Abstract
Chronic wounds often result from prolonged inflammation involving excessive polymorphonuclear leukocyte activity. Studies show that the ω-3 polyunsaturated fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) found in fish oils generate bioactive lipid mediators that reduce inflammation and polymorphonuclear leukocyte recruitment in numerous inflammatory disease models. This study’s purpose was to test the hypotheses that boosting plasma levels of EPA and DHA with oral supplementation would alter lipid mediator levels in acute wound microenvironments and reduce polymorphonuclear leukocyte levels. Eighteen individuals were randomized to 28 days of either EPA + DHA supplementation (Active Group) or placebo. After 28 days, the Active Group had significantly higher plasma levels of EPA (p < 0.001) and DHA (p < 0.001) than the Placebo Group and significantly lower wound fluid levels of two 15-lipoxygenase products of ω-6 polyunsaturated fatty acids (9-hydroxyoctadecadienoic acid [p=0.033] and 15-hydroxyeicosatrienoic acid [p=0.006]), at 24 hours postwounding. The Active Group also had lower mean levels of myeloperoxidase, a leukocyte marker, at 12 hours and significantly more reepithelialization on Day 5 postwounding. We suggest that lipid mediator profiles can be manipulated by altering polyunsaturated fatty acid intake to create a wound microenvironment more conducive to healing.
It is generally acknowledged that chronic wounds often result from a prolonged inflammatory response, which is associated with excessive polymorphonuclear leukocyte (PMN) activity in the microenvironment of the wound.1 Although inflammation is the essential initial stage in tissue regeneration, unresolved inflammation delays advancement to subsequent healing stages. Also, high numbers of PMNs found in chronically inflamed wounds secrete excessive amounts of proteases such as matrix met-alloproteinase-8 and neutrophil elastase that can cause tissue destruction and persistent inflammation.1 The bioactive lipid mediators derived from the long-chain n-3 polyunsaturated fatty acids (PUFA) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) acids found in fish oils have been shown to reduce inflammatory responses and PMN transmigration across the endothelium in several disease models of inflammation.2-4 However, the cellular and molecular mechanisms leading to inflammation reduction and PMN regulation are not fully understood and, to our knowledge, no studies have assessed whether supplementing the human diet with EPA and DHA can alter lipid mediator levels within the wound and thus, potentially limit local inflammatory responses.
The two main PUFA families, n-6 and n-3, are essential components of the phospholipid bilayer of cell membranes acting as pools of bioavailable PUFAs, affecting membrane fluidity and mediating the actions of membrane-bound enzymes and receptors.5 N-6 and n-3 PUFAs are precursors to differential types of bioactive lipids such as eicosanoids, lipoxins (LX), resolvins, and protectins that play important roles in regulating different aspects of the inflammatory response.2-4,6 It is generally considered that n-6-PUFA-derived lipid mediators are more proinflammatory than their counterparts generated from n-3 PUFA metabolism6,7 that are believed to elicit antiinflammatory protective effects. Western diets currently contain high n-6 : n-3 PUFA ratios in the range of 15–16 : 1, primarily due to the high consumption of n-6-rich vegetable oils, when compared with the recommended optimal ratio of approximately 4 : 1.7 Diets with high n-6 : n-3 ratios, in conjunction with genetic factors, have been associated with the increasing prevalence of many chronic inflammatory-related diseases such as rheumatoid arthritis, cardiovascular disease, and asthma.7
Extensive epidemiological studies report clinical improvements in a number of chronic inflammatory-related diseases with diets rich in the n-3 PUFAs EPA and DHA.7,8 In pursuit of explanations for the antiinflammatory effects of EPA and DHA, numerous studies have compared and contrasted the differential actions of the n-3- and n-6-derived metabolites. Eicosanoids are a family of active lipid mediators containing 20 carbons generated primarily by n-6 arachidonic acid (AA), but also by n-6 dihomogammalinolenic acid (DGLA) and n-3 EPA that wield various modulating effects on the inflammatory process.6,9,10 The eicosanoid family includes prostaglandins (PG), thromboxanes (TX), leukotrienes (LT), LX, and various hydroxy fatty acids (Figure 1). After being released from cell membranes by phospholipase A2 activation as a result of cell stressors (e.g., tissue injury), AA, DGLA, and EPA induce eicosanoid production by the lipoxygenase (LOX), cyclooxygenase (COX), and/or cytochrome P-450 enzymatic pathways. Although not all AA-derived eicosanoids are exclusively pro-inflammatory, most species promote and sustain greater inflammatory responses than those from EPA and DGLA metabolism.6,8
Figure 1.
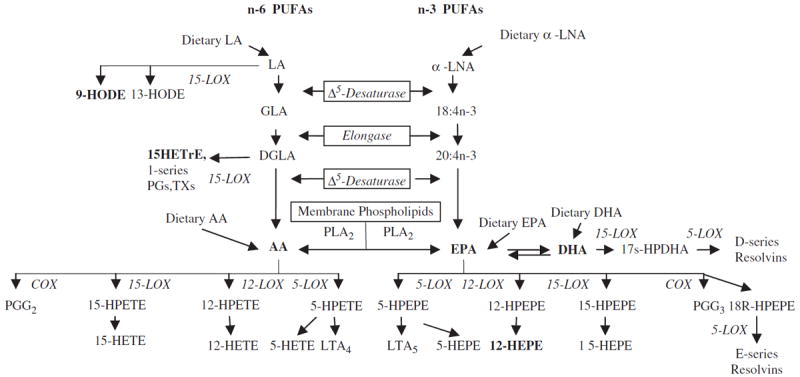
Polyunsaturated fatty acid (PUFA) pathways leading to their respective enzymatically derived mediators. LA, linolenic acid; α-LNA, α-linolenic acid; LOX, lipoxygenase; GLA, γ-linoleic acid; DGLA, dihomo-γ-linoleic acid; HETrE, hydroxyeicosatrienoic acid; PG, prostaglandins; TX, thromboxanes; AA, arachidonic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; COX, cyclooxygenase; HPETE, hydroperoxyeicosatetraenoic acid; HETE, hydroxyeicosatetraenoic acid; LT, leukotrienes; HPEPE, hydroperoxyeicosapentaenoic acid; HEPE, hydroxyeicosapentaenoic acid.
Hydroxy fatty acids are products of various LOX activities, with the 12/15-LOX enzymatic pathways of n-6 and n-3 PUFAs of special interest to the study of wound healing because of their regulatory influence on skin inflammation.9,11,12 Specific examples include the 15-LOX products of n-6 linoleic acid (LA), 13-hydroxyoctadecadienoic (HODE) acid (13-HODE), and 9-HODE, and the DGLA metabolite 15-hydroxyeicosatrienoic (15-HETrE), the 12-LOX product of AA 12-hydroxyeicosatetraenoic acid (12-HETE), and EPA 12-hydroxyeicosapentaenoic acid (12-HEPE). A recent study found that 9-HODE played a strong proinflammatory role in human skin under oxidative conditions,12 while 12-HETE was first reported to accrue in psoriatic lesions13 and was shown to exert chemoattractant actions and inflammatory effects in various cell types.11 Conversely, a study investigating alveolar macrophages reported that EPA-derived 12-HEPE was devoid of proinflammatory potencies.14 Finally, the novel di- and trihydroxy fatty acid mediators resolvins (RvE from EPA; RvD from DHA) and protectins (PD from DHA) have also displayed potent antiinflammatory actions in disease models such as peritonitis, colitis, and peridontitis.4,15
Overall, the collective findings from a plethora of studies investigating the antiinflammatory actions of EPA and DHA suggest that it may be possible to assuage the excessive inflammation and PMN activity associated with chronic wounds by increasing EPA and DHA consumption. In this study, we have set out to determine the in vivo effects of EPA + DHA oral supplementation in healthy young adults on levels of PUFA-derived lipid mediators in acute wounds utilizing a blister wound model. PMN activity and wound reepithelialization were also assessed. We hypothesized that the n-3 PUFA oral supplements could alter the bioactive lipid mediator levels in the wound and create a microenvironment more conducive to healing. This approach may prove to be a cost-effective adjuvant therapeutic to current treatment strategies for patients with chronically inflamed wounds.
MATERIALS AND METHODS
Procedure
This randomized, double-blind, repeated measures study was conducted at the Clinical Research Center (CRC) at The Ohio State University. A total of 18 healthy individuals between 18 and 45 years of age with the ability to understand English were enrolled for the study and assigned by computerized random sort to either the Active Group (EPA + DHA supplement) or Placebo Group. Individuals were excluded if they were taking nonsteroidal antiinflammatory or lipid-lowering drugs, nutritional supplements or corticosteroids or were current smokers, pregnant, or lactating. After Institutional Review Board approval and following a complete explanation of the study by the primary investigator (PI), informed written consent was obtained from eligible individuals. The study was in compliance with the ethical rules for human experimentation stated in the 1975 Declaration of Helsinki.
Visit one took place at the CRC at which time demographic data were collected. Body measurements consisted of height, weight, sagittal abdominal diameter, and body mass index. Verbal and written instructions were given to all participants in both groups to take five softgels and one 81 mg aspirin tablet at bedtime until study completion (Group 1—a total of 1.6 g of EPA and 1.2 g of DHA per day; Group 2—a total of 2.4 mL of mineral oil per day). Low-dose aspirin has been found to enhance the actions of resolvin species.4 A specific date to begin the softgels and aspirin was assigned to each participant. The FDA states that intakes up to 3 g/day of marine n-3 PUFAs are safe for inclusion in the diet.16 A similar EPA + DHA dose in our pilot work significantly increased plasma levels in healthy human subjects after 4 weeks,17 significantly reduced ex vivo proinflammatory cytokine production after 4 weeks18 and significantly reduced production of proinflammatory PGE2 by peripheral blood mononuclear cells after 12 weeks with no adverse effects.19 Mineral oil was chosen for the placebo because it is chemically inert and on ingestion the majority (98%) remains unabsorbed in the feces. All softgels were the same in appearance and packaged in like containers by J. R. Carlson Laboratories Inc. (Arlington Heights, IL). Participants were instructed to maintain their usual diets, but to exclude fish, seafood, kelp, and flaxseeds until study completion. Blood was collected for plasma fatty acid analysis after an 8-hour fast. Food frequency questionnaires (FFQ) were completed, which reflected micro- and macronutrients for the 3 months before study enrollment. Data from the FFQ allowed for baseline comparisons of nutrients important for efficient wound healing between the two groups.
Four weeks after beginning the softgels, each participant was admitted to the CRC on a scheduled morning and discharged the following morning (Visit two). Blood samples were collected and body measurements were recorded. Eight 8 mm blisters were created on the nondominant forearms to initiate inflammation and produce wound fluid for lipid mediator and PMN quantifications. The study design and variable measurement points are summarized in Figure 2.
Figure 2.

Study design. X=28 days of EPA/DHA supplement (EPA: 1.6 g/day; DHA: 1.2 g/day)+1aspirin (81 mg/day) taken at bedtime, ~X=28 days of placebo (mineral oil: 2.4 mL/day)1 + aspirin (81 mg/day) taken at bedtime. O1, bioactive lipid mediators, myeloperoxidase, salivary cortisol; O2, photos of healing, CRC, Clinical Research Center.
Blister initiation
A suction blister protocol was modeled after one utilized in studies at the National Institute of Allergy and Infectious Diseases and at The Ohio State University.20 The blister wound model has been used extensively to examine epidermal regeneration. The blister device consisted of two plastic templates containing eight circular orifices that measured 8 mm in diameter, which were taped to the volar surface of the nondominant forearm. A vacuum of 350 mm Hg was applied through a pump attached to a regulator until blisters formed ( ) (Electronic Diversities, Finksburg, MD). The dermoepidermal junction was separated by the gentle suction and eight sterile 8-mm blisters were formed. Fluid was aspirated from four of the blisters at 12 hours postblistering and the other four at 24 hours. A standard wound care protocol was initiated.
After discharge
Healing assessments of the superficial blister wound sites occurred at 15 minutes postblistering, and days 1, 2, 5, 10, and 15 days postblistering or until complete healing.
Plasma fatty acid assay
Plasma fatty acids were quantified by gas chromatography/mass spectrometry (GC/MS) at Dr. Martha Belury’s Laboratory at the College of Education and Human Ecology, OSU (Columbus, OH). Blood samples (1.0 mL) were collected in EDTA vacutainers, centrifuged (720×g, 30 minutes, at room temperature), plasma was collected and stored at −80 °C before analysis. Total plasma lipids were extracted with 2 : 1 (v/v) chloroform : methanol and 0.24 mL 0.88% KCL.21 Fatty acid methyl esters were prepared using tetramethylguandine at 100 °C22 and analyzed by gas chromatography using a 30-m Omegawax™ 320 fused silica capillary column (Supelco, Bellefonte, PA). Oven temperature started at 175 °C and increased at a rate of 3 °C/min until reaching 220 °C. Flow rate of the carrier gas helium was 30 mL/min. Retention times were compared with those of fatty acid methyl ester standards (Matreya, LLC, Pleasant Gap, PA; Supelco and Nu-Check Prep Inc., Elysian, MN). Results are reported as percent area of total fatty acids identified in plasma samples.
Stress assays
All subjects completed a Perceived Stress Scale on admission to the CRC (after 4 weeks of treatment or placebo) so that subjective stress levels could be assessed. Increased stress has been associated with changes in levels of inflammatory mediators and delayed wound healing and thus, is essential to consider in an in vivo wound healing study.20,23
Salivary cortisol was measured at 12 and 24 hours post-blistering to appraise the potential stressful effects of the blistering procedure. Saliva was obtained from a dental cotton roll and assayed using the solid-phase radio-immunoassay procedure (Diagnostic Products Corporation, Los Angeles, CA). Salivary cortisol is a valid and reliable measure of unbound hormone in the blood and reflects the physiological stress response.24
Analysis of lipid mediators
Lipid mediators in blister fluid and plasma were analyzed by liquid chromatography coupled to electrospray ionization tandem mass spectrometry (LC/ESI-MS/MS) as reported.25,26 Briefly, samples were collected, stored immediately at −80 °C and, at study end, shipped to the UK in dry ice. Samples (20–500 mL blister fluid; 500–1,000 mL plasma) were defrosted on ice and adjusted to 15% (v/v) methanol : water (final volume 3–4 mL). Internal standards, PGB2-d4 (40 μL, 1 ng/μL) and 12-HETE-d8 (40 μL, 1 ng/μL) (Cayman Chemicals, Ann Arbor, MI) were added and the pH of resulting solutions adjusted to 3.0 (0.1 M HCL). Acidified samples were immediately applied to preconditioned solid-phase cartridges (C18-E, Phenomenex, Macclesfield, UK) and lipid mediators eluted with methyl formate. LC/ESI-MS/MS analysis was performed on a Waters Alliance 2695 HPLC pump coupled to an ESI triple quadrupole Quattro Ultima mass spectrometer (Waters, Elstree, Hertsfordshire, UK). Chromatographic separation was performed on a C18 column (Luna, 5 μ, 150×2 mm; Phenomenex) and analytes monitored on multiple reaction monitoring mode as reported.25,27 Results are expressed as pg/mL (protein assay: Bio-Rad, Herts, UK).
PMN analysis
PMN quantification was achieved by measuring myeloperoxidase activity (MPO), a leukocyte marker enzyme.1,3 Quantifying PMN in wound fluid at 12 and 24 hours postwounding has been found to be a valid and reliable approach to objectively test the efficacy of interventions designed to reduce PMN infiltration.28 In brief, MPO activity in the blister fluid was measured using Meso Scale Discovery (MSD) Multi-Array 96-well Small Spot Human MPO plate. The detection reagent is the MSD Sulfo-Tag Human MPO Detection Antibody. Plates were read on MSD SECTOR Imager 6000. Serum samples (0.05–0.1 mL) were obtained from blisters formed by the suction blister device (Electronics Diversified, Finksburg, MD).
Macroscopic analysis of wound healing
Daily wound area yet to be healed was measured by a noncontact method using single camera digital photogrammetry and the wound measurement software (VeV MD, Vista Medical, Winnipeg, Manitoba, Canada). The wound perimeters were outlined on the computer with a cursor by the research assistant and the sum of the areas yet to be healed for all eight blisters was then calculated by the software program. Significant Pearson’s correlation coefficients showed an average of 0.88 in the PI’s previous wound healing study, signifying that the VeV measurement system showed high test–retest intrarater reliability.17
Statistical analysis
For the dependent variables of blister fluid lipid mediator levels, a linear censored data model was constructed. Treatment group was used as the independent variable, and each response was classified as being either observed or interval censored (for values that fell between zero and the limit of detection). Responses were assumed to be normally distributed conditional on treatment group. The analysis was performed using the LIFEREG procedure in SAS 9.2. Mann–Whitney tests assessed between-group comparisons of plasma lipid mediator levels (0 and 4 weeks). T-tests evaluated between-group comparisons of sociodemographic data, body measurements, plasma fatty acid levels (0 and 4 weeks), MPO levels in blister fluid (12 and 24 hours postblistering), total area yet to heal (cm2/day) for all eight blisters, and nutrient data. Paired t-tests assessed within-group comparisons of plasma fatty acid levels. The analyses were performed using Predictive Analytic Software, version 18.0. Significance levels were set a priori at α=0.05.
RESULTS
Anthropometric measures, demographics, nutritional, and stress influences
In this study designed to evaluate the effects of EPA + DHA oral supplementation on lipid mediator levels in acute blister wound fluid, data from 18 participants who completed the protocol were analyzed. Anthropometric measures and demographic characteristics describing the participants in the Active and Placebo groups are displayed in Table 1 with similar data for both groups.
Table 1.
Demographic characteristics and anthropometric measures of participants (N=18)
| Active (n=9)*,†,‡ | Placebo (n=9) *,†,‡ | |
|---|---|---|
| Age, mean years | 24 (5.4) | 28 (8.4) |
| Male (%) | 22 | 78 |
| White (%) | 89 | 78 |
| African American (%) | 0 | 11 |
| Asian (%) | 11 | 0 |
| Other (%) | 0 | 11 |
| Height (cm) | 168.9 (6.2) | 176.4 (10.2) |
| Weight (kg) | ||
| Baseline | 73.3 (20.3) | 79.4 (12.6) |
| 4 weeks | 73.5 (21.1) | 79.5 (13.2) |
| BMI (kg/m2) | ||
| Baseline | 25.8 (7.7) | 25.6 (3.8) |
| 4 weeks | 25.8 (7.9) | 25.6 (3.8) |
| SAD (cm) | ||
| Baseline | 19.3 (4.3) | 19.7 (1.7) |
| 4 weeks | 19.3 (3.8) | 20.0 (2.2) |
No significant differences between groups.
Analyzed with t-test.
Mean ± SD (all such values).
BMI, body mass index; SAD, sagittal abdominal diameter.
Baseline data regarding nutrients that could potentially influence wound healing revealed that although the Active Group consumed slightly lower mean daily levels of vitamin C, protein, kilocalories, and AA than the Placebo Group during the 3 months before the study, the differences were not statistically significant (Table 2).
Table 2.
Dietary characteristics of participants at baseline (N=18), mean (SD)
| FFQ data (visit 1) estimates daily intake for preceding 3 mos. | Active (n=9) | Placebo (n=9) |
|---|---|---|
| Mean vitamin C (mg/day) (SD) (RDAs 75–90 mg/day) | 111.8 (54.3) | 157.1 (102.7) |
| Mean protein g/day (SD) (RDAs 46–56 g/day)* | 88.4 (38.7) | 110.6 (30.6) |
| Mean kilocalories/day (SD) (RDAs 1,848–3,141 kcal/day)† | 2073.0 (623.2) | 2716.0 (760.1) |
| Mean EPA (g/day) (SD) | 0.03 (0.03) | 0.02 (0.01) |
| Mean DHA (g/day) (SD) (AI 0.50 g/day EPA + DHA)‡ | 0.07 (0.08) | 0.05 (0.03) |
| Mean AA (g/day) (SD) | 0.10 (0.10) | 0.20 (0.10) |
Based on 0.8 g/kg body weight for reference body weight for adults 18≥70 years of age.
Estimated energy requirements (EER) for men and women of 30 years of age. For each year below 30, add 7 kcal/day for women and 20 kcal/day for men. For each year above 30, subtract 7 kcal/day for women and 20 kcal/day for men. Requirements vary with BMI and physical activity level.
Source: International Society for Study of Fatty Acids and Lipids; Recommendations for Dietary Intake of Polyunsaturated Fatty Acids in Healthy Adults (2004).
Source: Food and Nutrition Board, Institute of Medicine, National Academies; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (2002/2005).
AA, arachidonic acid; AI, acceptable intake; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; RDA, recommended dietary allowances.
Statistical analyses of salivary cortisol levels (at 12 and 24 hours postblistering; Time 1 and Time 2, respectively) showed that participants in both groups were somewhat similar in their physiological reactions to stress (Table 3). Mean salivary cortisol levels at Time 2 were significantly higher than at Time 1 for the Active Group (t=−9.88, df=8, p < 0.001) and the Placebo Group (t=−7.57, df=8, p < 0.001), likely because Time 2 samples were collected in the morning when cortisol levels are typically higher than in the evening when Time 1 samples were collected. Interestingly, the interaction between treatment group and time was significant. At Time 2 (24 hours postblistering), cortisol levels were significantly higher for the Active Group when compared with the Placebo Group (t=3.38, df=16, p=0.004). However, salivary cortisol levels at 24 hours were not significantly correlated with the total wound area remaining to be reepithelialized on Days 1, 2, or 5, suggesting the elevated salivary cortisol levels noted in the Active Group at 24 hours postblistering had no effect on wound healing in the present study.
Table 3.
Stress characteristics of participants (N518), mean (SD)
| Active (n=9) | Placebo (n=9) | |
|---|---|---|
| Mean PSS scores (SD) | ||
| Higher score (0–40)=greater stress over preceding month | 13 (3.4) | 12 (6.5) |
| Mean salivary cortisol levels (μg/dL; SD) | ||
| Time 1 (12 hours postblistering—PM) | 0.04 (0.01) | 0.04 (0.01) |
| Time 2 (24 hours postblistering—AM) | 0.31 (0.08)*,† | 0.20 (0.06)*,† |
Significantly different from Time 1 (p < 0.001).
Significant between-group differences at Time 2 (p=0.004).
PSS, Perceived Stress Scale.
Plasma fatty acid levels
The within-group analysis showed that mean plasma fatty acid levels for the Active Group who consumed the EPA + DHA supplement were significantly higher at 4 weeks than at baseline for EPA (t=8.83, df=8, p < 0.001) and DHA (t=8.01, df=8, p < 0.001) and the total n-6 to n-3 ratio was significantly reduced (t=9.32, df=8, p < 0.001). Plasma levels of AA were also diminished at 4 weeks in the Active Group, but not significantly; however, the AA : EPA ratio was significantly lowered (t=5.77, df=8, p < 0.001), suggesting that rising EPA levels were the primary contributing factors to the change. Previous studies have also reported that an EPA dose similar to that of the current study resulted in significantly increased proportions in the plasma that occurred partly at the expense of AA.29 Collectively, the current study data indicated that the EPA + DHA supplements were taken appropriately by participants in the Active Group and that the dose and duration of supplementation were adequate to significantly raise plasma levels of EPA and DHA and significantly reduce both the total n-6 : n-3 and AA : EPA ratios from baseline values.
Interestingly, mean plasma DHA levels were significantly lower at 4 weeks for the Placebo Group when compared with baseline values (t=5.37, df=8, p=0.001), as were mean plasma AA levels (t=2.36, df=8, p=0.05). No significant changes were detected in the Placebo Group from baseline to 4 weeks for EPA levels or for AA : EPA or n-6 : n-3 ratios.
As expected, the between-group comparisons showed that at 4 weeks postenrollment, the Active Group who consumed the EPA + DHA supplements had significantly higher mean plasma levels of EPA (t=9.23, df=16, p < 0.001), DHA (t=9.71, df=16, p < 0.001) (Figure 3), and total n-3 PUFAs (t=9.20, df=16, p < 0.001), and significantly lower ratios of AA : EPA (t=−11.8, df=16, p < 0.001) (Figure 4) and n-6 : n-3 (t=−10.9, df=16, p < 0.001) than the Placebo Group.
Figure 3.
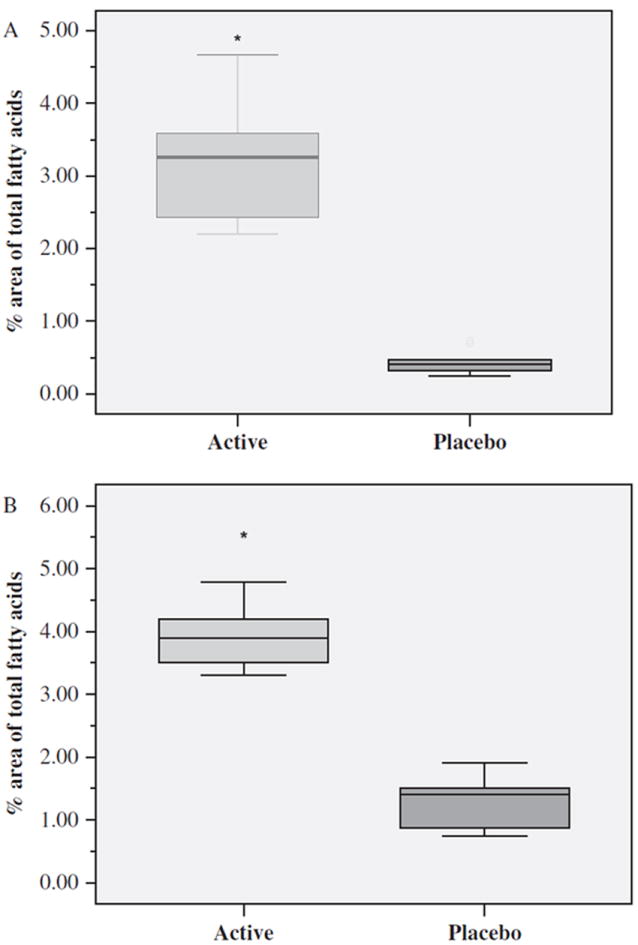
Mean distribution levels of plasma EPA (A) and DHA (B) after 4 weeks of supplement or placebo by Active (n=9) and Placebo (n=9) Groups expressed as percentage area of total fatty acids. Boxes define the middle 50% of the distribution for each group. The vertical line crossing each box defines the median. Upper and lower bars represent the largest and smallest values that are not outliers. *p < 0.001 Active vs. Placebo Group. EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
Figure 4.
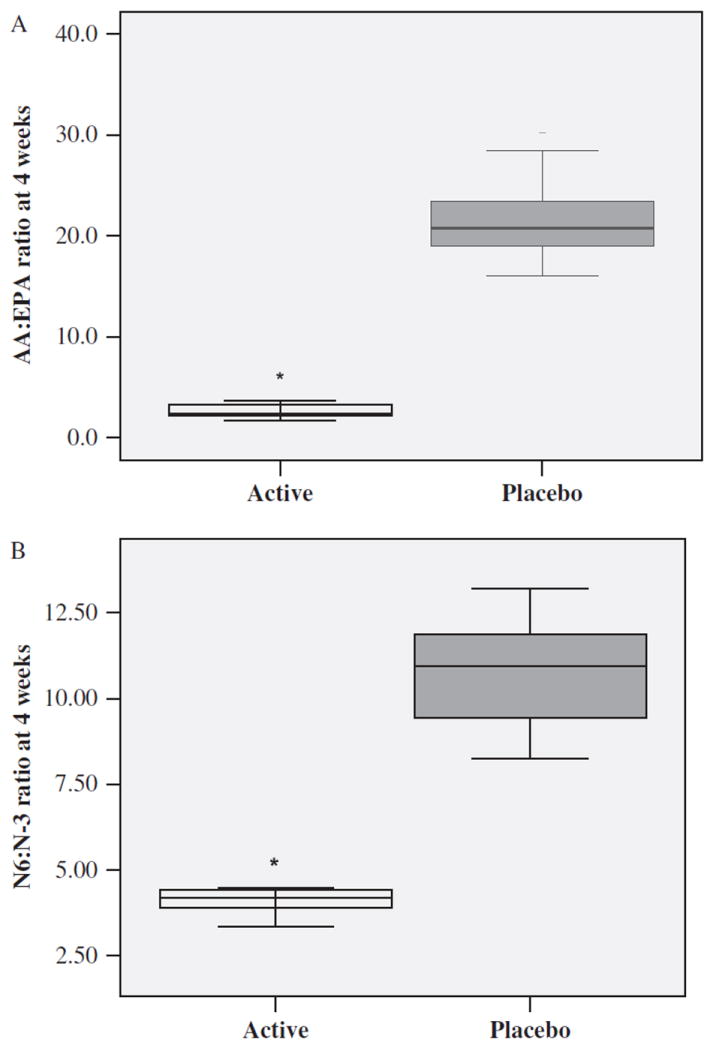
AA : EPA ratio (A) and n-6 : n-3 PUFA ratio (B) after 4 weeks of supplement or placebo by Active (n=9) and Placebo (n=9) Groups. Each box defines the middle 50% of the distribution. The vertical line crossing each box defines the median. Upper and lower bars represent the largest and the smallest values that are not outliers. *p < 0.001 Active vs. Placebo Group. AA, arachidonic acid; EPA, eicosapentaenoic acid; PUFA, polyunsaturated fatty acids.
Lipid mediators in blister wound fluid
The acute wound fluid collected at 12 and 24 hours post-blistering was pooled by group and time point and analyzed for PUFA-derived lipid mediators by LC/ESI-MS/MS. We detected 9- and 13-HODE, 12- and 15-HETE, 15-HETrE and 12- and 15-HEPE in the blister fluid (Table 5), which are comparable with the data shown in a recent study examining the sunburn response in human skin.26 Levels of two hydroxy fatty acids, namely the product of 12/15 LOX metabolism of n-6 LA, 9-HODE (χ2=4.55, df=1, p=0.03) and the 15-LOX product of n-6 DGLA, 15-HETrE (χ2=7.54, df=1, p=0.006), were identified in the blister fluid of the Active Group at 24 hours postblistering when compared with the Placebo Group. Furthermore, the Active Group had higher mean levels of 12-HEPE, a 12-LOX product of EPA, at 12 hours postblistering than the Placebo Group; a difference that approached statistical significance (χ2=3.18, df=1, p=0.07). Mean concentrations of 12-HETE were also higher in the Active Group than in the Placebo Group at both 12 and 24 hours post-blistering, while 15-HETE levels were lower at both time points in the Active Group (Table 5).
Table 5.
Levels of lipoxygenase-mediated hydroxy fatty acids in blister fluid and plasma in Active (n=9) and Placebo (n=9) Groups
| Hydroxyl fatty acids (pg/μL) | Blister fluid
|
Plasma
|
||||||
|---|---|---|---|---|---|---|---|---|
| 12 hours postblistering
|
24 hours postblistering
|
Baseline
|
28 days
|
|||||
| Active | Placebo | Active | Placebo | Active | Placebo | Active | Placebo | |
| 9-HODE | 6.32 (4.99) | 3.74 (2.95) | 1.67 (2.05)* | 3.51 (1.85) | 2.56 (0.82) | 2.21 (0.75) | 3.29 (2.58) | 3.09 (1.50) |
| 13-HODE | 37.09 (19.74) | 38.57 (26.21) | 22.10 (19.22) | 23.11 (13.84) | 4.04 (1.79) | 3.14 (1.95) | 2.93 (1.64) | 3.79 (1.50) |
| 5-HETE | — | — | — | — | 0.48 (0.20) | 0.60 (0.34) | 0.48 (0.51) | 0.61 (0.22) |
| 8-HETE | — | — | — | — | 0.19 (0.01) | 0.01 (0.003) | 0.08 (0.20) | 0.02 (0.006) |
| 11-HETE | — | — | — | — | 0.01 (0.003) | 0.01 (0.002) | 0.01 (0.004) | 0.01 (0.002) |
| 12-HETE | 15.09 (11.04) | 13.92 (9.67) | 8.26 (5.26) | 4.99 (2.51) | 0.06 (0.06) | 0.05 (0.03) | 0.05 (0.06) | 0.20 (0.42) |
| 15-HETE | 9.78 (4.12) | 15.24 (11.69) | 11.38 (10.84) | 11.92 (7.15) | 0.27 (0.13) | 0.19 (0.08) | 0.33 (0.32) | 0.24 (0.13) |
| 15-HETrE | 1.96 (2.10) | 1.45 (2.26) | —** | 2.99 (3.25) | — | — | — | — |
| 5-HEPE | — | — | — | — | — | 0.04 (0.02) | 0.21 (0.24)*** | — |
| 8-HEPE | — | — | — | — | — | — | 0.47 (0.47)*** | — |
| 11-HEPE | — | — | — | — | — | — | — | — |
| 12-HEPE | 1.61 (2.08) | 0.42 (0.38) | 0.57 (0.82) | 0.27 (0.17) | — | — | 0.25 (0.11)**** | 0.06 (0.08) |
| 15-HEPE | 1.00 (2.40) | — | — | — | — | — | 0.68 (0.64)*** | — |
| 18-HEPE | — | — | — | — | — | — | 0.89 (0.56)*** | — |
Values are mean (standard deviation).
Different from Placebo Group (p < 0.03).
Different from Placebo Group (p < 0.006).
Different from Placebo Group (p < 0.001).
Different from Placebo Group (p < 0.002).
HEPE, hydroxyeicosapentaenoic acid; HETE, hydroxyeicosatetraenoic acid; HETrE, hydroxyeicosatrienoic acid; HODE, hydroxyoctadecadienoic acid.
The analysis of suction blister fluid also revealed the presence of COX-mediated eicosanoids that included TXB2, TXB3, PGJ2, Δ12PGJ2, PGE2, PGF1α, PGF2α, PGD2, and PGD3. However, there were no significant between-group differences in any of these eicosanoid levels at either time point (Table 4). Although TXB2 was present at 12 hours postblistering in the Active Group, it was not present at 24 hours postblistering. Conversely, TXB3 was detected in higher concentrations at 24 hours postblistering than at 12 hours in the Active Group, while PGF2α was reduced by 24 hours. PGD2 and PGD3 were only identified in the Active Group at 24 hours postblistering.
Table 4.
Levels of cyclooxygenase-mediated eicosanoids in blister fluid and plasma in Active (n=9) and Placebo (n=9) Groups
| Eicosanoids (pg/μL) | Blister fluid
|
Plasma
|
||||||
|---|---|---|---|---|---|---|---|---|
| 12 hours postblistering
|
24 hours postblistering
|
Baseline
|
28 days
|
|||||
| Active | Placebo | Active | Placebo | Active | Placebo | Active | Placebo | |
| TXB2 | 2.18 (1.58) | 1.48 (1.39) | — | — | 0.37 (0.23) | 0.37 (0.27) | — | 0.32 (0.38) |
| TXB3 | 0.53 (0.77) | 0.16 (0.39) | 1.88 (3.72) | — | 0.22 (0.23) | 0.06 (0.11) | 0.30 (0.21) | — |
| PGJ2 | 1.21 (2.49) | 0.86 (1.41) | 9.28 (18.36) | 1.72 (2.29) | — | — | — | — |
| Δ 12PGJ2 | 1.05 (2.30) | 1.48 (1.66) | 4.56 (9.08) | 2.67 (0.62) | — | — | — | — |
| PGE1 | — | — | — | — | 0.50 (0.16) | 0.46 (0.14) | 0.43 (0.21) | 0.45 (0.14) |
| PGE2 | 0.59 (0.25) | 0.59 (0.34) | 0.52 (0.71) | 0.32 (0.03) | 0.33 (0.10) | 0.28 (0.11) | 0.29 (0.13) | 0.26 (0.16) |
| PGE3 | — | — | — | — | 0.35 (0.42) | 0.39 (0.47) | 0.92 (0.25)* | 0.20 (0.39) |
| PGF1α | 1.98 (1.28) | 0.85 (1.07) | 0.58 (0.93) | 1.01 (1.29) | 0.35 (0.25) | 0.28 (0.17) | 0.49 (0.60) | 0.34 (0.35) |
| PGF2α | 7.45 (5.28) | 6.82 (2.81) | 3.87 (4.47) | 2.12 (1.08) | 0.78 (0.42) | 0.74 (0.41) | 0.83 (0.57) | 0.90 (0.46) |
| 6-keto-PGF1α | — | — | — | — | 0.26 (0.34) | 0.23 (0.27) | 0.18 (0.25) | 0.22 (0.36) |
| PGD1 | — | — | — | — | 0.12 (0.25) | 0.05 (0.07) | 0.13 (0.20) | 0.15 (0.22) |
| PGD2 | — | — | 1.49 (2.46) | — | — | 0.01 (0.003) | — | — |
| PGD3 | — | — | 3.23 (6.42) | — | — | — | 0.01 (0.01)* | — |
Values are mean (standard deviation).
Different from Placebo Group change score (baseline to 28 days) (p < 0.001).
PGE, prostaglandin E.
Lipid mediators in plasma
The plasma collected at baseline and at 28 days was also pooled by group and time point (baseline and 28 days) and analyzed for PUFA-derived lipid mediators by LC/ESI-MS/MS. We detected 9- and 13-HODE, 5-, 8-, 11-, 12-, and 15-HETE, and 5-HEPE in the plasma at baseline (presupplementation) and 9- and 13-HODE, 5-, 8-, 11-, 12-, and 15-HETE and 5-, 8-, 12-, 15-, and 18-HEPE at 28 days (Table 5). After calculating mean change scores from 0 to 28 days for the groups, the data revealed that when compared with the Placebo Group, the Active Group had significantly higher plasma levels of 5-HEPE (Z=−3.645, p < 0.001), 8-HEPE (Z=−3.830, p < 0.001), 12-HEPE (Z=−3.070, p=0.002), 15-HEPE (Z=−3.824, p < 0.001), and 18-HEPE (Z=−3.821, p < 0.001) (Table 5).
Levels of TXB2, TXB3, PGE1, PGE2, PGE3, dihydro-keto-PGE1, PGF1α, PGF2α, dihydro-keto-PGF1α, and 6-keto-PGF1α, PGD1, PGD2, and PGD3 were also detected in plasma. Following a similar pattern that suggested a push toward certain EPA-derived products in the Active Group, the data revealed a significantly higher mean change score for PGE3 (Z=−2.433, p < 0.001) and PGD3 (Z=−3.866, p < 0.001) than in the Placebo Group. No significant between-group differences were detected in any other PUFA product change score (Table 4).
MPO in blister wound fluid
Mean MPO levels within the acute blister fluid, indicating leukocyte activity, were lower for the Active Group at 12 hours postblistering when compared with the Placebo Group (Figure 5); however, the differences were not statistically significant.
Figure 5.
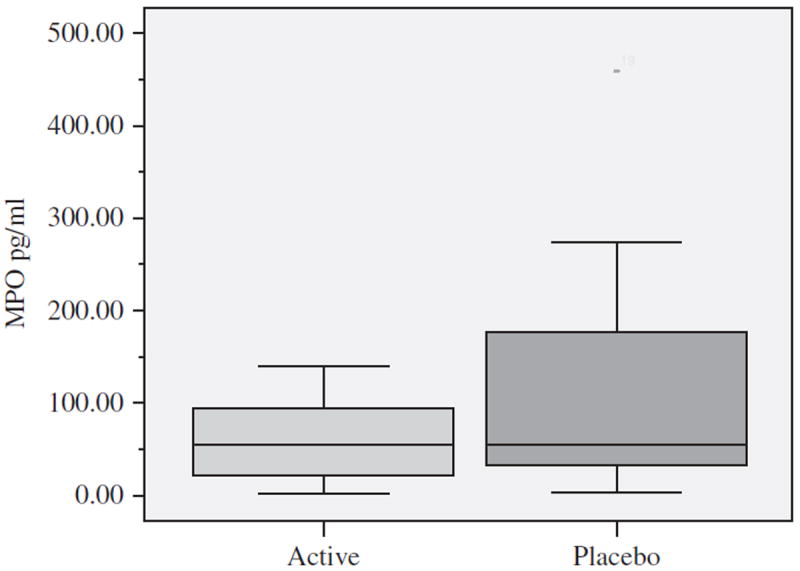
Mean myeloperoxidase (MPO) levels, a leukocyte marker enzyme, in blister wound fluid at 12 hours postblistering by Active (n=9) and Placebo (n=9) Groups expressed in pg/mL. Each box defines the middle 50% of the distribution. The vertical line crossing each box defines the median. Upper and lower bars represent the largest and the smallest values that are not outliers.
Wound reepithelialization
Figure 6 illustrates that the mean of the total wound area remaining to be reepithelialized for the Active Group (n=6), measured in cm2, was significantly less than the Placebo Group (n=6) on Day 5 postblistering (t=2.28, df=10, p=0.046). There were no significant group differences in total wound area on Day 1 or Day 2 postblistering between the Active (n=9) and Placebo (n=9) Groups.
Figure 6.
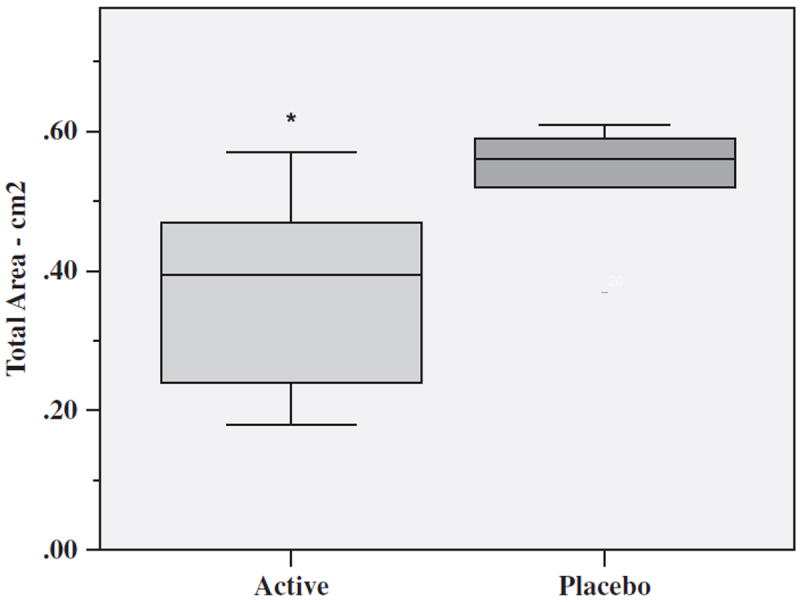
Total mean area of blisters remaining to reepithelialize on Day 5 for Active (n=6) and Placebo (n=6) Groups expressed in cm2. Each box defines the middle 50% of the distribution. The vertical line crossing each box defines the median. Upper and lower bars represent the largest and smallest values that are not outliers. *p=0.046 Active vs. Placebo Group.
Several participants were unable to keep scheduled appointments for wound photographs on postblister Days 8 and 10, preventing accurate comparisons of total area remaining to be reepithelialized on those days and total days to complete healing between the two groups.
DISCUSSION
The purpose of this study was to evaluate the effects of EPA + DHA supplementation on lipid mediators of inflammation and PMN in the acute wound microenvironment in healthy, young adults. Our data indicate that increasing EPA + DHA intake with oral supplements results in significant increases in EPA and DHA plasma levels compared with baseline values, significant decreases in total n-6 : n-3 and AA : EPA ratios, significantly higher plasma concentrations of selected EPA metabolites of LOX and COX pathways when compared with the Placebo Group and significant changes in levels of certain hydroxy fatty acids of the n-6 and n-3 metabolic 12/15 LOX pathways in acute wound fluid. By analyzing the fluid collected from blister wounds created on the study participants’ forearms, we find that the Active Group, who consumed the EPA + DHA oral supplements for 28 days, has significantly lower mean levels of 9-HODE and 15-HETrE at 24 hours postblistering than the Placebo Group and higher mean levels of 12-HEPE at 12 hours postblistering. These mediators have been associated with inflammation regulation. For example, 9-HODE, a product of 15-LOX n-6 LA metabolism, has showed a robust proinflammatory effect in a rat wound-healing model.30 Moch et al.30 reported that 3 days after 9-HODE was locally administered in the rat model of granulation tissue formation, PMN and macrophage numbers were significantly enhanced in the whole tissue, whereas the lymphocyte numbers were diminished, suggesting that 9-HODE has proinflammatory activity. A subsequent study found that 9-HODE induced a chemotactic response of both human and bovine PMNs in vitro.31 Additionally, Hattori et al.12 recently reported that 9-HODE–G2A signaling plays proinflammatory roles in skin under oxidative conditions. Taken together, these studies suggest that suppressing 9-HODE production may result in a reduction in the inflammatory response and PMN chemotaxis, which is in alignment with our data showing lower levels of MPO in the wound fluid, a leukocyte marker enzyme, in the Active Group when compared with the Placebo Group, at 12 hours postblistering, although this finding is not statistically significant (p=0.18).
Our data also illustrate that levels of the15-LOX product of DGLA, 15-HETrE, an inhibitor of 5-LOX, the enzyme converting AA to LTB4 and EPA to LTB5,11 are significantly lower in the Active Group at 24 hours post-blistering when compared with the Placebo Group. The ability of EPA + DHA oral supplements to affect this change in blister fluid levels of DGLA- and LA-derived metabolites of 15-LOX (9-HODE and 15-HETrE, respectively) is an interesting finding in that LA and DGLA are found abundantly in human skin.11 A similar trend is noted for the 15-LOX product of n-6 AA, 15-HETE, which is lower in the Active Group at 12 hours (9.8 ± 4.1 pg/μL blister fluid) and 24 hours (11.4 ± 10.8 pg/μL blister fluid) postblistering than in the Placebo Group (15.2 ± 11.7 and 11.9 ± 7.2 pg/μL blister fluid, 12 and 24 hours, respectively).
We also report a between-group difference in the 12-LOX EPA-derived 12-HEPE that approaches statistical significance, with the Active Group having higher mean levels at 12 hours postblistering than the Placebo Group. 12-HEPE was associated with an antiinflammatory profile in a study by Rose et al.,14 which assessed the impact of EPA on rabbit alveolar macrophages. That study reported a dose-dependent suppressed generation of the proinflammatory, potent neutrophil chemoattractant LTB4 and 12-HETE, a 12-LOX AA metabolite, in parallel with increased levels of the EPA-derived LTB5, and 12-HEPE, which were found to be devoid of proinflammatory potencies while sustaining antagonistic potencies.14 LTs are eicosanoids of the 5-LOX pathway of AA and EPA metabolism. Although our data support the findings reported in the Rose’s study14 related to EPA-derived 12-HEPE, we cannot make LT comparisons because LTs are not detected in the blister fluid of our study participants. It could be that LTs are in an area of the wound bed that our suction blister method cannot access. However, LTB4 has been detected in the suction blister fluid of certain skin conditions characterized by chronic inflammation.32 Alternatively, the lipid mediator profile expressed in vivo in the acute wound microenvironment in response to EPA supplementation may be different from that expressed in chronically inflamed skin or in in vitro animal models.
Our study findings of undetectable levels of LTs in the blister fluid, but lower levels of MPO in the Active Group when compared with the Placebo Group at 12 hours post-blistering, suggest that the reduction in leukocyte recruitment in the early inflammatory stage of healing occurs via other EPA/DHA-related mechanisms. One possibility involves the EPA metabolite of the COX pathway, PGD3. A recent study reported that PGD3, which we only discover in the blister fluid and plasma of the Active Group, inhibited the migration of neutrophils across endothelial cells.2 Together, our blister fluid data imply that the EPA + DHA supplements push an EPA profile in the wound microenvironment that includes less of the 15-LOX-derived products of n-6 LA metabolism (9-HODE), n-6 DGLA metabolism (15-HETrE), and AA metabolism (15-HETE) and more of the 12-LOX-derived product of n-3 EPA metabolism (12-HEPE).
The plasma eicosanoid findings in the current study also indicate a swing toward an n-3 EPA profile in the Active Group, who has significantly higher positive change scores (0–28 days) for the 5-LOX product 5-HEPE, the 12-LOX product 12-HEPE, the 15-LOX product 15-HEPE, and the COX products PGE3 and PGD3 when compared with the Placebo Group and greater concentrations of TXB3. The data show that it is possible to manipulate systemic levels of lipid mediators associated with inflammation reduction by increasing EPA + DHA intake, which may be beneficial when there is a heightened or prolonged inflammatory response (e.g., chronic wounds, surgery, trauma). Likewise, increased formation of 12- and 15-HEPE, PGD3 and TXB3 in the blister fluid of the Active Group suggests that systemic concentrations of EPA affect local levels and that EPA is preferentially diverted to LOX and COX pathways for selected eicosanoid synthesis. These findings are aligned with other studies showing improved fatty acid and eicosanoid patterns with the administration of lipid emulsions containing n-3 PUFAs.33,34
Furthermore, recent studies have showed that not only can EPA + DHA generate antiinflammatory eicosanoid patterns, they also give rise to the newly described COX-and LOX-generated mediators of inflammation, resolvins, and protectins that have strong antiinflammatory, proresolving actions involving PMN regulation.4,15 Their production is enhanced with low-dose aspirin (81 mg),15 and, thus, is the reason that aspirin was prescribed for both groups in the present study. It has also been reported that EPA and DHA are available for sites of acute inflammation directly from circulation and rapidly appear in inflammatory exudates to be converted to the resolvins (RvE, RvD) and protectins.3 Although we find that RvE and RvD levels are too low to be detected in 1 mL of plasma or blister fluid in the current study, we do detect the precursor of RvE, 18-HEPE, in the Active Group. Given that EPA and DHA are the precursors to several potent antiinflammatory lipid mediators, increasing EPA + DHA bioavailability with supplementation (+aspirin) may reduce the excessive inflammation found in chronic wounds and, thus, facilitate healing progression.
Our study data reveal that on Day 5 postblister formation, the Active Group has less total wound area remaining to be healed than that of the Placebo Group. We note no significant differences in total area to be healed on Days 1 or 2 between the two groups. These findings suggest that between Days 2 and 5 there is an enhanced reepithelialization in the Active Group. This effect could be as a result of the altered 12/15 LOX eicosanoid pattern and the lower MPO levels noted at 12 hours postblistering that suggest a dampening of the early PMN response to tissue injury and hasten inflammation resolution. Alternatively, the EPA + DHA + aspirin could increase local levels of resolvins and protectins that promote wound epithelialization by facilitating inflammation resolution, but are just not detected in the current study because of insufficient fluid samples. Past in vitro and in vivo studies in animal models have reported contradictory findings when comparing the effects of n-6 and n-3 PUFA administration on tissue repair. We have previously shown that EPA + DHA had little effect on acute wound reepithelialization; however, aspirin was not a part of that supplemental regimen.17 Other studies have suggested positive,35 negative,36 and no influences37 on various cell activities related to tissue regeneration. Interestingly, Shingel et al.38 recently reported a stimulation of dermal angiogenesis and tissue repair in complex wounds in a porcine model with a topical n-3 PUFA-rich solid emulsion gel. Few studies have evaluated the effects of EPA + DHA supplementation on wound reepithelialization in humans and of those, most have tested dietary formulas with EPA and other macro-nutrients that were administered via enteral or parenteral feedings. For instance, Farreras et al.39 reported that enteral nutrition supplemented with n-3 PUFAs and arginine increased hydroxyproline synthesis and improved wound healing in patients undergoing surgery for gastric cancer.
In conclusion, previous studies have showed that prolonged inflammation and excessive PMN activity in the wound microenvironment are associated with chronic wounds. Our study investigating acute wounds in healthy, young adults shows that it is possible to adjust the levels of local and systemic lipid mediators of inflammation with EPA + DHA oral supplementation, which may encourage inflammation resolution, PMN down-regulation and wound reepithelialization. These effects may be more profound in a chronic wound with protracted inflammation. Furthermore, considering that many patients with chronic wounds have nutritional deficiencies such as suboptimal caloric intake and lower than recommended daily intake of protein, vitamin C, and zinc, it is likely they consume nominal amounts of EPA and DHA.40 Additional clinical studies are needed to clarify the cellular and molecular effects of EPA + DHA on chronic wound healing before they are recommended as adjuncts to current treatment protocols. Nevertheless, the current study provides new evidence that boosting EPA + DHA intake with oral supplements can push an EPA + DHA lipid mediator profile in the acute wound microenvironment. If EPA + DHA supplementation can produce similar effects in the chronic wound and change the local milieu to one that is more favorable to healing, then it could prove to be a cost-effective treatment for this escalating, costly public health problem.
Acknowledgments
J.C.M. was supported by the Midwest Nursing Research Society, New Investigator Seed Grant Award. K.M. was supported by the Yorkshire Cancer Research (Pump Priming Grant). The technical assistance of Andrew Healey, University of Bradford Analytical Centre, is acknowledged.
Glossary
- AA
Arachidonic acid
- COX
Cyclooxygenase
- DGLA
Dihomogammalinolenic acid
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- HEPE
Hydroxyeicosapentaenoic acid
- HETE
Hydroxyeicosatetraenoic acid
- HETrE
Hydroxyeicosatrienoic acid
- HODE
Hydroxyoctadecadienoic acid
- LA
Linoleic acid
- LOX
Lipoxygenase
- LT
Leukotrienes
- LX
Lipoxins
- MPO
Myeloperoxidase
- PG
Prostaglandins
- PMN
Polymorphonuclear leukocytes
- PUFA
Polyunsaturated fatty acids
- TX
Thromboxane
References
- 1.Moor AN, Vachon DJ, Gould LJ. Proteolytic activity in wound fluids and tissues derived from chronic venous leg ulcers. Wound Repair Regen. 2009;17:832–9. doi: 10.1111/j.1524-475X.2009.00547.x. [DOI] [PubMed] [Google Scholar]
- 2.Tull SP, Yates CM, Maskrey BH, O’Donnell VB, Madden J, Grimble RF, Calder PC, Nash GB, Rainger GE. Omega-3 fatty acids and inflammation: novel interactions reveal a new step in neutrophil recruitment. PLoS Biol. 2009;7:e1000177. doi: 10.1371/journal.pbio.1000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J Immunol. 2008;181:8677–87. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–37. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calder PC. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2007;77:327–35. doi: 10.1016/j.plefa.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Gilroy DW. Eicosanoids and the endogenous control of acute inflammatory resolution. Int J Biochem Cell Biol. 2010;42:524–8. doi: 10.1016/j.biocel.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233:674–88. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 8.Sijben J, Calder P. Differential immunomodulation with long-chain n-3 PUFA in health and chronic disease. Proc Nutr Soc. 2007;66:237–59. doi: 10.1017/S0029665107005472. [DOI] [PubMed] [Google Scholar]
- 9.Fogh K, Kragballe K. Eicosanoids in inflammatory skin diseases. Prostaglandins Other Lipid Mediat. 2000;63:43–54. doi: 10.1016/s0090-6980(00)00096-4. [DOI] [PubMed] [Google Scholar]
- 10.Moreno JJ. Differential effects of arachidonic and eicosapen-taenoic acid-derived eicosanoids on polymorphonuclear transmigration across endothelial cell cultures. J Pharmacol Exp Ther. 2009;33:1111–7. doi: 10.1124/jpet.109.157891. [DOI] [PubMed] [Google Scholar]
- 11.Ziboh VA, Cho Y, Mani I, Xi S. Biological significance of essential fatty acids/prostanoids/lipoxygenase-derived monohydroxy fatty acids in the skin. Arch Pharm Res. 2002;25:747–58. doi: 10.1007/BF02976988. [DOI] [PubMed] [Google Scholar]
- 12.Hattori T, Obinata H, Ogawa A, Kishi M, Tatei K, Ishikawa O, Izumi T. G2A plays proinflammatory roles in human keratinocytes under oxidative stress as a receptor for 9-hydroxyoctadecadienoic acid. J Invest Dermatol. 2008;128:1123–33. doi: 10.1038/sj.jid.5701172. [DOI] [PubMed] [Google Scholar]
- 13.Hammarstrom S, Hamberg M, Samuelsson B, Duell EA, Stawiski M, Voorhees JJ. Increased concentrations of nonesterified arachidonic acid, 12L-hydroxy-5,8,10,14-eicosatetraenoic acid, prostaglandin E2, and prostaglandin F2alpha in epidermis of psoriasis. Proc Natl Acad Sci USA. 1975;72:5130–4. doi: 10.1073/pnas.72.12.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose F, Kiss L, Grimminger F, Mayer K, Grandel U, Seeger W, Bieniek E, Sibelius U. E. coli hemolysin-induced lipid mediator metabolism in alveolar macrophages: impact of eicosapentaenoic acid. Am J Physiol Lung Cell Mol Physiol. 2000;279:L100–9. doi: 10.1152/ajplung.2000.279.1.L100. [DOI] [PubMed] [Google Scholar]
- 15.Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. 2007;282:9323–34. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 16.American Heart Association Nutrition Committee. Lichten-stein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 17.McDaniel JC, Belury M, Ahijevych K, Blakely W. Omega-3 fatty acids effect on wound healing. Wound Repair Regen. 2008;16:337–45. doi: 10.1111/j.1524-475X.2008.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996;63:116–22. doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- 19.Rees D, Miles E, Banerjee T, Wells S, Roynette C, Wahle K, Calder PC. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: a comparison of young and older men. Am J Clin Nutr. 2006;83:331–42. doi: 10.1093/ajcn/83.2.331. [DOI] [PubMed] [Google Scholar]
- 20.Kuhns DB, DeCarlo E, Hawk DM, Gallin JI. Dynamics of the cellular and humoral components of the inflammatory response elicited in skin blisters in humans. J Clin Invest. 1992;89:1734–40. doi: 10.1172/JCI115775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 22.Shantha NC, Decker EA, Hennig B. Comparison of methylation methods for the quantitation of conjugated linoleic acid isomers. J AOAC Int. 1993;76:644–9. [Google Scholar]
- 23.Glaser R, Kiecolt-Glaser JK, Marucha PT, MacCallum RC, Laskowski BF, Malarkey WB. Stress-related changes in pro-inflammatory cytokine production in wounds. Arch Gen Psychiat. 1999;56:450–6. doi: 10.1001/archpsyc.56.5.450. [DOI] [PubMed] [Google Scholar]
- 24.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994;19:313–33. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 25.Masoodi M, Mir AA, Petasis NA, Serhan CN, Nicolaou A. Simultaneous lipidomic analysis of three families of bioactive lipid mediators leukotrienes, resolvins, protectins and related hydroxy-fatty acids by liquid chromatography/electrospray ionisation tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:75–83. doi: 10.1002/rcm.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhodes LE, Gledhill K, Masoodi M, Haylett AK, Brownrigg M, Thody AJ, Tobin DJ, Nicolaou A. The sunburn response in human skin is characterized by sequential eicosanoid profiles that may mediate its early and late phases. FASEB J. 2009;23:3947–56. doi: 10.1096/fj.09-136077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masoodi M, Nicolaou A. Lipidomic analysis of twenty-seven prostanoids and isoprostanes by liquid chromatography/electrospray tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:3023–9. doi: 10.1002/rcm.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–55. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 29.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83(Suppl. 6):1467S–76S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 30.Moch D, Schewe T, Kuhn H, Schmidt D, Buntrock P. The linoleic acid metabolite 9DS-hydroxy-10,12(E,Z)-octadecadienoic acid is a strong proinflammatory mediator in an experimental wound healing model of the rat. Biomed Biochim Acta. 1990;49:201–7. [PubMed] [Google Scholar]
- 31.Henricks PA, Engels F, van der Vliet H, Nijkamp FP. 9- and 13-hydroxy-linoleic acid possess chemotactic activity for bovine and human polymorphonuclear leukocytes. Prostaglandins. 1991;41:21–7. doi: 10.1016/0090-6980(91)90101-k. [DOI] [PubMed] [Google Scholar]
- 32.Barr RM, Brain S, Camp RD, Cilliers J, Greaves MW, Mallet AI, Misch K. Levels of arachidonic acid and its metabolites in the skin in human allergic and irritant contact dermatitis. Br J Dermatol. 1984;111:23–8. doi: 10.1111/j.1365-2133.1984.tb04012.x. [DOI] [PubMed] [Google Scholar]
- 33.Mayer K, Fegbeutel C, Hattar K, Sibelius U, Kramer HJ, Heuer KU, Temmesfeld-Wollbrück B, Gokorsch S, Grimminger F, Seeger W. Omega-3 vs. omega-6 lipid emulsions exert differential influence on neutrophils in septic shock patients: impact on plasma fatty acids and lipid mediator generation. Intensive Care Med. 2003;29:1472–81. doi: 10.1007/s00134-003-1900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimm H, Mertes N, Goeters C, Schlotzer E, Mayer K, Grimminger F, Fürst P. Improved fatty acid and leukotriene pattern with a novel lipid emulsion in surgical patients. Eur J Nutr. 2006;45:55–60. doi: 10.1007/s00394-005-0573-8. [DOI] [PubMed] [Google Scholar]
- 35.Ruthig DJ, Meckling-Gill KA. Both (n-3) and (n-6) fatty acids stimulate wound healing in the rat intestinal epithelial cell line, IEC-6. J Nutr. 1999;129:1791–8. doi: 10.1093/jn/129.10.1791. [DOI] [PubMed] [Google Scholar]
- 36.Scardino ME, Swaim SF, Sartin EA, Hoffman CE, Oglivie GK, Hanson RA, Coolman SL, Davenport DJ. The effects of omega-3 fatty acid diet enrichment on wound healing. Vet Dermatol. 1999;10:283–90. doi: 10.1046/j.1365-3164.1999.00148.x. [DOI] [PubMed] [Google Scholar]
- 37.Gercek A, Yildirim O, Konya D, Bozkurt S, Ozgen S, Kilic T, Sav A, Pamir N. Effects of parenteral fish-oil emulsion (omegaven) on cutaneous wound healing in rats treated with dexamethasone. J Parenter Enteral Nutr. 2007;31:161–6. doi: 10.1177/0148607107031003161. [DOI] [PubMed] [Google Scholar]
- 38.Shingel KI, Faure MP, Azoulay L, Roberge C, Deckelbaum RJ. Solid emulsion gel as a vehicle for delivery of polyunsaturated fatty acids: implications for tissue repair, dermal angiogenesis and wound healing. J Tissue Eng Regen Med. 2008;2:383–93. doi: 10.1002/term.101. [DOI] [PubMed] [Google Scholar]
- 39.Farreras N, Artigas V, Cardona D, Rius X, Trias M, Gonzalez JA. Effect of early postoperative enteral immunonutrition on wound healing in patients undergoing surgery for gastric cancer. Clin Nutr. 2005;24:55–65. doi: 10.1016/j.clnu.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Tobon J, Whitney JD, Jarrett M. Nutritional status and wound severity of overweight and obese patients with venous leg ulcers: a pilot study. J Vasc Nurs. 2008;26:43–52. doi: 10.1016/j.jvn.2007.12.002. [DOI] [PubMed] [Google Scholar]


