Abstract
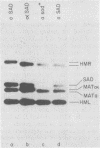
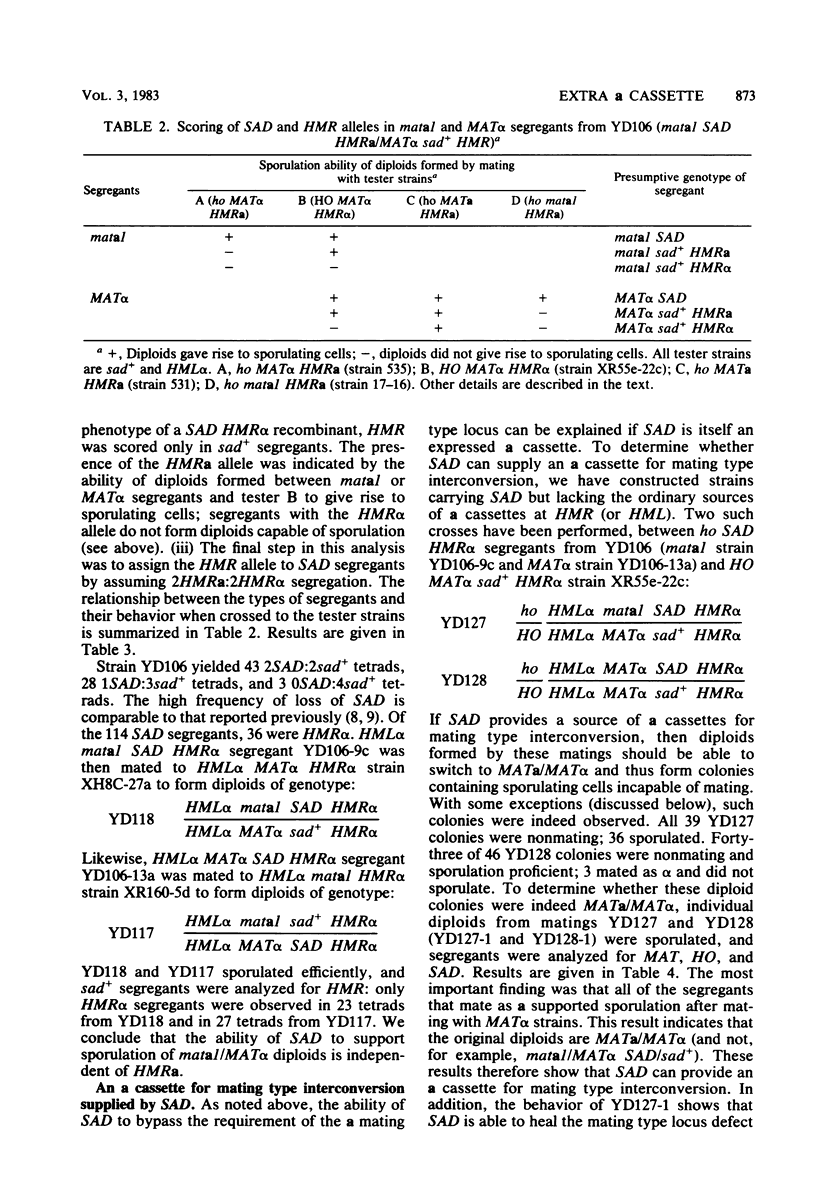
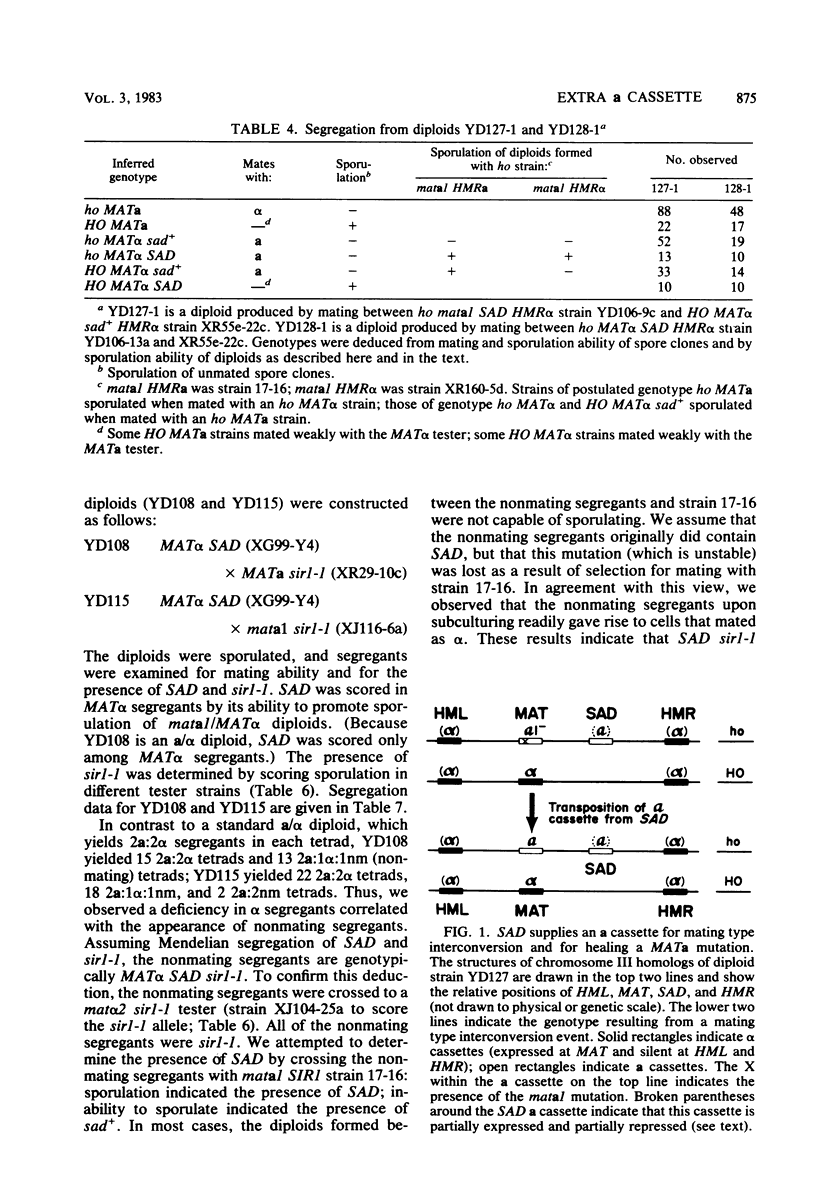
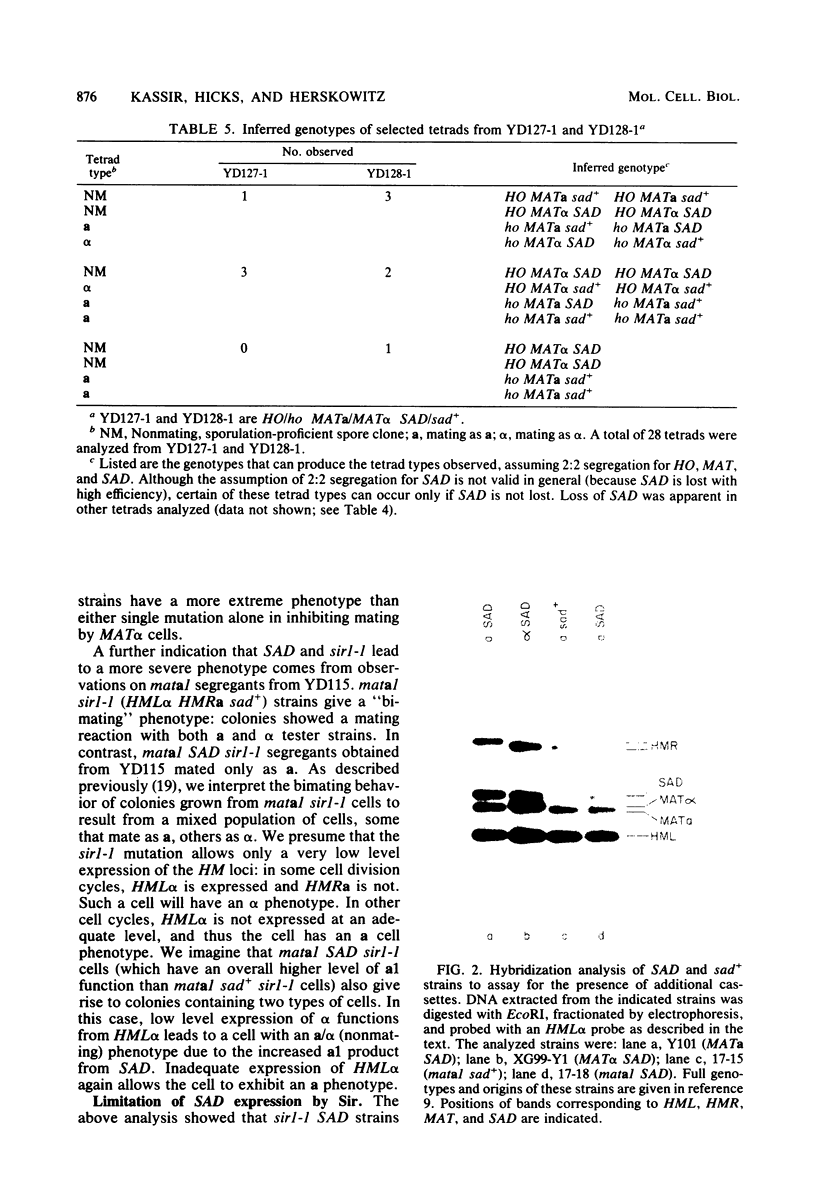
Sporulation of Saccharomyces cerevisiae ordinarily requires the a1 function of the a mating type locus. SAD is a dominant mutation that allows strains lacking a1 (MAT alpha/MAT alpha and mata1/MAT alpha diploids) to sporulate. We provide functional and physical evidence that SAD is an extra cassette in the yeast genome, distinct from those at HML, MAT, and HMR. The properties of SAD strains indicate that the a cassette at SAD produces a limited amount of a1 product, sufficient for promoting sporulation but not for inhibiting mating and other processes. These conclusions come from the following observations. (i) SAD did not act by allowing expression of HMRa: mata1/MAT alpha diploids carrying SAD and only alpha cassettes at HML and HMR sporulated efficiently. (ii) SAD acted as an a cassette donor in HML alpha HMR alpha strains and could heal a mata1 mutation to MATa as a result of mating type interconversion. (iii) The genome of SAD strains contained a single new cassette locus, as determined by Southern hybridization. (iv) Expression of a functions from the SAD a cassette was limited by Sir: sir- SAD strains exhibited more extreme phenotypes than SIR SAD strains. This observation indicates that SAD contains not only cassette information coding for a1 (presumably from HMRa) but also sites for Sir action.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Errede B., Cardillo T. S., Sherman F., Dubois E., Deschamps J., Wiame J. M. Mating signals control expression of mutations resulting from insertion of a transposable repetitive element adjacent to diverse yeast genes. Cell. 1980 Nov;22(2 Pt 2):427–436. doi: 10.1016/0092-8674(80)90353-0. [DOI] [PubMed] [Google Scholar]
- Feinstein S. C., Ross S. R., Yamamoto K. R. Chromosomal position effects determine transcriptional potential of integrated mammary tumor virus DNA. J Mol Biol. 1982 Apr 15;156(3):549–565. doi: 10.1016/0022-2836(82)90266-2. [DOI] [PubMed] [Google Scholar]
- Haber J. E., George J. P. A mutation that permits the expression of normally silent copies of mating-type information in Saccharomyces cerevisiae. Genetics. 1979 Sep;93(1):13–35. doi: 10.1093/genetics/93.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks J. B., Herskowitz I. Interconversion of Yeast Mating Types I. Direct Observations of the Action of the Homothallism (HO) Gene. Genetics. 1976 Jun;83(2):245–258. doi: 10.1093/genetics/83.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks J. B., Strathern J. N., Herskowitz I. Interconversion of Yeast Mating Types III. Action of the Homothallism (HO) Gene in Cells Homozygous for the Mating Type Locus. Genetics. 1977 Mar;85(3):395–405. doi: 10.1093/genetics/85.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks J., Strathern J. N., Klar A. J. Transposable mating type genes in Saccharomyces cerevisiae. Nature. 1979 Nov 29;282(5738):478–473. doi: 10.1038/282478a0. [DOI] [PubMed] [Google Scholar]
- Kassir Y., Simchen G. Regulation of mating and meiosis in yeast by the mating-type region. Genetics. 1976 Feb;82(2):187–206. doi: 10.1093/genetics/82.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., Fogel S., Macleod K. MAR1-a Regulator of the HMa and HMalpha Loci in SACCHAROMYCES CEREVISIAE. Genetics. 1979 Sep;93(1):37–50. doi: 10.1093/genetics/93.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., Fogel S., Radin D. N. Switching of a mating-type a mutant allele in budding yeast Saccharomyces cerevisiae. Genetics. 1979 Jul;92(3):759–776. doi: 10.1093/genetics/92.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., Strathern J. N., Broach J. R., Hicks J. B. Regulation of transcription in expressed and unexpressed mating type cassettes of yeast. Nature. 1981 Jan 22;289(5795):239–244. doi: 10.1038/289239a0. [DOI] [PubMed] [Google Scholar]
- Mackay V., Manney T. R. Mutations affecting sexual conjugation and related processes in Saccharomyces cerevisiae. I. Isolation and phenotypic characterization of nonmating mutants. Genetics. 1974 Feb;76(2):255–271. doi: 10.1093/genetics/76.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. A., Tatchell K., Hall B. D., Astell C., Smith M. A position effect in the control of transcription at yeast mating type loci. Nature. 1981 Jan 22;289(5795):244–250. doi: 10.1038/289244a0. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. A., Tatchell K. The structure of transposable yeast mating type loci. Cell. 1980 Mar;19(3):753–764. doi: 10.1016/s0092-8674(80)80051-1. [DOI] [PubMed] [Google Scholar]
- Rine J., Herskowitz I. The trans action of HMRa in mating type interconversion. Mol Gen Genet. 1980;180(1):99–105. doi: 10.1007/BF00267357. [DOI] [PubMed] [Google Scholar]
- Rine J., Sprague G. F., Jr, Herskowitz I. rme1 Mutation of Saccharomyces cerevisiae: map position and bypass of mating type locus control of sporulation. Mol Cell Biol. 1981 Oct;1(10):958–960. doi: 10.1128/mcb.1.10.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rine J., Strathern J. N., Hicks J. B., Herskowitz I. A suppressor of mating-type locus mutations in Saccharomyces cerevisiae: evidence for and identification of cryptic mating-type loci. Genetics. 1979 Dec;93(4):877–901. doi: 10.1093/genetics/93.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman H., Sands S. M. Heterogeneity of Clones of Saccharomyces Derived from Haploid Ascospores. Proc Natl Acad Sci U S A. 1953 Mar;39(3):171–179. doi: 10.1073/pnas.39.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sprague G. F., Jr, Rine J., Herskowitz I. Control of yeast cell type by the mating type locus. II. Genetic interactions between MAT alpha and unlinked alpha-specific STE genes. J Mol Biol. 1981 Dec 5;153(2):323–335. doi: 10.1016/0022-2836(81)90281-3. [DOI] [PubMed] [Google Scholar]
- Strathern J. N., Blair L. C., Herskowitz I. Healing of mat mutations and control of mating type interconversion by the mating type locus in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3425–3429. doi: 10.1073/pnas.76.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathern J. N., Newlon C. S., Herskowitz I., Hicks J. B. Isolation of a circular derivative of yeast chromosome III: implications for the mechanism of mating type interconversion. Cell. 1979 Oct;18(2):309–319. doi: 10.1016/0092-8674(79)90050-3. [DOI] [PubMed] [Google Scholar]
- Strathern J. N., Spatola E., McGill C., Hicks J. B. Structure and organization of transposable mating type cassettes in Saccharomyces yeasts. Proc Natl Acad Sci U S A. 1980 May;77(5):2839–2843. doi: 10.1073/pnas.77.5.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathern J., Hicks J., Herskowitz I. Control of cell type in yeast by the mating type locus. The alpha 1-alpha 2 hypothesis. J Mol Biol. 1981 Apr 15;147(3):357–372. doi: 10.1016/0022-2836(81)90488-5. [DOI] [PubMed] [Google Scholar]