [99]. Recently, it was reported that non-small cell lung carcinoma (NSCLC) patients also possess E17K mutation in Akt1 although the frequency is very less [100]. Kim et al. has confirmed that Akt1 polymorphism might be used as a prognostic marker for NSCLS [101]. However, Sung et al reported that polymorphism in Akt2 and Akt3 is not associated by NSCLC [102].
3.4 Pancreatic cancer
Akt activation due to mutation of Kras or PTEN pathway is a potent survival signal in pancreatic cancer cell lines [103] and correlates with the aggressiveness of tumor. Specifically, Akt2 is amplified in 60% of pancreatic cancer [104] and has been associated with increased growth and invasiveness in human ductal pancreatic cancer. There is a significant increase in the expression of Akt2 and pAkt(p<0.01) in pancreatic ductal adenocarcinoma (PDAC) [105]. Akt2 is considered a prognostic marker for PDAC. Inhibition of Akt2 signaling by PI3K inhibitor results in the reduction in growth of pancreatic cancer cells in vitro and in vivo, by induction of apoptosis. Therefore Akt2 can be an important therapeutic target for treatment of pancreatic cancer [106]. It has been reported that Akt isoforms show differential role in pancreatic cancer, whereas Akt2 is responsible for poor prognosis, Akt1 is associated with favorable prognosis of pancreatic cancer [105]. As mentioned above PHLPPs are responsible for dephosphorylation of Akt thus inactivating it. Nitschie et al. studied the expression of PHLPP1 and PHLPP2 in pancreatic cancer and observed a reduction in the expression of these phosphatases in PDAC as compared to normal pancreas. It was also reported that increased expression of PHLPP1 results in reduction in tumor growth confirming that it is the loss of phosphorylation of Akt2, that causes reduced growth of cancer cells [42].
4. TGFβ-Akt signalling crosstalk regulates aberrant cell survival in CRC metastasis
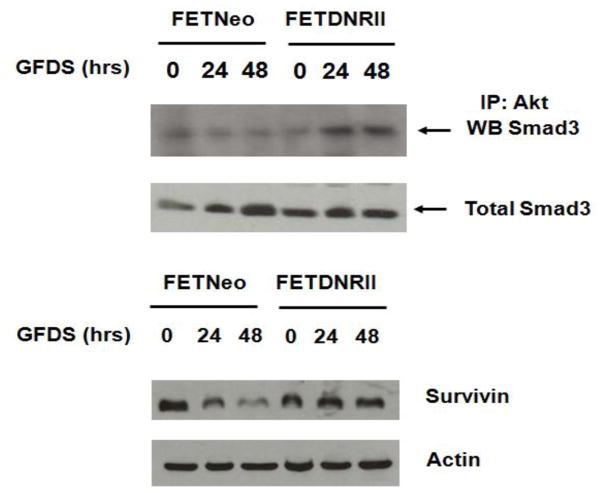
TGFβ signalling plays a critical role in cell survival and metastasis. TGFβ inhibitory/tumor suppressor responses are decreased with increasing malignant progression and in advanced-stages, TGFβ promotes invasion and metastasis. Substantial reports from our laboratory have established a loss or epigenetic silencing of TGFβ receptor expression in a wide range of cancer [107–110]. The mechanisms of epigenetic loss of TGFβ receptors and their therapeutic implications have been reviewed in details [111]. Recently, we have demonstrated that reconstitution of TGFβ receptor expression in CRC cell lines with attenuated TGFβ receptor signalling resulted in the inhibition of metastatic colonization from primary colon tumors to lungs and liver. Significantly, invasion at the primary colon tumor was not prevented indicating that the effect of receptor reconstitution was on the ability to form progressively growing metastatic deposits at liver and/or lungs rather than preventing the initial steps in metastasis [47]. It has been shown that TGFβ mediated apoptosis can be inhibited by insulin in hepatocytes and that the PI3K/Akt pathway (which is activated by insulin stimulation) is involved in this protective effect conferred by insulin [112–114]. Although additional signalling pathways might contribute to TGFβ induced responses, Smads are the only direct effectors known to act as transcription factors and therefore represent the most direct mediators for the transmission of TGFβ signalling from the cell surface to the nucleus [115]. In systemic screening for Akt-associated proteins, Smad3 was identified as a binding partner [116, 117]. We have shown previously that endogenous cellular TGFβ signalling increases apoptosis during growth factor deprivation stress (GFDS) in CRC cells [118]. Further, the loss of autocrine TGFβ in colon cancer cells with attenuated TGFβ signalling resulted in increased PI3K/Akt activation and survivin expression, as well as resistance to GFDS-induced apoptosis [118]. Interestingly, poorly metastatic FET colon cancer cells transfected with the neomycin vector (designated FETNeo) were compared with the highly metastatic FETDNRII cells that have been stably transfected with a dominant negative RII construct to knockout TGFβ signalling [119]. In Figure 4 (unpublished data), the upper panel shows that relatively little Smad3 binds to Akt when TGFβ signalling is intact or in the absence of GFDS (“0” time for FETDNRII). When stress as reflected by GFDS is initiated there is rapid induction of complex formation. In Figure 4, the lower panel shows that when GFDS is initiated in TGFβ autocrine competent cells there is a rapid loss of survivin expression. In contrast, loss of autocrine activity and Smad3/Akt complex formation results in the induction of survivin expression.
Figure 4.
Loss of autocrine TGFβ leads to complex formation between Akt& Smad3 during stress (unpublished data)
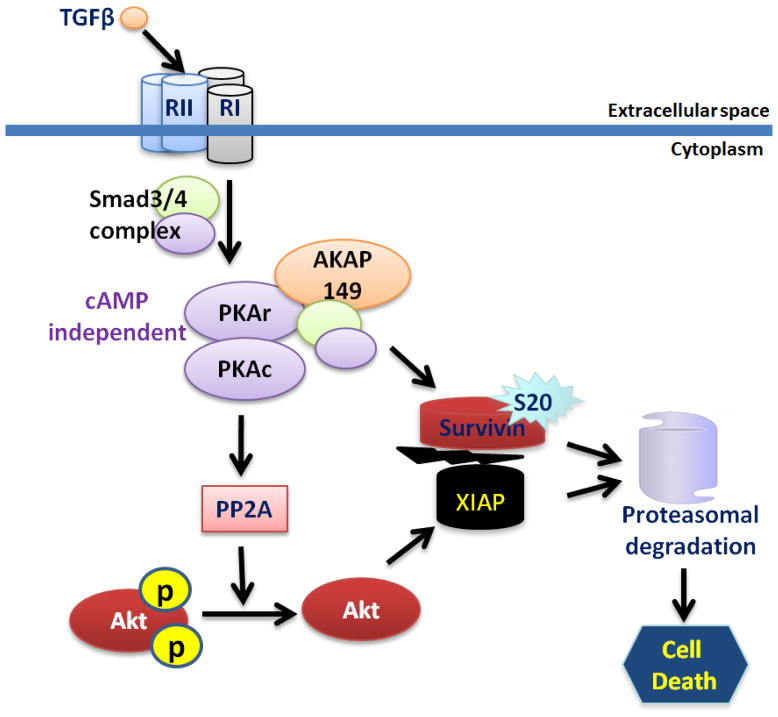
The linkage of this apoptotic mechanism in human colon cancer cell lines to repression of survivin expression may be of significance, because survivin overexpression is strongly associated with poor prognosis in colon cancer [118]. Survivin/XIAP complexes that mediate caspase inhibition have been shown to be a key cell survival mechanism enabling metastasis process [23, 120]. The complex is critical for stabilization of XIAP to inhibit caspases. Survivin and XIAP are in separate mitochondrial compartments, but are released by mitochondria into the cytoplasm in response to stress (such as metastatic growth in a foreign microenvironment) to promote cell survival. We recently identified a novel TGFβ/PKA signalling transduceome by which TGFβ mediated cyclic AMP independent PKA activation lead to the disruption and subsequent destabilization of the survivin/XIAP complex to enable cell death due to PP2A mediated inhibition of Akt phosphorylation of a stabilizing XIAP site (S87) and direct phosphorylation of survivin at S20 (Figure 5). Moreover, we have shown that Akt phosphorylation was higher in the highly metastatic CRC cells with attenuated TGFβ receptor. However, reconstitution of TGFβ receptors in these CRC cells lead to a reduction in Akt phosphorylation followed by a reduction in metastatic colonization [121].
Figure 5.
TGFβ/PKA mediated Akt regulation leading to cell death in CRC
5. Clinical advancement of Akt as a therapeutic target
5.1 MK-2206
It is a potent allosteric kinase inhibitor of Akt. It binds to the pleckstrin homology(PH) domain of Akt and thus inhibits its phosphorylation and translocation to the plasma membrane thus inactivating Akt. It is presently in phase II clinical trial in colon cancer and breast cancer. It is being used in combination with EGFR and IGFR inhibitors in leukemia, breast, colon cancer and malignant gliomas [122]. A recent study showed that MK-2206 has antitumor role in breast cancer in vivo, which was further augmented in the presence of paclitaxel [123]. This combination of MK-2206 and paclitaxel is important since paclitaxel is used in the treatment of breast cancer. A report on nasopharyngeal carcinoma (NPC) reveals that MK-2206 inhibits growth of NPC cells by inhibiting Akt and mTOR signaling. MK-2206 treatment results in reduction in survival of pediatric cancer cell lines in vitro and in vivo [124]. In T cell acute lymphoblastic leukemia it has been demonstrated that MK-2206 results in induction of apoptosis, autophagy and cell cycle arrest [125]. The treatment of MK-2206 in various cancer conditions as a single agent or in combination with other inhibitors has been effective in diminishing the tumor growth and reducing cell survival of cancer cells.
5.2 Perifosine
It is an allosteric inhibitor that binds the PH domain of Akt thus preventing the translocation to plasma membrane [126]. It inhibits the activation of Akt signaling thus reducing cell survival in many cancer conditions like multiple myeloma, T-cell acute lymphoblastic leukemia, NSCLC, prostate and ovarian carcinoma [127–130]. It has been reported that perifosine in combination with curcumin results in increased cell death and decreased cell proliferation of CRC cells in vivo and in vitro. It is believed that this combination inhibits Akt and NF-kB signalling and increases endoplasmic stress and thus plays an anti-tumorigenic role [131]. The role of perifosine was studied in hepatocellular carcinoma and it was found that this Akt inhibitor results in increased apoptosis by affecting the phosphorylation of ERK and JNK [132]. In a study in hepatocellular carcinoma it was observed that perifosine in combination with cisplatin increases apoptosis by increasing the expression of Bax and by reduction in the expression of Bcl-2 [132]. A recent report by Richardson et. al. has revealed that periforsine in combination with bortezomib shows promising results in patients suffering from multiple myeloma with a response rate of 40% [129].
5.3 Deguelin
It is a plant product derived from rotenone, which is used as an important chemotherapeutic agent. It plays anti-tumorigenic role by affecting multiple pathways, such as the inhibition of PI3K/Akt pathway, suppressing the expression of cyclooxygenase 2 (COX2) and cyclin D1 expression [133]. Deguelin causes increase in apoptosis both in vivo and in vitro in a dose and time dependent manner by inhibiting phosphorylation of Akt in lung cancer. It is a heat shock protein 90 (Hsp90) inhibitor with potent anti-angiogenic activity [134]. This drug inhibits Akt activity both via PI3K dependent and PI3K independent pathways [135]. Deguelin suppresses the growth of colon cancer cells in mice by modulating the NF-kB and Wnt signalling pathway. However, it is a potent inhibitor of complex-1 of electron transport chain and its long-term use can result in Parkinson’s disease thus making it incompetent for clinical trials [134]. Li et. al. studied the role of deguelin in murine myeloma cell proliferation and observed that it plays a pro-apoptotic role by upregulation of Bax, down-regulation of Bcl2 and activation of caspase3 [136].
5.4 A443654 and Akti1/2
A443654 is a potent ATP competitive inhibitor of Akt activity. It inhibits all isoforms of Akt with equal potency. Another Akt inhibitor Akti1/2, which is an ATP non-competitive inhibitor, inhibits Akt1 and 2 but not Akt3 [137]. Both these inhibitors are being studied in chronic lymphocytic leukemia (CLL). Treatment of CLL cells with these inhibitors results in reduced expression of MCL-1, which is a critical survival protein in CLL cells [125]. It has also been shown that A443654 increases phosphorylation of Akt at Ser473 thus resulting in increase in Akt activity. This increase in Akt activity is considered as a feedback response of the cell to maintain activation of Akt [138].
5.5 GSK690693
It is a potent ATP competitive Akt inhibitor that inhibits phosphorylation of downstream targets. It is given intravenously to patients with hematological malignancy [139]. Alt more et. al has studied the effect of this inhibitor on mouse model that develop spontaneous athymic lymphoma, endometrial carcinoma or ovarian cancer. Since all these mouse models had activated Akt, treatment with GSK690693 results in reduction in tumor progression, phosphorylation of Akt substrates, cell proliferation and increased apoptosis [140]. This Akt kinase inhibitor has been shown to reduce tumor growth in xenograft models for ovarian, prostate and breast tumors [141]. It has been reported that long term use of this GSK690693 inhibits glycogen synthesis and activates glycogenolysis thus resulting in hyperglycemia as one of the major side effects thus making this drug unfit for further clinical trials [142]. It is being used in combination with other inhibitors, such as mTOR inhibitor and have shown to induce cell death in non-small lung cancer cells [143].
5.6 HSP90 inhibitors
HSP90 is a molecular chaperone which plays an important role in the stability and post-translational maturation and activation of some of the proteins which are implicated in oncogenesis [144]. HSP90 is responsible for maturation of certain cell signalling proteins such as Raf Serine kinases, CDK4 and receptor tyrosine kinases [145]. It has been reported that HSP90 is overexpressed in number of cancers and in virally-transformed cells [146]. HSP90 is a dimer and contains an ATP binding pocket, which is required for its optimal activity. It was reported that amino acid residues (229-309) of Akt are responsible for binding to HSP90. The formation of Akt –HSP90 complex stabilizes Akt activity by preventing its dephosphorylation from PP2A. Stabilization of Akt in phosphorylated state prevents apoptosis in the normal cells making them tumorigenic [147]. Thus, HSP90 has now become an attractive target in anti-cancer therapy. Multiple small molecule inhibitors are being used to inhibit HSP90-client protein interaction that leads to client protein degradation [148]. Whitesell et. al reported that benaoquinoneanamycins like geldanamycin inhibit HSP90 by binding at the ATP binding pocket and thus release the client protein (such as Akt) and directs it to proteasome for degradation, thus resulting in shortening of half-life of Akt from 36 h to 12 h [149]. Geldanamycin is a naturally occurring drug, which is produced by microorganisms and is an HSP90 inhibitor which is being used in combination with chemotherapy and radiotherapy for tumor suppression in preclinical models. In human neuroblastoma and multiple myeloma studies, HSP90 inhibitor was combined with Akt inhibitor perifosine demonstrating increased anti-tumor activity [150, 151]. It is presently in phase II clinical trial for Her2-positive breast cancer in combination with Herceptin [152].
6. Conclusions
As discussed in this review, Akt acts a nodal onco-protein critical for regulating cell survival and metastasis of cancer cells. Thus, targeting Akt as a potential anti-metastatic therapeutic strategy either as a single agent or in combination holds significant promise.
Highlights.
Emerging roles of Akt isoforms in different cancer-types
Importance of Akt-driven aberrant cell survival mechanism that regulate metastasis
TGFβ/PKA signaling transduceome regulate Akt-mediated survival and metastasis
Current Akt inhibitors in clinical trials
Acknowledgments
Support for the work came from National Institutes of Health to Dr. Michael G. Brattain (2R01 CA054807-21, 5RO1 CA034432-26, 5 R01 CA038173-25 and 5 R01 CA072001-14)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stein U, Walther W, Arlt F, Schwabe H, Smith J, Fichtner I, Birchmeier W, Schlag PM. Nat Med. 2009;15(1):59–67. doi: 10.1038/nm.1889. [DOI] [PubMed] [Google Scholar]
- 2.Johnson SM, Gulhati P, Rampy BA, Han Y, Rychahou PG, Doan HQ, Weiss HL, Evers BM. J Am Coll Surg. 2010;210(5):767–776. 776–768. doi: 10.1016/j.jamcollsurg.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christofori G. Nature. 2006;441(7092):444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 4.Stein U, Schlag PM. Recent Results Cancer Res. 2007;176:61–80. doi: 10.1007/978-3-540-46091-6_7. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen DX, Bos PD, Massague J. Nat Rev Cancer. 2009;9(4):274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 6.Weigelt B, Peterse JL, van ’t Veer LJ. Nat Rev Cancer. 2005;5(8):591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 7.Duffy MJ, van Dalen A, Haglund C, Hansson L, Holinski-Feder E, Klapdor R, Lamerz R, Peltomaki P, Sturgeon C, Topolcan O. Eur J Cancer. 2007;43(9):1348–1360. doi: 10.1016/j.ejca.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Vansant JP, Johnson DH, O’Donnell DM, Stewart JR, Sonin AH, McCook BM, Powers TA, Salk DJ, Frist WH, Sandler MP. Clin Nucl Med. 1992;17(6):431–438. doi: 10.1097/00003072-199206000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Bernards R, Weinberg RA. Nature. 2002;418(6900):823. doi: 10.1038/418823a. [DOI] [PubMed] [Google Scholar]
- 10.Fearon ER, Vogelstein B. Cell. 1990;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 11.Takayama T, Miyanishi K, Hayashi T, Sato Y, Niitsu Y. J Gastroenterol. 2006;41(3):185–192. doi: 10.1007/s00535-006-1801-6. [DOI] [PubMed] [Google Scholar]
- 12.Vogelstein B, Kinzler KW. Nat Med. 2004;10(8):789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 13.Mehlen P, Puisieux A. Nat Rev Cancer. 2006;6(6):449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 14.Cory S, Adams JM. Nat Rev Cancer. 2002;2(9):647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasula SM, Ashwell JD. Mol Cell. 2008;30(2):123–135. doi: 10.1016/j.molcel.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Youle RJ, Strasser A. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 17.Grad JM, Zeng XR, Boise LH. Curr Opin Oncol. 2000;12(6):543–549. doi: 10.1097/00001622-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Del Bufalo D, Biroccio A, Leonetti C, Zupi G. FASEB J. 1997;11(12):947–953. doi: 10.1096/fasebj.11.12.9337147. [DOI] [PubMed] [Google Scholar]
- 19.Pinkas J, Martin SS, Leder P. Mol Cancer Res. 2004;2(10):551–556. [PubMed] [Google Scholar]
- 20.Subhawong AP, Nassar H, Halushka MK, Illei PB, Vang R, Argani P. Mod Pathol. 2010;23(8):1089–1096. doi: 10.1038/modpathol.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaux DL, Silke J. Nat Rev Mol Cell Biol. 2005;6(4):287–297. doi: 10.1038/nrm1621. [DOI] [PubMed] [Google Scholar]
- 22.Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvesen GS, Reed JC. EMBO J. 1999;18(19):5242–5251. doi: 10.1093/emboj/18.19.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehrotra S, Languino LR, Raskett CM, Mercurio AM, Dohi T, Altieri DC. Cancer Cell. 2010;17(1):53–64. doi: 10.1016/j.ccr.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawasaki H, Toyoda M, Shinohara H, Okuda J, Watanabe I, Yamamoto T, Tanaka K, Tenjo T, Tanigawa N. Cancer. 2001;91(11):2026–2032. doi: 10.1002/1097-0142(20010601)91:11<2026::aid-cncr1228>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 25.Kelly RJ, Lopez-Chavez A, Citrin D, Janik JE, Morris JC. Mol Cancer. 2011;10:35. doi: 10.1186/1476-4598-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwasa T, Okamoto I, Suzuki M, Nakahara T, Yamanaka K, Hatashita E, Yamada Y, Fukuoka M, Ono K, Nakagawa K. Clin Cancer Res. 2008;14(20):6496–6504. doi: 10.1158/1078-0432.CCR-08-0468. [DOI] [PubMed] [Google Scholar]
- 27.Nakahara T, Kita A, Yamanaka K, Mori M, Amino N, Takeuchi M, Tominaga F, Hatakeyama S, Kinoyama I, Matsuhisa A, Kudoh M, Sasamata M. Cancer Res. 2007;67(17):8014–8021. doi: 10.1158/0008-5472.CAN-07-1343. [DOI] [PubMed] [Google Scholar]
- 28.Altomare DA, Testa JR. Oncogene. 2005;24(50):7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 29.Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. Cancer Treat Rev. 2004;30(2):193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Nat Rev Drug Discov. 2005;4(12):988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 31.Manning BD, Cantley LC. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheng S, Qiao M, Pardee AB. J Cell Physiol. 2009;218(3):451–454. doi: 10.1002/jcp.21616. [DOI] [PubMed] [Google Scholar]
- 33.Bader AG, Kang S, Zhao L, Vogt PK. Nat Rev Cancer. 2005;5(12):921–929. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- 34.Engelman JA, Luo J, Cantley LC. Nat Rev Genet. 2006;7(8):606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 35.Vivanco I, Sawyers CL. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 36.Yang HW, Shin MG, Lee S, Kim JR, Park WS, Cho KH, Meyer T, Do Heo W. Mol Cell. 2012;47(2):281–290. doi: 10.1016/j.molcel.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiner OD, Neilsen PO, Prestwich GD, Kirschner MW, Cantley LC, Bourne HR. Nat Cell Biol. 2002;4(7):509–513. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vivanco I, Rohle D, Versele M, Iwanami A, Kuga D, Oldrini B, Tanaka K, Dang J, Kubek S, Palaskas N, Hsueh T, Evans M, Mulholland D, Wolle D, Rajasekaran S, Rajasekaran A, Liau LM, Cloughesy TF, Dikic I, Brennan C, Wu H, Mischel PS, Perera T, Mellinghoff IK. Proc Natl Acad Sci U S A. 2010;107(14):6459–6464. doi: 10.1073/pnas.0911188107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Georgescu MM. Genes Cancer. 2010;1(12):1170–1177. doi: 10.1177/1947601911407325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wan X, Helman LJ. Oncogene. 2003;22(50):8205–8211. doi: 10.1038/sj.onc.1206878. [DOI] [PubMed] [Google Scholar]
- 41.Gao T, Furnari F, Newton AC. Mol Cell. 2005;18(1):13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Nitsche C, Edderkaoui M, Moore RM, Eibl G, Kasahara N, Treger J, Grippo PJ, Mayerle J, Lerch MM, Gukovskaya AS. Gastroenterology. 2012;142(2):377–387. e371–375. doi: 10.1053/j.gastro.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Weiss HL, Rychahou P, Jackson LN, Evers BM, Gao T. Oncogene. 2009;28(7):994–1004. doi: 10.1038/onc.2008.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuo YC, Huang KY, Yang CH, Yang YS, Lee WY, Chiang CW. J Biol Chem. 2008;283(4):1882–1892. doi: 10.1074/jbc.M709585200. [DOI] [PubMed] [Google Scholar]
- 45.Suganuma M, Fujiki H, Suguri H, Yoshizawa S, Hirota M, Nakayasu M, Ojika M, Wakamatsu K, Yamada K, Sugimura T. Proc Natl Acad Sci U S A. 1988;85(6):1768–1771. doi: 10.1073/pnas.85.6.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li W, Chen Z, Zong Y, Gong F, Zhu Y, Lv J, Zhang J, Xie L, Sun Y, Miao Y, Tao M, Han X, Xu Z. Cancer Lett. 304(2):117–127. doi: 10.1016/j.canlet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Chowdhury S, Howell GM, Rajput A, Teggart CA, Brattain LE, Weber HR, Chowdhury A, Brattain MG. PLoS One. 2011;6(5):e19335. doi: 10.1371/journal.pone.0019335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu P, Cheng H, Roberts TM, Zhao JJ. Nat Rev Drug Discov. 2009;8(8):627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cantley LC. Science. 2002;296(5573):1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 50.Chin YR, Toker A. Cell Signal. 2009;21(4):470–476. doi: 10.1016/j.cellsig.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toker A, Yoeli-Lerner M. Cancer Res. 2006;66(8):3963–3966. doi: 10.1158/0008-5472.CAN-06-0743. [DOI] [PubMed] [Google Scholar]
- 52.Brodbeck D, Cron P, Hemmings BA. J Biol Chem. 1999;274(14):9133–9136. doi: 10.1074/jbc.274.14.9133. [DOI] [PubMed] [Google Scholar]
- 53.Nakatani K, Thompson DA, Barthel A, Sakaue H, Liu W, Weigel RJ, Roth RA. J Biol Chem. 1999;274(31):21528–21532. doi: 10.1074/jbc.274.31.21528. [DOI] [PubMed] [Google Scholar]
- 54.Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA. J Biol Chem. 1997;272(50):31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 55.Borgatti P, Martelli AM, Tabellini G, Bellacosa A, Capitani S, Neri LM. J Cell Physiol. 2003;196(1):79–88. doi: 10.1002/jcp.10279. [DOI] [PubMed] [Google Scholar]
- 56.Calera MR, Martinez C, Liu H, Jack AK, Birnbaum MJ, Pilch PF. J Biol Chem. 1998;273(13):7201–7204. doi: 10.1074/jbc.273.13.7201. [DOI] [PubMed] [Google Scholar]
- 57.Kupriyanova TA, Kandror KV. J Biol Chem. 1999;274(3):1458–1464. doi: 10.1074/jbc.274.3.1458. [DOI] [PubMed] [Google Scholar]
- 58.Yang ZZ, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, Hemmings BA. J Biol Chem. 2003;278(34):32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- 59.Santi SA, Lee H. Am J Physiol Cell Physiol. 298(3):C580–591. doi: 10.1152/ajpcell.00375.2009. [DOI] [PubMed] [Google Scholar]
- 60.Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, Hay N. Genes Dev. 2003;17(11):1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okano J, Gaslightwala I, Birnbaum MJ, Rustgi AK, Nakagawa H. J Biol Chem. 2000;275(40):30934–30942. doi: 10.1074/jbc.M004112200. [DOI] [PubMed] [Google Scholar]
- 62.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Science. 2001;292(5522):1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 63.Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM, Szabolcs M, de Jong R, Oltersdorf T, Ludwig T, Efstratiadis A, Birnbaum MJ. Mol Cell Biol. 2005;25(5):1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dummler B, Hemmings BA. Biochem Soc Trans. 2007;35(Pt 2):231–235. doi: 10.1042/BST0350231. [DOI] [PubMed] [Google Scholar]
- 65.Zhou W, Fu XQ, Liu J, Yu HG. Regul Pept. 2012;176(1–3):13–21. doi: 10.1016/j.regpep.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 66.Chau NM, Ashcroft M. Breast Cancer Res. 2004;6(1):55–57. doi: 10.1186/bcr739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bacus SS, Altomare DA, Lyass L, Chin DM, Farrell MP, Gurova K, Gudkov A, Testa JR. Oncogene. 2002;21(22):3532–3540. doi: 10.1038/sj.onc.1205438. [DOI] [PubMed] [Google Scholar]
- 68.Heron-Milhavet L, Khouya N, Fernandez A, Lamb NJ. Histol Histopathol. 2011;26(5):651–662. doi: 10.14670/HH-26.651. [DOI] [PubMed] [Google Scholar]
- 69.Roy HK, Olusola BF, Clemens DL, Karolski WJ, Ratashak A, Lynch HT, Smyrk TC. Carcinogenesis. 2002;23(1):201–205. doi: 10.1093/carcin/23.1.201. [DOI] [PubMed] [Google Scholar]
- 70.Chung DC. Gastroenterology. 2000;119(3):854–865. doi: 10.1053/gast.2000.16507. [DOI] [PubMed] [Google Scholar]
- 71.Lynch HT, Smyrk TC. N Engl J Med. 1998;338(21):1537–1538. doi: 10.1056/NEJM199805213382109. [DOI] [PubMed] [Google Scholar]
- 72.Yoeli-Lerner M, Toker A. Cell Cycle. 2006;5(6):603–605. doi: 10.4161/cc.5.6.2561. [DOI] [PubMed] [Google Scholar]
- 73.Yoeli-Lerner M, Yiu GK, Rabinovitz I, Erhardt P, Jauliac S, Toker A. Mol Cell. 2005;20(4):539–550. doi: 10.1016/j.molcel.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 74.Feng J, Tamaskovic R, Yang Z, Brazil DP, Merlo A, Hess D, Hemmings BA. J Biol Chem. 2004;279(34):35510–35517. doi: 10.1074/jbc.M404936200. [DOI] [PubMed] [Google Scholar]
- 75.Arboleda MJ, Lyons JF, Kabbinavar FF, Bray MR, Snow BE, Ayala R, Danino M, Karlan BY, Slamon DJ. Cancer Res. 2003;63(1):196–206. [PubMed] [Google Scholar]
- 76.Coffer PJ, Woodgett JR. Eur J Biochem. 1992;205(3):1217. [PubMed] [Google Scholar]
- 77.Morini M, Mottolese M, Ferrari N, Ghiorzo F, Buglioni S, Mortarini R, Noonan DM, Natali PG, Albini A. Int J Cancer. 2000;87(3):336–342. [PubMed] [Google Scholar]
- 78.Rychahou PG, Kang J, Gulhati P, Doan HQ, Chen LA, Xiao SY, Chung DH, Evers BM. Proc Natl Acad Sci U S A. 2008;105(51):20315–20320. doi: 10.1073/pnas.0810715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuan ZQ, Sun M, Feldman RI, Wang G, Ma X, Jiang C, Coppola D, Nicosia SV, Cheng JQ. Oncogene. 2000;19(19):2324–2330. doi: 10.1038/sj.onc.1203598. [DOI] [PubMed] [Google Scholar]
- 80.Ericson K, Gan C, Cheong I, Rago C, Samuels Y, Velculescu VE, Kinzler KW, Huso DL, Vogelstein B, Papadopoulos N. Proc Natl Acad Sci U S A. 2010;107(6):2598–2603. doi: 10.1073/pnas.0914018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee MW, Kim DS, Lee JH, Lee BS, Lee SH, Jung HL, Sung KW, Kim HT, Yoo KH, Koo HH. Cancer Sci. 2011;102(10):1822–1828. doi: 10.1111/j.1349-7006.2011.02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang S, Basson MD. Am J Physiol Cell Physiol. 2011;300(3):C657–670. doi: 10.1152/ajpcell.00377.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berwick DC, Hers I, Heesom KJ, Moule SK, Tavare JM. J Biol Chem. 2002;277(37):33895–33900. doi: 10.1074/jbc.M204681200. [DOI] [PubMed] [Google Scholar]
- 84.Thamilselvan V, Craig DH, Basson MD. FASEB J. 2007;21(8):1730–1741. doi: 10.1096/fj.06-6545com. [DOI] [PubMed] [Google Scholar]
- 85.Itoh N, Semba S, Ito M, Takeda H, Kawata S, Yamakawa M. Cancer. 2002;94(12):3127–3134. doi: 10.1002/cncr.10591. [DOI] [PubMed] [Google Scholar]
- 86.Khaleghpour K, Li Y, Banville D, Yu Z, Shen SH. Carcinogenesis. 2004;25(2):241–248. doi: 10.1093/carcin/bgg195. [DOI] [PubMed] [Google Scholar]
- 87.Guertin DA, Sabatini DM. Cancer Cell. 2007;12(1):9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 88.Gulhati P, Cai Q, Li J, Liu J, Rychahou PG, Qiu S, Lee EY, Silva SR, Bowen KA, Gao T, Evers BM. Clin Cancer Res. 2009;15(23):7207–7216. doi: 10.1158/1078-0432.CCR-09-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johnson SM, Gulhati P, Rampy BA, Han Y, Rychahou PG, Doan HQ, Weiss HL, Evers BM. J Am Coll Surg. 210(5):767–776. 776–768. doi: 10.1016/j.jamcollsurg.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yuan ZQ, Feldman RI, Sussman GE, Coppola D, Nicosia SV, Cheng JQ. J Biol Chem. 2003;278(26):23432–23440. doi: 10.1074/jbc.M302674200. [DOI] [PubMed] [Google Scholar]
- 91.Jung HH, Park YH, Jun HJ, Kong J, Kim JH, Kim JA, Yun J, Sun JM, Won YW, Lee S, Kim ST, Ahn JS, Im YH. Mol Cancer Res. 8(7):1037–1047. doi: 10.1158/1541-7786.MCR-09-0469. [DOI] [PubMed] [Google Scholar]
- 92.Newby JC, Johnston SR, Smith IE, Dowsett M. Clin Cancer Res. 1997;3(9):1643–1651. [PubMed] [Google Scholar]
- 93.Zhou BP, Hu MC, Miller SA, Yu Z, Xia W, Lin SY, Hung MC. J Biol Chem. 2000;275(11):8027–8031. doi: 10.1074/jbc.275.11.8027. [DOI] [PubMed] [Google Scholar]
- 94.Lindberg K, Helguero LA, Omoto Y, Gustafsson JA, Haldosen LA. Breast Cancer Res. 2011;13(2):R43. doi: 10.1186/bcr2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chung S, Yao J, Suyama K, Bajaj S, Qian X, Loudig OD, Eugenin EA, Phillips GR, Hazan RB. Oncogene. 2012 doi: 10.1038/onc.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cristiano BE, Chan JC, Hannan KM, Lundie NA, Marmy-Conus NJ, Campbell IG, Phillips WA, Robbie M, Hannan RD, Pearson RB. Cancer Res. 2006;66(24):11718–11725. doi: 10.1158/0008-5472.CAN-06-1968. [DOI] [PubMed] [Google Scholar]
- 97.Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ. Science. 1999;286(5445):1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- 98.Romashkova JA, Makarov SS. Nature. 1999;401(6748):86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 99.Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, Hostetter G, Boguslawski S, Moses TY, Savage S, Uhlik M, Lin A, Du J, Qian YW, Zeckner DJ, Tucker-Kellogg G, Touchman J, Patel K, Mousses S, Bittner M, Schevitz R, Lai MH, Blanchard KL, Thomas JE. Nature. 2007;448(7152):439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 100.Sung JS, Park KH, Kim ST, Kim YH. Genomics Inform. 2012;10(3):167–174. doi: 10.5808/GI.2012.10.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim JH, Kim H, Lee KY, Choe KH, Ryu JS, Yoon HI, Sung SW, Yoo KY, Hong YC. Hum Mol Genet. 2006;15(7):1181–1186. doi: 10.1093/hmg/ddl033. [DOI] [PubMed] [Google Scholar]
- 102.Sung JS, Han SG, Whang YM, Shin ES, Lee JW, Lee HJ, Ryu JS, Choi IK, Park KH, Kim JS, Shin SW, Chu EK, Kim YH. Lung Cancer. 2008;61(3):301–308. doi: 10.1016/j.lungcan.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 103.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Cell. 1998;95(1):29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 104.Schlieman MG, Fahy BN, Ramsamooj R, Beckett L, Bold RJ. Br J Cancer. 2003;89(11):2110–2115. doi: 10.1038/sj.bjc.6601396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamamoto S, Tomita Y, Hoshida Y, Morooka T, Nagano H, Dono K, Umeshita K, Sakon M, Ishikawa O, Ohigashi H, Nakamori S, Monden M, Aozasa K. Clin Cancer Res. 2004;10(8):2846–2850. doi: 10.1158/1078-0432.ccr-02-1441. [DOI] [PubMed] [Google Scholar]
- 106.Ng SSW, Tsao MS, Chow S, Hedley DW. Cancer Res. 2000;60(19):5451–5455. [PubMed] [Google Scholar]
- 107.Chowdhury S, Howell GM, Teggart CA, Chowdhury A, Person JJ, Bowers DM, Brattain MG. J Biol Chem. 2011;286(35):30937–30948. doi: 10.1074/jbc.M110.212035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ammanamanchi S, Brattain MG. J Biol Chem. 2004;279(31):32620–32625. doi: 10.1074/jbc.M402691200. [DOI] [PubMed] [Google Scholar]
- 109.Ammanamanchi S, Freeman JW, Brattain MG. J Biol Chem. 2003;278(37):35775–35780. doi: 10.1074/jbc.M305961200. [DOI] [PubMed] [Google Scholar]
- 110.Huang W, Zhao S, Ammanamanchi S, Brattain M, Venkatasubbarao K, Freeman JW. J Biol Chem. 2005;280(11):10047–10054. doi: 10.1074/jbc.M408680200. [DOI] [PubMed] [Google Scholar]
- 111.Chowdhury S, Ammanamanchi S, Howell GM. Mol Cell Pharmacol. 2009;1(1):57–70. doi: 10.4255/mcpharmacol.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Buenemann CL, Willy C, Buchmann A, Schmiechen A, Schwarz M. Carcinogenesis. 2001;22(3):447–452. doi: 10.1093/carcin/22.3.447. [DOI] [PubMed] [Google Scholar]
- 113.Chen RH, Su YH, Chuang RL, Chang TY. Oncogene. 1998;17(15):1959–1968. doi: 10.1038/sj.onc.1202111. [DOI] [PubMed] [Google Scholar]
- 114.Tanaka S, Wands JR. Cancer Res. 1996;56(15):3391–3394. [PubMed] [Google Scholar]
- 115.Kretschmer A, Moepert K, Dames S, Sternberger M, Kaufmann J, Klippel A. Oncogene. 2003;22(43):6748–6763. doi: 10.1038/sj.onc.1206791. [DOI] [PubMed] [Google Scholar]
- 116.Conery AR, Cao Y, Thompson EA, Townsend CM, Jr, Ko TC, Luo K. Nat Cell Biol. 2004;6(4):366–372. doi: 10.1038/ncb1117. [DOI] [PubMed] [Google Scholar]
- 117.Remy I, Montmarquette A, Michnick SW. Nat Cell Biol. 2004;6(4):358–365. doi: 10.1038/ncb1113. [DOI] [PubMed] [Google Scholar]
- 118.Wang J, Yang L, Yang J, Kuropatwinski K, Wang W, Liu XQ, Hauser J, Brattain MG. Cancer Res. 2008;68(9):3152–3160. doi: 10.1158/0008-5472.CAN-07-5348. [DOI] [PubMed] [Google Scholar]
- 119.Ongchin M, Sharratt E, Dominguez I, Simms N, Wang J, Cheney R, LeVea C, Brattain M, Rajput A. J Surg Res. 2009;156(2):250–256. doi: 10.1016/j.jss.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 120.Dohi T, Xia F, Altieri DC. Mol Cell. 2007;27(1):17–28. doi: 10.1016/j.molcel.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Simms N, Rajput A, Sharratt EA, Ongchin M, Teggart CA, Wang J, Brattain MG. BMC Cancer. 2012;12(1):221. doi: 10.1186/1471-2407-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cheng Y, Zhang Y, Zhang L, Ren X, Huber-Keener KJ, Liu X, Zhou L, Liao J, Keihack H, Yan L, Rubin E, Yang JM. Mol Cancer Ther. 2012;11(1):154–164. doi: 10.1158/1535-7163.MCT-11-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sangai T, Akcakanat A, Chen H, Tarco E, Wu Y, Do KA, Miller TW, Arteaga CL, Mills GB, Gonzalez-Angulo AM, Meric-Bernstam F. Clin Cancer Res. 2012;18(20):5816–5828. doi: 10.1158/1078-0432.CCR-12-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gorlick R, Maris JM, Houghton PJ, Lock R, Carol H, Kurmasheva RT, Kolb EA, Keir ST, Reynolds CP, Kang MH, Billups CA, Smith MA. Pediatr Blood Cancer. 2011;59(3):518–524. doi: 10.1002/pbc.23412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.de Frias M, Iglesias-Serret D, Cosialls AM, Coll-Mulet L, Santidrian AF, Gonzalez-Girones DM, de la Banda E, Pons G, Gil J. Haematologica. 2009;94(12):1698–1707. doi: 10.3324/haematol.2008.004028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pinton G, Manente AG, Angeli G, Mutti L, Moro L. PLoS One. 2012;7(5):e36856. doi: 10.1371/journal.pone.0036856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Floryk D, Thompson TC. Cancer Lett. 2008;266(2):216–226. doi: 10.1016/j.canlet.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ma Z, Gibson SL, Byrne MA, Zhang J, White MF, Shaw LM. Mol Cell Biol. 2006;26(24):9338–9351. doi: 10.1128/MCB.01032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Richardson PG, Wolf J, Jakubowiak A, Zonder J, Lonial S, Irwin D, Densmore J, Krishnan A, Raje N, Bar M, Martin T, Schlossman R, Ghobrial IM, Munshi N, Laubach J, Allerton J, Hideshima T, Colson K, Poradosu E, Gardner L, Sportelli P, Anderson KC. J Clin Oncol. 2011;29(32):4243–4249. doi: 10.1200/JCO.2010.33.9788. [DOI] [PubMed] [Google Scholar]
- 130.Yang X, Fraser M, Moll UM, Basak A, Tsang BK. Cancer Res. 2006;66(6):3126–3136. doi: 10.1158/0008-5472.CAN-05-0425. [DOI] [PubMed] [Google Scholar]
- 131.Chen MB, Wu XY, Tao GQ, Liu CY, Chen J, Wang LQ, Lu PH. Int J Cancer. 2012;131(11):2487–2498. doi: 10.1002/ijc.27548. [DOI] [PubMed] [Google Scholar]
- 132.Chiarini F, Del Sole M, Mongiorgi S, Gaboardi GC, Cappellini A, Mantovani I, Follo MY, McCubrey JA, Martelli AM. Leukemia. 2008;22(6):1106–1116. doi: 10.1038/leu.2008.79. [DOI] [PubMed] [Google Scholar]
- 133.Nair AS, Shishodia S, Ahn KS, Kunnumakkara AB, Sethi G, Aggarwal BB. J Immunol. 2006;177(8):5612–5622. doi: 10.4049/jimmunol.177.8.5612. [DOI] [PubMed] [Google Scholar]
- 134.Kim WY, Chang DJ, Hennessy B, Kang HJ, Yoo J, Han SH, Kim YS, Park HJ, Seo SY, Mills G, Kim KW, Hong WK, Suh YG, Lee HY. Cancer Prev Res (Phila) 2008;1(7):577–587. doi: 10.1158/1940-6207.CAPR-08-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chun KH, Kosmeder JW, 2nd, Sun S, Pezzuto JM, Lotan R, Hong WK, Lee HY. J Natl Cancer Inst. 2003;95(4):291–302. doi: 10.1093/jnci/95.4.291. [DOI] [PubMed] [Google Scholar]
- 136.Li Z, Wu J, Wu C, Jiang J, Zheng X, Xu B, Li M. Oncol Lett. 2012;4(4):677–681. doi: 10.3892/ol.2012.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gilot D, Giudicelli F, Lagadic-Gossmann D, Fardel O. Chem Biol Interact. 2010;188(3):546–552. doi: 10.1016/j.cbi.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 138.Han EK, Leverson JD, McGonigal T, Shah OJ, Woods KW, Hunter T, Giranda VL, Luo Y. Oncogene. 2007;26(38):5655–5661. doi: 10.1038/sj.onc.1210343. [DOI] [PubMed] [Google Scholar]
- 139.Levy DS, Kahana JA, Kumar R. Blood. 2009;113(8):1723–1729. doi: 10.1182/blood-2008-02-137737. [DOI] [PubMed] [Google Scholar]
- 140.Altomare DA, Zhang L, Deng J, Di Cristofano A, Klein-Szanto AJ, Kumar R, Testa JR. Clin Cancer Res. 16(2):486–496. doi: 10.1158/1078-0432.CCR-09-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rhodes N, Heerding DA, Duckett DR, Eberwein DJ, Knick VB, Lansing TJ, McConnell RT, Gilmer TM, Zhang SY, Robell K, Kahana JA, Geske RS, Kleymenova EV, Choudhry AE, Lai Z, Leber JD, Minthorn EA, Strum SL, Wood ER, Huang PS, Copeland RA, Kumar R. Cancer Res. 2008;68(7):2366–2374. doi: 10.1158/0008-5472.CAN-07-5783. [DOI] [PubMed] [Google Scholar]
- 142.Crouthamel MC, Kahana JA, Korenchuk S, Zhang SY, Sundaresan G, Eberwein DJ, Brown KK, Kumar R. Clin Cancer Res. 2009;15(1):217–225. doi: 10.1158/1078-0432.CCR-08-1253. [DOI] [PubMed] [Google Scholar]
- 143.Jeong EH, Choi HS, Lee TG, Kim HR, Kim CH. Tuberc Respir Dis (Seoul) 2012;72(4):343–351. doi: 10.4046/trd.2012.72.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mahalingam D, Swords R, Carew JS, Nawrocki ST, Bhalla K, Giles FJ. Br J Cancer. 2009;100(10):1523–1529. doi: 10.1038/sj.bjc.6605066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu W, Marcu M, Yuan X, Mimnaugh E, Patterson C, Neckers L. Proc Natl Acad Sci U S A. 2002;99(20):12847–12852. doi: 10.1073/pnas.202365899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Flandrin P, Guyotat D, Duval A, Cornillon J, Tavernier E, Nadal N, Campos L. Cell Stress Chaperones. 2008;13(3):357–364. doi: 10.1007/s12192-008-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sato S, Fujita N, Tsuruo T. Proc Natl Acad Sci U S A. 2000;97(20):10832–10837. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Neckers L. Trends Mol Med. 2002;8(4 Suppl):S55–61. doi: 10.1016/s1471-4914(02)02316-x. [DOI] [PubMed] [Google Scholar]
- 149.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Proc Natl Acad Sci U S A. 1994;91(18):8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim S, Kang J, Hu W, Evers BM, Chung DH. Int J Cancer. 2003;103(3):352–359. doi: 10.1002/ijc.10820. [DOI] [PubMed] [Google Scholar]
- 151.Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Kung AL, Davies FE, Morgan G, Akiyama M, Shringarpure R, Munshi NC, Richardson PG, Hideshima T, Chauhan D, Gu X, Bailey C, Joseph M, Libermann TA, Rosen NS, Anderson KC. Blood. 2006;107(3):1092–1100. doi: 10.1182/blood-2005-03-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Modi S, Stopeck AT, Gordon MS, Mendelson D, Solit DB, Bagatell R, Ma W, Wheler J, Rosen N, Norton L, Cropp GF, Johnson RG, Hannah AL, Hudis CA. J Clin Oncol. 2007;25(34):5410–5417. doi: 10.1200/JCO.2007.11.7960. [DOI] [PubMed] [Google Scholar]