Abstract
Objective
To determine if, in children, plasma glial fibrillary acidic protein (GFAP) is associated with brain injury during extracorporeal membrane oxygenation (ECMO) and with mortality.
Design
Prospective, observational study.
Setting
Pediatric intensive care unit in an urban tertiary care academic center.
Patients
Neonatal and pediatric ECMO patients (n=22).
Interventions
Serial blood sampling for GFAP measurements.
Measurements and Main Results
Prospective patients age 1 day-18 years who required ECMO from April 2008 to August 2009 were studied. GFAP was measured using an electrochemiluminescent immunoassay developed at Johns Hopkins. Control samples were collected from 99 healthy children (0.5-16 years) and 59 NICU infants without neurologic injury. In controls, the median GFAP concentration was 0.055 ng/mL (IQR: 0-0.092 ng/mL) and the 95th percentile of GFAP was 0.436ng/mL. In ECMO patients, plasma GFAP was measured at 6, 12 and every 24 hours after cannulation. We enrolled 22 children who underwent ECMO. Median age was 7 days (IQR, 2 days-9 years), and primary ECMO indication was: cardiac failure, 6/22 (27.3%), respiratory failure, 12/22 (54.5%), ECPR, 3/22 (13.6%), and sepsis, 1/22 (4.6%). Seven of 22 (32%) patients developed acute neurologic injury (intracranial hemorrhage, brain death or cerebral edema diagnosed by imaging). Fifteen of 22 (68%) survived to hospital discharge. In the ECMO group, peak GFAP levels were higher in children with brain injury than those without (median, 5.9 vs 0.09ng/mL, p=0.04) and in non-survivors compared to survivors to discharge (median, 5.9 vs 0.09ng/mL, p=0.04). The odds ratio (OR) for brain injury for GFAP >0.436ng/mL vs normal was 11.5 (95%CI: 1.3-98.3) and the OR for mortality was 13.6 (95%CI: 1.7-108.5).
Conclusions
High GFAP during ECMO is significantly associated with acute brain injury and death. Brain injury biomarkers may aid in outcome prediction and neurologic monitoring of ECMO patients to improve outcomes and benchmark new therapies.
Keywords: ECMO, extracorporeal membrane oxygenation, GFAP, glial fibrillary acidic protein, biochemical marker, intracranial hemorrhage
Introduction
Extracorporeal membrane oxygenation (ECMO) is used in over 1,000 children each year for severe cardiopulmonary failure (1). Survival varies by age and underlying diagnosis and ranges from 32%-42% for extracorporeal cardiopulmonary resuscitation (ECPR) (1, 2, 3) to 50%-65% in children excluding ECPR (1, 4-8) and as high as 75%-80% for selected neonatal populations (1, 4, 9). ECMO is associated with high risk for neurologic injury, including intracranial hemorrhage, brain infarction and brain death (1, 10, 11). Major causes for neurologic injury are thought to be related to disturbances of cerebral blood flow (CBF) at the time of right neck vessel cannulation (12, 13), altered cerebral autoregulation during the recovery phase from hypoxia (14) and during venoarterial (VA) ECMO non-pulsatile flow (15), profound hypoxia and shock encountered by the critically-ill patient around the time of cannulation and exposure to systemic heparinization. Poor neurologic outcomes have been reported in 10% to as many as 60% of survivors (6, 11, 16-25), and brain death is declared in 1%-11% of newborns and children who undergo ECMO for a variety of indications (1, 4, 26, 27). Several predictors of neurologic outcome post-ECMO have been proposed, including neuroimaging findings (10, 24, 28), seizure activity during the ECMO course (29), abnormal electroencephalograms during ECMO (24, 30, 31) and somatosensory evoked potentials (24). To our knowledge, there is a single prior study that examined the association between a biochemical marker of brain injury, S100-B, and development of intracranial hemorrhage in infants who required ECMO after open-heart surgery (32). S100-B, though, is not specific to the brain and has been shown to be elevated in shock and after cardiopulmonary by-pass due to extracerebral sources (33-35). We hypothesized that glial fibrillary acidic protein (GFAP), a brain-specific biochemical marker, is associated with brain injury and neurologic and survival outcomes of children post-ECMO.
GFAP is a class III intermediate filament protein that is specific to astrocytes and is upregulated in reactive gliosis after central nervous system injury (36). As a blood biomarker, GFAP was first evaluated to diagnose acute stroke in adults (37, 38), and it has since been applied to other clinical scenarios that carry a high risk for brain injury. Alone or in combination with other brain injury biomarkers, GFAP has been shown to be a predictor for neurologic injury and outcomes after acute stroke (37-39), cardiac arrest (40, 41) and traumatic brain injury (42-46).
As the quality of neurologic exams is limited during pediatric ECMO by sedation, and serial brain imaging is only practical for those children who are candidates for head ultrasound, a simple, fast, blood biomarker of brain injury would be immediately useful to clinicians for monitoring neurologic status in children on ECMO. In this study, we evaluated the association of plasma GFAP levels with acute neurologic injury, neurologic outcome, and survival in pediatric patients undergoing ECMO.
Methods
Study Design
This is a prospective observational cohort study of children who underwent ECMO in a 26-bed Pediatric Intensive Care Unit (PICU) of a single tertiary care, academic pediatric center, from April 2008 to August 2009. Patients <18 years who required ECMO for any indication were eligible for this study. This cohort was initiated for the study of coagulation-related risk factors for neurologic injury during ECMO, with a secondary aim to investigate brain injury biomarkers in this population. Exclusion criteria were: history of heparin induced thrombocytopenia and use of direct thrombin inhibitors for anticoagulation during ECMO. Parents or legal guardians were approached for consent after patient stabilization, within the first 6 hours after ECMO cannulation, only when present in the PICU. No consent was conducted over the phone. Demographic, clinical, laboratory, imaging and survival data were collected for each enrolled subject. The ECMO circuit consisted of: custom-packed 1/4 or 3/8-inch flexible polyvinylchloride tubing (Medtronic, Minneapolis, MN) with a silicone reservoir, a bladderbox (Johns Hopkins Hospital, Baltimore, MD), a 0.8 m2 – 4.5 m2 membrane oxygenator (Medtronic, Minneapolis, MN), a heat exchanger (Medtronic, Minneapolis, MN) and a roller pump (Sorin Cardiovascular U.S.A., Arvada, CO). This study was approved by the Johns Hopkins Institutional Review Board.
Biomarker sampling and analysis
Venous blood samples (5mL in sodium citrate 3.2%) were collected at 6, 12, and 24 hours after initiation of ECMO and then daily until ECMO discontinuation. After separation by centrifugation within one hour, platelet-poor plasma was stored at −80°C. Fifty microliters were used for GFAP measurements in undiluted duplicate plasma samples using an electrochemiluminescent sandwich immunoassay developed on the MesoScale Discovery platform (MesoScale Discovery, Gaithersburg, MD) at Johns Hopkins University and based on the assay of Petzold et al (47). The monoclonal anti-GFAP blend SMI-26 (Covance, Princeton, NJ) was used at 100 ng per well as capture antibody in standard bind plates (MesoScale Discovery) coated either by the manufacturer or in our laboratory with overnight incubation in phosphate buffered saline (PBS). Polyclonal anti-GFAP (Dako, Carpinteria, CA) that was directly conjugated with Sulfo-Tag (MesoScale Discovery) was used for detection at 1 μg/mL in PBS. Plates were read with a Sector Imager 2400 (MesoScale Discovery). Standard curves were constructed with purified GFAP (Calbiochem, La Jolla, CA) in 1% bovine serum albumin (BSA) (SeraCare Life Sciences, Milford, MA). After experiments to determine the optimal antibody concentrations, plate type, and blocking material, the final assay for GFAP values had a standard curve with a linear range of quantification from 0.040-40.0 ng/mL. Our GFAP assay had a lower limit of detection of 0.011 ng/mL as defined by two standard deviations above the background of blank wells (n= 19 experiments). Values <0.040 ng/mL were reported as 0. The signal to noise ratio was 1.17 at 0.01 ng/mL (n= 19 experiments). The lower limit of quantification, defined as the lowest dilution with a calculated concentration ±20% of a known concentration, was 0.040 ng/mL (n= 3 experiments). Inter-assay precision was 2.4% at 10 ng/mL and 3.4% at 0.156 ng/mL (n= 21 experiments). Plasma spiked with GFAP shows 49.8%±22.9% recovery at 10 ng/mL, when compared to a standard curve generated in BSA. Validation of the GFAP assay was conducted using discarded diagnostic specimens from normal and positive controls. This GFAP assay validation study was approved in a separate application by the Johns Hopkins Institutional Review Board with a waiver of consent.
Outcome Measures
The primary independent variable was plasma GFAP elevation above the 95th percentile of normal values in children. The primary outcome was development of acute neurologic injury during ECMO, defined as intracranial hemorrhage, brain infarction or cerebral edema diagnosed by brain imaging and/or neurologic examination by a pediatric neurologist consistent with brain death, while the patient was on ECMO support. It is our institutional protocol to obtain daily head ultrasounds for newborns and infants with open fontanelles; older children had brain computed tomography or magnetic resonance imaging (MRI) studies obtained at the discretion of their physicians. All imaging studies were reviewed by pediatric radiologists as part of routine clinical care. The secondary outcomes were neurologic outcome and survival to discharge from hospital. Neurologic outcome was measured using the pediatric cerebral performance category (PCPC) (48, 49). The PCPC is a six-point scale developed from the Glasgow Outcome Scale to assess changes in cognitive abilities in pediatric intensive care. The six PCPC categories are 1: normal, age-appropriate neurodevelopmental functioning; 2: mild cerebral disability, 3: moderate cerebral disability; 4: severe cerebral disability; 5: coma or vegetative state and 6: brain death (48, 49). A trained pediatric critical care physician assigned PCPC retrospectively by conducting a chart review of the patient condition at admission to the hospital and at discharge from hospital. Good neurologic outcome was defined a priori as PCPC of 1 or 2 at discharge from hospital, or no change from PCPC at hospital admission.
Statistical Analysis
Exploratory descriptive data analysis was conducted to examine patient and ECMO course characteristics, to describe the distribution of GFAP values among subjects and to determine the proportion of subjects in whom GFAP levels were above the 95th percentile. The Kruskal-Wallis test was used to compare GFAP concentrations across age categories in normal controls.
Patients were divided into two categories for each outcome: those with and without acute neurologic injury, good versus poor neurologic outcomes at hospital discharge, and survivors versus non-survivors at hospital discharge. The Mann-Whitney U test was used to compare the median peak GFAP between these groups. Fisher’s exact test was used to compare percent of cases above and below 95th percentile of peak GFAP between the groups.
Logistic regression with clustering by patient was used to estimate odds of brain injury and death using all serial GFAP data points. For the clustered analysis, subjects were coded ashaving no acute neurologic injury until brain injury by imaging or a first neurologic exam consistent with brain death was observed. Subsequent observations were coded as having acute neurologic injury. The odds ratio (OR) and 95% confidence intervals (CIs) are provided. A p-value of 0.05 was considered significant. Statistical analysis was conducted using STATA 10.0 (StataCorp, College Station, TX, 2007).
Results
Patient Characteristics
Twenty two of 46 eligible patients were enrolled; 18 patients’ parents were not present in the PICU or we were not able to conduct a full consent discussion during the 6-hour consent window, 5 parents declined consent and one patient had no samples available for testing. Demographic and clinical characteristics of the 22 evaluated patients are presented in Table 1.
Table 1.
Patient and ECMO Course Characteristics (n=22)
| Patient Characteristics | ECMO Course Characteristics | ||
|---|---|---|---|
| Age, median (range) | 10d (2d-16y) | Primary indication for ECMO, n(%) | |
| Male, n(%) | 12 (54.5) | Cardiac failure | 6 (27.3) |
| Race, n(%) | Respiratory failure | 12 (54.5) | |
| African American | 12 (54.5) | Cardiac arrest/ECPR | 3 (13.6) |
| Caucasian | 7 (31.8) | Sepsis | 1 (4.6) |
| Other | 3 (13.6) | Mode of extracorporeal support, n(%)a | |
| Hispanic, n(%) | 2 (9.1) | VA-ECMO | 17 (77.3) |
| Weight (kg), median (IQR) | 3.8 (3.1, 6.9) | VV-ECMO | 3 (13.6) |
| Illness category, n(%) | VV to VA-ECMO | 2 (9.1) | |
| Medical cardiac | 5 (22.7) | Complications on ECMO, n(%) | |
| Medical noncardiac | 14 (63.6) | Acute neurologic injury | 7 (31.8) |
| Surgical cardiac | 1 (4.6) | Intracranial hemorrhage | 5 (22.7) |
| Surgical noncardiac | 2 (9.1) | Severe cerebral edema | 1 (4.6) |
| PICU length of stay, median (IQR) | 15d (8d-23d) | Brain death | 1 (4.6) |
| Hospital length of stay, median (IQR) | 30d (19d-53d) | Pulmonary hemorrhage | 2 (9.1) |
| Mortality prior to PICU discharge, n(%) | 7 (31.8) | Renal replacement therapy, n(%) | 14 (63.6) |
| ECMO to ANI diagnosis, median (IQR) | 82h (69h-104h) | Duration of ECMO support, median (IQR) | 114h (86h-12d) |
ECMO: extracorporeal membrane oxygenation; IQR: interquartile range; VA: veno-arterial; VV: veno-venous; PICU: pediatric intensive care unit; ANI: acute neurologic injury; d: days; h: hours; y: years.
All ECMO cannulations were performed via the right jugular vein and right carotid artery for VA-ECMO and via the right jugular vein using a double lumen cannula for VV-ECMO. None of the patients enrolled in this study underwent direct intracardiac cannulation.
Individual patient characteristics and outcomes are presented in Table 2. Seven of 22 patients (31.8%) suffered an acute neurologic injury during ECMO based on our a priori definition. Using baseline and discharge PCPC, 14/22 (63.6%) patients had good neurologic outcome and 8/22 (36.4%) patients had poor neurologic outcome: one had a PCPC >2 and 7 died. Causes of death included: brain death (1 patient), large intracranial hemorrhage (2 patients), and withdrawal of mechanical support for medical futility in face of irreversible multisystem organ failure (4 patients).
Table 2.
Patient, ECMO and neurologic outcomes characteristics
| Patient | Primary Diagnosis | ECMO Indication |
ECMO Duration |
#GFAP Measure ments |
Peak GFAP |
ANI Diagnosed during ECMO | ANI Diagnostic Modality |
ECMO to ANI Diagnosis |
ECMO to GFAP >95th percentile |
Baseline PCPC |
Discharge PCPC |
Neurologic Outcome |
PICU Survival |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Liver failure, CPA post-transplant |
Cardiac disease |
4d | 5 | 5.86 | Unilateral IVH, intraparenchymal (basal ganglia, right temporal and occipital) hemorrhage,2 cm midline shift, edema |
HCT | 104h | 24h | 1 | 6 | Poor | No |
| 2 | Dilated cardiomyopathy |
Cardiac disease |
3d | 4 | 20.5 | Frontoparietal 4×5×4cm intra- parenchymal hemorrhage, 2.5cm mildline shift, unilateral SDH, SAH, IVH |
HUS | 11h | 19h | 2 | 6 | Poor | No |
| 3 | Dilated cardiomyopathy |
Cardiac disease |
5d | 3 | 0.053 | No | - | - | - | 1 | 1 | Good | Yes |
| 4 | PPHN | Respiratory disease |
6d | 4 | 0.164 | No | - | - | - | 1 | 2 | Good | Yes |
| 5 | Septic shock | Sepsis | 1d | 1 | 0a | No | - | - | - | 1 | 6 | Poor | No |
| 6 | PPHN | Respiratory disease |
5d | 8 | 0.088 | No | - | - | - | 2 | 2 | Good | Yes |
| 7 | PPHN | Respiratory disease |
4d | 5 | 0.081 | No | - | - | - | 1 | 1 | Good | Yes |
| 8 | Dilated Cardiomyopathy |
Cardiac disease |
1d | 2 | 0.162 | No | - | - | - | 1 | 1 | Good | Yes |
| 9 | PPHN | Respiratory disease |
7d | 7 | 0.062 | No | - | - | - | 1 | 2 | Good | Yes |
| 10 | Critical aortic stenosis |
Cardiac disease |
12d | 6 | 0.194 | No | - | - | - | 1 | 1 | Good | Yes |
| 11 | PPHN | Respiratory disease |
30d | 16 | 1.43 | No | - | - | 12hb | 1 | 3 | Poor | Yes |
| 12 | Diaphragmatic eventration |
Respiratory disease |
14d | 11 | 0.398 | Unilateral cerebellar hemisphere, 1.5× 2cm intraparenchymal hemorrhage |
HUS | 82h | - | 1 | 6 | Poor | No |
| 13 | TOF, Pulmonary atresia |
ECPR | 4d | 3 | 0.090 | No | - | - | - | 1 | 2 | Good | Yes |
| 14 | Status asthmaticus | ECPR | 4d | 2 | 44.9 | Rapid progression to brain death | BD exam | 64h | 12h | 1 | 6 | Poor | No |
| 15 | PPHN | Respiratory disease |
12d | 3 | 0.795 | No | - | - | 10dc | 1 | 2 | Good | Yes |
| 16 | PPHN | Respiratory disease |
15d | 11 | 0.111 | Small SDH along the falx cerebri | HUS | 10d | - | 1 | 1 | Good | Yes |
| 17 | PPHN | Respiratory disease |
4d | 3 | 0.155 | No | - | - | - | 1 | 1 | Good | Yes |
| 18 | CDH | Respiratory disease |
18d | 13 | 0.400 | No | - | - | - | 1 | 6 | Poor | No |
| 19 | Truncus arteriosus | Cardiac disease |
4d | 2 | 0a | No | - | - | - | 1 | 1 | Good | Yes |
| 20 | PPHN | Respiratory disease |
3d | 1 | 0a | Unilateral subependymal grade I IVH | HUS | 69h | - | 1 | 1 | Good | Yes |
| 21 | CDH | Respiratory disease |
17d | 8 | 0a | No | - | - | - | 1 | 1 | Good | Yes |
| 22 | Neonatal enterovirus sepsis |
ECPR | 8d | 8 | 14.6 | Severe diffuse cerebral edema | HUS | 2h | 6h | 3 | 6 | Poor | No |
ECMO: extracorporeal membrane oxygenation, ANI: acute neurologic injury, GFAP: glial fibrillary acidic protein, PCPC: Pediatric Cerebral Performance Category, PICU: pediatric intensive care unit, CPA: cardiopulmonary arrest, IVH: intraventricular hemorrhage, SDH: subdural hemorrhage, SAH: subarachnoid hemorrhage, HCT: head computed tomography, HUS: head ultrasound, PPHN: persistent pulmonary hypertension of the newborn, TOF: tetralogy of Fallot, ECPR: extracorporeal cardiopulmonary resuscitation, CDH: congenital diaphragmatic hernia.
Denotes values below the lower limit of quantification (LLQ=0.040 ng/mL)
Normal serial HUS during ECMO. Brain MRI 6 weeks post-ECMO decannulation: few old intraventricular and intraparenchymal hemorrhagic foci
Normal serial HUS during ECMO. Brain MRI 2 weeks post-ECMO decannulation: unilateral unilobar focal encephalomalacia consistent with prior ischemic event
The median duration of ECMO was 12 days (IQR: 5 days-17 days). The median number of GFAP measurements per patient was 4.5 (range: 1-16). Seventeen patients had ≥3 measurements, three patients had two measurements and two patients had only one measurement.
GFAP Results: Controls
In normal pediatric controls with no known neurologic injury, GFAP levels had a median of 0.055 ng/mL (IQR: 0-0.092 ng/mL). GFAP levels were similar across age categories (newborns: median 0.041 ng/mL [IQR: 0-0.096 ng/mL], 6 months – 4 years: median 0.046 ng/mL [IQR: 0-0.117 ng/mL], 5 years – 16 years: median 0.057 ng/mL [IQR: 0-0.088 ng/mL], p-value=0.7). A 95th percentile cutoff for normal values in children (plasma GFAP ≤0.436 ng/mL) was determined using samples from 158 infants and children: 59 newborns <4 days of life in the neonatal intensive care unit without known genetic disorders or intracranial pathology and 99 healthy children 6 months to 16 years of age who presented to the Johns Hopkins pediatric outpatient clinic for well-child visits. We further validated our assay for the detection of neurologic injury in patients with brain tumor resection (n=13), brain biopsy (n=3) and stroke (n=12). GFAP levels in positive controls samples were overall 1-55 fold higher than for normal controls.
GFAP Results: ECMO Patients
The median initial GFAP level within 12 hours after starting ECMO was 0.07 ng/mL (IQR: 0 - 0.155 ng/mL). There were 3/22 patients with abnormal GFAP concentrations in the first 24 hours after cannulation – two were patients who suffered cardiac arrest and underwent ECPR with VA cannulation of right neck vessels and one was a patient without known neurologic injury who was placed on venovenous (VV) ECMO via a double-lumen right jugular vein cannula. All other 19/22 patients had low GFAP levels in the first 24 hours after cannulation. The median GFAP level on the last ECMO day for those children with ≥3 samples was 0.07 ng/mL (IQR: 0.053 - 0.795 ng/mL; n=17). Peak GFAP concentrations were similar comparing newborns with children and infants >30 days (0.155 ng/mL vs 0.162 ng/mL, respectively, p-value=0.48).
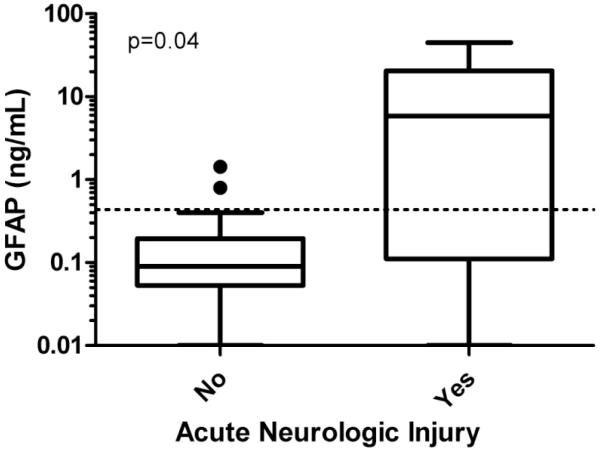
Median peak GFAP levels were significantly higher in children with acute neurologic injury diagnosed during the ECMO course than those without (5.9 vs 0.09ng/mL, p=0.04) (Figure 1), in children with poor vs good neurologic outcome (3.6 ng/mL vs 0.09 ng/mL, p<0.01), and in non-survivors compared to survivors to hospital discharge (5.9 ng/mL vs 0.09 ng/mL, p=0.04). Serial GFAP concentrations in patients with and without acute neurologic injury are displayed in Figure 2.
Figure 1.
Peak plasma GFAP concentrations in children on ECMO with and without acute neurologic injury (n=22)
GFAP: glial fibrillary acidic protein; ECMO: extracorporeal membrane oxygenation. Please note logarithmic scale on Y axis.
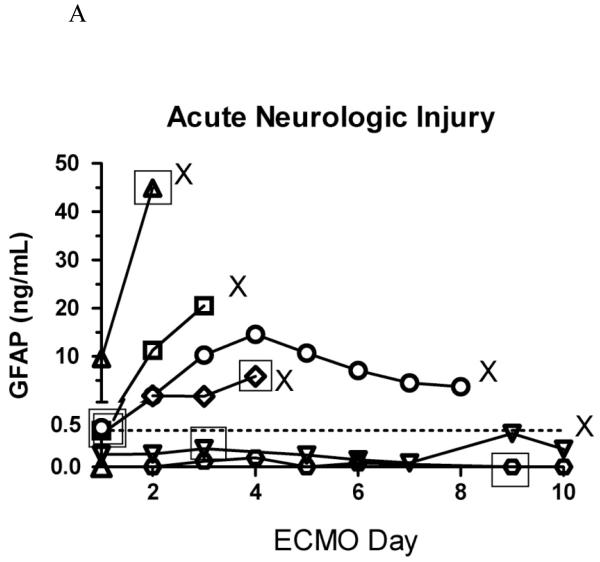
Figure 2.
A. Serial plasma GFAP concentrations in children on ECMO with acute neurologic injury (n=7).
X represents death in the pediatric intensive care unit; open squares represent the time of diagnosis of acute neurologic injury closest in time (within 24 hours) to the last GFAP measurement; dashed line marks the 95th percentile of normal controls. Note only 6 open squares – one patient had a diagnostic head ultrasound 48 hours after the only GFAP measurement (GFAP measurement 24 hours prior to diagnosis is missing). Please note different scales in Figures 2A. and 2B.
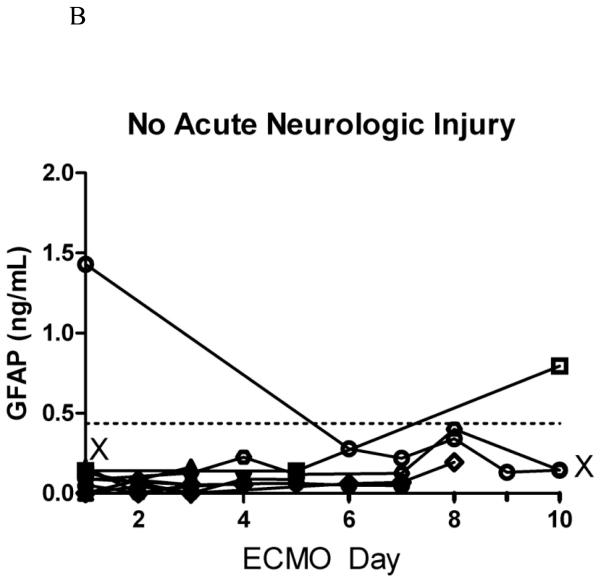
B. Serial plasma GFAP concentrations in children on ECMO without acute neurologic injury (n=15).
X represents death in the pediatric intensive care unit; dashed line marks the 95th percentile of normal controls. Please note different scales in Figures 2A. and 2B.
Peak plasma GFAP concentrations >95th percentile for normal controls (i.e., >0.436 ng/mL) were noted in 6/22 (27.3%) patients. The proportion of patients with acute neurologic injury was higher in patients with peak GFAP >0.436 ng/mL than in those with peak GFAP ≤0.436 ng/mL (4/6, 66.7% vs 3/16, 18.8%; p=0.054). Poor neurologic outcome was seen more frequently in patients with peak GFAP>0.436 ng/mL than in those with normal GFAP measurements (5/6, 83.3% vs 3/16, 18.8%; p<0.01). Similarly, hospital mortality was higher in patients with peak GFAP>0.436 ng/mL than in those with normal GFAP measurements (4/6. 66.7% vs 3/16, 18.8%, p=0.054).
To account for repeated measures per patient, we evaluated the association of all 126 serial GFAP levels with the outcomes using logistic regression clustered by patient. The odds of acute neurologic injury given elevated GFAP (>0.436ng/ml) were 11.5 (95%CI: 1.3-98.3). Similar statistically significant results were found for poor neurologic outcome (OR: 25.7, 95%CI: 2.2-298.5) and hospital mortality (OR: 13.6, 95%CI: 1.7-108.5).
After adjusting for neonatal status (≤30 days), the odds of acute neurologic injury remained significantly higher in patients with abnormally elevated plasma GFAP compared to patients with normal GFAP (adjusted OR, 15.7, 95% CI, 1.8-139.9). In the subgroup of 17 newborns and infants who had daily head ultrasounds performed throughout the duration of ECMO, the unadjusted OR for acute neurologic injury was 22.3 (95%CI: 2.0-245.9).
Although exploratory, this analysis yields an area under receiver operating characteristic curve (AUC) in an acceptable range for acute neurologic injury (AUC, 0.72, 95%CI, 0.50-0.94), poor neurologic outcome (AUC, 0.78, 95%CI, 0.58-0.97), and death (AUC, 0.72, 95%CI, 0.50-0.94).
Elevations in GFAP correlated temporally with the imaging diagnosis of brain injury during ECMO. Abnormal GFAP levels (>0.436 ng/mL) were observed 1-2 days prior to the imaging diagnosis of severe acute neurologic injury or brain death in 2/4 patients. GFAP levels remained normal in three patients with acute neurologic injury diagnosed by head ultrasound during ECMO, including a patient with a small subdural hematoma and good neurologic function at discharge (PCPC=1), a patient with grade I intraventricular hemorrhage and good neurologic function at discharge (PCPC=1) and a patient with a small right cerebellar hemorrhage who developed multisystem organ failure and ultimately expired (Table 2). While we can speculate that in the first two patients we found no elevations in GFAP as the lesions were minor and extraparenchymal in location, we do not have a good explanation for a lack in GFAP “response” to a cerebellar intraparenchymal hemorrhage in the third patient. Of note, cause of death in this latter patient was not related to neurologic injury but rather to multisystem organ failure and withdrawal of support due to medical futility.
There were two patients without a diagnosis of acute neurologic injury during ECMO but with peak GFAP >95th percentile on the first and the 10th day of ECMO, respectively (Table 2). These were newborns with normal daily head ultrasounds throughout the ECMO course. However, at 6 weeks and 2 weeks after ECMO decannulation, respectively, one patient was found to have small old intraventricular and intraparenchymal hemorrhagic foci and the other had findings of unilateral unilobar focal encephalomalacia consistent with prior ischemic event on brain MRI.
This initial cohort of ECMO patients included three patients who underwent extracorporeal cardiopulmonary resuscitation (ECPR): one survived with good neurologic outcome and had normal GFAP levels throughout the ECMO course (median GFAP, 0.07 ng/mL, IQR: 0.05-0.09 ng/mL); two patients had poor outcomes: one suffered a hypoxic pulseless electrical activity cardiac arrest due to status asthmaticus and evolved to brain death (median GFAP, 27.2 ng/mL, IQR: 9.5-44.9 ng/mL) and one patient developed severe cerebral edema, was successfully decannulated from ECMO, but eventually support was withdrawn for multisystem organ failure and medical futility (median GFAP: 5.8 ng/mL, IQR, 2.8-10.5 ng/mL).
Discussion
ECMO is a procedure with high risk for brain injury, including intracranial hemorrhage, brain infarction and brain death (1, 10, 11). The means for timely assessment of such injuries in patients on ECMO are often lacking. While acute neurologic insult is of great concern in critically-ill patients, no brain injury biomarker is available yet for routine clinical practice, although many coordinated efforts are ongoing (40, 50). The plasma GFAP biomarker used in this study has many advantages, such as: high specificity to brain, easy to obtain samples for small blood volumes, fast processing, precise quantification, low cost and minimal technical expertise required for the assay. Serial GFAP measurements could thus be used to monitor neurologic status and response to potential neuroprotective interventions, aid in the prompt diagnosis of acute brain injury and predict outcomes.
This study demonstrates that plasma concentrations of GFAP are associated with brain injury in children on ECMO. Serial GFAP concentrations appeared stable over time in the absence of neurologic insults and were significantly elevated in patients who were diagnosed with brain injury during ECMO. The majority of patients (19/22) had normal GFAP levels in the first 24 hours after ECMO cannulation, suggesting that cannulation of right jugular vein +/− the right carotid artery is not accompanied by injury leading to reactive gliosis and GFAP elevations. GFAP concentrations were elevated in four patients prior to a diagnosis of brain injury: two patients were diagnosed with acute neurologic injury while on ECMO and two patients had imaging evidence of prior brain ischemia or hemorrhage after ECMO decannulation. This may be particularly important for infants with intraparenchymal lesions that cannot be detected by transfontanellar sonography, thus providing false reassurance to clinicians. While there is evidence that serum GFAP concentrations can discriminate between ischemic and hemorrhagic stroke in adults within specific time windows after onset of symptoms (39, 51, 52) and volume of lesions (38), in our small cohort, we could not determine if plasma GFAP could discriminate among different types of brain injury (e.g., hemorrhagic vs ischemic, local vs global). Future, larger studies of patients undergoing ECMO with standardized imaging protocols and serial blood biomarker measurements are needed to address these important questions.
Acute neurologic injury is found more frequently in ECPR patients compared to ECMO for other indications (1, 27). Recent studies report 73% survival to hospital discharge in pediatric ECPR patients (53), with 75%-78% of survivors having favorable neurologic outcomes (27, 53). Acute neurologic injury occurs in 22% of pediatric ECPR patients; of these, 89% die prior to hospital discharge (27). In our study, we found normal serial GFAP levels in one patient who underwent ECPR and survived with good neurologic outcome. In contrast, two children who underwent ECPR and subsequently developed severe hypoxic brain injury, had plasma GFAP levels 20 to 100 times higher than the 95th percentile for normal children. To our knowledge, this is the first report of plasma GFAP as a potential predictive biomarker for ECPR: two prior studies of GFAP after cardiac arrest excluded patients who underwent ECPR (40, 41). However, these data are very preliminary and no further inferences can be made at this time.
This study has several limitations. Neurologic assessment and brain imaging were not standardized. According to our a priori definitions, for purposes of analysis, we assumed that no child developed brain injury until demonstrated by routine brain imaging or a first neurologic examination consistent with brain death. Thus, it is possible that neurologic injury is reflected in GFAP levels prior to imaging or clinical diagnosis and that the sensitivity of GFAP is underestimated. Also, the variable timing of GFAP measurements and diagnostic studies may have limited the ability to assess sensitivity. We used as cutoff plasma GFAP concentration of 0.436 ng/mL, the 95th percentile for plasma GFAP measured in newborns, infants and children free of neurologic injury. This is higher than the cutoff used in most adult studies 0.1-0.3 ng/mL (40, 43-45), which may have led to underestimation of sensitivity. Further, it is not known if the dilution of endogenous protein concentrations that occurs by adding the volume of the ECMO circuit to the patient’s blood volume may underestimate the sensitivity of this biomarker. Finally, this preliminary analysis shows that GFAP may be a predictor of mortality or poor neurologic outcome defined by PCPC; however, only limited adjustment for potential confounding factors was possible with this small cohort and OR estimates are imprecise as reflected by the wide CIs. Missing data points for GFAP levels may make our analysis less accurate.
More detailed study is needed to assess the performance of GFAP as a biomarker of acute neurologic injury during ECMO. Standardized imaging and neurologic exams would need to be coordinated with sample collection to more precisely establish the temporal relationship between elevations of GFAP and brain injury. The rigorous demonstration of this temporal relationship would advance GFAP as an adjunct to brain imaging for the surveillance of new or ongoing acute neurologic injury in ECMO patients. In situations where brain imaging is difficult, serial GFAP testing could be used as a screening test to triage patients with elevated values to imaging.
Conclusions
This series of 22 pediatric patients who underwent ECMO for various indications in a single institution provides preliminary data to support the use of plasma GFAP, a brain-specific protein, to detect acute neurologic injury in this high risk population. Studies specifically designed to assess the diagnostic performance of single or multi-panel blood biomarkers are needed to further investigate the utility of brain-specific biomarkers for acute and ongoing brain injury in children and also as a monitoring tool for neuroprotective therapies employed after occurrence of a neurologic insult.
Acknowledgments
Research support: The project described was supported by Grants Number UL1 RR 025005 and 1KL2RR025006-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp. (MMB). WS acknowledges support from the Johns Hopkins Clinical Hematology Development Program 5K12HL087169, 5R01HL091759, and the NHLBI Proteomics and Genomics Hands-on Workshop 5T15HL086386.
Abbreviations
- ECMO
extracorporeal membrane oxygenation
- ECPR
extracorporeal cardiopulmonary resuscitation
- GFAP
glial fibrillary acidic protein
- PICU
pediatric intensive care unit
- OR
odds ratio
- CI
confidence interval
- IQR
interquartile range
Footnotes
Financial disclosures
The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest
The authors have no conflicts of interest relevant to this article.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).Conrad SA, Rycus PT, Dalton H. Extracorporeal Life Support Registry Report 2004. ASAIO J. 2005 Jan-Feb;51(1):4–10. doi: 10.1097/01.mat.0000151922.67540.e9. [DOI] [PubMed] [Google Scholar]
- (2).Morris MC, Wernovsky G, Nadkarni VM. Survival outcomes after extracorporeal cardiopulmonary resuscitation instituted during active chest compressions following refractory in-hospital pediatric cardiac arrest. Pediatr Crit Care Med. 2004 Sep;5(5):440–6. doi: 10.1097/01.pcc.0000137356.58150.2e. [DOI] [PubMed] [Google Scholar]
- (3).Alsoufi B, Al-Radi OO, Nazer RI, Gruenwald C, Foreman C, Williams WG, Coles JG, Caldarone CA, Bohn DG, Van Arsdell GS. Survival outcomes after rescue extracorporeal cardiopulmonary resuscitation in pediatric patients with refractory cardiac arrest. J Thorac Cardiovasc Surg. 2007 Oct;134(4):952–959.e2. doi: 10.1016/j.jtcvs.2007.05.054. [DOI] [PubMed] [Google Scholar]
- (4).Haines NM, Rycus PT, Zwischenberger JB, Bartlett RH, Undar A. Extracorporeal Life Support Registry Report 2008: neonatal and pediatric cardiac cases. ASAIO J. 2009 Jan-Feb;55(1):111–6. doi: 10.1097/MAT.0b013e318190b6f7. [DOI] [PubMed] [Google Scholar]
- (5).Rajagopal SK, Almond CS, Laussen PC, Rycus PT, Wypij D, Thiagarajan RR. Extracorporeal membrane oxygenation for the support of infants, children, and young adults with acute myocarditis: A review of the Extracorporeal Life Support Organization registry. Crit Care Med. 2009 Sep 28; doi: 10.1097/CCM.0b013e3181bc8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Lequier L, Joffe AR, Robertson CM, Dinu IA, Wongswadiwat Y, Anton NR, Ross DB, Rebeyka IM, Western Canadian Complex Pediatric Therapies Program Follow-up Group Two-year survival, mental, and motor outcomes after cardiac extracorporeal life support at less than five years of age. J Thorac Cardiovasc Surg. 2008 Oct;136(4):976–983.e3. doi: 10.1016/j.jtcvs.2008.02.009. [DOI] [PubMed] [Google Scholar]
- (7).Gupta M, Shanley TP, Moler FW. Extracorporeal life support for severe respiratory failure in children with immune compromised conditions. Pediatr Crit Care Med. 2008 Jul;9(4):380–5. doi: 10.1097/PCC.0b013e318172d54d. [DOI] [PubMed] [Google Scholar]
- (8).Green TP, Moler FW, Goodman DM. Probability of survival after prolonged extracorporeal membrane oxygenation in pediatric patients with acute respiratory failure . Extracorporeal Life Support Organization. Crit Care Med. 1995;23:1132–1139. doi: 10.1097/00003246-199506000-00021. [DOI] [PubMed] [Google Scholar]
- (9).UK Collaborative ECMO Trial Group UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. Lancet. 1996;348:75–82. [PubMed] [Google Scholar]
- (10).Cilley RE, Zwischenberger JB, Andrews AF, Bowerman RA, Roloff DW, Bartlett RH. Intracranial haemorrhage during extracorporeal membrane oxygenation in neonates. Pediatrics. 1986;78:699–704. [PubMed] [Google Scholar]
- (11).Cengiz P, Seidel K, Rycus PT, et al. Central nervous system complications during pediatric extracorporeal life support: incidence and risk factors. Crit Care Med. 2005;33(12):2817–24. doi: 10.1097/01.ccm.0000189940.70617.c3. [DOI] [PubMed] [Google Scholar]
- (12).Hunter CJ, Blood AB, Bishai JM, Hickerson AD, Wall DD, Peverini RL, Power GG, Hopper AO. Cerebral blood flow and oxygenation during venoarterial and venovenous extracorporeal membrane oxygenation in the newborn lamb. Pediatr Crit Care Med. 2004 Sep;5(5):475–81. doi: 10.1097/01.pcc.0000130992.73123.bc. [DOI] [PubMed] [Google Scholar]
- (13).Van Heijst A, Liem D, Hopman J, Van Der Staak F, Sengers R. Oxygenation and hemodynamics in left and right cerebral hemispheres during induction of veno-arterial extracorporeal membrane oxygenation. J Pediatr. 2004 Feb;144(2):223–8. doi: 10.1016/j.jpeds.2003.11.006. [DOI] [PubMed] [Google Scholar]
- (14).Short BL, Walker LK, Traystman RJ. Impaired cerebral autoregulation in the newborn lamb during recovery from severe, prolonged hypoxia, combined with carotid artery and jugular vein ligation. Crit Care Med. 1994 Aug;22(8):1262–8. doi: 10.1097/00003246-199408000-00010. [DOI] [PubMed] [Google Scholar]
- (15).Taylor GA, Martin GR, Short BL. Cardiac determinants of cerebral blood flow during extracorporeal membrane oxygenation. Invest Radiol. 1989 Jul;24(7):511–6. doi: 10.1097/00004424-198907000-00001. [DOI] [PubMed] [Google Scholar]
- (16).Glass P, Miller M, Short B. Morbidity for survivors of extracorporeal membrane oxygenation: Neurodevelopmental outcome at 1 year of age. Pediatrics. 1989;83:72–8. [PubMed] [Google Scholar]
- (17).Adolph V, Ekelund C, Smith C, Starrett A, Falterman K, Arensman R. Developmental outcome of neonates treated with extracorporeal membrane oxygenation. J Pediatr Surg. 1990;25:43–6. doi: 10.1016/s0022-3468(05)80162-9. [DOI] [PubMed] [Google Scholar]
- (18).Schumacher RE, Palmer TW, Roloff MD, LaClaire PA, Bartlett RH. Follow-up of infants treated with extracorporeal membrane oxygenation for newborn respiratory failure. Pediatrics. 1991;87:451–7. [PubMed] [Google Scholar]
- (19).Hofkosh D, Thompson AE, Nozza RJ, Kemp SS, Bowen A, Feldman HM. Ten years of extracorporeal membrane oxygenation: Neurodevelopmental outcome. Pediatrics. 1991;87:549–55. [PubMed] [Google Scholar]
- (20).Glass P, Wagner AE, Papero HP, et al. Neurodevelopmental status at age five years of neonates treated with extracorporeal membrane oxygenation. J Pediatr. 1995;127:447–57. doi: 10.1016/s0022-3476(95)70082-x. [DOI] [PubMed] [Google Scholar]
- (21).Vaucher YE, Dudell GG, Bejar R, Gist K. Predictors of early childhood outcome in candidates for extracorporeal membrane oxygenation. J Pediatr. 1996;128:109–17. doi: 10.1016/s0022-3476(96)70439-0. [DOI] [PubMed] [Google Scholar]
- (22).Taylor AK, Cousins R, Butt WW. The long-term outcome of children managed with extracorporeal life support: an institutional experience. Crit Care Resusc. 2007 Jun;9(2):172–7. [PubMed] [Google Scholar]
- (23).Hanekamp MN, Mazer P, van der Cammen-van Zijp MH, van Kessel-Feddema BJ, Nijhuis-van der Sanden MW, Knuijt S, Zegers-Verstraeten JL, Gischler SJ, Tibboel D, Kollée LA. Follow-up of newborns treated with extracorporeal membrane oxygenation: a nationwide evaluation at 5 years of age. Crit Care. 2006;10(5):R127. doi: 10.1186/cc5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Amigoni A, Pettenazzo A, Biban P, Suppiej A, Freato F, Zaramella P, Zacchello F. Neurologic outcome in children after extracorporeal membrane oxygenation: prognostic value of diagnostic tests. Pediatr Neurol. 2005 Mar;32(3):173–9. doi: 10.1016/j.pediatrneurol.2004.10.002. [DOI] [PubMed] [Google Scholar]
- (25).Ibrahim AE, Duncan BW, Blume ED, Jonas RA. Long-term follow-up of pediatric cardiac patients requiring mechanical circulatory support. Ann Thorac Surg. 2000 Jan;69(1):186–92. doi: 10.1016/s0003-4975(99)01194-7. [DOI] [PubMed] [Google Scholar]
- (26).Thiagarajan RR, Laussen PC, Rycus PT, Bartlett RH, Bratton SL. Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children. Circulation. 2007 Oct 9;116(15):1693–700. doi: 10.1161/CIRCULATIONAHA.106.680678. [DOI] [PubMed] [Google Scholar]
- (27).Barrett CS, Bratton SL, Salvin JW, Laussen PC, Rycus PT, Thiagarajan RR. Neurological injury after extracorporeal membrane oxygenation use to aid pediatric cardiopulmonary resuscitation. Pediatr Crit Care Med. 2009 Jul;10(4):445–51. doi: 10.1097/PCC.0b013e318198bd85. [DOI] [PubMed] [Google Scholar]
- (28).Lazar EL, Abramson SJ, Weinstein S, Stolar CGH. Neuroimaging of brain injury in neonates treated with extracorporeal membrane oxygenation: lessons learned from serial examinations. J Pediatr Surg. 1994;29:186–91. doi: 10.1016/0022-3468(94)90315-8. [DOI] [PubMed] [Google Scholar]
- (29).Campbell LR, Bunyapen C, Gangarosa ME, Cohen M, Kanto WP. Significance of seizures associated with extracorporeal membrane oxygenation. J Pediatr. 1991;119:789–92. doi: 10.1016/s0022-3476(05)80304-x. [DOI] [PubMed] [Google Scholar]
- (30).Graziani LJ, Streletz LJ, Baumgart S, Cullen J, McKee LM. Predictive value of neonatal electroencephalograms before and during extracorporeal membrane oxygenation. J Pediatr. 1994;125:969–75. doi: 10.1016/s0022-3476(05)82017-7. [DOI] [PubMed] [Google Scholar]
- (31).Korinthenberg R, Kachel W, Koelfen W, Schultze C, Varnholt V. Neurological findings in newborn infants after extracorporeal membrane oxygenation, with special reference to the EEG. Dev Med Child Neurol. 1993;35:249–57. doi: 10.1111/j.1469-8749.1993.tb11630.x. [DOI] [PubMed] [Google Scholar]
- (32).Gazzolo D, Masetti P, Meli M, Grutzfeld D, Michetti F. Elevated S100B protein as an early indicator of intracranial haemorrhage in infants subjected to extracorporeal membrane oxygenation. Acta Paediatr. 2002;91(2):218–21. doi: 10.1080/080352502317285243. [DOI] [PubMed] [Google Scholar]
- (33).Anderson RE, Hansson LO, Nilsson O, Liska J, Settergren G, Vaage J. Increase in serum S100A1-B and S100BB during cardiac surgery arises from extracerebral sources. Ann Thorac Surg. 2001 May;71(5):1512–7. doi: 10.1016/s0003-4975(01)02399-2. [DOI] [PubMed] [Google Scholar]
- (34).Jönsson H, Johnsson P, Bäckström M, Alling C, Dautovic-Bergh C, Blomquist S. Controversial significance of early S100B levels after cardiac surgery. BMC Neurol. 2004 Dec 16;4(1):24. doi: 10.1186/1471-2377-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Routsi C, Stamataki E, Nanas S, Psachoulia C, Stathopoulos A, Koroneos A, Zervou M, Jullien G, Roussos C. Increased levels of serum S100B protein in critically ill patients without brain injury. Shock. 2006 Jul;26(1):20–4. doi: 10.1097/01.shk.0000209546.06801.d7. [DOI] [PubMed] [Google Scholar]
- (36).Panickar KS, Norenberg MD. Astrocytes in cerebral ischemic injury: morphological and general considerations. Glia. 2005 Jun;50(4):287–98. doi: 10.1002/glia.20181. Review. [DOI] [PubMed] [Google Scholar]
- (37).Niebroj-Dobosz I, Rafalowska J, Lukasiuk M, Pfeffer A, Mossakowski MJ. Immunochemical analysis of some proteins in cerebrospinal fluid and serum of patients with ischemic strokes. Folia Neuropathol. 1994;32:129–37. [PubMed] [Google Scholar]
- (38).Herrmann M, Vos P, Wunderlich MT, de Bruijn CH, Lamers KJ. Release of glial tissue-specific proteins after acute stroke: A comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke. 2000 Nov;31(11):2670–7. doi: 10.1161/01.str.31.11.2670. [DOI] [PubMed] [Google Scholar]
- (39).Dvorak F, Haberer I, Sitzer M, Foerch C. Characterisation of the diagnostic window of serum glial fibrillary acidic protein for the differentiation of intracerebral haemorrhage and ischaemic stroke. Cerebrovasc Dis. 2009;27(1):37–41. doi: 10.1159/000172632. [DOI] [PubMed] [Google Scholar]
- (40).Kaneko T, Kasaoka S, Miyauchi T, Fujita M, Oda Y, Tsuruta R, Maekawa T. Serum glial fibrillary acidic protein as a predictive biomarker of neurological outcome after cardiac arrest. Resuscitation. 2009 Jul;80(7):790–4. doi: 10.1016/j.resuscitation.2009.04.003. [DOI] [PubMed] [Google Scholar]
- (41).Hayashida H, Kaneko T, Kasaoka S, Oshima C, Miyauchi T, Fujita M, Oda Y, Tsuruta R, Maekawa T. Comparison of the Predictability of Neurological Outcome by Serum Procalcitonin and Glial Fibrillary Acidic Protein in Postcardiac-Arrest Patients. Neurocrit Care. 2009 Dec 22; doi: 10.1007/s12028-009-9318-5. [DOI] [PubMed] [Google Scholar]
- (42).Wiesmann M, Steinmeier E, Magerkurth O, Linn J, Gottmann D, Missler U. Outcome prediction in traumatic brain injury: comparison of neurological status, CT findings, and blood levels of S100B and GFAP. Acta Neurol Scand. 2009 Oct 5; doi: 10.1111/j.1600-0404.2009.01196.x. [DOI] [PubMed] [Google Scholar]
- (43).Pelinka LE, Kroepfl A, Schmidhammer R, et al. Glial fibrillary acidic protein in serum after traumatic brain injury and multiple trauma. J Trauma. 2004;57:1006–12. doi: 10.1097/01.ta.0000108998.48026.c3. [DOI] [PubMed] [Google Scholar]
- (44).Pelinka LE, Kroepfl A, Leixnering M, et al. GFAP versus S100B in serum after traumatic brain injury: relationship to brain damage and outcome. J Neurotrauma. 2004;21:1553–61. doi: 10.1089/neu.2004.21.1553. [DOI] [PubMed] [Google Scholar]
- (45).Nylén K, Ost M, Csajbok LZ, Nilsson I, Blennow K, Nellgård B, Rosengren L. Increased serum-GFAP in patients with severe traumatic brain injury is related to outcome. J Neurol Sci. 2006 Jan 15;240(1-2):85–91. doi: 10.1016/j.jns.2005.09.007. [DOI] [PubMed] [Google Scholar]
- (46).Fraser DD, Close TE, Rose KL, Ward R, Mehl M, Farrell C, Lacroix J, Creery D, Kesselman M, Stanimirovic D, Hutchison JS, for the Canadian Critical Care Translational Biology Group Severe traumatic brain injury in children elevates glial fibrillary acidic protein in cerebrospinal fluid and serum. Pediatr Crit Care Med. 2010 Jul 9; doi: 10.1097/PCC.0b013e3181e8b32d. [DOI] [PubMed] [Google Scholar]
- (47).Petzold A, Keir G, Green AJ, Giovannoni G, Thompson EJ. An ELISA for glial fibrillary acidic protein. J Immunol Methods. 2004 Apr;287(1-2):169–77. doi: 10.1016/j.jim.2004.01.015. [DOI] [PubMed] [Google Scholar]
- (48).Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992 Jul;121(1):68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- (49).Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000 Jul;28(7):2616–20. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- (50).Laskowitz DT, Kasner SE, Saver J, Remmel KS, Jauch EC, BRAIN Study Group Clinical usefulness of a biomarker-based diagnostic test for acute stroke: the Biomarker Rapid Assessment in Ischemic Injury (BRAIN) study. Stroke. 2009 Jan;40(1):77–85. doi: 10.1161/STROKEAHA.108.516377. [DOI] [PubMed] [Google Scholar]
- (51).Foerch C, Curdt I, Yan B, Dvorak F, Hermans M, Berkefeld J, et al. Serum glial fibrillary acidic protein as a biomarker for intracerebral haemorrhage in patients with acute stroke. J Neurol Neurosurg Psychiatry. 2006;77:181–4. doi: 10.1136/jnnp.2005.074823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Unden J, Strandberg K, Malm J, Campbell E, Rosengren L, Stenflo J, et al. Explorative investigation of biomarkers of brain damage and coagulation system activation in clinical stroke differentiation. J Neurol. 2009;256:72–7. doi: 10.1007/s00415-009-0054-8. [DOI] [PubMed] [Google Scholar]
- (53).Prodhan P, Fiser RT, Dyamenahalli U, Gossett J, Imamura M, Jaquiss RD, Bhutta AT. Outcomes after extracorporeal cardiopulmonary resuscitation (ECPR) following refractory pediatric cardiac arrest in the intensive care unit. Resuscitation. 2009 Oct;80(10):1124–9. doi: 10.1016/j.resuscitation.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]