Abstract
Amyloid fibrils formed by the 40-residue β-amyloid peptide (Aβ1–40) are highly polymorphic, with molecular structures that depend on the details of growth conditions. Underlying differences in physical properties are not well understood. Here, we investigate differences in growth kinetics and thermodynamic stabilities of two Aβ1–40 fibril polymorphs for which detailed structural models are available from solid state nuclear magnetic resonance (NMR) studies. Rates of seeded fibril elongation in the presence of excess soluble Aβ1–40 and shrinkage in the absence of soluble Aβ1–40 are determined with atomic force microscopy (AFM). From these rates, we derive polymorph-specific values for the soluble Aβ1–40 concentration at quasi-equilibrium, from which relative stabilities can be derived. The AFM results are supported by direct measurements by ultraviolet absorbance, using a novel dialysis system to establish quasi-equilibrium. At 24° C, the two polymorphs have significantly different elongation and shrinkage kinetics but similar thermodynamic stabilities. At 37° C, differences in kinetics are reduced, and thermodynamic stabilities are increased significantly. Fibril length distributions in AFM images provide support for an intermittent growth model, in which fibrils switch randomly between an "on" state (capable of elongation) and an "off" state (incapable of elongation). We also monitor interconversion between polymorphs at 24° C by solid state NMR, showing that the two-fold symmetric "agitated" ( ) polymorph is more stable than the three-fold symmetric "quiescent"
) polymorph is more stable than the three-fold symmetric "quiescent"  polymorph. Finally, we show that the two polymorphs have significantly different rates of fragmentation in the presence of shear forces, a difference that helps explain the observed predominance of the
polymorph. Finally, we show that the two polymorphs have significantly different rates of fragmentation in the presence of shear forces, a difference that helps explain the observed predominance of the  structure when fibrils are grown in agitated solutions.
structure when fibrils are grown in agitated solutions.
Introduction
Alzheimer’s disease (AD) is characterized by the formation of neurotoxic β-amyloid (Aβ) plaques in brain tissue. The amyloid deposits contain Aβ fibrils with primarily 40-residue (Aβ1–40) and 42-residue (Aβ1–42) sequences. In vitro studies have shown that Aβ fibrils formed under various experimental conditions possess distinct molecular structures.1–7 Although a variety of Aβ1–40 and Aβ1–42 fibril structures have been characterized in varying levels of detail,3,4,8–19 kinetic and thermodynamic differences among these structures that may influence the observed dependence of molecular structure on growth conditions are not fully characterized. In particular, our laboratory has shown that the predominant Aβ1–40 fibril morphology that develops de novo (i.e., in the absence of pre-existing fibril seeds) is strongly affected by the presence or absence of agitation of the Aβ1–40 solution.2 At 24° C, pH 7.4, and low ionic strength, the majority of fibrils that form in a quiescent solution are single filaments with an apparent periodic twist about the fibril growth axis, as in Fig. 1A. Under the same buffer conditions, but with agitation of the solution, the majority of fibrils occur as bundles of multiple filaments, as in Fig. 1B. "Quiescent"  and "agitated"
and "agitated"  Aβ1–40 fibrils have distinct 13C chemical shifts in solid state nuclear magnetic resonance (NMR) spectra2–4 and distinct mass-per-length values,2,5,6,20 in addition to their distinct appearances in transmission electron microscope (TEM) images. According to detailed structural models developed from solid state NMR and electron microscopy data,
Aβ1–40 fibrils have distinct 13C chemical shifts in solid state nuclear magnetic resonance (NMR) spectra2–4 and distinct mass-per-length values,2,5,6,20 in addition to their distinct appearances in transmission electron microscope (TEM) images. According to detailed structural models developed from solid state NMR and electron microscopy data,  fibrils have approximate three-fold rotational symmetry about the fibril growth axis,4 while protofilaments within
fibrils have approximate three-fold rotational symmetry about the fibril growth axis,4 while protofilaments within  fibrils have two-fold rotational symmetry.3 It has been unclear whether
fibrils have two-fold rotational symmetry.3 It has been unclear whether  and
and  fibrils have different thermodynamic stabilities or different growth kinetics, and it has been unclear why the presence or absence of gentle agitation has such a profound structural effect.
fibrils have different thermodynamic stabilities or different growth kinetics, and it has been unclear why the presence or absence of gentle agitation has such a profound structural effect.
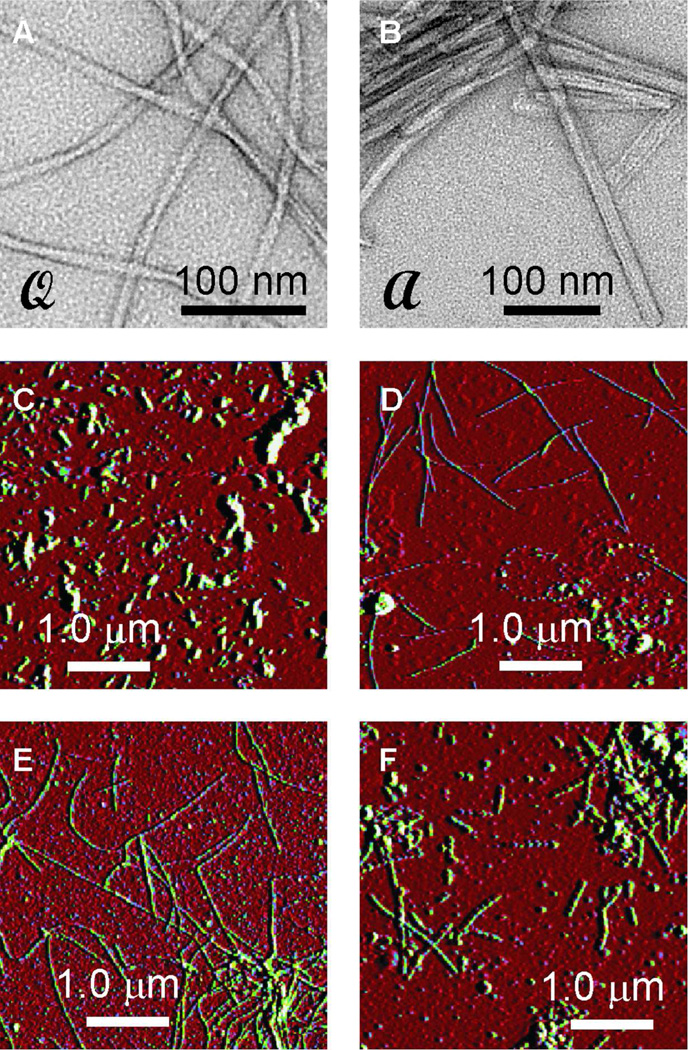
Figure 1.
(A,B) Negatively stained TEM images of "quiescent" and "agitated" Aβ1–40 fibrils (called  and
and  fibrils), showing their distinct morphologies. These fibrils resulted from seeded growth at 24° C, using 50 µM initial monomer concentrations. (C,D) Representative AFM images of
fibrils), showing their distinct morphologies. These fibrils resulted from seeded growth at 24° C, using 50 µM initial monomer concentrations. (C,D) Representative AFM images of  fibrils from elongation measurements after 0 and 20 min incubation, respectively. Measurements were done at 24° C with 50 µM soluble Aβ1–40 concentration. (E,F) Representative AFM images of
fibrils from elongation measurements after 0 and 20 min incubation, respectively. Measurements were done at 24° C with 50 µM soluble Aβ1–40 concentration. (E,F) Representative AFM images of  fibrils from shrinkage measurements at 24° C after 2 hr and 46 hr incubation, respectively. Vertical scales represent AFM feedback error signals, not height.
fibrils from shrinkage measurements at 24° C after 2 hr and 46 hr incubation, respectively. Vertical scales represent AFM feedback error signals, not height.
As demonstrated by others,7,21–25 thermodynamic stabilities of amyloid fibrils, including dependences of thermodynamic stabilities on amino acid substitutions and polymorphism, can be assessed from direct measurements of the concentration of peptide monomers that are in quasi-equilibrium with the fibrils. An alternative approach is to measure the elongation rates ke of fibrils in the presence of excess monomers and the shrinkage rates ks in the absence of monomers. 26–30 Assuming that fibrils elongate or shrink by addition or subtraction of monomers, and with other reasonable assumptions (see Discussion section), the average fibril length L is expected to follow an equation of the form
| (1) |
where M is the time-dependent monomer concentration. Note that ke can have units of nm/s-µM, while ks can have units of nm/s. At quasi-equilibrium (dL/dt = 0), the monomer concentration is MQE = ks/ke. This indirect approach to assessment of thermodynamic stability has several potential advantages, including insensitivity to chemical impurities, no requirement for separation of fibrils from monomers, no requirement that a quasi-equilibrium state be reached, and ability to measure small values of MQE. This approach also yields interesting kinetic parameters.
In this paper, we describe the use of atomic force microscopy (AFM) to measure the time dependences of Aβ1–40 fibril length distributions in the presence of various Aβ1–40 monomer concentrations, allowing ks and ke to be determined. We report measurements for  and
and  fibrils, allowing the thermodynamic stabilities and kinetic properties of the two Aβ1–40 fibril polymorphs to be compared. The time dependences of fibril length distributions provide evidence for intermittent growth of individual fibrils,30–32 possibly due to structural transitions of fibril ends between "on" and "off" states. We show that values of MQE determined from the AFM measurements are in good agreement with direct measurements of soluble Aβ1–40 by ultraviolet (UV) absorbance, using a dialysis technique that separates soluble species from fibrils without uncertainties inherent in separation by centrifugation. We find that
fibrils, allowing the thermodynamic stabilities and kinetic properties of the two Aβ1–40 fibril polymorphs to be compared. The time dependences of fibril length distributions provide evidence for intermittent growth of individual fibrils,30–32 possibly due to structural transitions of fibril ends between "on" and "off" states. We show that values of MQE determined from the AFM measurements are in good agreement with direct measurements of soluble Aβ1–40 by ultraviolet (UV) absorbance, using a dialysis technique that separates soluble species from fibrils without uncertainties inherent in separation by centrifugation. We find that  and
and  fibrils have similar thermodynamic stabilities (i.e., similar values of MQE) despite their different molecular structures, but significantly different elongation and shrinkage kinetics. At 24° C under our buffer conditions, MQE values determined by AFM and by UV (MQE,AFM and MQE,UV) suggest that
fibrils have similar thermodynamic stabilities (i.e., similar values of MQE) despite their different molecular structures, but significantly different elongation and shrinkage kinetics. At 24° C under our buffer conditions, MQE values determined by AFM and by UV (MQE,AFM and MQE,UV) suggest that  fibrils are slightly more stable than
fibrils are slightly more stable than  fibrils, a finding that is supported by direct measurements of structural interconversion using solid state NMR. Finally, we discuss factors that may contribute to the observed dependence of fibril structure on growth conditions, and show that differences in susceptibility to fragmentation under shear forces are an important factor.
fibrils, a finding that is supported by direct measurements of structural interconversion using solid state NMR. Finally, we discuss factors that may contribute to the observed dependence of fibril structure on growth conditions, and show that differences in susceptibility to fragmentation under shear forces are an important factor.
In the preceding introduction and in the following sections, we use the term "quasi-equilibrium" to describe a state in which fibrils with a particular morphology and molecular structure are effectively in a steady state with soluble Aβ1–40 (monomer and possibly small oligomers). Such a state is not necessarily a true equilibrium state, because this particular fibril structure may not be the most stable fibril structure. In principle, the quasi-equilibrium state could evolve slowly toward a state in which the fibrils have converted to a more stable structure. However, as shown below, such evolution is practically unobservable in the absence of seeds of the more stable structure, because values of MQE are so small that nucleation of the more stable structure is inefficient and because different fibril polymorphs do not interconvert by internal structural rearrangements. The existence of quasi-equilibrium states for individual fibril polymorphs is a prerequisite for studies of the type described below.
Materials and Methods
Fibril elongation measurements
Aβ1–40was prepared by solid-phase peptide synthesis, using an Applied Biosystems 433A automated synthesizer, and purified by high-performance liquid chromatography (HPLC), using a Beckman-Coulter model 125P solvent pump module and model 168 detector, a Zorbax 300SB-C3 column (Agilent), and a H2O/acetonitrile gradient with 1.0% trifluoroacetic acid. Purity was assessed by electrospray ionization mass spectrometry (1100 MSD, Hewlett-Packard) and found to be better than 95%. After purification, the peptide was lyophilized and stored at −20° C.  fibrils were prepared by seeded growth, using one of the solid state NMR samples described by Paravastu et al4. as the original source of seeds. A portion of the NMR sample (∼0.5 mg of fibrils) was added to 1 ml of incubation buffer (10 mM phosphate, pH 7.4, 0.01% NaN3), sonicated to break the fibrils into short fragments (Branson model S-250A sonifier with tapered 1/8" microtip horn, lowest power, 10% duty factor, 2 min), and diluted to 5 ml in incubation buffer in a vertical 20 ml tube. Purified, lyophilized Aβ1–40 was dissolved in dimethyl sulfoxide (DMSO) to a concentration of 8 mM. An aliquot of DMSO-solubilized Aβ1–40 was then added to the 5 ml seed solution to produce a 100 µM Aβ1–40 concentration (not including seeds) and immediately mixed by vortexing. After 24 hr of quiescent incubation at ambient temperature, the solution gelled due to growth and entanglement of fibrils. The gel was readily disrupted by vortexing. The predominant fibril morphology was confirmed by TEM to match the "twisted" morphology described by Paravastu et al.4 An aliquot of these fibrils was then used as seeds for a second generation of seeded growth. Fibrils from the second generation were used in experiments described below.
fibrils were prepared by seeded growth, using one of the solid state NMR samples described by Paravastu et al4. as the original source of seeds. A portion of the NMR sample (∼0.5 mg of fibrils) was added to 1 ml of incubation buffer (10 mM phosphate, pH 7.4, 0.01% NaN3), sonicated to break the fibrils into short fragments (Branson model S-250A sonifier with tapered 1/8" microtip horn, lowest power, 10% duty factor, 2 min), and diluted to 5 ml in incubation buffer in a vertical 20 ml tube. Purified, lyophilized Aβ1–40 was dissolved in dimethyl sulfoxide (DMSO) to a concentration of 8 mM. An aliquot of DMSO-solubilized Aβ1–40 was then added to the 5 ml seed solution to produce a 100 µM Aβ1–40 concentration (not including seeds) and immediately mixed by vortexing. After 24 hr of quiescent incubation at ambient temperature, the solution gelled due to growth and entanglement of fibrils. The gel was readily disrupted by vortexing. The predominant fibril morphology was confirmed by TEM to match the "twisted" morphology described by Paravastu et al.4 An aliquot of these fibrils was then used as seeds for a second generation of seeded growth. Fibrils from the second generation were used in experiments described below.
 fibrils were prepared by diluting DMSO-solubilized Aβ1–40 to 230 µM in incubation buffer. A 5 ml volume of the Aβ1–40 solution was incubated in a 20 ml tube, lying horizontally on an orbital platform shaker (VWR model DS-500E). The agitation frequency (roughly 1 Hz) was adjusted to produce a "sloshing" motion of the solution along the length of the tube. A visible precipitate of Aβ1–40 fibrils developed within 12 hr. The predominant fibril morphology was confirmed by TEM to match the "striated ribbon" morphology described previously by Petkova et al. 2,3 These
fibrils were prepared by diluting DMSO-solubilized Aβ1–40 to 230 µM in incubation buffer. A 5 ml volume of the Aβ1–40 solution was incubated in a 20 ml tube, lying horizontally on an orbital platform shaker (VWR model DS-500E). The agitation frequency (roughly 1 Hz) was adjusted to produce a "sloshing" motion of the solution along the length of the tube. A visible precipitate of Aβ1–40 fibrils developed within 12 hr. The predominant fibril morphology was confirmed by TEM to match the "striated ribbon" morphology described previously by Petkova et al. 2,3 These fibrils were then used as seeds for growth of a second generation of
fibrils were then used as seeds for growth of a second generation of  fibrils, at 100 µM Aβ1–40 concentration. Fibrils from the second generation were used in experiments described below.
fibrils, at 100 µM Aβ1–40 concentration. Fibrils from the second generation were used in experiments described below.
For fibril elongation measurements by AFM at ambient temperature (24° C), a 25 µl aliquot of fibril solution (either  or
or  ) was added to 1.0 ml of incubation buffer. For measurements at 37°C, 6.25 µl of fibril solution was added to 0.5 ml of incubation buffer. The diluted fibril solution was then sonicated for 2 min on an ice bath, and was kept at 24° C or 37° C for at least 10 min for temperature equilibration. DMSO-solubilized Aβ1–40 was then added to reach the desired final concentrations, i.e., 25 µM, 50 µM and 75 µM for measurements at 24° C and 13 µM, 27 µM and 40 µM for measurements at 37°C. Fibrils were allowed to elongate, and 20 µl aliquots were taken at various time points for AFM imaging.
) was added to 1.0 ml of incubation buffer. For measurements at 37°C, 6.25 µl of fibril solution was added to 0.5 ml of incubation buffer. The diluted fibril solution was then sonicated for 2 min on an ice bath, and was kept at 24° C or 37° C for at least 10 min for temperature equilibration. DMSO-solubilized Aβ1–40 was then added to reach the desired final concentrations, i.e., 25 µM, 50 µM and 75 µM for measurements at 24° C and 13 µM, 27 µM and 40 µM for measurements at 37°C. Fibrils were allowed to elongate, and 20 µl aliquots were taken at various time points for AFM imaging.
Note that the precise values of fibril seed concentrations and seed lengths do not affect the experiments described below, because lengths of individual fibrils (rather than total fibril mass) are measured by AFM. Final DMSO concentrations in all experiments were <2% by volume.
Fibril shrinkage measurements
Fibril shrinkage was measured by AFM on freshly prepared fibrils under dialysis conditions, using the apparatus depicted in Fig. S1 of the Supporting Information. To prepare the fibrils, 0.5 mg of DMSO-solubilized Aβ1–40 was added to a solution of seeds in incubation buffer to produce a 50 µM Aβ1–40 concentration (not including the seeds). Fibril growth was then allowed to proceed for 3 hr at 24° C or 1 hr at 37°C. Fibrils were then pelleted by ultracentrifugation for 1 hr at 432,000 × g and 4° C (Beckman-Coulter Optima ultracentrifuge, TLA100.2 rotor). The residual Aβ1–40 concentration in the supernatant was determined by analytical HPLC, as described below, to ensure that fibril growth was essentially complete. The pellet was resuspended in 1 ml of incubation buffer, thoroughly mixed, and diluted to 25 ml in incubation buffer. A 1 ml aliquot of the diluted solution, containing approximately 0.02 mg of fibrils, was transferred to a dialysis tube (Spectrum Laboratories Float-A-Lyzer G2, 300 kDa MWCO, 1 ml volume) and dialyzed against 30 ml of incubation buffer in a 50 ml Falcon tube. Magnetic stir bars were placed both inside the dialysis tube and in the buffer reservoir. The entire dialysis system was flushed with a gentle N2 flow to prevent oxidation of Aβ1–40. Importantly, the N2 flow was low enough that there was negligible evaporation of total buffer volume during the dialysis period. Aliquots of the solution inside the dialysis tube were taken at various time points for AFM imaging.
The stir bar within the dialysis tube prevented fibrils from settling to the bottom of the tube during shrinkage experiments. The stir bar rotated at roughly 0.3 Hz. At this low stirring rate, fragmentation of fibrils due to shear forces was negligible. For measurements at 37° C, the entire dialysis system was heated and thermally insulated as shown in Fig. S1. Temperature was stabilized at 37° ± 3°C for the duration of these measurements, as monitored by a thermometer in the buffer reservoir.
AFM measurements
For each image, a 20 µl aliquot of fibril solution was deposited on a freshly cleaved, dry mica surface. The solution was adsorbed for 1 min before blotting by tissue paper. The mica surface was then washed once with 500 µl of deionized water and dried under a gentle N2 flow. For aliquots containing short fibrils (first time point in elongation experiments), one additional washing step was required in order to remove buffer salts from the mica surface that otherwise interfered with fibril length measurements. AFM images were recorded in tapping mode using a Veeco MultiMode instrument and Nanoscope IV controller, equipped with Veeco DMASP tips. The tip oscillation frequency was typically 250 kHz, with a drive amplitude of 100–150 mV and a detector setpoint of approximately 0.6 V. Images typically contained 512 × 512 points in a 8.0 × 8.0 µm area, scanned at 1.0 µm/s rate. Height, feedback error signal, and phase images were recorded simultaneously. Error signal images were used for fibril length measurements and are shown in the figures (except where indicated), because these images did not require baseline subtraction.
To measure fibril length distributions, images from the AFM software were imported into ImageJ.33 All objects in an image with fibrillar appearance (i.e., with obvious asymmetry and with the expected apparent width and height) that were separated from other fibrils and that were fully contained within the image field were selected for measurements. Lengths were measured manually with the standard ImageJ freehand line selection tool. Lengths of curved fibrils were obtained by summing the lengths of straight sections.
Measurement of MQE by UV absorbance
Values of MQE, representing soluble Aβ1–40 concentration at quasi-equilibrium, were also measured directly by UV absorbance in an analytical HPLC system. For these measurements, fibrils were prepared as for fibril shrinkage experiments, and the same dialysis system was used. After ultracentrifugation, fibril pellets were resuspended in 2 mL of incubation buffer and mixed thoroughly. A 1 ml aliquot, containing approximately 0.25 mg of fibrils, was transferred to a dialysis tube without further dilution and dialyzed against 30 ml of incubation buffer for approximately four days. The dialysis system was flushed continuously with a gentle N2 flow to prevent oxidation of Aβ1–40. Aliquots of 0.5 ml were taken from the buffer reservoir and subjected to analytical HPLC measurements at 0, 24, 48, 72 and 96 hr after the beginning of the dialysis process. Under these conditions, we found that quasi-equilibrium was established by 72 hr. HPLC measurements (Beckman-Coulter model 125P solvent pump module, model 168 detector, Vydac 218TP104 reverse-phase C18 column) used a linear H2O/acetonitrile gradient (from 10% to 90% acetonitrile in 40 min) with 0.1% triflouroacetic acid. The Aβ1–40 peak eluted at approximately 16 min, as confirmed using matrix-assisted laser-desorption-ionization time-of-flight mass spectrometry (Axima-CFR, Shimadzu). At 24° C, five independent dialysis experiments were performed, starting from fresh fibrils each time. At 37° C, two independent dialysis experiments were performed. Absorbance peak volumes at 214 nm were determined using the Beckman-Coulter 32 Karat software package. The corresponding Aβ1–40 concentrations were determined from a standard working curve, generated by a series of HPLC measurements on solutions with known concentrations in the 0.5–10 µM range (prepared from DMSO-solubilized Aβ1–40). The smallest Aβ1–40 concentration that could be measured reliably was roughly 0.1 µM.
TEM measurements
TEM images were recorded with an FEI Morgagni microscope operating at 80 kV. For negatively stained images, a 10 µl drop of fibril solution was absorbed for 2 min on a glow-discharged carbon film, supported by lacey carbon on a 300 mesh copper TEM grid. After blotting, the grid was rinsed twice with deionized water, and then stained with 10 µl of 3% uranyl acetate for 30 s. The stained solution was blotted, and the grid was dried in air before imaging.
Solid state NMR
Solid state 15N and 13C NMR spectra of isotopically labeled fibrils were acquired at ambient temperature in a 9.39 T field (100.4 MHz 13C NMR frequency), using a Varian InfinityPlus spectrometer, a Varian 3.2 mm magic-angle spinning (MAS) NMR probe, and a 9.0 kHz MAS frequency. Standard 1H-15N and 1H-13C cross-polarization conditions and 1H decoupling conditions were employed, with a 1 s delay between scans.
Results
Fibril morphologies are preserved in seeded growth
Figs. 1A and 1B show TEM images of Aβ1–40 fibrils after seeded growth in fibril elongation measurements described below. Fibrils grown from  or
or  fibril seeds retain the characteristic "twisted" and "striated ribbon" morphologies described previously.2,4 These images verify that the fibrils grown from seeds in our experiments were morphologically homogeneous. TEM images of fibrils used as seeds (before sonication) are shown in Fig. S2 of the Supporting Information. Solid state NMR measurements described below provide additional evidence for morphological homogeneity in our samples. From TEM images, we estimate that morphological homogeneity exceeds 90%.
fibril seeds retain the characteristic "twisted" and "striated ribbon" morphologies described previously.2,4 These images verify that the fibrils grown from seeds in our experiments were morphologically homogeneous. TEM images of fibrils used as seeds (before sonication) are shown in Fig. S2 of the Supporting Information. Solid state NMR measurements described below provide additional evidence for morphological homogeneity in our samples. From TEM images, we estimate that morphological homogeneity exceeds 90%.
We emphasize that the terms " fibrils" and "
fibrils" and " fibrils" refer only to the conditions under which seed fibrils were originally prepared. Fibril elongation and shrinkage experiments described below were performed under quiescent conditions for both polymorphs.
fibrils" refer only to the conditions under which seed fibrils were originally prepared. Fibril elongation and shrinkage experiments described below were performed under quiescent conditions for both polymorphs.
Fibril elongation and shrinkage at 24° C monitored by AFM
AFM has been used extensively by other groups to study the growth and morphology of Aβ fibrils and to identify intermediate species in the assembly process.6,34–38 Here, we use AFM to measure time-dependent length distributions of Aβ1–40 fibrils that grow from seeds with specific structures, after the seeds are added to an Aβ1–40 solution with a specific initial concentration. We also use AFM to measure length distributions when fibrils with a specific structure are placed in a monomer-free solution, so that the fibrils dissolve toward the quasi-equilibrium state. Our AFM images were recorded in air, after adsorption of aliquots of fibril-containing Aβ1–40 solutions to mica as described above. In principle, time-dependent length distributions could be obtained from images that were recorded in situ (i.e., images of fibrils on the mica surface under the Aβ1–40 solution). In our hands, this alternative approach is precluded by instrumental instabilities over long imaging times, fibril fragmentation and desorption induced by the AFM tip, and image degradation due to adherence of Aβ1–40 fibrils or other aggregates to the AFM tip. In addition, when fibril elongation and shrinkage are monitored in situ, the possibility exists that interactions with the mica (or other substrate) surface may affect the observations.
Figs. 1C and 1D show representative AFM images of aliquots of a seeded Aβ1–40 solution, taken immediately after seeding (Fig. 1C) and 20 min later (Fig. 1D). In this case,  seeds were used, and the initial soluble Aβ1–40 concentration was 50 µM. It is apparent that the seeded solution contained only short fibril fragments initially, and that fibrils longer than 1 µm grew from these fragments. In control experiments, no fibrils were observed by TEM or AFM after incubation of an unseeded 50 µM Aβ1–40 solution for 30 min. Figs. 1E and 1F show representative AFM images of a
seeds were used, and the initial soluble Aβ1–40 concentration was 50 µM. It is apparent that the seeded solution contained only short fibril fragments initially, and that fibrils longer than 1 µm grew from these fragments. In control experiments, no fibrils were observed by TEM or AFM after incubation of an unseeded 50 µM Aβ1–40 solution for 30 min. Figs. 1E and 1F show representative AFM images of a  fibril solution after being placed in a monomer-free solution, under dialysis conditions described above. Average fibril lengths after 2 hr incubation (Fig. 1E) are clearly greater than after 46 hr incubation (Fig. 1F).
fibril solution after being placed in a monomer-free solution, under dialysis conditions described above. Average fibril lengths after 2 hr incubation (Fig. 1E) are clearly greater than after 46 hr incubation (Fig. 1F).
Fibrils in Fig. 1D appear to have uniform diameters, with no smaller aggregates associated with them, suggesting an absence of lateral nucleation of Aβ1–40 aggregates. In addition, the apparent fibril diameter did not change significantly over 20 min, indicating that linear elongation, rather than self-association or lateral expansion of filaments was the predominant process.
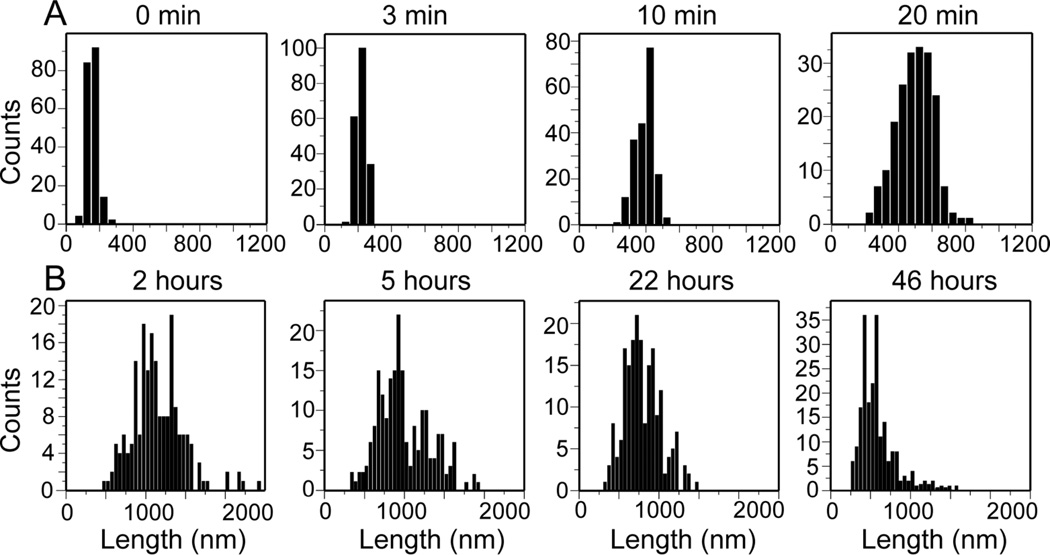
Figs. 2A and 2B show histograms of length distributions determined from multiple AFM images under the conditions in Figs. 1C–1F, for elongation and shrinkage experiments, respectively. Elongation and shrinkage experiments were performed for both  and
and  fibrils at 24° C, with initial monomer concentrations of 25 µM, 50 µM, and 75 µM in the elongation experiments. Additional AFM images and length histograms from these experiments are shown in Figs. S3 and S4 of the Supporting Information. Interestingly, in all elongation experiments, in addition to the expected monotonic increase in the average fibril length, we observed that the width of the length distribution increased with increasing incubation time. This observation is discussed further below.
fibrils at 24° C, with initial monomer concentrations of 25 µM, 50 µM, and 75 µM in the elongation experiments. Additional AFM images and length histograms from these experiments are shown in Figs. S3 and S4 of the Supporting Information. Interestingly, in all elongation experiments, in addition to the expected monotonic increase in the average fibril length, we observed that the width of the length distribution increased with increasing incubation time. This observation is discussed further below.
Figure 2.
Experimental histograms of  fibril lengths from elongation measurements at 24° C with 50 µM soluble Aβ1–40 concentration (A) and from shrinkage measurements (B). Each histogram contains length measurements for 200 individual filaments in the corresponding AFM images.
fibril lengths from elongation measurements at 24° C with 50 µM soluble Aβ1–40 concentration (A) and from shrinkage measurements (B). Each histogram contains length measurements for 200 individual filaments in the corresponding AFM images.
Kinetics and thermodynamics from AFM and UV
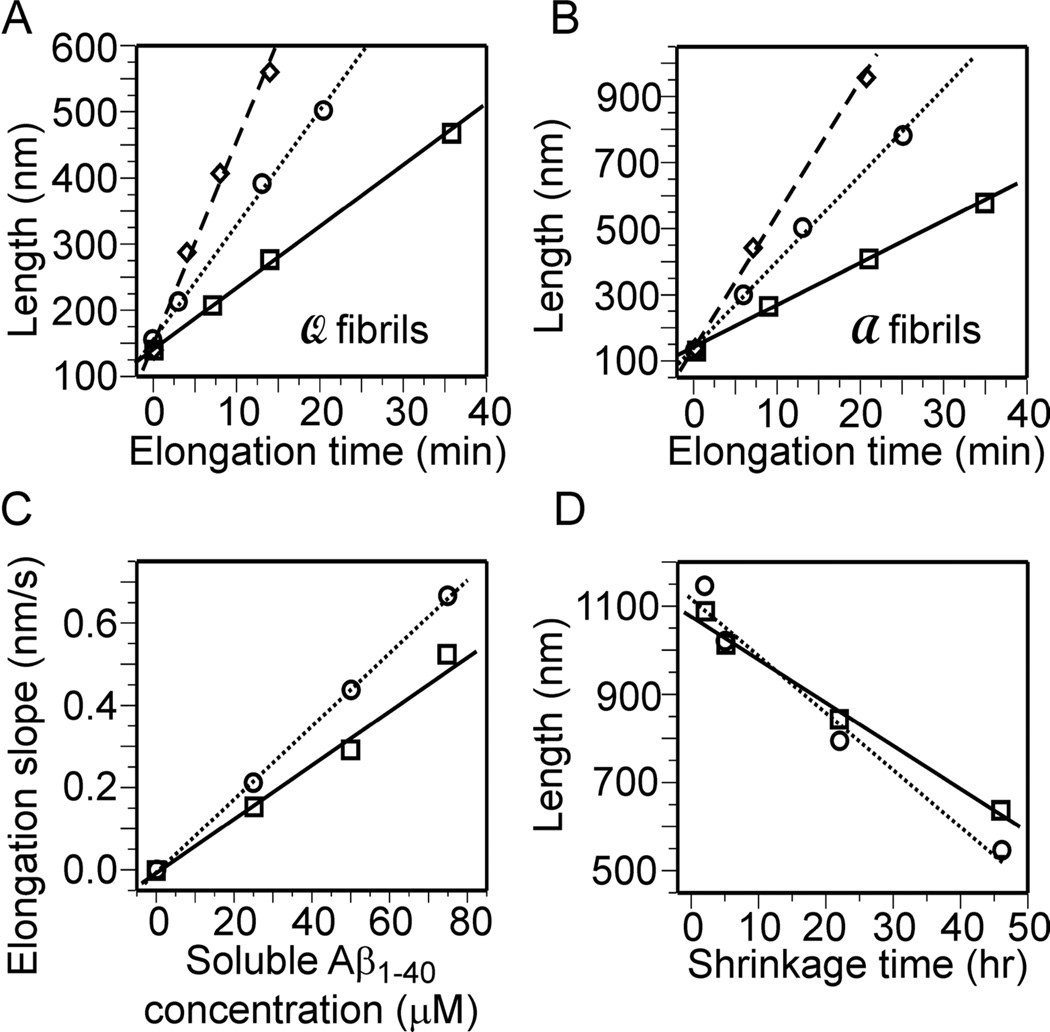
Figs. 3A and 3B show plots of average fibril lengths as a function of incubation time in elongation experiments. For each value of the initial Aβ1–40 monomer concentration, fibril lengths increase linearly with time. As shown in Fig. 3C, the slope increases linearly with monomer concentration. (Linear fits in Fig. 3C were constrained to pass through the origin, an approximation that is justified by the small experimental values of ks.) Fig. 3D shows that the average fibril length decreases linearly with incubation time in shrinkage experiments. Slopes from Figs. 3C and 3D represent values of ke and ks in Eq. (1). These values are given in Table 1, along with values of MQE,AFM = ks/ke. Interestingly, elongation and shrinkage rates for  fibrils are both significantly larger than the corresponding rates for
fibrils are both significantly larger than the corresponding rates for  fibrils, but the values of MQE,AFM are nearly the same. Error limits on ks and ke represent standard errors reported by the Origin 6.0 software (OriginLab Corp.) used to fit the data. Given these error limits, the resulting values of MQE,AFM are not significantly different.
fibrils, but the values of MQE,AFM are nearly the same. Error limits on ks and ke represent standard errors reported by the Origin 6.0 software (OriginLab Corp.) used to fit the data. Given these error limits, the resulting values of MQE,AFM are not significantly different.
Figure 3.
(A,B) Plots of experimental average fibril lengths and best-fit lines as a function of elongation time for  and
and  Aβ1–40 fibrils. Experiments were done with 25 µM (squares and solid lines), 50 µM (circles and dotted lines) and 75 µM (diamonds and dashed lines) soluble Aβ1–40 concentrations. (C) Experimental elongation slopes as a function of soluble Aβ1–40 concentration and best-fit lines for
Aβ1–40 fibrils. Experiments were done with 25 µM (squares and solid lines), 50 µM (circles and dotted lines) and 75 µM (diamonds and dashed lines) soluble Aβ1–40 concentrations. (C) Experimental elongation slopes as a function of soluble Aβ1–40 concentration and best-fit lines for  (squares and solid lines) and
(squares and solid lines) and  (circles and dotted lines) fibrils. (D) Plots of experimental average fibril lengths and best-fit lines as a function of shrinkage time for
(circles and dotted lines) fibrils. (D) Plots of experimental average fibril lengths and best-fit lines as a function of shrinkage time for  (squares and solid lines) and
(squares and solid lines) and  (circles and dotted lines) fibrils. Experiments were done at 24° C. Experimental errors are approximately equal to the symbol sizes.
(circles and dotted lines) fibrils. Experiments were done at 24° C. Experimental errors are approximately equal to the symbol sizes.
Table 1.
Kinetic parameters and quasi-equilibrium Aβ1–40 monomer concentrations for  and
and  fibrils determined by AFM and UV absorbance.
fibrils determined by AFM and UV absorbance.
| temperature | fibril type | ke (nm/s-µM) |
ks (nm/s) |
MQE,AFM (µM) |
MQE,UV (µM)a,b |
|---|---|---|---|---|---|
| 24°C | (6.07±0.23) | (2.71±0.19) | |||
| 0.45±0.04 | 0.50±0.08 | ||||
| X 10−3 | X 10−3 | ||||
| (8.68±0.11) | (3.44±0.36) | ||||
| 0.40±0.04 | 0.34±0.06 | ||||
| X 10−3 | X 10−3 | ||||
| (35.5±1.8) | (1.89±0.13) | ||||
| 0.053±0.005 | ---- | ||||
| X 10−3 | X 10−3 | ||||
| 37°C | (35.0±2.0) | (2.21±0.02) | |||
| 0.063±0.004 | ---- | ||||
| X 10−3 | X 10−3 |
Uncertainties in MQE,UV represent the standard deviation from five independent measurements.
MQE,UV at 37° C was below our detection limit.
Linear dependence of the average fibril length on time is predicted by Eq. 1, provided that M does not change appreciably over the measurement time, which is the case in our experiments. Linear dependence of the elongation rate on soluble peptide concentration is expected under conditions that are explained in the Discussion section.
Quasi-equilibrium solubilities were also determined from dialysis experiments under conditions similar to the fibril shrinkage experiments, but with a larger initial quantity of fibrils in the dialysis tube, so that quasi-equilibrium between fibrillar and soluble Aβ1–40 was established over the entire dialysis system within 72 hr. Values of MQE,UV in Table 1 were then determined from UV absorbance of the solution outside the dialysis tube, using analytical HPLC. HPLC traces are shown in Fig. S5 of the Supporting Information. Values of MQE,AFM and MQE,UV agree to within the errors in each measurement. The combined results from AFM and UV suggest that  fibrils may be more stable than
fibrils may be more stable than  fibrils. This suggestion is supported by solid state NMR data described below.
fibrils. This suggestion is supported by solid state NMR data described below.
In elongation experiments, the average fibril lengths increased by 400–800 nm during the measurement time. Given an initial 20-fold excess of soluble Aβ1–40 over fibrillar Aβ1–40 and an initial average fibril length of 150 nm, this implies that 20% or less of the soluble Aβ1–40was consumed during the measurement time. In principle, consumption of soluble Aβ1–40 would cause the data in Figs. 3 A and 3B to depart from linearity. Under our conditions, consumption of soluble Aβ1–40 affects slopes determined by linear fits to the data by 5% or less. (In preliminary experiments, departure from linearity was observed at longer times, particularly at the higher soluble Aβ1–40 concentrations.)
In shrinkage experiments, the volume of the buffer reservoir and the initial quantity of fibrils were chosen so that the Aβ1–40 monomer concentration would be approximately 0.15 µM if all fibrils dissolved completely. Based on the measured length distributions, less than half of the total fibril mass dissolved during shrinkage measurements. Control experiments shown in Fig. S5 of the Supporting Information confirmed that monomeric Aβ1–40 diffused out of the dialysis tube on the time scale of 2 hr, much less than the time scale for fibril shrinkage. Thus, the shrinkage kinetics were not affected by the presence of the dialysis membrane between the fibrils and the buffer reservoir.
Temperature dependence of kinetics and thermodynamics
Fibril elongation and shrinkage experiments were also performed at 37° C. Fibril length distributions from elongation experiments are shown in Fig. S6 of the Supporting Information. Plots of the dependences of average fibril lengths on incubation time and the dependence of the elongation slope on initial monomer concentration are shown in Fig. 4. As shown in Table 1, values of ks decrease by factors of roughly 1.5 and values of ke increase by factors of 4–6 as temperature increases from 24° C to 37° C. As a result, values of MQE,AFM decrease by factors of 6–9, and become less than the 0.1 µM detection limit of our UV/HPLC measurements. Indeed, attempts to measure MQE,AV were unsuccessful (see Fig. S5). At 37° C, the difference between MQE,AFM values for  and
and  fibrils is not larger than experimental uncertainties.
fibrils is not larger than experimental uncertainties.
Figure 4.
Similar plots as in Fig. 3, but for experiments done at 37°C. Soluble Aβ1–40 concentrations in panels A and B are 13.3 µM (squares and solid lines), 26.7 µM (circles and dotted lines) and 40.0 µM (diamonds and dashed lines).
Simulations of intermittent fibril elongation
As shown in Fig. 2, Figs. S3, and S5, the widths of fibril length distributions in elongation experiments clearly increase with increasing incubation time. If elongation and shrinkage rates of individual fibrils are constant (with these rates defined as the probabilities of adding or subtracting a monomer in a very small time interval, divided by the time interval and multiplied by the length change λ per monomer), then one expects the average fibril length to increase as (keM-ks)t, where t is the incubation time and M is the monomer concentration, and the width of the length distribution to increase as [λ(keM + ks )t]1/2. Thus, the ratio of the width of the distribution to the average fibril length should decrease with increasing incubation time in elongation experiments, contrary to our experimental results. This observation suggests that elongation rates of individual fibrils are not constant.
To explain the observed length distributions, we invoke a model in which individual filaments switch between "on" and "off states, with correlation time τc and fractional occupancies fon and foff = 1 - fon. In the "on" state, fibrils elongate at the rate kon = keM/fon, where ke is the experimentally determined value; in the "off" state, fibrils do not elongate. This model is supported by previous studies of Aβ and glucagon fibrils, which provided direct evidence for intermittent fibril growth from in situ fluorescence microscopy 31,32 and AFM30 imaging. To estimate the values of fon and τc for Aβ1–40 fibril growth under our experimental conditions, we simulated fibril length distributions as follows: (i) In each elongation simulation, we calculated the growth of 200 independent fibrils, with an initial length distribution equal to the experimental length distribution at t = 0; (ii) The initial state of each fibril was randomly chosen to be "on" or "off", with probabilities fon and foff; (iii) The incubation period was divided into time steps δt = 1 s. At each time step, the state of the fibril was allowed to change, based on random numbers, with probabilities equal to [1–exp(–t'/τc)]foff for changing from "on" to "off" and [1 – exp(–t'/ τc )]fon for changing from "off" to "on", where t' is the time since the previous change in state; (iv) The fibril length at time t was then equal to its initial length plus non(t)konδt, where non(t) is the number of time steps in which the fibril was in the "on" state up to time t; (v) For each experimental condition and each choice of fon and τc, the results of 50 independent simulations, with different random number choices, were averaged. The shrinkage rate ks was not included in these simulations, because shrinkage is negligible on the time scale of our elongation experiments. Simulated length distributions were then compared with experimental distributions by calculating the total squared deviation Δ2 between simulated and experimental length histograms at all incubation times for a given experimental condition.
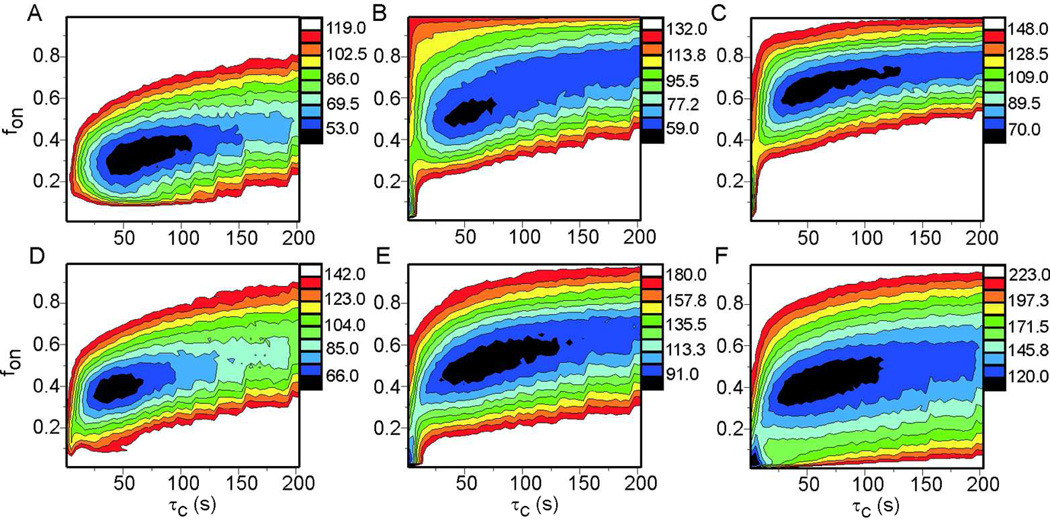
Fig. 5 compares experimental and best-fit simulated length distributions at 9, 21 and 35 min incubation times for  fibrils with 25 µM initial soluble Aβ1–40 concentration at 24° C. Figs. S4 and S6 of the Supporting Information show the full set of experimental and simulated distributions. Agreement between experiments and simulations is good, indicating that the intermittent elongation model can explain the observed broadening of fibril length distributions. Fig. 6 and Fig. S7 of the Supporting Information show contour plots of Δ as a function of τc and fon, from which the best-fit parameters and their uncertainties given in Table 2 were determined. Best-fit values of fon are in the 0.3–0.7 range. Values of τc are in the 10–70 s range, with the smaller values occurring at 37° C.
fibrils with 25 µM initial soluble Aβ1–40 concentration at 24° C. Figs. S4 and S6 of the Supporting Information show the full set of experimental and simulated distributions. Agreement between experiments and simulations is good, indicating that the intermittent elongation model can explain the observed broadening of fibril length distributions. Fig. 6 and Fig. S7 of the Supporting Information show contour plots of Δ as a function of τc and fon, from which the best-fit parameters and their uncertainties given in Table 2 were determined. Best-fit values of fon are in the 0.3–0.7 range. Values of τc are in the 10–70 s range, with the smaller values occurring at 37° C.
Figure 5.
Experimental (A) and best-fit simulated (B) histograms of  fibril lengths in elongation measurements with 25 µM soluble Aβ1–40 concentration (τc = 41 s and fon = 0.36 in simulations).
fibril lengths in elongation measurements with 25 µM soluble Aβ1–40 concentration (τc = 41 s and fon = 0.36 in simulations).
Figure 6.
Contour plots of the deviation between experimental and simulated histograms of Aβ1–40 fibril length distributions in fibril elongation measurements at 24° C, as a function of the correlation time (τc) and elongation fraction (fon) assumed in the simulations. (A,B,C)  fibrils with 25 µM, 50 µM, and 75 µM soluble Aβ1–40 concentrations, respectively. (D,E,F)
fibrils with 25 µM, 50 µM, and 75 µM soluble Aβ1–40 concentrations, respectively. (D,E,F)  fibrils with 25 µM, 50 µM, and 75 µM soluble Aβ1–40 concentrations, respectively. Each contour plot represents the average of 50 independent simulations with different random numbers to determine switching of fibrils between "on" and "off" elongation states. In each plot, the contour level increment was set to where was the minimum total squared deviation between simulated and experimental histograms.
fibrils with 25 µM, 50 µM, and 75 µM soluble Aβ1–40 concentrations, respectively. Each contour plot represents the average of 50 independent simulations with different random numbers to determine switching of fibrils between "on" and "off" elongation states. In each plot, the contour level increment was set to where was the minimum total squared deviation between simulated and experimental histograms.
Table 2.
Best-fit elongation fraction (fon) and correlation time (rc) values in simulations of Aβ1–40 fibril elongation measurements.
| temperature | fibril type | initial monomer concentration (µM) |
fona | τc (s)a |
|---|---|---|---|---|
| 24° C | 25 | 0.35±0.09 | 77±35 | |
| 50 | 0.57±0.07 | 61±21 | ||
| 75 | 0.69±0.07 | 65±40 | ||
| 25 | 0.39±0.05 | 37±18 | ||
| 50 | 0.49±0.09 | 57±42 | ||
| 75 | 0.41±0.08 | 57±40 | ||
| 37° C | 13 | 0.40±0.10 | 19±9 | |
| 27 | 0.42±0.17 | 12±8 | ||
| 40 | 0.61±0.11 | 47±32 | ||
| 13 | 0.45±0.13 | 23±12 | ||
| 27 | 0.39±0.19 | 15±7 | ||
| 40 | 0.63±0.18 | 50±40 |
Uncertainties represent ranges of variation for individual parameters within which the total squared deviation between experimental and simulated length histograms does not exceed .
Structural inter conversion monitored by solid state NMR
At 24°C, values of MQE derived from both AFM and UV measurements are smaller for  fibrils than for
fibrils than for  fibrils, although the uncertainties in these measurements preclude a definite conclusion regarding the relative thermodynamic stabilities of the two polymorphs. Based on results in Table 1, the ratio of the MQE value for
fibrils, although the uncertainties in these measurements preclude a definite conclusion regarding the relative thermodynamic stabilities of the two polymorphs. Based on results in Table 1, the ratio of the MQE value for  fibrils to the MQE value for
fibrils to the MQE value for  fibrils may be as large as 2.0 at 24° C. Assuming that the chemical potential for soluble Aβ1–40 is given by the expression µS = µS* +RTln(M/M *), where M is the concentration in solution and µS* is the chemical potential at a reference concentration M* , and assuming that the chemical potential of Aβ1–40 in a specific fibril polymorph at quasi-equilibrium equals the chemical potential in solution at concentration MQE, the difference in chemical potentials in
fibrils may be as large as 2.0 at 24° C. Assuming that the chemical potential for soluble Aβ1–40 is given by the expression µS = µS* +RTln(M/M *), where M is the concentration in solution and µS* is the chemical potential at a reference concentration M* , and assuming that the chemical potential of Aβ1–40 in a specific fibril polymorph at quasi-equilibrium equals the chemical potential in solution at concentration MQE, the difference in chemical potentials in  and
and  fibrils is ΔµF = RTln[MQE
fibrils is ΔµF = RTln[MQE /MQE
/MQE ]. Using values in Table 1, ΔµF may be as large as 0.8 kcal/mol or as small as −0.08 kcal/mol at 24°C.
]. Using values in Table 1, ΔµF may be as large as 0.8 kcal/mol or as small as −0.08 kcal/mol at 24°C.
Provided that ΔµF ≠ 0, a 1:1 mixture of  and
and  fibrils should evolve toward the more stable structure by gradual net shrinkage of the less stable fibrils and net elongation of the more stable fibrils. Eventually, the less stable fibrils should disappear (even if ΔµF is very small, because it is always thermodynamically favorable to transfer Aβ1–40 molecules from a less stable to a more stable polymorph, assuming that the chemical potentials are independent of fibril length). The time scale for this process can be estimated as follows: Assuming that the 1:1 mixture contains equal numbers of
fibrils should evolve toward the more stable structure by gradual net shrinkage of the less stable fibrils and net elongation of the more stable fibrils. Eventually, the less stable fibrils should disappear (even if ΔµF is very small, because it is always thermodynamically favorable to transfer Aβ1–40 molecules from a less stable to a more stable polymorph, assuming that the chemical potentials are independent of fibril length). The time scale for this process can be estimated as follows: Assuming that the 1:1 mixture contains equal numbers of  and
and  fibrils, a steady-state monomer concentration will be established in which the average number of monomers in the more stable fibrils increases at the same rate at which the average number of monomers in the less stable fibrils decreases. One can show that this rate (in monomers per second) equals ηAηQ(keQksA−keAksQ)/(ηQkeQ+ηAkeA) = kmix , where subscripts A and Q refer to the two polymorphs and η is the number of monomers per nm. From Table 1, with ηA = 4.2/nm and ηQ = 6.3/nm (corresponding to two-fold and three-fold symmetric structures with a 0.48 nm repeat distance), the value of kmix may be roughly 5 × 10−4 s−1 , corresponding to a time of roughly 2 × 106 s, or 22 days, for
fibrils, a steady-state monomer concentration will be established in which the average number of monomers in the more stable fibrils increases at the same rate at which the average number of monomers in the less stable fibrils decreases. One can show that this rate (in monomers per second) equals ηAηQ(keQksA−keAksQ)/(ηQkeQ+ηAkeA) = kmix , where subscripts A and Q refer to the two polymorphs and η is the number of monomers per nm. From Table 1, with ηA = 4.2/nm and ηQ = 6.3/nm (corresponding to two-fold and three-fold symmetric structures with a 0.48 nm repeat distance), the value of kmix may be roughly 5 × 10−4 s−1 , corresponding to a time of roughly 2 × 106 s, or 22 days, for  fibrils to shrink by 150 nm. The actual time scale is sensitive to the precise values of ke and ks.
fibrils to shrink by 150 nm. The actual time scale is sensitive to the precise values of ke and ks.
To test for interconversion between  and
and  fibril structures experimentally, we recorded solid state 13C and 15N NMR spectra of
fibril structures experimentally, we recorded solid state 13C and 15N NMR spectra of  fibrils,
fibrils,  fibrils, and a mixture of the two polymorphs. For these measurements, Aβ1–40 was synthesized with uniform 15N and 13C labeling of a single residue, namely I32. Labeled
fibrils, and a mixture of the two polymorphs. For these measurements, Aβ1–40 was synthesized with uniform 15N and 13C labeling of a single residue, namely I32. Labeled  fibrils were grown from seeds. Labeled
fibrils were grown from seeds. Labeled  fibrils were grown de novo, with agitation as described above. Fibrils (3 mg of each polymorph) were pelleted by ultracentrifugation (435,000 × g, 3 hr) and loaded into separate MAS rotors by centrifugation (14,000 × g). After recording NMR spectra of each polymorph separately, the fibrils were removed from the rotors, resuspended together in 200 µl of incubation buffer, pelleted again, and reloaded into a single MAS rotor. NMR spectra of the mixture were recorded immediately. Fibrils were then removed from the rotor, resuspended in 500 µl of incubation buffer, sonicated to produce fragments with lengths of approximately 50 nm (Branson model S-250A sonifier with tapered 1/8" microtip horn, lowest power, 10% duty cycle, 10 min), and incubated at 24° C for 35 days. During the incubation period, the fibrils were sonicated again for 2 min on days 3, 6, 9, and 12. Sonication was performed to ensure that all fibrils remained short, thereby accelerating the interconversion process. After 35 days, fibrils were pelleted again and reloaded into a single MAS rotor. Due to sample losses during unpacking and repacking of the MAS rotor, the final set of spectra required a total of 5.5 days of signal averaging. Earlier spectra were recorded in less than one day.
fibrils were grown de novo, with agitation as described above. Fibrils (3 mg of each polymorph) were pelleted by ultracentrifugation (435,000 × g, 3 hr) and loaded into separate MAS rotors by centrifugation (14,000 × g). After recording NMR spectra of each polymorph separately, the fibrils were removed from the rotors, resuspended together in 200 µl of incubation buffer, pelleted again, and reloaded into a single MAS rotor. NMR spectra of the mixture were recorded immediately. Fibrils were then removed from the rotor, resuspended in 500 µl of incubation buffer, sonicated to produce fragments with lengths of approximately 50 nm (Branson model S-250A sonifier with tapered 1/8" microtip horn, lowest power, 10% duty cycle, 10 min), and incubated at 24° C for 35 days. During the incubation period, the fibrils were sonicated again for 2 min on days 3, 6, 9, and 12. Sonication was performed to ensure that all fibrils remained short, thereby accelerating the interconversion process. After 35 days, fibrils were pelleted again and reloaded into a single MAS rotor. Due to sample losses during unpacking and repacking of the MAS rotor, the final set of spectra required a total of 5.5 days of signal averaging. Earlier spectra were recorded in less than one day.
15N NMR spectra are shown in Fig. 7A. The backbone amide 15N chemical shifts for I32 in  and
and  fibrils differ by 2.65 ppm, allowing signal contributions from the two polymorphs to be resolved in spectra of the mixture. Deconvolution of the 15N NMR spectra shows that the ratio
fibrils differ by 2.65 ppm, allowing signal contributions from the two polymorphs to be resolved in spectra of the mixture. Deconvolution of the 15N NMR spectra shows that the ratio  fibril mass to
fibril mass to  fibril mass in the mixture was initially 1.3:1.0. After 35 days of incubation at 24° C, this ratio changed to 2.6:1.0. Thus,
fibril mass in the mixture was initially 1.3:1.0. After 35 days of incubation at 24° C, this ratio changed to 2.6:1.0. Thus,  fibrils have greater thermodynamic stability than
fibrils have greater thermodynamic stability than  fibrils under our experimental conditions, as suggested by the values of MQE in Table 1. The long time scale required for interconversion (>35 days for completion) is consistent with the estimate derived above.
fibrils under our experimental conditions, as suggested by the values of MQE in Table 1. The long time scale required for interconversion (>35 days for completion) is consistent with the estimate derived above.
Figure 7.
(A) Solid state 15N NMR spectra of Aβ1–40 fibrils, with uniform 15N and 13C labeling of I32. Spectra of  fibrils,
fibrils,  fibrils, and a mixture of
fibrils, and a mixture of  and
and  fibrils after 0 and 35 days of incubation at 24° C are shown. Dashed blue and red lines are Gaussian lineshapes with full-width-at-half-maximum equal to 2.22 ppm, fitted to 15N signals of
fibrils after 0 and 35 days of incubation at 24° C are shown. Dashed blue and red lines are Gaussian lineshapes with full-width-at-half-maximum equal to 2.22 ppm, fitted to 15N signals of  and
and  fibrils, respectively. Blue and red vertical lines indicate peak positions for signals from
fibrils, respectively. Blue and red vertical lines indicate peak positions for signals from  and
and  fibrils. Spectra of the mixture indicate gradual transfer of Aβ1–40 from
fibrils. Spectra of the mixture indicate gradual transfer of Aβ1–40 from  fibrils to
fibrils to  fibrils, confirming the greater thermodynamic stability of
fibrils, confirming the greater thermodynamic stability of  fibrils under these experimental conditions. (B) Solid state 13C NMR spectra of the same samples. (C) Expansions of carbonyl regions of the 13C NMR spectra. (D) Expansions of aliphatic regions of the 13C NMR spectra. (Note that up to 5% of the 15N NMR signal amplitude and up to 33% of the carbonyl 13C NMR signal amplitude arises from natural-abundance 15N and 13C at residues other than I32.)
fibrils under these experimental conditions. (B) Solid state 13C NMR spectra of the same samples. (C) Expansions of carbonyl regions of the 13C NMR spectra. (D) Expansions of aliphatic regions of the 13C NMR spectra. (Note that up to 5% of the 15N NMR signal amplitude and up to 33% of the carbonyl 13C NMR signal amplitude arises from natural-abundance 15N and 13C at residues other than I32.)
13C NMR spectra are shown in Figs. 7A, 7B, and 7C. Most 13C sites in I32 have nearly identical chemical shifts in the two polymorphs. The largest difference occurs at the backbone carbonyl site. Although carbonyl 13C NMR lines of  and
and  in Fig. 7C indicate a greater ratio of
in Fig. 7C indicate a greater ratio of  fibril mass to
fibril mass to  fibril mass after 35 days, consistent with conclusions from the 15N NMR spectra. In the aliphatic region of the 13C NMR spectra (Fig. 7D), α-carbon signals of I32 appear near 58 ppm, with a 0.7 ppm difference between chemical shifts of
fibril mass after 35 days, consistent with conclusions from the 15N NMR spectra. In the aliphatic region of the 13C NMR spectra (Fig. 7D), α-carbon signals of I32 appear near 58 ppm, with a 0.7 ppm difference between chemical shifts of  and
and  fibrils. After 35 days, the peak position of the α-carbon line in the spectrum of the mixture agrees well with the peak position in the spectrum of
fibrils. After 35 days, the peak position of the α-carbon line in the spectrum of the mixture agrees well with the peak position in the spectrum of  fibrils, again consistent with conclusions from the 15N NMR spectra.
fibrils, again consistent with conclusions from the 15N NMR spectra.
Polymorphic-specific susceptibility of Aβ1–40 fibrils to fragmentation
As discussed by others, fibril growth can be influenced by mechanical properties, especially susceptibility to fragmentation, since fragmentation creates new fibril ends and therefore accelerates the net growth of fibril mass.39,40 To test for differences in susceptibility to fragmentation, we exposed  and
and  fibrils to shear forces, by placing fibril solutions in vials that contained rapidly rotating stir bars. As shown in Fig. S8,
fibrils to shear forces, by placing fibril solutions in vials that contained rapidly rotating stir bars. As shown in Fig. S8,  fibrils were found to be significantly more susceptible to fragmentation by shear forces than were
fibrils were found to be significantly more susceptible to fragmentation by shear forces than were  fibrils, breaking into <1 µm segments within 15 min (compared with several hours for
fibrils, breaking into <1 µm segments within 15 min (compared with several hours for  fibrils). This result may arise from the fact that fragmentation of
fibrils). This result may arise from the fact that fragmentation of  fibrils requires disruption of ∼2/3 as many intermolecular interactions, including backbone hydrogen bonds and sidechain-sidechain interactions, as does fragmentation of
fibrils requires disruption of ∼2/3 as many intermolecular interactions, including backbone hydrogen bonds and sidechain-sidechain interactions, as does fragmentation of  fibrils.
fibrils.
Discussion
Summary of results
Our results show that AFM images can be used to quantify time-dependent Aβ1–40 fibril length distributions, allowing the determination of rates of fibril extension and shrinkage in the presence and absence of excess soluble Aβ1–40. Growth rates are proportional to the concentration of excess soluble Aβ1–40. The soluble Aβ1–40 concentration at quasi-equilibrium, MQE, is then given by the ratio of rate constants for shrinkage and extension, ks/ke. Values of MQE determined in this way are in good agreement with direct measurements by UV absorbance, providing validation for both approaches. In principle, the AFM approach has several advantages: (i) it is not necessary for a quasi-equilibrium state to be reached, as long as ks and ke are measurable; (ii) it is not necessary for fibrils to be separated from soluble species; (iii) both kinetic information and thermodynamic information are obtained; (iv) small values of MQE, below the detectable limit of our UV measurements, can be determined; (v) impurities that do not interact with Aβ1–40 fibrils but may interfere with UV measurements, such as low levels of oxidized or racemized Aβ1–40, do not affect the AFM measurements. For UV absorbance measurements, we have developed a simple dialysis approach that obviates the need to separate soluble from fibrillar peptide (by ultracentrifugation, filtration, or some other method) and allows periodic monitoring of the fibril dissolution process.
Results in Table 1 show that two Aβ1–40 fibril polymorphs with distinct molecular structures have very similar values of MQE, and hence similar thermodynamic stabilities. However, at 24° C, values of ke and (possibly) ks are significantly greater for  fibrils than for
fibrils than for  fibrils. This difference in kinetics may be related to structural differences. In particular,
fibrils. This difference in kinetics may be related to structural differences. In particular,  fibrils and
fibrils and  fibrils have mass-per-length values of approximately 27 kDa/nm and 18 kDa/nm, respectively, corresponding to molecular structures with three-fold or two-fold symmetry about the fibril growth axis.2–4,20 If the rates of addition of Aβ1–40 monomers to the ends of the two polymorphs were the same, values of ke (in length units, rather than monomer units) would be in a 3:2 ratio, close to experimental observations. At 37° C, values of ke and ks are very similar, indicating that this simple structural argument has limited validity.
fibrils have mass-per-length values of approximately 27 kDa/nm and 18 kDa/nm, respectively, corresponding to molecular structures with three-fold or two-fold symmetry about the fibril growth axis.2–4,20 If the rates of addition of Aβ1–40 monomers to the ends of the two polymorphs were the same, values of ke (in length units, rather than monomer units) would be in a 3:2 ratio, close to experimental observations. At 37° C, values of ke and ks are very similar, indicating that this simple structural argument has limited validity.
Both AFM and UV measurements suggest that  fibrils may be more stable than
fibrils may be more stable than  fibrils at 24° C, by up to 0.8 kcal/mol. Direct measurements of interconversion between the two polymorphs, monitored by solid state NMR spectroscopy over a period of 35 days as shown in Fig. 7, confirm that
fibrils at 24° C, by up to 0.8 kcal/mol. Direct measurements of interconversion between the two polymorphs, monitored by solid state NMR spectroscopy over a period of 35 days as shown in Fig. 7, confirm that  fibrils are more stable.
fibrils are more stable.
Values of MQE are roughly eight times smaller at 37° C than at 24° C, an observation that is consistent with stabilization of Aβ1–40 fibril structures by hydrophobic interactions, as indicated by early biochemical studies41–43 and later structural studies.3,4,8,44 The lower values at the more physiologically relevant temperature may have implications for fibril formation in AD. Soluble Aβ1–40 and Aβ1–42 concentrations in brain tissue of AD patients have been reported to be roughly 5–10 nM.45 Of course, we expect quasi-equilibrium solubilities to be affected also by ionic strength, association with membrane surfaces, macromolecular crowding, and other physiological factors.
Kinetic model
Fibril growth rates are much less than diffusion-limited rates. With a translational diffusion constant for Aβ1–40 of 1.5 × 10−6 cm2/s,46 the diffusion-limited rate of attachment of new monomers to the ends of a fibril can be estimated to be roughly 1 × 103/s-µM.47 With a repeat distance of 0.48 nm in a cross-β amyloid structure, and with either two or three monomers per repeat unit, the diffusion-limited value of ke is roughly 200 nm/s-µM, much greater than experimentally observed values in Table 1. Therefore, only a small fraction (∼10−4) of collisions between monomers and fibril ends lead to fibril growth. Given that Aβ1–40 is conformationally disordered in its soluble state and becomes conformationally ordered in its fibrillar state, it seems likely that monomers bind initially to fibril ends in a somewhat disordered state and in a transient manner, before adopting the fibrillar conformation. Such behavior is observed in simulations of monomer/fibril interactions,48–50 and may be related to the "dock-lock" mechanism of fibril growth proposed by Esler et al.29 (see below).
A simple kinetic scheme that corresponds to transient binding of disordered monomers before their incorporation into the fibril structure is the following:
| (2) |
where M represents a free monomer, Fn represents a fibril containing n structured monomers, and M*Fn represents a fibril containing n structured monomers with an additional unstructured monomer bound to its end. Scheme (2) implies
| (3) |
Under conditions of steady-state growth, . Defining the total fibril concentration to be and the total concentration of fibril-bound unstructured monomers to be , and assuming [F1] to be negligible, Eq. (3) then implies
| (4) |
The net fibril growth rate kgrowth (in monomers per unit time) is the difference between the total rate of conversion from M*Fn to Fn+1 and the total rate of conversion from Fn+1 to M*Fn, summed over n:
| (5a) |
| (5b) |
The expression for kgrowth in Eq. (5b) is analogous to standard expressions for rates of enzymatic catalysis, with [M] taking the place of the substrate concentration and [Ft] taking the place of the total enzyme concentration.51 In the limit that [M] is sufficiently small that fibril ends are not saturated with unstructured monomers (i.e., k1[M] << k−1), yet sufficiently large that fibril elongation dominates over fibril shrinkage, Eq. (5b) predicts that the fibril growth rate is proportional to the monomer concentration, in agreement with our experiments (see Figs. 3C and 4C). In the context of this kinetic model, and assuming k2 >> k−2, our measured values of ke and ks correspond to and ηks ≈ k−2, where η is the number of monomers per nm. The fraction of fibrils with a bound, unstructured monomer is, which can not be determined from ke and ks.
We note that the foregoing analysis assumes that fibrils have only one actively growing end, an assumption that can be easily removed. For simplicity, we assume that fibril ends accommodate only one unstructured monomer, although the experimentally-based structural models for  and
and  fibrils and experimental mass-per-length data indicate multiple monomers per repeat unit. More complicated kinetic models can treat a situation in which two or more unstructured monomers must bind before the structurally ordered fibril length can increase. In the absence of information about the molecular structure at fibril ends, which is likely to differ from the bulk structure, we have not pursued such models.
fibrils and experimental mass-per-length data indicate multiple monomers per repeat unit. More complicated kinetic models can treat a situation in which two or more unstructured monomers must bind before the structurally ordered fibril length can increase. In the absence of information about the molecular structure at fibril ends, which is likely to differ from the bulk structure, we have not pursued such models.
Intermittent fibril elongation
Fibril length distributions increase in width with increasing time in elongation experiments, more rapidly than expected if elongation were proceeding at a constant rate. As shown in Figs. 5, S4, and S6, the length distributions can be explained by assuming that fibrils grow intermittently, as observed for Aβ1–40 and other fibrils in earlier studies.30–32 A likely physical mechanism for intermittent elongation is that molecules at the fibril ends occasionally adopt a stable structure that differs from the bulk structure and can not propagate, effectively capping the fibril ends. Fits to the length distributions in Figs. 6, S7, and Table 2 indicate that fibrils may be capped in this way 20–80% of the time, with no clear correlation between fon and monomer concentration, temperature, or fibril structure. The correlation time for switching between elongating and capped states is 10–70 s, apparently somewhat smaller at 37° C than at 24° C, suggesting that switching between states is a thermally activated process. Values of ks in Table 1 indicate that structured Aβ1–40 monomers dissociate from fibrils at 1–2 × 10−2 s−1 rates in the absence of soluble Aβ1–40, corresponding to lifetimes that are comparable to the values of τc in Table 2.
Intermittent elongation could be included in the kinetic scheme discussed above by adding a capped state M†Fn that exchanges with M*Fn at forward and reverse rates on the order of 1/τc but does not convert to Fn+1. It is worth emphasizing that the existence of capped states and intermittent elongation does not invalidate the equality of ks/ke and MQE, because the quasi-equilibrium solubility is determined by time-averaged elongation and shrinkage rates that already include intermittency.
Factors influencing fibril polymorphism
One motivation for investigating polymorph-specific thermodynamics and kinetics of Aβ1–40 fibril formation is to improve our understanding of how certain growth conditions favor certain polymorphs in de novo (i.e., unseeded) fibril preparations. In particular, the predominant fibril structure in de novo preparations has two-fold symmetry when the Aβ1–40 solution is agitated during fibril growth and three-fold symmetry when the solution is quiescent, all other conditions being the same (pH 7.4, 24° C, 10 mM phosphate buffer).2 In principle, the predominant structure under a given set of experimental conditions could be determined by kinetic factors (i.e., rates of nucleation and elongation) or by thermodynamic stability. Experiments described above show that the thermodynamic stabilities and elongation rates of  and
and  fibrils are rather similar, indicating that these factors do not explain why
fibrils are rather similar, indicating that these factors do not explain why  or
or  fibrils predominate under specific conditions. On the other hand,
fibrils predominate under specific conditions. On the other hand,  and
and  fibrils have significantly different susceptibilities to fragmentation under shear forces. In agitated solutions, where shear forces are similar in magnitude to those in experiments shown in Fig. S8, preferential fragmentation of
fibrils have significantly different susceptibilities to fragmentation under shear forces. In agitated solutions, where shear forces are similar in magnitude to those in experiments shown in Fig. S8, preferential fragmentation of  fibrils is expected to accelerate the net growth of these fibrils relative to the net growth of
fibrils is expected to accelerate the net growth of these fibrils relative to the net growth of  fibrils, leading to the predominance of
fibrils, leading to the predominance of  fibrils. The predominance of
fibrils. The predominance of  fibrils in quiescent solutions, where shear forces are negligible, is explicable if the intrinsic nucleation rate of
fibrils in quiescent solutions, where shear forces are negligible, is explicable if the intrinsic nucleation rate of  fibrils exceeds that of
fibrils exceeds that of  fibrils.
fibrils.
It is additionally possible that nucleation of  fibrils occurs at interfaces, either between the Aβ1–40 solution and air or between the peptide solution and the walls of the tube. Under agitated growth conditions (see Materials and Methods), the surface-to-volume ratio of the solution is increased, potentially accelerating interface-dependent nucleation.
fibrils occurs at interfaces, either between the Aβ1–40 solution and air or between the peptide solution and the walls of the tube. Under agitated growth conditions (see Materials and Methods), the surface-to-volume ratio of the solution is increased, potentially accelerating interface-dependent nucleation.
The similarity of thermodynamic stabilities of  and
and  fibrils helps explain why polymorphism is a prevalent phenomenon in studies of amyloid fibrils. Similar MQE values (i.e., similar values of ke/ks, which in turn leads to small values of kmix) for structurally distinct fibrils imply that interconversion among polymorphs is a slow process, especially after fibrils grow to micron-scale lengths. Thus, a mixture of polymorphs will not evolve to a structurally homogeneous state on the time scale of typical in vitro experiments. The low MQE values of various polymorphs also imply that, once a quasi-equilibrium state is reached, nucleation of a new polymorph (even a more thermodynamically stable one) will occur at a very low rate. It is therefore essentially impossible for a sample of amyloid fibrils to evolve to its true equilibrium state on a realistic time scale, unless the most thermodynamically stable fibril structures are already present in the sample.
fibrils helps explain why polymorphism is a prevalent phenomenon in studies of amyloid fibrils. Similar MQE values (i.e., similar values of ke/ks, which in turn leads to small values of kmix) for structurally distinct fibrils imply that interconversion among polymorphs is a slow process, especially after fibrils grow to micron-scale lengths. Thus, a mixture of polymorphs will not evolve to a structurally homogeneous state on the time scale of typical in vitro experiments. The low MQE values of various polymorphs also imply that, once a quasi-equilibrium state is reached, nucleation of a new polymorph (even a more thermodynamically stable one) will occur at a very low rate. It is therefore essentially impossible for a sample of amyloid fibrils to evolve to its true equilibrium state on a realistic time scale, unless the most thermodynamically stable fibril structures are already present in the sample.
Comparisons with previous kinetic and thermodynamic studies
Values of MQE for Aβ1–40 fibrils, also called "critical concentrations", have been determined previously by several groups, either from direct measurements of soluble Aβ1–40 concentrations at quasi-equilibrium 7,23–25,52,53 or from kinetic measurements based on surface plasmon resonance 26,27 (SPR) or quartz crystal microbalance28 (QCM) data. Reported values cover a wide range, from below 0.1 µM 24,26 to above 10 µM.25,53 Some of this variation may be due to incomplete establishment of quasi-equilibrium, incomplete separation of aggregated Aβ1–40 from soluble Aβ1–40, or other experimental uncertainties. In addition, it is likely that different studies involved different polymorphs and that fibrils were structurally heterogeneous in some of these studies. Although we find that  and
and  fibrils have similar MQE values, other polymorphs may have significantly different values. Kodali et al. have observed values ranging from 0.23 µM to 16 µM for distinct Aβ1–40 fibril polymorphs in phosphate buffer at 37° C.
fibrils have similar MQE values, other polymorphs may have significantly different values. Kodali et al. have observed values ranging from 0.23 µM to 16 µM for distinct Aβ1–40 fibril polymorphs in phosphate buffer at 37° C.
Previous kinetic studies of seeded Aβ1–40 fibril growth have also yielded variable results. The linear dependence of elongation rate on soluble Aβ1–40 concentration in our experiments has been seen in most previous studies, 26–28,46 but not all. From quasi-elastic light scattering data, Lomakin et al. inferred an elongation rate (ηke) equal to 6.5 × 10−5 µM−1 s−1 in 0.1 M HCl,54 significantly smaller than the values in our experiments near neutral pH. Kinetic data from SPR 26,27 and QCM28 studies do not provide estimates of absolute ke and ks values, due to the unknown coverages and lengths of fibrils in those studies. Using fluorescence microscopy, Ban et al. observed intermittent Aβ1–40 fibril growth, with an average elongation rate of approximately 5 nm/s at pH 7.5, 37° C, and 50 µM soluble Aβ1–40 concentration,31 which corresponds to a ke value roughly three times larger than values in Table 1. By analyzing hydrogen/deuterium exchange data attributed to continual dissociation and reassociation of monomers at fibril ends under quasi-equilibrium conditions, Sanchez et al. derived a dissociation/reassociation rate of 0.6 s–1 for Aβ1–40 fibrils at pH 7.0 and 28° C.55 The data analysis described by Sanchez et al. assumes a fibril structure with only one Aβ1–40 molecule per 0.48 nm repeat. With two or three molecules per repeat, their analysis would imply ks values of roughly 0.6 nm/s or 0.9 nm/s, much larger than values in Table 1.
In measurements of the association of radio-labeled Aβ1–40 with preformed fibrils, and with an initial concentration of the radio-labeled peptide equal to 100 pM in phosphate-buffered saline at room temperature, Esler et al. observed a biphasic association process.29 Subsequent dissociation rates were also found to be biphasic and to depend on the association time. Similar behavior was observed for association/dissociation with amyloid in brain tissue. These observations led to the proposal of a "dock-lock" mechanism for fibril growth, in which monomers bound to the ends of fibrils are initially in a "docked" state that is in dynamic equilibrium with soluble monomers, and slowly convert to a more tightly bound "locked" state. In principle, the dock-lock mechanism can be described by Scheme (2) above. However, quantitative aspects of the results reported by Esler et al. appear to be inconsistent with our own results. In particular, at 100 pM soluble Aβ1–40 concentration, the time scale for adding one molecule to a fibril would be ∼100 hr according to ke values in Table 1 (assuming ks = 0), whereas the "locked" state develops within ∼1 hr in the experiments of Esler et al. In addition, under our experimental conditions, MQE >> 100 pM, so that fibrils would shrink rather than elongate.
Conclusion
Results described above for two Aβ1–40 fibril polymorphs demonstrate the efficacy of AFM as a means of quantifying polymorph-specific fibril elongation and shrinkage kinetics and quasi-equilibrium solubilities. The same methods can be applied to other amyloid-forming peptides and proteins. At 24° C, pH 7.4, and low ionic strength,  fibrils have greater elongation and shrinkage rates than
fibrils have greater elongation and shrinkage rates than  fibrils but similar solubilities (approximately 0.4 µM). Solid state NMR spectra show that a mixture of
fibrils but similar solubilities (approximately 0.4 µM). Solid state NMR spectra show that a mixture of  and
and  fibrils evolves toward pure
fibrils evolves toward pure  fibrils over a period of more than 35 days, indicating that
fibrils over a period of more than 35 days, indicating that  fibrils have greater thermodynamic stability under these conditions. At 37° C, elongation rates are increased and solubilities are reduced to about 0.06 µM for both polymorphs. Analysis of fibril length distributions during elongation suggests that growth is intermittent, with individual fibrils switching randomly between "on" and "off" states on the 10–70 s time scale. Finally,
fibrils have greater thermodynamic stability under these conditions. At 37° C, elongation rates are increased and solubilities are reduced to about 0.06 µM for both polymorphs. Analysis of fibril length distributions during elongation suggests that growth is intermittent, with individual fibrils switching randomly between "on" and "off" states on the 10–70 s time scale. Finally,  fibrils are significantly more susceptible than
fibrils are significantly more susceptible than  fibrils to fragmentation by shear forces, a fact that contributes to the predominance of
fibrils to fragmentation by shear forces, a fact that contributes to the predominance of  fibrils when Aβ1–40 fibrils are grown de novo in agitated solutions.
fibrils when Aβ1–40 fibrils are grown de novo in agitated solutions.
Supplementary Material
Acknowledgement
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. We thank Dr. Wai-Ming Yau for synthesizing and purifying Aβ1–40.
Footnotes
Supporting Information Available:
Diagram of dialysis apparatus (Fig. S1), TEM images of parent  and
and  fibrils which were used as seeds (Fig. S2), additional representative AFM images from fibril elongation experiments (Fig. S3), experimental and simulated histograms of fibril length distributions at 24° C (Fig. S4), dialysis control results and analytical HPLC traces to measure MQE,UV values (Fig. S5), experimental and simulated histograms of fibril length distributions at 37° C (Fig. S6), contour plots of fits between experimental and simulated fibril length distributions at 37° C (Fig. S7), and AFM images from fibril fragmentation experiments (Fig. S8). This material is available free of charge via the Internet at http://pubs.acs.org.
fibrils which were used as seeds (Fig. S2), additional representative AFM images from fibril elongation experiments (Fig. S3), experimental and simulated histograms of fibril length distributions at 24° C (Fig. S4), dialysis control results and analytical HPLC traces to measure MQE,UV values (Fig. S5), experimental and simulated histograms of fibril length distributions at 37° C (Fig. S6), contour plots of fits between experimental and simulated fibril length distributions at 37° C (Fig. S7), and AFM images from fibril fragmentation experiments (Fig. S8). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Petkova AT, Buntkowsky G, Dyda F, Leapman RD, Yau WM, Tycko R. J. Mol Biol. 2004;335:247. doi: 10.1016/j.jmb.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 2.Petkova AT, Leapman RD, Guo ZH, Yau WM, Mattson MP, Tycko R. Science. 2005;307:262. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- 3.Petkova AT, Yau WM, Tycko R. Biochemistry. 2006;45:498. doi: 10.1021/bi051952q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paravastu AK, Leapman RD, Yau WM, Tycko R. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18349. doi: 10.1073/pnas.0806270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldsbury CS, Wirtz S, Muller SA, Sunderji S, Wicki P, Aebi U, Frey P. J. Struct. Biol. 2000;130:217. doi: 10.1006/jsbi.2000.4259. [DOI] [PubMed] [Google Scholar]
- 6.Goldsbury C, Frey P, Olivieri V, Aebi U, Muller SA. J. Mol. Biol. 2005;352:282. doi: 10.1016/j.jmb.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Kodali R, Williams AD, Chemuru S, Wetzel R. J. Mol. Biol. 2010;401:503. doi: 10.1016/j.jmb.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertini I, Gonnelli L, Luchinat C, Mao JF, Nesi A. J. Am. Chem. Soc. 2011;133:16013. doi: 10.1021/ja2035859. [DOI] [PubMed] [Google Scholar]
- 9.Meinhardt J, Sachse C, Hortschansky P, Grigorieff N, Fandrich M. J. Mol. Biol. 2009;386:869. doi: 10.1016/j.jmb.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt M, Sachse C, Richter W, Xu C, Fandrich M, Grigorieff N. Proc. Natl. Acad. Sci. U. S. A. 2009;106:19813. doi: 10.1073/pnas.0905007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del Amo JML, Schmidt M, Fink U, Dasari M, Fandrich M, Reif B. Angew. Chem.-Int. Edit. 2012;51:6136. doi: 10.1002/anie.201200965. [DOI] [PubMed] [Google Scholar]
- 12.Luhrs T, Ritter C, Adrian M, Riek-Loher D, Bohrmann B, Doeli H, Schubert D, Riek R. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17342. doi: 10.1073/pnas.0506723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parthasarathy S, Long F, Miller Y, Xiao YL, McElheny D, Thurber K, Ma BY, Nussinov R, Ishii Y. J. Am. Chem. Soc. 2011;133:3390. doi: 10.1021/ja1072178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheidt HA, Morgado I, Rothemund S, Huster D. J. Biol. Chem. 2012;287:2017. doi: 10.1074/jbc.M111.308619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato T, Kienlen-Campard P, Ahmed M, Liu W, Li HL, Elliott JI, Aimoto S, Constantinescu SN, Octave JN, Smith SO. Biochemistry. 2006;45:5503. doi: 10.1021/bi052485f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torok M, Milton S, Kayed R, Wu P, McIntire T, Glabe CG, Langen R. J. Biol. Chem. 2002;277:40810. doi: 10.1074/jbc.M205659200. [DOI] [PubMed] [Google Scholar]
- 17.McDonald M, Box H, Bian W, Kendall A, Tycko R, Stubbs G. J. Mol. Biol. 2012;423:454. doi: 10.1016/j.jmb.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang R, Hu XY, Khant H, Ludtke SJ, Chiu W, Schmid MF, Frieden C, Lee JM. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4653. doi: 10.1073/pnas.0901085106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller Y, Ma B, Nussinov R. Chem. Rev. 2010;110:4820. doi: 10.1021/cr900377t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen B, Thurber KR, Shewmaker F, Wickner RB, Tycko R. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14339. doi: 10.1073/pnas.0907821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Nuallain B, Shivaprasad S, Kheterpal I, Wetzel R. Biochemistry. 2005;44:12709. doi: 10.1021/bi050927h. [DOI] [PubMed] [Google Scholar]
- 22.Shivaprasad S, Wetzel R. J. Biol. Chem. 2006;281:993. doi: 10.1074/jbc.M505091200. [DOI] [PubMed] [Google Scholar]
- 23.Williams AD, Shivaprasad S, Wetzel R. J. Mol. Biol. 2006;357:1283. doi: 10.1016/j.jmb.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 24.Komatsu H, Feingold-Link E, Sharp KA, Rastogi T, Axelsen PH. J. Biol. Chem. 2010;285:41843. doi: 10.1074/jbc.M110.165068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sengupta P, Garai K, Sahoo B, Shi Y, Callaway DJE, Maiti S. Biochemistry. 2003;42:10506. doi: 10.1021/bi0341410. [DOI] [PubMed] [Google Scholar]
- 26.Hasegawa K, Ono K, Yamada M, Naiki H. Biochemistry. 2002;41:13489. doi: 10.1021/bi020369w. [DOI] [PubMed] [Google Scholar]
- 27.Cannon MJ, Williams AD, Wetzel R, Myszka DG. Anal. Biochem. 2004;328:67. doi: 10.1016/j.ab.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Kotarek JA, Johnson KC, Moss MA. Anal. Biochem. 2008;378:15. doi: 10.1016/j.ab.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Esler WP, Stimson ER, Jennings JM, Vinters HV, Ghilardi JR, Lee JP, Mantyh PW, Maggio JE. Biochemistry. 2000;39:6288. doi: 10.1021/bi992933h. [DOI] [PubMed] [Google Scholar]
- 30.Kellermayer MSZ, Karsai A, Benke M, Soos K, Penke B. Proc. Natl. Acad. Sci. U. S. A. 2008;105:141. doi: 10.1073/pnas.0704305105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ban T, Hoshino M, Takahashi S, Hamada D, Hasegawa K, Naiki H, Goto Y. J. Mol. Biol. 2004;344:757. doi: 10.1016/j.jmb.2004.09.078. [DOI] [PubMed] [Google Scholar]
- 32.Ferkinghoff-Borg J, Fonslet J, Andersen CB, Krishna S, Pigolotti S, Yagi H, Goto Y, Otzen D, Jensen MH. Phys. Rev. E. 2010:82. doi: 10.1103/PhysRevE.82.010901. [DOI] [PubMed] [Google Scholar]
- 33.Schneider CA, Rasband WS, Eliceiri KW. Nat. Methods. 2012;9:671. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harper JD, Lieber CM, Lansbury PT. Chem. Biol. 1997;4:951. doi: 10.1016/s1074-5521(97)90303-3. [DOI] [PubMed] [Google Scholar]
- 35.Harper JD, Wong SS, Lieber CM, Lansbury PT. Chem. Biol. 1997;4:119. doi: 10.1016/s1074-5521(97)90255-6. [DOI] [PubMed] [Google Scholar]
- 36.Stine WB, Snyder SW, Ladror US, Wade WS, Miller MF, Perun TJ, Holzman TF, Krafft GA. J Protein Chem. 1996;15:193. doi: 10.1007/BF01887400. [DOI] [PubMed] [Google Scholar]
- 37.Kowalewski T, Holtzman DM. Proc. Natl. Acad. Sci. U. S. A. 1999;96:3688. doi: 10.1073/pnas.96.7.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nichols MR, Moss MA, Reed DK, Lin WL, Mukhopadhyay R, Hoh JH, Rosenberry TL. Biochemistry. 2002;41:6115. doi: 10.1021/bi015985r. [DOI] [PubMed] [Google Scholar]
- 39.Collins SR, Douglass A, Vale RD, Weissman JS. PLoS. Biol. 2004;2:1582. doi: 10.1371/journal.pbio.0020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knowles TPJ, Waudby CA, Devlin GL, Cohen SIA, Aguzzi A, Vendruscolo M, Terentjev EM, Welland ME, Dobson CM. Science. 2009;326:1533. doi: 10.1126/science.1178250. [DOI] [PubMed] [Google Scholar]
- 41.Halverson K, Fraser PE, Kirschner DA, Lansbury PT. Biochemistry. 1990;29:2639. doi: 10.1021/bi00463a003. [DOI] [PubMed] [Google Scholar]
- 42.Hilbich C, Kisterswoike B, Reed J, Masters CL, Beyreuther K. J. Mol. Biol. 1991;218:149. doi: 10.1016/0022-2836(91)90881-6. [DOI] [PubMed] [Google Scholar]
- 43.Hilbich C, Kisterswoike B, Reed J, Masters CL, Beyreuther K. J. Mol. Biol. 1992;228:460. doi: 10.1016/0022-2836(92)90835-8. [DOI] [PubMed] [Google Scholar]
- 44.Antzutkin ON, Balbach JJ, Leapman RD, Rizzo NW, Reed J, Tycko R. Proc. Natl. Acad. Sci. U. S. A. 2000;97:13045. doi: 10.1073/pnas.230315097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Dickson DW, Trojanowski JQ, Lee VMY. Exp. Neurol. 1999;158:328. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- 46.Tseng BP, Esler WP, Clish CB, Stimson ER, Ghilardi JR, Vinters HV, Mantyh PW, Lee JP, Maggio JE. Biochemistry. 1999;38:10424. doi: 10.1021/bi990718v. [DOI] [PubMed] [Google Scholar]
- 47.Zhou GQ, Zhong WZ. Eur. J. Biochem. 1982;128:383. [PubMed] [Google Scholar]
- 48.Nguyen PH, Li MS, Stock G, Straub JE, Thirumalai D. Proc. Natl. Acad. Sci. U. S. A. 2007;104:111. doi: 10.1073/pnas.0607440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Brien EP, Okamoto Y, Straub JE, Brooks BR, Thirumalai D. J. Phys. Chem. B. 2009;113:14421. doi: 10.1021/jp9050098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takeda T, Klimov DK. Biophys. J. 2009;96:4428. doi: 10.1016/j.bpj.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Creighton TE. Proteins: Structures and Molecular Properties. 2nd ed. W.H. Freeman; New York: 1992. [Google Scholar]
- 52.O’Nuallain B, Wetzel R. Proc. Natl. Acad. Sci. U. S. A. 2002;99:1485. doi: 10.1073/pnas.022662599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tjernberg LO, Pramanik A, Bjorling S, Thyberg P, Thyberg J, Nordstedt C, Berndt KD, Terenius L, Rigler R. Chem. Biol. 1999;6:53. doi: 10.1016/S1074-5521(99)80020-9. [DOI] [PubMed] [Google Scholar]
- 54.Lomakin A, Chung DS, Benedek GB, Kirschner DA, Teplow DB. Proc. Natl. Acad. Sci. U. S. A. 1996;93:1125. doi: 10.1073/pnas.93.3.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanchez L, Madurga S, Pukala T, Vilaseca M, Lopez-Iglesias C, Robinson CV, Giralt E, Carulla N. J. Am. Chem. Soc. 2011;133:6505. doi: 10.1021/ja1117123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.