Abstract
Most systemic cancer therapies target tumor cells directly though there is increasing interest in targeting the tumor stroma that can comprise a substantial portion of the tumor mass. We report here a synergy between two T cell therapies, one directed against the stromal tumor vasculature and the other directed against antigens expressed on the tumor cell. Simultaneous transfer of genetically engineered syngeneic T cells expressing a chimeric antigen receptor targeting the Vascular Endothelial Growth Factor Receptor-2 (VEGFR-2; KDR) that is over expressed on tumor vasculature and T cells specific for the tumor antigens gp100 (PMEL), TRP-1 (TYRP1), or TRP-2 (DCT) synergistically eradicated established B16 melanoma tumors in mice and dramatically increased the tumor-free survival of mice compared to treatment with either cell type alone or T cells coexpressing these two targeting molecules. Host lymphodepletion prior to cell transfer was required to mediate the anti-tumor effect. The synergistic antitumor response was accompanied by a significant increase in the infiltration and expansion and/or persistence of the adoptively transferred tumor antigen-specific T cells in the tumor microenvironment and thus enhanced their anti-tumor potency. The data presented here emphasize the possible beneficial effects of combining anti-angiogenic with tumor-specific immunotherapeutic approaches for the treatment of patients with cancer.
Keywords: Adoptive cell therapy, anti-angiogenesis, gene therapy, anti-VEGFR2 chimeric antigen receptor, tumor-specific TCR
Introduction
The adoptive transfer of autologous tumor-infiltrating lymphocytes (TIL) or peripheral blood T cells genetically engineered with conventional T cell receptors (TCR) or chimeric antigen receptors (CAR) to recognize cancer antigens can result in durable objective regression in patients with a variety of cancer types including metastatic melanoma, sarcomas, lymphomas, and neuroblastoma (1-7). Preclinical and clinical studies have identified multiple inhibitory mechanisms evolved by tumors to escape from immune surveillance including the maintenance of an immune inhibitory tumor microenvironment (8-10). Furthermore, solid tumor cells can be heterogeneous; thus, inhibiting one target will affect some, but not all, tumor cells. Ongoing tumor angiogenesis can mediate immune suppressive activity through down regulation of adhesion molecules on the vascular endothelium that are involved in leukocyte interactions and inhibit leukocyte extravasation into the tumor (11-14). However, most anti-angiogenic cancer monotherapies selectively targeting tumor vasculature have limited clinical benefit for patients with advanced malignancies due to redundancies of angiogenesis factors and pathways (15-17). Recently we have shown that adoptive transfer of T cells engineered with a chimeric antigen receptor (CAR) against VEGFR-2, overexpressed in tumor vasculature transiently inhibited tumor growth in mice with a variety of established tumor types.
Studies in murine models of ACT demonstrated that tumor-specific T cells if administered with IL-2 and a vaccine that stimulates the transferred cells can result in tumor regression in mice but also rarely provide long-term cure and tumor-free survival (19, 20). Here we show that simultaneous attack against specific cancer antigens and the tumor vasculature by coadministration of syngeneic T cells transduced with a vascular-specific anti-VEGFR2 CAR along with cells expressing a tumor-specific TCR resulted in a synergistic anti-tumor effect and prolonged tumor-free survival of mice with established cancers. These results open new possibilities for the application of this combination ACT for the treatment of a wide variety of cancer types.
Materials and Methods
Mice and cell culture assays
All animal studies were performed in accordance with the Animal Care and Use Committee guidelines of the NIH and were conducted under protocols approved by the Animal Care and Use Committee of the NCI. Details of the mouse strains and cell culture conditions used in this paper are described in Supplementary Methods.
Recombinant retroviral vectors, vaccinia viral vaccines
The recombinant retroviral vector constructs used in this study are schematically illustrated in Figure 3A and are described in the corresponding figure legend and Supplementary Methods. Details of the molecular sequences and methods used to generate these retroviral constructs have been described elsewhere (18, 21-23). The recombinant vaccinia viral vaccines (rVVs) expressing human gp100 (hgp100) or mouse TRP1 or TRP2 were used as vaccines in adoptive transfer experiments in this study were described elsewhere (19, 24).
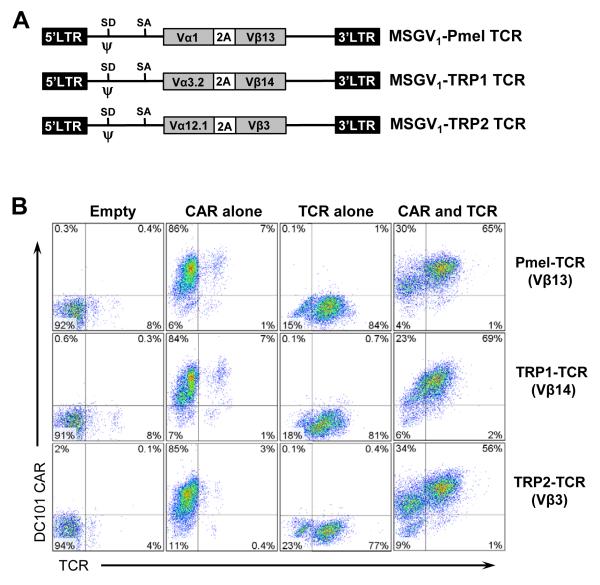
Figure 3. Retroviral transduction of mouse T lymphocytes.
A, schematic representation of tumor antigen-specific TCRs expressing recombinant retroviral vectors used in this study. In the MSGV1 retroviral vectors expressing the codon optimized Pmel TCR (Vα1, Vβ13), TRP-1 TCR (Vα3.2, Vβ14), or TRP-2 TCR (Vα12.1, Vβ3) recognizing the murine gp100, tyrosinase-related-protein-1 (TRP-1), or tyrosinase-related-protein-2 (TRP-2) antigens respectively, the corresponding Vα and Vβ cDNAs were fused together using the picornavirus foot-and-mouth disease virus 2A sequence. SD, splice donor; SA, splice acceptor; LTR, long terminal repeat; PA2, polyadenylation signal. B, enriched splenic CD3+ T cells from C57BL/6 mice were stimulated for 2 days with ConA and IL-7 and then transduced with an empty or anti-VEGFR2 CAR retroviral vector. The next day, cells were transduced with retroviral vector expressing one of the TCRs or left untransduced and analyzed by FACS tw days later. Cells were also costained for CD3ε expression using allo phycocyanin (APC) conjugated rat anti-mouse CD3ε. FACS data showing the percentage of cells in gated CD3+ viable T cells are presented. Data are representative of 2 independent experiments.
Retroviral transduction of mouse T lymphocytes
The anti-VEGFR2 CAR (DC101 CAR) expressing retroviral vector supernatant was produced from a stable phoenix Eco producer clone as described previously (18). All other retroviral vector supernatants were generated in Platinum Eco packaging cell line stably expressing retroviral GAG and POL proteins as described elsewhere (18). CD3+ T cells were purified from the splenocytes of WT mice using Dynal mouse T cell negative isolation kit (Invitrogen Corp). WT T cells were stimulated for 24-48 hours with 2 μg/mL Concanavalin A (Sigma) and 1 ng/mL recombinant mouse IL-7 (R&D Systems) in mouse T cell media as described in Supplementary Methods. Tg-Pmel-1 splenocytes were cultured for 2 days in the mouse T cell media in the presence of 1 μM/L hgp10025–33 peptide. Two days post stimulation T cells were transduced with retroviral vectors as previously described (18). Cultured cells were adoptively transferred 3-5 days post transduction (> 95% CD8+ T cells).
Transgene analysis by flow cytometry
Expression of the anti-VEGFR2 CAR (DC101 CAR) on retrovirally transduced mouse T cells was detected by flow cytometry with soluble mouse VEGFR2-hIgG.Fc protein (R&D Systems), followed by staining with a phycoerythrin (PE)-labeled goat anti-human IgG.Fc (α-hIgG.Fc) antibody (eBioscience) as described previously (18). Expression of SP6 CAR on transduced mouse T cells was detected as described previously (18). Transduction efficiency of Pmel TCR, TRP1 TCR, and TRP2 TCR in CD3+ T cells was determined by staining the cells with the fluorescein isothiocyanate (FITC) conjugated antibodies against Vβ13, Vβ14, and Vβ3 respectively (BD Phamingen, San Diego, CA). Cells were also costained for CD3ε expression using allo phycocyanin (APC) conjugated rat anti-mouse CD3ε. Flow cytometry acquisitions were performed on a FACSCalibur and analyzed with FlowJo software (TreeStar, Eugene, OR).
Cytokine release assays
Transduced mouse T cells were tested for specific reactivity against target cells using standard overnight coculture IFN-γ release assays as previously described (18). The. MB49 (mouse bladder tumor cell line negative for the expression of gp100 antigen and VEGFR-2) and MB49-Flk1 cells expressing full length coding sequence of mouse VEGFR-2 (18) were pulsed with 0-1 μM hgp10025–33 or 1 μM irrelevant influenza nucleoprotein peptide (NP) were used as targets.
Adoptive T cell transfer
Six to eight week-old C57BL/6 mice (n = 5-9 for all groups) were injected subcutaneously with 5 × 105 B16 melanoma cells. After 10 to 14 days, they were irradiated with 5 Gy of total-body irradiation (TBI) and treated intravenously (i.v.) with transduced or untransduced WT and/or Pmel T cells as indicated in the results. All treated mice received 2.2 × 105 IU rhIL-2 twice a day intraperitoneally for 3 consecutive days. Where indicated mice also received a single dose of 2×107 plaque-forming units (PFU) of recombinant vaccinia viral vaccine (rVV) coding for the relevant antigen (hgp100 or mouse TRP-1 or TRP-2) recognized by transferred T cells at the time of cell transfer in order to systematically modulate the intensity of antigen restimulation in vivo. All tumor measurements were performed in a blinded, randomized fashion and performed independently at least twice with similar results.
Enumeration of adoptively transferred T cells
Spleens and tumors from 3 mice in each treatment and control groups were harvested at the indicated time points and single cell suspensions were made by crushing the tissues through a 40 μ cell strainer. Splenocytes were obtained after red blood cell lysis. Dead cells were removed from the tumor suspensions by centrifugation with Lympholyte M. (Cedarlane Laboratories). Cells were then stained with FITC conjugated anti-rat Thy1.1 (CD90.1) and PE labeled anti-mouse Ly5.1 antibodies (both from BD Biosciences). The absolute numbers of Ly5.1+ and Thy1.1+ cells were calculated by multiplying the absolute cell count by the total percentage of Ly5.1+ and Thy1.1+ cells. Fold changes in the absolute number of Thy1.1+ Pmel T cells in spleen or tumor tissues was calculated by dividing the absolute numbers of Thy1.1+ cells per spleen or per gram tumor of mice receiving a mixture of anti-VEGFR2 CAR transduced T cells and Tg-Pmel T cells with that of mice treated with a mixture of SP6 CAR transduced T cells and Tg-Pmel T cells.
Statistical analysis
Tumor growth slopes were compared using Wilcoxon rank sum test. P values less than 0.05 were considered significant. Student’s t tests were used to test for significant differences in enumeration assays. A P value of 0.05 or lower was considered significant.
Results
Anti-VEGFR2 CAR expression and ex vivo functional integrity of retrovirally engineered Tg-Pmel T cells
Anti-VEGFR2 CAR (DC101 CAR) transduction resulted in CAR expression in approximately 92% (range 83-95%) of T cells derived from wild type (Wt) mice (Wt/DC101CAR)and 85% (range 78-92%) of Tg-Pmel T cells (Tg-Pmel/DC101CAR) (Fig. 1A).Both the Wt/DC101 CAR and the Tg-Pmel/DC101 CAR T cells specifically secreted IFN-γ when cocultured with the VEGFR-2 expressing MB49-Flk1 cells, but failed to respond to VEGFR-2 negative MB49 cells (Fig. 1B). Similarly both the untransduced and the anti-VEGFR2 CAR transduced Tg-Pmel T cells secreted IFN-γ in response to MB49 tumor cells that were pulsed with hgp10027-33 peptide but not to those pulsed with an irrelevant peptide. These results suggest that anti-VEGFR2 CAR expressing Pmel T cells (Tg-Pmel/DC101 CAR) retained their native TCR function while genetically modified to confer dual specificity through a MHC-unrestricted chimeric receptor. When the targeting specificities were present on different effector T cells (Tg-Pmel + DC101 CAR) they could independently recognize their target antigen and generate an IFN-γ response, which was greater than that obtained with effector T cells possessing both the targeting specificities.
Figure 1. Anti-VEGFR2 CAR expression and ex vivo functional integrity of retrovirally engineered Tg-Pmel T cells.
A, CD3+ T cells from splenocytes of Wt or transgenic Pmel mice were stimulated with ConA and IL-7 or 1 μM hgp10025–33 peptide respectively for 2 days in T cell media containing 30 IU /mL rhIL-2 and transduced with retroviral vectors expressing an anti-VEGFR2 CAR (DC101 CAR) or an empty vector. Two days later T cells were analyzed for expression of the DC101 CAR and Pmel TCR (measured by Vβ13 staining) by FACS. Cells were also costained for CD3ε expression using allo phycocyanin (APC) conjugated rat anti-mouse CD3ε. CD3+ viable T cells were gated. Representative FACS data from 3 experiments showing the percentage of cells in each quadrant are shown. B, Two days after transduction, 105 effector mouse T cells were cocultured with indicated target cells at 1:1 ratio for 18 hours. Where indicated 2 effector T cell types were mixed in equal numbers (each 5 × 104) and cocultured with 105 target cells. Target cells were pulsed with indicated concentrations of either hgp10025-33 peptide or irrelevant control peptide prior to coculture. Culture supernatants were assayed for secreted IFN-γ by ELISA. The data shown are representative of two independent experiments.
In vivo functional activity of anti-VEGFR CAR transduced Tg-Pmel T cells
We next treated groups of mice bearing 10-12 day-old B16 melanoma with different numbers of Tg-Pmel T cells engineered to express an anti-VEGFR-2 CAR or a control vector, or a mixture of Tg-Pmel T cells and the Wt open repertoire T cells transduced with an anti-VEGFR2 CAR or a control vector.
As shown in Figure 2A, tumors in groups receiving no treatment or 107 empty vector transduced T cells grew steadily and 106 or 105 anti-VEGFR2 CAR (DC101 CAR) transduced T cells had little or no effect on B16 tumor growth. However, as demonstrated previously (18) 107 anti-VEGFR2 CAR engineered open repertoire T cells mediated a significant anti-tumor effect compared to the no treatment group and to the group treated with 107 empty vector transduced T cells (P = 0.002 and 0.001 respectively; Fig. 2A, left panel). The Tg-Pmel T cells mediated significant but transient tumor growth inhibition at all the dose levels tested compared to the no treatment group (P = 0.01; 0.002; 0.002 for 105, 106, and 107 T cells respectively). Notably, transduction of the anti-VEGFR2 CAR into Tg-Pmel cells did not enhance the anti-tumor efficacy compared to Tg-Pmel or anti-VEGFR2 CAR T cells alone. In contrast, a synergistic antitumor effect mediating complete durable regression of tumors was seen in mice treated with a mixture of Tg-Pmel T cells and anti-VEGFR-2 CAR engineered open repertoire T cells (Tg-Pmel + DC101 CAR) at all cell doses studied compared to those treated with same number of Tg-Pmel or anti-VEGFR2 CAR transduced Wt T cells alone (P values are shown in Fig. 2A). Tumors in mice treated with a single dose 107, 106, or 105 Tg-Pmel T cells mixed with 107 but not with 106 VEGFR2 CAR transduced T cells regressed completely by day 15-21 post treatment. These results demonstrated that the presence of anti-VEGFR2 CAR T cells significantly enhanced the anti-tumor efficacy of tumor-specific TCR expressing T cells provided these two targeting molecules with different specificities were present on two different T cells rather than coexpressed on a single T cell. Furthermore, while administration of tumor antigen-specific rVV did not affect the anti-tumor efficacy of the anti-VEGFR2 CAR transduced Tg-Pmel or the Wt T cells, it significantly enhanced the tumor treatment effect (P = 0.008) and tumor-free survival mediated by the 106 Tg-Pmel T cells administered in conjunction with 5 × 106 anti-VEGFR2 CAR transduced Wt T cells (Fig. 2B). These results suggest that the functionality of the tumor antigen-specific TCR was greatly reduced when they were enforced to possess dual specificity against a completely different target cell type, in this case the VEGFR-2 expressing cells in the tumor environment.
Figure 2. Cotransfer of anti-VEGFR2 CAR transduced open repertoire T cells and Tg-Pmel T cells induced durable tumor regression compared to Tg-Pmel transduced with anti-VEGFR2 CAR.
A, groups of 5 C57BL/6 mice bearing B16 tumors were sublethally irradiated with 5 Gy TBI and treated with 105, 106, or 107 Tg-Pmel T cells, open repertoire T cells from Wt mice transduced with an empty vector or an anti-VEGFR CAR, or Tg-Pmel T cells transduced with an anti-VEGFR2 CAR. Some groups received a combination of Tg-Pmel T cells and anti-VEGFR2 CAR transduced open repertoire T cells. Control groups received neither T cells nor vaccine nor rhIL-2. All treatment groups received a single dose of 2 × 107 pfu vaccinia virus expressing hgp100 antigen and 2 daily doses of 2.2 × 105 IU rhIL-2 per dose for 3 consecutive days. Serial, blinded tumor measurements were obtained and the products of perpendicular diameters were plotted ± SEM. The data shown are representative of three independent experiments. B, groups of 5 C57BL/6 mice bearing B16 tumors were sublethally irradiated with 5 Gy TBI and treated with different numbers and combinations of T cells as indicated in the figure. Tumor area (left) and survival (right) in mice receiving various treatments compared with untreated controls are shown. All treatment groups received rhIL-2 for 3 days. Where indicated some groups received hgp100 expressing vaccinia virus vaccine in conjunction with cell transfer. The data shown are representative of 2 independent experiments.
Notably, in mice receiving a mixture of 106 Tg-Pmel and 5 × 106 anti-VEGFR2 CAR engineered T cells tumor growth was significantly inhibited without the administration of tumor antigen-specific viral vaccine compared to the control groups receiving 106 Tg-Pmel T cells or 106 Wt T cells mixed with 5 × 106 empty vector transduced Wt T cells without rVV administration or rVV alone without T cells or any treatment (P = 0.01, 0.007, 0.003, and 0.009 respectively) and also to 106 Wt T cells mixed with 5 × 106 empty vector transduced Wt T cells transferred together with rVV (P = 0.007; Fig. 2B). However, their tumor treatment effect was comparable or equal to groups receiving 106 Tg-Pmel T cells mixed with 5 × 106 empty vector transduced Wt T cells and rVV or 106 Wt T cells mixed with 5 × 106 anti-VEGFR2 CAR transduced Wt T cells administered in conjunction with or without rVV expressing hgp100 antigen. Importantly the tumor treatment efficacy was significantly enhanced in mice treated with a mixture of Tg-Pmel T cells and anti-VEGFR2 CAR T cells in conjunction with rVV compared to group receiving the same T cell mixture but no vaccine (P = 0.008) and resulted in complete tumor regression and long-term tumor-free survival of mice (Fig. 2B).
In vivo tumor treatment efficacy of combination therapy using anti-VEGFR CAR and/or tumor antigen-specific TCR transduced open repertoire T cells
We next evaluated whether the results obtained in our previous experiments were restricted to Tg-Pmel T cells or could be extended to open repertoire T cells genetically engineered to express an MHC class I or II restricted antigen-specific TCR. We used MSGV-based retroviral vectors expressing MHC class I restricted TCRs recognizing the gp100 or TRP-2 antigens or a MHC class II restricted TCR recognizing the TRP-1 antigen (Fig. 3A). All three antigens are widely expressed on the B16 mouse melanoma. We transduced C57BL/6 splenocytes with an empty vector or an anti-VEGFR2 CAR vector and/or one of the retroviral constructs expressing a TCR shown in Figure 3A. On day 3 post transduction, the anti-VEGFR2 CAR was expressed in 85-95% of the T cells (Fig. 3B). Similarly, in three independent experiments all three retroviral constructs expressing TCR genes resulted in expression of the respective TCR in 74 to 87% of cells when transduced alone. If the anti-VEGFR-2 CAR expressing T cells were cotransduced with retroviral vectors expressing one of the TCRs, the coexpression of the Pmel TCR, TRP-1 TCR, and TRP2 TCR was detectable in 54 to 75% of cells (Fig. 3B).
Adoptive transfer of a mixture of T cells transduced with an antigen-specific TCR and the anti-VEGFR2 CAR induced a pronounced synergistic inhibitory effect on tumor growth and increased the tumor-free survival of mice compared to treatment with only one of these transduced T cells or those cotransduced to express both of the TCR and the CAR (Fig. 4). Although the 106 TCR or 5 × 106 anti-VEGFR2 transduced T cells transiently controlled the tumor growth, the treatment effect was ineffective beyond 3 weeks. Empty vector transduced T cells and low numbers (106) of anti-VEGFR2 CAR transduced T cells failed to control tumor growth similar to the no treatment group.
Figure 4. Simultaneous transfer of anti-VEGFR2 CAR and TCR engineered mouse T cells induced regression of established syngeneic tumors in mice and increased their tumor-free survival.
Enriched splenic CD3+ T cells obtained from C57BL/6 mice were stimulated for 2 days with ConA and IL-7 and then transduced with an empty or anti-VEGFR2 CAR retroviral vector. The next day, cells were transduced with retroviral vector expressing one of the TCRs or left untransduced. Two days later transduced T cells were adoptively transferred into B16 tumor bearing C57BL/6 mice (5 mice per group) as indicated in the figure. Animals received 5 Gy TBI prior to T cell transfer and concurrently received vaccine and rhIL-2 as described in legend for Figure 2A. Serial, blinded tumor measurements were obtained and the products of perpendicular diameters were plotted ± SEM. The data shown are representative of 2 independent experiments.
Coadministration of anti-VEGR2 CAR transduced T cells enhanced the effective infiltration and persistence of the adoptively transferred tumor-specific T cells in the tumor
Next we studied whether the enhanced synergistic anti-tumor effect of combination therapy with tumor-specific T cells and the anti-VEGFR2 CAR T cells was due to their increased infiltration and/or persistence at the tumor site. Mice bearing 10 day-old B16 melanoma were lymphodepleted by 5 Gy TBI prior to treatment and intravenously injected with either 106 untransduced Wt or Tg-Pmel T cells expressing the congenic marker Thy1.1 mixed with 5 × 106 Ly5.1 marker positive T cells engineered with an irrelevant control CAR (SP6 CAR specific for a synthetic hapten) or an anti-VEGFR2 CAR together with vaccinia virus vaccine expressing the gp100 antigen. Single cell preparations of spleen and tumor samples obtained from 3 mice in each group on 3, 6, and 9 days post T cell transfer and were analyzed by flow cytometry to determine the total number of adoptively transferred Thy1.1+ and Ly5.1+ T cells. Representative FACS data showing the percentage of viable Thy1.1+ and Ly5.1+ T cells in spleen and tumor tissues of one mouse in each group are presented in Figure 5. The average absolute numbers of viable Thy1.1+ and Ly5.1+ T cells in spleen and tumor (normalized to per gram tumor) tissues of 3 mice in each group are presented in Figure 6A. Both the percentage and number of Tg-Pmel T cells (Thy1.1+) in spleen and tumor were increased at all-time points if they were cotransferred with the anti-VEGFR2 CAR engineered T cells (Ly5.1+) but not with T cells expressing a control SP6 CAR (Ly5.1+).
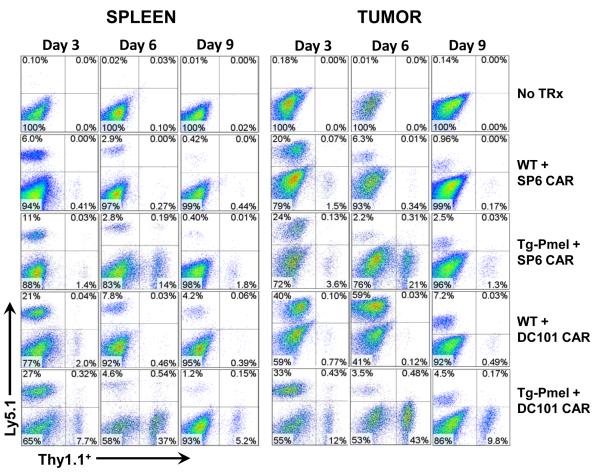
Figure 5. Tg-Pmel T cells effectively infiltrate and persist in vivo in the tumor if adoptively transferred in conjunction with anti-VEGFR2 CAR transduced T cells.
C57BL/6 mice bearing B16 melanoma tumor were sublethally irradiated with 5 Gy TBI and treated with 106 Thy1.1+ Wt T cells or 106 Thy1.1+ Tg-Pmel T cells mixed with 5 × 106 Ly5.1+ syngeneic open repertoire Wt T cells transduced with a retroviral vector expressing the control CAR (SP6 CAR) or an anti-VEGFR2 CAR (DC101 CAR). Animals concurrently received a single dose of 2 × 107 pfu vaccinia virus expressing hgp100 and twice daily rhIL-2 administration for 3 days. Control group received no treatment. Tumors and spleens of 3 mice from each group were excised at different time points post therapy and single cell suspensions were made as described in materials and methods. Cell preparations were stained with FITC-labeled anti-rat Thy1.1 and PE-labeled mouse anti-mouse Ly5.1 antibodies and analyzed by flow cytometry. Representative flow cytometry data from single cell preparations of spleen and tumor tissues from one mouse in each group obtained on day 3, 6, and 9 post T cell treatment indicating the percentage Thy1.1+ and Ly5.1+ cells gated in the total viable cell population are shown.
Figure 6. Enhanced early infiltration and durable persistence of Tg-Pmel T cells adoptively transferred in conjunction with anti-VEGFR2 CAR transduced T cells.
C57BL/6 mice bearing B16 melanoma tumor were sublethally irradiated with 5 Gy TBI. Mice received T cell treatments as described in Figure 5. Single cell preparations from tumors and spleens of 3 mice from each group were prepared and analyzed by flow cytometry as described in Figure 5. The absolute numbers of Thy1.1+ and Ly5.1+ cells were determined by multiplying the % Thy1.1+ and Ly5.1+ cells obtained by FACS by the total number of viable cells. A, pooled data obtained from three mice from each group collected at indicated time points post ACT showing the absolute numbers of Thy1.1+ and Ly5.1+ cells in spleen and tumor tissues. B, fold changes in the absolute numbers of Thy1.1+ in the spleen and tumor tissues of mice treated with a mixture of 106 Tg-Pmel and 5 × 106 anti-VEGFR2 CAR (DC101 CAR) transduced Wt T cells compared to that of mice treated with a mixture of 106 Tg-Pmel and 5 × 106 SP6 CAR (control CAR) transduced Wt T cells. The data shown are representative of 2 independent experiments.
In our ACT model, we administered vaccinia virus expressing hgp100 vaccine in order to systematically modulate the intensity of antigen restimulation of Pmel T cells, which resulted in similar systemic distribution in the spleen and tumor tissues. Even though the percentage values of Pmel in spleen and tumor tissues on day 6 post-ACT are close, there was a 10 fold increase in the total number of transferred Pmel T cells in tumor compared to spleen suggesting that vaccine induced an increase in their proliferative capacity and likely facilitated their rapid expansion during a second stimulation when they encountered the target antigen on the tumor. Unlike Pmel T cells, the activation and proliferation of DC101 CAR T cells is solely dependent on the target antigen VEGFR-2 expressed by the tumor vasculature, which resulted in accumulation of DC101 CAR T cells in the tumor but not in spleen.
Notably, on day 3 post therapy, the absolute number of Thy1.1+ Tg-Pmel T cells was 25.3 and 12.7 fold more in the spleen and tumor tissues respectively if the mice were coadministered with anti-VEGFR2 CAR transduced T cells compared to those treated together with control CAR (SP6 CAR) transduced T cells (Fig. 6B). Interestingly, the anti-VEGFR2 CAR T cells (Ly5.1+) failed to persist both in the spleen and the tumor tissues if they were cotransferred with Tg-Pmel T cells, but not with the Wt T cells, despite their effective infiltration on day 3 post transfer. These results suggest that the anti-VEGFR2 CAR expressing T cells facilitated the trafficking and early infiltration of the tumor-specific T cells and enhanced their persistence at the tumor site.
Discussion
Adoptive cell transfer (ACT) using T cells reactive with tumor antigens can mediate the regression of established tumor masses in both mouse models as well as in the human(1, 19, 20, 25-29). In the human, ACT using tumor infiltrating lymphocytes that target tumor antigens following a lymphodepleting regimen can mediate the regression of widely metastatic melanoma in 50 to 70% of patients including up to 40% of patients who exhibit durable complete responses (1, 7, 25, 26, 30-32). ACT using T cells genetically engineered to express either conventional T cell receptors (TCR) or chimeric antigen receptors (CAR) can mediate tumor regression in patients with melanoma, lymphomas, and synovial cell sarcomas, and neuroblastoma (2-6). These studies have provided definitive evidence that T cells targeting specific tumor antigens can mediate tumor destruction.
Therapeutic attempts to target tumor vasculature using anti-angiogenic agents have had limited success in the treatment of tumors in both mouse models and in the human (15, 17, 33-36) possibly due to the multiple redundant pathways involved in angiogenesis (15, 17). Multiple studies have shown that anti-angiogenic agents can remodel the tumor vasculature and improve drug penetration into tumors (37, 38) and also enhance active immunotherapy through increasing the infiltration of immune cells into tumors (39). In a prior study we showed that the administration of an anti-VEGF antibody could improve the infiltration of adoptively transferred anti-gp100 transgenic T cells into a growing B16 melanoma tumor mass and could enhance the anti-tumor effects of this T cell transfer (14). In a recent study low-dose TNF-α treatment was shown to mediate inflammatory vessel remodeling and enhanced the infiltration of CD8+ effector cells and increased survival of spontaneous pancreatic neuroendocrine tumors bearing RIP1-Tag5 transgenic mice (40). In a similar study low dose anti-VEGFR2 antibody therapy was shown to enhance the anti-tumor efficacy of T-cell activation induced by a whole cancer cell vaccine therapy in a CD8+ T-cell–dependent manner in both immune-tolerant and immunogenic murine breast cancer models (41). We have developed an alternate approach to target the tumor vasculature using lymphocytes genetically engineered to express a CAR that targets VEGFR-2 on tumor vasculature. ACT using these genetically modified lymphocytes could inhibit the growth of five different vascularized murine tumors in two different mouse strains (18). T cells transduced with this anti-VEGFR2 chimeric antigen receptor exhibited durable and increased infiltration of T cells into tumor, which correlated with their anti-tumor effect. The ability to use ACT to target tumor antigens expressed on tumor cells as well as the tumor vasculature led to the current study attempting to simultaneously target these two tumor elements.
In these experiments we used the anti-VEGFR 2 CAR that we previously described (18). The specific tumor antigen reactive T-cells we utilized included the transgenic Pmel cells reactive with the gp-100 antigen, as well as wild type cells transduced with either the anti gp100 TCR, the anti-TRP-1 TCR, or the anti-TRP-2 T-cell receptor. Mice treated with a mixture of T-cells transduced with an anti-VEGFR2 CAR and the tumor antigen specific TCR exhibited a superior anti-tumor effect compared to those treated with T-cells expressing only one of these targeting molecules or those modified to co-express both targeting molecules. These effects were synergistic since the dose of the anti-VEGFR2 CAR cells had moderate impact when used alone, but could help mediate complete tumor regression when combined with the antigen specific cells that alone had only a transient impact on tumors (Fig. 2A, middle and right panels).
Furthermore, our study showed increased numbers of adoptively transferred tumor antigen-specific T cells within the tumor if they were coadministered with T cells targeted against the tumor vasculature. However, it is not known for certain whether the increased numbers of transferred antigen-specific T cells within the tumor were because of increased infiltration, increased proliferation, or increased retention in the tumor after the initial entry, although it is likely that all factors played a role. Even though the SP6 CAR alone transduced T cells reach the tumor similar to anti-VEGFR2 CAR cotransduced T cells at the earlier time point, they failed to proliferate and persist due to the absence of antigenic stimulation. Our results showed that provision of a CAR with a predefined specificity against VEGFR-2 facilitated their retention at the tumor site and conferred proliferative potential.
Of interest was our finding that the coexpression of the anti-VEGFR2 CAR and antigen-specific receptors on the same cell was not effective in mediating tumor regression, despite the demonstration in vitro that both receptors were expressed on the cells and were functionally competent in vitro. It is possible that if anti-VEGFR2 CAR and tumor-specific TCRs are engaged one after the other, with several hours delay, T cells with dual receptors may be in a refractory state after recognition one of the targets. In a clinical trial in neuroblastoma patients treated with EBV specific T-cells engineered with a CAR against the tumor associated antigen GD2, cells expressing both receptors survived longer than T-cells that expressed the same chimeric receptor alone (42). However, our ability to study whether these T cells with dual specificity could recognize two antigens present in two different anatomical locations in vivo was complicated by the administration of a vaccinia viral vaccine expressing the hgp100 antigen in our ACT that impacted on the activation and proliferation, trafficking potential, and functionality of the transferred Pmel T cells in vivo.
Emerging evidence has supported a hypothesis that tumor vasculature may undergo morphological normalization following anti-angiogenic treatment, whereby immature and leaky blood vessels are pruned and the remaining vasculature is remodeled so that they more closely resemble the normal vasculature (37, 38). The “normalized” blood vessels have increased transport capability, which more than compensates for the decrease in the total number of patent blood vessels and allows efficient entry of drugs and possibly immune cells as well (38). In our previous study (18) we demonstrated that DC101 CAR transduced T cells specifically released IFN-γ in vitro in response to most of the VEGFR-2 positive cell lines and primary endothelial cells tested. The amount of IFN-γ secretion was highly correlated with the level of VEGFR-2 expressed on target cells. Furthermore, immunofluorescence analysis of the tumor samples taken from mice treated with DC101-CAR transduced Thy1.1+ T cells on day 4 post ACT contained more Thy1.1+ T cells and a reduced number of CD31+ endothelial cells compared to those treated with empty vector transduced cells. However, our efforts to date to directly observe the anti-VEGFR2 CAR transduced T cell-mediated destruction of tumor vasculature in vivo through measurements of microvessel density have been unsuccessful due to the extensive tumor necrosis resulting from our treatment. The anti-angiogenic therapy with anti-VEGFR2 CAR cells could act as an auxiliary therapy that reduced the number of suppressor cell populations in the tumor environment including but not limited to the myeloid suppressor cells and regulatory T cells (Treg) that are known to express VEGFR-2 (36, 43-48). Notably, other studies have shown that the effect of anti-angiogenic drugs such as sunitinib and anti-VEGF antibodies was mediated partly through the elimination of VEGFR2 expressing myeloid suppressor cells and Treg in the tumor environment in addition to targeting the tumor endothelial cells (13, 49, 50).
No significant adverse effects were seen in mice treated with T cells engineered with an anti-VEGFR2 CAR in this and previous studies (18) under conditions where significant antitumor effects were seen. In this study, no signs of morbidity or mortality were seen in animals treated simultaneously with genetically engineered T cells expressing the anti-VEGFR2 CAR and a tumor antigen-specific TCR except those receiving mixture of TRP2 TCR transduced T cells and anti-VEGFR2 CAR engineered T cells. Notwithstanding these and previous observations (18), the potential adverse effects of anti-angiogenic immunotherapy have to be carefully considered in future experiments.
In conclusion, using an established mouse model of melanoma, we have demonstrated antitumor effects of a dual targeting adoptive therapy strategy of simultaneously attacking the tumor and tumor vasculature using genetically modified T cells. The strategy is particularly meaningful for the treatment of solid tumors with known antigenic signatures and may encourage the clinical application of combined immunotherapy and anti-angiogenic therapy in the future.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and with funds generously provided by the Milstein Family Foundation. We thank Douglas Palmer, Zulmarie Franco, and David Jones (Surgery Branch, NCI) for helping us with the animal studies.
Footnotes
The authors disclose no potential conflicts of interest.
References
- (1).Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–24. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–45. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- (10).Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, et al. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest. 2011;121:4015–29. doi: 10.1172/JCI45862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Dirkx AE, Oude Egbrink MG, Kuijpers MJ, van der Niet ST, Heijnen VV, Bouma-ter Steege JC, et al. Tumor angiogenesis modulates leukocyte-vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res. 2003;63:2322–9. [PubMed] [Google Scholar]
- (12).Griffioen AW. Anti-angiogenesis: making the tumor vulnerable to the immune system. Cancer Immunol Immunother. 2008;57:1553–8. doi: 10.1007/s00262-008-0524-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- (14).Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–80. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–9. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- (16).Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- (17).Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–34. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Chinnasamy D, Yu Z, Theoret MR, Zhao Y, Shrimali RK, Morgan RA, et al. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J Clin Invest. 2010;120:3953–68. doi: 10.1172/JCI43490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–80. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Palmer DC, Balasubramaniam S, Hanada K, Wrzesinski C, Yu Z, Farid S, et al. Vaccine-stimulated, adoptively transferred CD8+ T cells traffic indiscriminately and ubiquitously while mediating specific tumor destruction. J Immunol. 2004;173:7209–16. doi: 10.4049/jimmunol.173.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Abad JD, Wrzensinski C, Overwijk W, de Witte MA, Jorritsma A, Hsu C, et al. T-cell receptor gene therapy of established tumors in a murine melanoma model. J Immunother. 2008;31:1–6. doi: 10.1097/CJI.0b013e31815c193f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–85. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Singh V, Ji Q, Feigenbaum L, Leighty RM, Hurwitz AA. Melanoma progression despite infiltration by in vivo-primed TRP-2-specific T cells. J Immunother. 2009;32:129–39. doi: 10.1097/CJI.0b013e31819144d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Overwijk WW, Lee DS, Surman DR, Irvine KR, Touloukian CE, Chan CC, et al. Vaccination with a recombinant vaccinia virus encoding a “self” antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4(+) T lymphocytes. Proc Natl Acad Sci U S A. 1999;96:2982–7. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–12. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Gattinoni L, Powell DJ, Jr., Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–93. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–74. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–75. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–40. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, et al. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–64. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- (34).Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757–63. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- (35).Kuenen BC, Rosen L, Smit EF, Parson MR, Levi M, Ruijter R, et al. Dose-finding and pharmacokinetic study of cisplatin, gemcitabine, and SU5416 in patients with solid tumors. J Clin Oncol. 2002;20:1657–67. doi: 10.1200/JCO.2002.20.6.1657. [DOI] [PubMed] [Google Scholar]
- (36).Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–21. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- (37).Jain RK. Delivery of molecular medicine to solid tumors. Science. 1996;271:1079–80. doi: 10.1126/science.271.5252.1079. [DOI] [PubMed] [Google Scholar]
- (38).Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- (39).Dirkx AE, Oude Egbrink MG, Castermans K, van der Schaft DW, Thijssen VL, Dings RP, et al. Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. FASEB J. 2006;20:621–30. doi: 10.1096/fj.05-4493com. [DOI] [PubMed] [Google Scholar]
- (40).Johansson A, Hamzah J, Payne CJ, Ganss R. Tumor-targeted TNFalpha stabilizes tumor vessels and enhances active immunotherapy. Proc Natl Acad Sci U S A. 2012;109:7841–6. doi: 10.1073/pnas.1118296109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109:17561–6. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Duignan IJ, Corcoran E, Pennello A, Plym MJ, Amatulli M, Claros N, et al. Pleiotropic stromal effects of vascular endothelial growth factor receptor 2 antibody therapy in renal cell carcinoma models. Neoplasia. 2011;13:49–59. doi: 10.1593/neo.101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Larrivee B, Pollet I, Karsan A. Activation of vascular endothelial growth factor receptor-2 in bone marrow leads to accumulation of myeloid cells: role of granulocyte-macrophage colony-stimulating factor. J Immunol. 2005;175:3015–24. doi: 10.4049/jimmunol.175.5.3015. [DOI] [PubMed] [Google Scholar]
- (45).Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–31. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- (46).Suzuki H, Onishi H, Wada J, Yamasaki A, Tanaka H, Nakano K, et al. VEGFR2 is selectively expressed by FOXP3high CD4+ Treg. Eur J Immunol. 2010;40:197–203. doi: 10.1002/eji.200939887. [DOI] [PubMed] [Google Scholar]
- (47).Udagawa T, Puder M, Wood M, Schaefer BC, D’Amato RJ. Analysis of tumor-associated stromal cells using SCID GFP transgenic mice: contribution of local and bone marrow-derived host cells. FASEB J. 2006;20:95–102. doi: 10.1096/fj.04-3669com. [DOI] [PubMed] [Google Scholar]
- (48).Zhang N, Fang Z, Contag PR, Purchio AF, West DB. Tracking angiogenesis induced by skin wounding and contact hypersensitivity using a Vegfr2-luciferase transgenic mouse. Blood. 2004;103:617–26. doi: 10.1182/blood-2003-06-1820. [DOI] [PubMed] [Google Scholar]
- (49).Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol. 2011;11:856–61. doi: 10.1016/j.intimp.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14:6674–82. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.