Abstract
OBJECTIVES
The prevalence of celiac disease (CD) varies greatly, potentially because of incomplete ascertainment of cases and small study samples with limited statistical power. Previous reports indicate that the incidence of CD is increasing. We examined the prevalence of CD in a well-defined US county.
METHODS
Population-based study in Olmsted County, Minnesota, US. Using the infrastructure of the Rochester Epidemiology Project, medical, histopathology, and CD serology records were used to identify all new cases of CD in Olmsted County since 2000. Age- and sex-specific and adjusted (to the US white 2000 population) incidence rates for CD were estimated. Clinical presentation at diagnosis was also assessed.
RESULTS
Between 2000 and 2010, 249 individuals (157 female or 63%, median age 37.9 years) were diagnosed with CD in Olmsted County. The overall age- and sex-adjusted incidence of CD in the study period was 17.4 (95% confidence interval [CI] = 15.2–19.6) per 100,000 person-years, increasing from 11.1 (95% CI=6.8–15.5) in 2000–2001 to 17.3 (95% CI=13.3–21.3) in 2008–2010. The temporal trend in incidence rates was modeled as a two-slope pattern, with the incidence leveling off after 2004. Based on the two classic CD symptoms of diarrhea and weight loss, the relative frequency of classical CD among incident cases decreased over time between 2000 and 2010 (p=0.044).
CONCLUSION
The incidence of CD has continued to increase in the past decade in a North American population.
Keywords: autoimmunity, celiac, coeliac, epidemiology, prevalence
INTRODUCTION
Celiac disease (CD) is a chronic immune-mediated disease characterized by small intestinal inflammation.(1) Triggered by gluten exposure in genetically sensitive individuals,(2) this disease is associated with excess mortality as well as a number of complications, including type 1 diabetes(3) and lymphoproliferative disease.(4, 5) These and other high-risk groups, such as anemia, osteoporosis, and irritable bowel syndrome (IBS), have been identified(6) and patients in these risk groups are increasingly tested for CD. Even so, the prevalence of diagnosed CD is low. In a recent European multicenter study the prevalence of diagnosed CD was 0.18%; however, if Finland is excluded, where awareness is especially high, only 0.07% of the population had been diagnosed with CD.(7) Most previous American data indicate a true overall CD prevalence based on screening of about or slightly less than 1%.(8–12)
Prevalence studies based exclusively on serologic CD screening often report higher figures.(6) However, real differences may also exist between regions(7) and races, as higher levels of CD have also been shown outside Europe and the US among populations of predominantly European extraction (e.g., New Zealand and Australia).(13, 14)
Some prevalence data imply a true increase in CD or celiac autoimmunity over time,(15–17) but data are scarce on the actual incidence of CD.(18, 19) Such data are important in that temporal trends in CD incidence could provide insight into the environmental factors that trigger CD, as well as serve in planning for the health care needs of CD patients. For instance, the “Swedish epidemic,” with a sudden increase in CD incidence,(20) helped identify the interplay between gluten introduction and breastfeeding as important for the risk of CD in early age.(21) In this regard, we previously documented a strong increase in CD incidence in one US county in the 1990s(18) but it is unknown whether this increase was transient. The primary aim of this study was to estimate the incidence of CD in Olmsted County, Minnesota between 2000 and 2010.
METHODS
Setting
Olmsted County is situated in the upper Midwest, where whites make up over 85% of the population.(22) This community is ideally suited for epidemiologic studies because the Rochester Epidemiologic Project (REP) collects essentially all data on medical care for Olmsted County residents, focusing on the two major providers of health care in the community: the Mayo Clinic and its component hospitals (Saint Mary’s and Rochester Methodist) along with the Olmsted Medical Center with its affiliated community hospital and outpatient clinics. This medical records linkage system, which has been described elsewhere,(23) was used in our previous paper on CD trends in the area.(18) Using the infrastructure of the REP, comprehensive (inpatient and outpatient) community medical records, together with CD serology and histopathology data, were reviewed to estimate the incidence of diagnosed CD in this well-defined geographic region. Because of this unique medical records linkage system, complete ascertainment of diagnosed CD cases is possible.
Several data sources were used to identify patients with CD: the REP medical index, as was used in previous studies(9, 18, 24), active recruitment of patients known to be undergoing assessment and potential diagnosis of the disease at the time of their clinical visit, and the electronic medical record (the Mayo Clinic Life Sciences System, begun in 1994), which also contains information on disease symptoms, histopathology records, and serology data. We also reviewed the patient charts of all individuals with a diagnosis of either CD (ICD-9: 579.0) or DH (ICD-9: 694.0).
All of the above searches included a check for Olmsted County residency based on zip codes. Because the county boundary passes through some small towns, the patient’s street address was reviewed in some instances to determine if he or she lived within Olmsted County.
Data collection
Following approval by the Institutional Review Boards of Mayo Clinic and the Olmsted Medical Center, one co-author (CvD) extracted data on CD from the clinical records and then classified patients according to age, sex, classical symptoms (presence of diarrhea or weight loss)(25), and year of diagnosis. In accordance with Minnesota law, patients who declined to provide authorization for the use of their medical records for research purposes were not included in this study (or any other medical study).
Statistical analyses
Incidence rates of clinically-diagnosed CD were estimated using the number of new cases in each age-, sex-, and calendar year-specific stratum as the numerator, with corresponding denominators derived from annual census figures for Olmsted County, assuming the entire population was at risk(22). Age- and sex-adjusted incidence rates were computed based on direct standardization against the 2000 US white population. We estimated 95% confidence intervals (CIs) assuming the Poisson distribution for numbers of cases. Multiple variable Poisson regression was used to examine the association of age, sex, and calendar year with incidence rates. Measured per individual year, both age and calendar year were fit as continuous variables in the modeling process, with smoothing loess plots applied to assess their functional form. For brevity, we tabulated incidence rates in Table 2 according to age categories (0–3, 4–18, 19–44, 45–64, and ≥65 years) and periods of 3-year intervals (except for the first two years, 2000–01, which overlap with our previously published incidence study).(18) A logistic regression model (using a generalized logit link function) was employed to assess the association of calendar year on the proportion of incidence cases presenting with classical symptoms (diarrhea with or without weight loss(25)) over the study period, adjusted for age and sex. The different models used were selected based on simple plots of the data in order to reflect the observed patterns in the data.
Table 2.
Temporal trends of celiac disease in patients with diarrhea or weight loss (classical symptoms)
| CD Symptoms | 2000–01 (n=26) |
2002–04 (n=67) |
2005–07 (n=83) |
2008–10 (n=73) |
Temporal trend p- value |
|
|---|---|---|---|---|---|---|
| Diarrhea WITHOUT Weight Loss | ||||||
| Proportion of Cases with Symptom† | ||||||
| Total | 4 (15%) | 22 (33%) | 16 (19%) | 23 (32%) | 0.557 | |
| Incidence of Symptom-Related CD‡ | ||||||
| Total | 1.6 (0.0–3.2) | 5.6 (3.2–7.9) | 4.4 (2.2–6.5) | 5.5 (3.2–7.7) | 0.136 | |
| Females | 2.4 (0.0–5.1) | 6.5 (3.0–10.1) | 4.1 (1.2–6.9) | 6.3 (3.0–9.6) | ||
| Males | 0.8 (0.0–2.3) | 4.6 (1.6–7.7) | 4.9 (1.4–8.5) | 4.8 (1.6–8.1) | ||
| Diarrhea WITH Weight Loss | ||||||
| Proportion of Cases with Symptom† | ||||||
| Total | 4 (15%) | 4 (6%) | 4 (5%) | 2 (3%) | 0.044 | |
| Incidence of Symptom-Related CD‡ | ||||||
| Total | 1.9 (0.0–3.8) | 1.0 (0.0–1.9) | 1.2 (0.0–2.3) | 0.6 (0.0–1.3) | 0.168 | |
| Females | 1.7 (0.0–4.2) | 1.0 (0.0–2.4) | 1.6 (0.0–3.3) | 0.6 (0.0–1.7) | ||
| Males | 2.2 (0.0–5.3) | 0.9 (0.0–2.2) | 0.8 (0.0–2.3) | 0.5 (0.0–1.4) | ||
Frequency of classical symptoms in CD cases summarized with counts and percentages and tested for a temporal trend using a generalized logit model after adjusting for age and gender.
Incidence described with age-adjusted (and sex-adjusted for bolded “Total” results) incidence rates and 95% confidence intervals (per 100,000 person-years adjusted to the US white population in 2000), with rates tested for a temporal trend via Poisson regression after adjusting for age and gender.
All analyses were performed using SAS/STAT software, version 9.2 (SAS Institute, Cary, NC. A two-sided alpha level of 0.05 was used to determine statistical significance.
RESULTS
Study subjects
From 2000 to 2010, 249 individuals were diagnosed with CD in Olmsted County. Some 22 of these had been identified through our family screening study in 2001–05.(26) Of the 249, 157 (63%) were females. The median age at diagnosis was 37.9 (range, 1.2–84.9 years). Concerning presenting symptoms, 79 (32%) patients had diarrhea and 25 (10%) had weight loss, although only 14 (6%) had both diarrhea and weight loss at CD diagnosis and were therefore classified as having “classical” CD.
Of the 248 individuals with more extensive data available on symptoms, 99 (40%) had abdominal pain/cramping, 69 (28%) had anemia, 41 (17%) suffered from malaise, and 36 reported nausea/vomiting (15%). A positive family history for CD was seen in 72 individuals (29%). Of individuals tested for either tissue transglutaminase (TTG) or endomysial antibodies (EMA), 89% (166/187) were positive for at least one of these antibodies. Supplementary Table 1 shows the prevalence of CD in Olmsted county on Jan 1, 2010.
Temporal trends in CD incidence
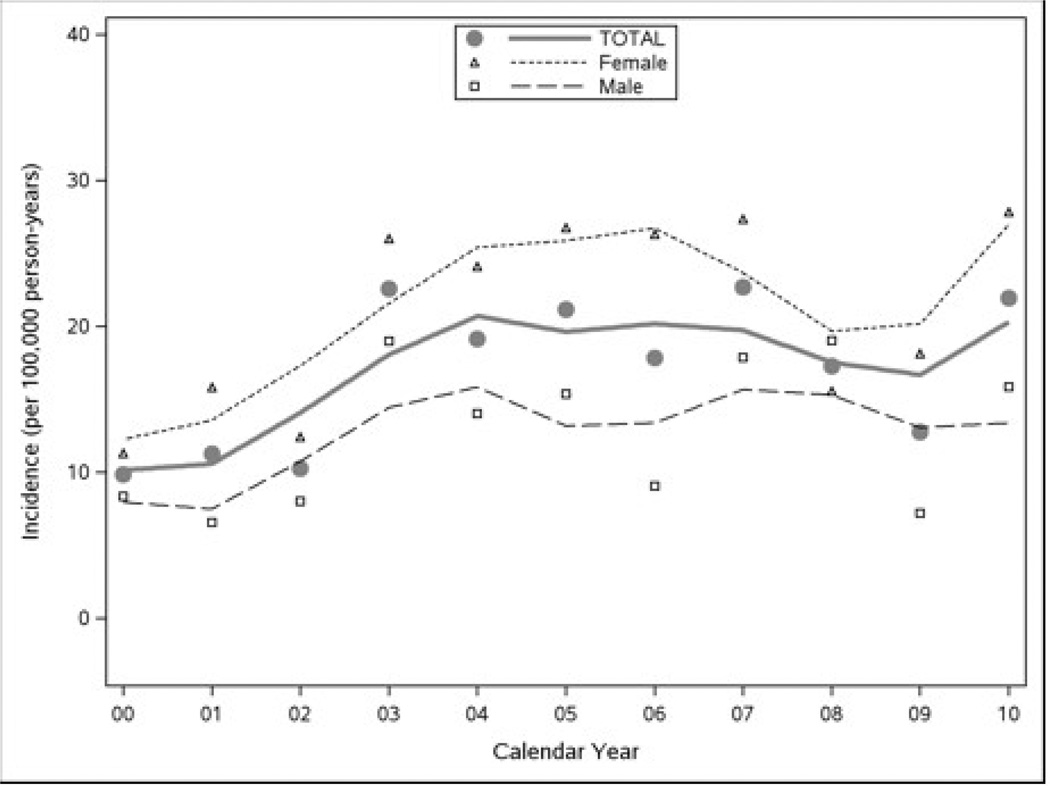
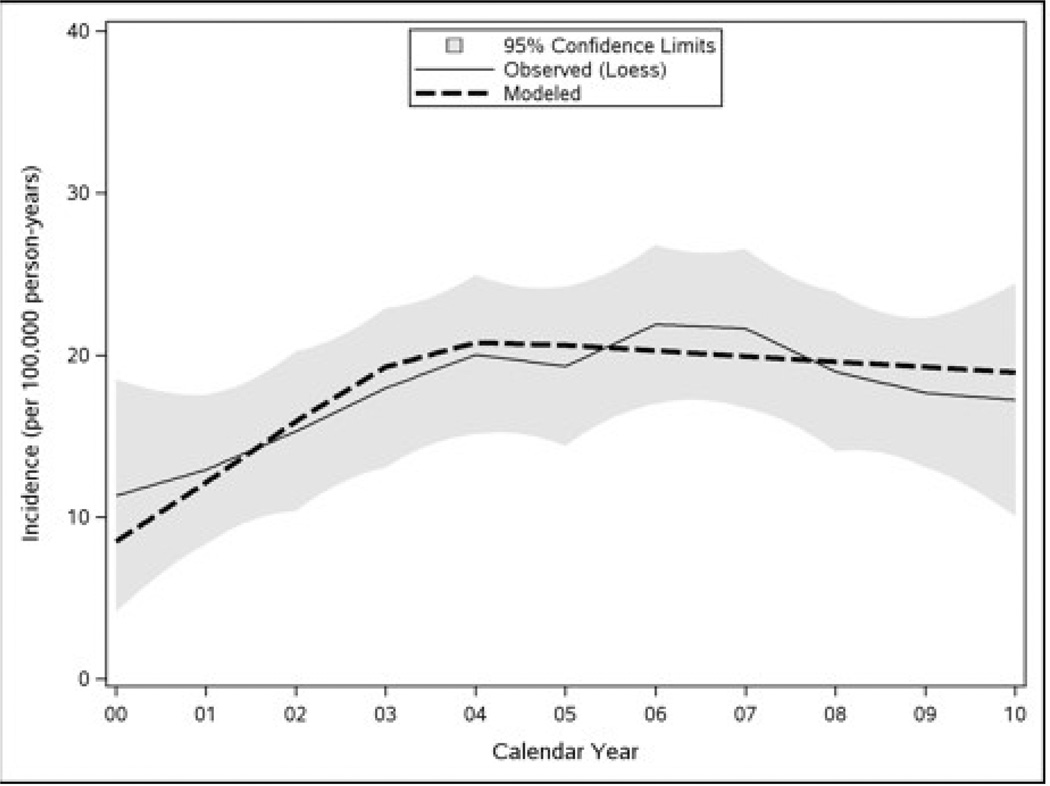
The overall age- and sex-adjusted incidence of clinically diagnosed CD over the study period was 17.4 (95% CI = 15.2–19.6) per 100,000 person-years (p-y), increasing from 11.1 (95% CI = 6.8–15.5) per 100,000 p-y in 2000–2001 to 17.3 (95% CI = 13.3–21.3) per 100,000 p-y in 2008–2010 (Table 1 and Figure 1). Various options for the functional form of the relationship with calendar year were considered based on Figure 2. In particular, a loess smooth of incidence rate was plotted against calendar year to explore the linearity of their relationship. Because the incidence of CD appeared to increase in the first several years and then flatten after that (Figure 2, thin solid line), a model that assumed a linear increase from years 2000 to 2004 and a leveling off thereafter (Figure 2, thick dashed line) was eventually adopted. Based on a comparison of log-likelihood statistics, the model with this two-slope time trend provided a significantly better fit of the data than a model with a linear (one-slope) time effect (p=0.021).
Table 1.
Incidence of celiac disease in Olmsted County, MN according to age and sex (2000–2010).
| Period | ||||
|---|---|---|---|---|
| Age group | 2000–2001 (n=26) n (rate*) |
2002–2004 (n=67) n (rate*) |
2005–2007 (n=83) n (rate*) |
2008–2010 (n=73) n (rate*) |
| Females | ||||
| 0–3 | 0 (0.0) | 1 (9.1) | 1 (8.6) | 0 (0.0) |
| 4–18 | 2 (7.3) | 7 (16.2) | 9 (19.9) | 15 (31.8) |
| 19–44 | 5 (10.1) | 17 (21.9) | 28 (34.5) | 21 (24.8) |
| 45–64 | 8 (29.0) | 9 (20.8) | 12 (26.5) | 4 (8.5) |
| 65–85 | 2 (15.0) | 7 (33.5) | 5 (22.8) | 4 (17.5) |
| All ages | 14.2 (7.4–21.0) | 21.4 (14.8–28.0) | 26.8 (19.7–33.9) | 20.1 (14.1–26.1) |
| Males | ||||
| 0–3 | 0 (0.0) | 0 (0.0) | 2 (16.8) | 0 (0.0) |
| 4–18 | 3 (10.3) | 7 (15.3) | 4 (8.3) | 9 (18.0) |
| 19–44 | 2 (4.2) | 11 (14.6) | 6 (7.6) | 7 (8.5) |
| 45–64 | 2 (7.7) | 7 (17.2) | 9 (21.1) | 8 (18.0) |
| 65–85 | 2 (19.7) | 1 (6.3) | 7 (41.9) | 5 (28.7) |
| All ages | 8.1 (2.6–13.5) | 13.5 (8.3–18.8) | 15.9 (9.9–22.0) | 14.9 (9.3–20.5) |
| Total | ||||
| 0–3 | 0 (0.0) | 1 (4.5) | 3 (12.8) | 0 (0.0) |
| 4–18 | 5 (8.8) | 14 (15.7) | 13 (13.9) | 24 (24.7) |
| 19–44 | 7 (7.2) | 28 (18.3) | 34 (21.2) | 28 (16.8) |
| 45–64 | 10 (18.6) | 16 (19.0) | 21 (23.9) | 12 (13.1) |
| 65–85 | 4 (17.0) | 8 (21.7) | 12 (31.1) | 9 (22.4) |
| All ages | 11.1 (6.8–15.5) | 17.7 (13.4–22.0) | 21.1 (16.5–25.7) | 17.3 (13.3–21.3) |
Age-specific results summarized with count and incidence rates (per 100,000 person-years, unadjusted), while bolded across-all-age results are described with the age-adjusted (and sex-adjusted for “Total” section) incidence rates and 95% confidence intervals.
Rate = incidence rate per 100,000 person-years adjusted to the US white population in 2000.
Figure 1.
Rates of CD Incidence in Olmsted County, MN from 2000 to 2010 (Loess Smooth).
Figure 2.
Modeling the Temporal Trend in CD Incidence between 2000 and 2010
Modeled incidence of CD, assuming a linear increase from 2000 to 2004 and constant thereafter, is shown in comparison with a loess smooth of the observed incidence (with shaded 95% confidence limits).
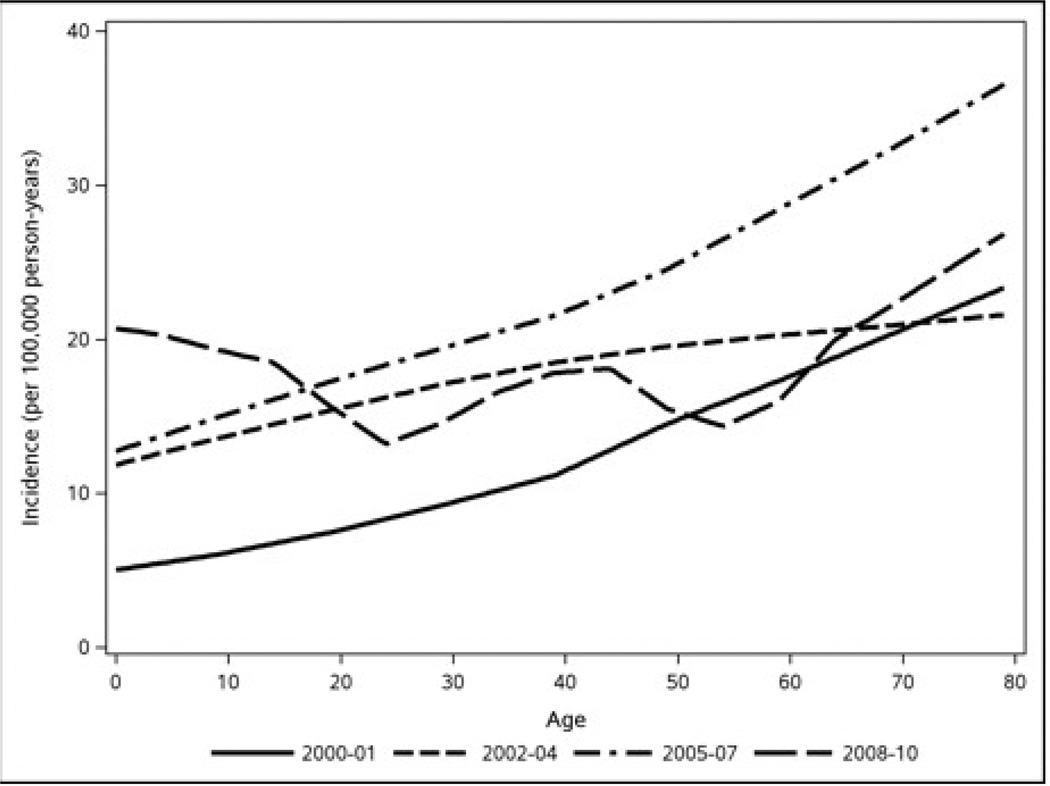
In the two-slope model a higher incidence of CD was associated with female sex (p<0.001) and increasing age (p=0.034). Over the entire period, the age-adjusted incidence of CD in females and males was 21.3 (95% CI = 18.0–24.7) and 13.6 (95% CI = 10.8–16.5) per 100,000 p-y, respectively (p<0.001, adjusted for age and calendar year effects) (Table 1). With the possible exception of the last period (2008–2010), in which incidence rates were relatively high in individuals aged 4–18 years (Table 1 and Figure 3), the incidence of CD tended to be highest in the older age groups (45–64 years and 65–85 years). Controlling for sex and calendar year effects, the CD incidence rate increased about 6.5% (95% CI = 0.5%–12.8%) per 10-year increment in age (p=0.034).
Figure 3.
Age Trends in CD Incidence by Calendar Year (Loess Smooth).
CD incidence according to patient characteristics
Neither the percentage nor the incidence of CD characterized by diarrhea without weight loss increased significantly over the study period (p=0.557 and 0.136, respectively, adjusted for age and sex) (Table 2). In contrast, and as illustrated in Figure 4, the proportion of cases presenting with both diarrhea and weight loss decreased significantly over time (15% in 2000–2001 down to 3% in 2008–2010, p =0.044), although this did not result in a significant drop in the incidence of classical CD over the study period (p=0.168)
Figure 4.
Temporal Trend in the Proportion of Classical Symptoms in Incidence CD Cases (Regression Lines).
DISCUSSION
This study is potentially the most complete assessment of the recent incidence of CD in a North American population. Between 2000 and 2010, some 249 individuals were diagnosed with CD in Olmsted County. The corresponding overall incidence was 17.4 per 100,000 p-y, which, to our knowledge, is one of the highest figures reported anywhere in a carefully enumerated population that includes both adults and children. Adding to this our recent National Health and Nutrition Examination Survey (NHANES) data showing an overall prevalence of CD of 0.71% in the US,(11) it is evident that CD is swiftly becoming a major health problem in this country. Although earlier efforts have been made to identify CD through screening of risk groups(8) and certain age strata,(9) as well as through screening in a primary care setting(27) or in connection with upper endoscopy,(28, 29) few studies(18) have used an integrative approach to determine the incidence of CD.
We found that the previously described increase in CD incidence (0.9 per 100,000 p-y in the 1960s, rising to 3.3 per 100,000 p-y in the 1990s) has not abated.(18) As documented in the present study, the incidence of CD has continued to rise since then but seems to have leveled off at about 20 per 100,000 p-y after 2004. Although the high (and increasing) incidence may partly be explained by high physician awareness and large-scale efforts to screen at-risk groups (increasing detection rate), we believe that other factors play a substantial role since the rising incidence in the 1990s, when serological screening tools (e.g., endomysial and tissue transglutaminase antibodies) became readily available, was not followed by a drop in incidence after 2000, as would be expected if the Olmsted County population had merely been swept of unrecognized prevalent CD cases. Still we cannot rule out that external factors influenced case-finding. For instance it is possible that the publication of the first major US study of celiac seroprevalence in 2003(8) led to heightened awareness of CD and contributed to the rise in CD incidence seen in 2004–2006.
Several studies have shown changing incidence in CD.(15–17, 19, 20) Considering the predominance of a Caucasian population in Olmsted County now, as in the 1950s, the rise of CD cannot be explained by a change in the underlying genetic makeup of the community. Instead, an environmental factor(s) is likely. Because we(19, 30) and others(31) have found an association between infectious disease (especially gastroenteritis) and CD, a changing pattern in infections may have contributed to the rise in CD in Olmsted County. Another explanation concerns amount, timing, and frequency of gluten consumption. For instance, Ivarsson et al(21) reported that high amounts of gluten increased the risk of CD. Unfortunately, we have no data on gluten consumption in individuals from Olmsted County, although gluten enriched foods (e.g., pizza, bagels, and high protein and high fiber bread) are increasingly ingested in the US. (32)
In contrast to Swedish data showing very high incidence rates in children <2 years of age between 1985 and 1995 (about 200 per 100,000 p-y),(20) we found a low incidence of CD in young US children. Interestingly, however, high incidence figures in the most recent period (2008–2010) were noted in the age groups born in the years of the Swedish epidemic (late 1980s and early 1990s).(33) Therefore, we cannot rule out that the same environmental factors have been present both in Sweden and in the US.
The majority of newly diagnosed CD patients were females. This sex pattern is consistent with previous data.(34, 35) The female predominance is noteworthy given that population-based screening in our area has previously found a more neutral female-male ratio.(9, 11) Some data suggest that sex affects the clinical presentation of CD,(36, 37) with women more often diagnosed with CD, whereas men remain undiagnosed. During their fertile years, women are also more likely to encounter health care and incidentally be diagnosed with CD.
Previous data from our center(18) and elsewhere(38) have otherwise established that classical CD (diarrhea and weight loss) has become less prominent. The current study was able to confirm that the proportion of newly diagnosed patients with both diarrhea and weight loss decreased from 2000 to 2010 but the absolute incidence of diarrhea-associated CD did not decrease. This observation suggests that the lower proportion of diarrhea in CD patients is probably due to testing of new risk groups,(39) such as patients with other immune-mediated diseases(40, 41) or osteoporosis,(42) first-degree relatives,(26) or a more extensive use of CD serology to identify early onset patients before classical symptoms can develop. Yet, the persistence of a steady rate of more classic disease may suggest that the true incidence of CD may be increasing.
The main strength of this paper is its population-based design. The well-established collaboration between the major health care providers in the area should virtually guarantee that we have identified all cases with diagnosed CD. Moreover, we had access not only to clinical registers but also to serology and histopathology data to ascertain patients. Finally, the large number of diagnosed cases means that we could better estimate the incidence rates (i.e. calculate narrow CIs). This study has some limitations that need to be considered when interpreting the results. The predominant population of Olmsted County consists of non-Hispanic whites and thus the number of Hispanics, Blacks and Asians are limited. We cannot therefore extrapolate our findings to these latter populations. CD has a marked predominance among non-Hispanic whites and may be less common among other minority groups.(11) We were also unable to determine the relative incidence of both diagnosed and undiagnosed CD since this was not a screening study. Finally, Olmsted county is dominated by the Mayo Clinic, which is also the main provider of health care for the county inhabitants. There is a strong interest in CD at the Mayo clinic, and it cannot be ruled out that this has influenced the diagnostic rate of CD in the Olmsted county.
In conclusion, the increase in incidence of CD between 1950 and 2001 has continued in the past decade but probably has leveled since 2004. The high CD incidence (17 per 100,000 p-y in the past decade) points towards a change in environmental exposures, potentially responsible for triggering CD not only in children but also, and particularly, in adults.
Supplementary Material
STUDY HIGHLIGHTS.
WHAT IS THE CURRENT KNOWLEDGE
The prevalence of celiac disease varies greatly
Previous reports indicate that the incidence of celiac disease is increasing
There is a paucity of research on CD incidence in the past decade
WHAT IS NEW HERE
The incidence of celiac disease has continued to increase in the past decade in a North American population, but seems to have levelled off after 2004
The absolute frequency of classical celiac disease did not change
However, the relative frequency of classical celiac disease among incident cases has decreased
Acknowledgments
Grant Support (Funding):
JFL was supported by grants from The Swedish Society of Medicine, the Swedish Research Council – Medicine (522-2A09-195), the Swedish Celiac Society, and the Fulbright commission.
ART was supported by the American College of Gastroenterology Junior Faculty Development Award
The work at Mayo Clinic was supported by a grant from the National Institutes of Health – DK057892 and made possible by the Rochester Epidemiology Project (R01 AG034676 from the National Institute on Aging). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- CD
Celiac disease
- CI
Confidence interval
- HR
Hazard ratio
- VA
Villous atrophy
Footnotes
Independence (role of the sponsors): None of the funders had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Potential competing interests: None
Writing Assistance: None.
Author contributions:
ICMJE criteria for authorship read and met: JFL, ART, CTD, JM, ARZ, BDL, JAM.
Agree with the manuscript’s results and conclusions: JFL, ART, CTD, JM, ARZ, BDL, JAM.
Designed the experiments/the study: JFL, JM, ARZ, BDL, JAM.
Collected data: CTD and JAM
Analyzed the data: JM, ARZ, BDL
Wrote the first draft of the paper: JFL.
Contributed to the writing of the paper: ART, CTD, JM, ARZ, BDL, JAM.
Contributed to design of study and interpretation of the data analyses: JFL, ART, CTD, JM, ARZ, BDL, JAM.
Interpretation of data; approved the final version of the manuscript: JFL, ART, CTD, JM, ARZ, BDL, JAM.
Supervised the project: JAM and JFL.
Obtained funding: JAM.
REFERENCES
- 1.Walker MM, Murray JA. An update in the diagnosis of coeliac disease. Histopathology. 2010 doi: 10.1111/j.1365-2559.2010.03680.x. [DOI] [PubMed] [Google Scholar]
- 2.Sollid LM, Thorsby E. HLA susceptibility genes in celiac disease: genetic mapping and role in pathogenesis. Gastroenterology. 1993;105:910–922. doi: 10.1016/0016-5085(93)90912-v. [DOI] [PubMed] [Google Scholar]
- 3.Ludvigsson JF, Ludvigsson J, Ekbom A, et al. Celiac Disease and Risk of Subsequent Type 1 Diabetes: A general population cohort study of children and adolescents. Diabetes Care. 2006;29:2483–2488. doi: 10.2337/dc06-0794. [DOI] [PubMed] [Google Scholar]
- 4.West J, Logan RF, Smith CJ, et al. Malignancy and mortality in people with coeliac disease: population based cohort study. Bmj. 2004;329:716–719. doi: 10.1136/bmj.38169.486701.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elfstrom P, Granath F, Ekstrom Smedby K, et al. Risk of Lymphoproliferative Malignancy in Relation to Small Intestinal Histopathology Among Patients With Celiac Disease. J Natl Cancer Inst. 2011;103:436–444. doi: 10.1093/jnci/djq564. [DOI] [PubMed] [Google Scholar]
- 6.Dube C, Rostom A, Sy R, et al. The prevalence of celiac disease in average-risk and at-risk Western European populations: a systematic review. Gastroenterology. 2005;128:S57–S67. doi: 10.1053/j.gastro.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Mustalahti K, Catassi C, Reunanen A, et al. The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med. 2010;42:587–595. doi: 10.3109/07853890.2010.505931. [DOI] [PubMed] [Google Scholar]
- 8.Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 9.Godfrey JD, Brantner TL, Brinjikji W, et al. Morbidity and mortality among older individuals with undiagnosed celiac disease. Gastroenterology. 2010;139:763–769. doi: 10.1053/j.gastro.2010.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill I, Fasano A, Schwartz R, et al. The prevalence of celiac disease in at-risk groups of children in the United States. J Pediatr. 2000;136:86–90. doi: 10.1016/s0022-3476(00)90055-6. [DOI] [PubMed] [Google Scholar]
- 11.Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012;107:1538–1544. doi: 10.1038/ajg.2012.219. quiz 1537, 1545. [DOI] [PubMed] [Google Scholar]
- 12.Katz KD, Rashtak S, Lahr BD, et al. Screening for celiac disease in a north american population: sequential serology and gastrointestinal symptoms. Am J Gastroenterol. 2011;106:1333–1339. doi: 10.1038/ajg.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook HB, Burt MJ, Collett JA, et al. Adult coeliac disease: prevalence and clinical significance. J Gastroenterol Hepatol. 2000;15:1032–1036. doi: 10.1046/j.1440-1746.2000.02290.x. [DOI] [PubMed] [Google Scholar]
- 14.Hovell CJ, Collett JA, Vautier G, et al. High prevalence of coeliac disease in a population-based study from Western Australia: a case for screening? Med J Aust. 2001;175:247–250. doi: 10.5694/j.1326-5377.2001.tb143555.x. [DOI] [PubMed] [Google Scholar]
- 15.Lohi S, Mustalahti K, Kaukinen K, et al. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007;26:1217–1225. doi: 10.1111/j.1365-2036.2007.03502.x. [DOI] [PubMed] [Google Scholar]
- 16.Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137:88–93. doi: 10.1053/j.gastro.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catassi C, Kryszak D, Bhatti B, et al. Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Ann Med. 2010;42:530–538. doi: 10.3109/07853890.2010.514285. [DOI] [PubMed] [Google Scholar]
- 18.Murray JA, Van Dyke C, Plevak MF, et al. Trends in the identification and clinical features of celiac disease in a North American community, 1950–2001. Clin Gastroenterol Hepatol. 2003;1:19–27. doi: 10.1053/jcgh.2003.50004. [DOI] [PubMed] [Google Scholar]
- 19.Riddle MS, Murray JA, Porter CK. The Incidence and Risk of Celiac Disease in a Healthy US Adult Population. Am J Gastroenterol. 2012;107:1248–1255. doi: 10.1038/ajg.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivarsson A, Persson LA, Nystrom L, et al. Epidemic of coeliac disease in Swedish children [see comments] Acta Paediatr. 2000;89:165–171. doi: 10.1080/080352500750028771. [DOI] [PubMed] [Google Scholar]
- 21.Ivarsson A, Hernell O, Stenlund H, et al. Breast-feeding protects against celiac disease. Am J Clin Nutr. 2002;75:914–921. doi: 10.1093/ajcn/75.5.914. [DOI] [PubMed] [Google Scholar]
- 22.US_Census. [Date of access: Jan 19, 2012]; http://www.co.olmsted.mn.us/yourgovernment/demographics/Documents/2010data/Population_1970_2010.pdf;
- 23.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 24.Talley NJ, Valdovinos M, Petterson TM, et al. Epidemiology of celiac sprue: a community-based study. Am J Gastroenterol. 1994;89:843–846. [PubMed] [Google Scholar]
- 25.Ludvigsson JF, Leffler DA, Bai JC, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62:43–52. doi: 10.1136/gutjnl-2011-301346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubio-Tapia A, Van Dyke CT, Lahr BD, et al. Predictors of family risk for celiac disease: a population-based study. Clin Gastroenterol Hepatol. 2008;6:983–987. doi: 10.1016/j.cgh.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catassi C, Kryszak D, Louis-Jacques O, et al. Detection of Celiac disease in primary care: a multicenter case-finding study in North America. Am J Gastroenterol. 2007;102:1454–1460. doi: 10.1111/j.1572-0241.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 28.Hopper AD, Cross SS, Hurlstone DP, et al. Pre-endoscopy serological testing for coeliac disease: evaluation of a clinical decision tool. Bmj. 2007;334:729. doi: 10.1136/bmj.39133.668681.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green PH, Shane E, Rotterdam H, et al. Significance of unsuspected celiac disease detected at endoscopy. Gastrointest Endosc. 2000;51:60–65. doi: 10.1016/s0016-5107(00)70389-0. [DOI] [PubMed] [Google Scholar]
- 30.Welander A, Tjernberg AR, Montgomery SM, et al. Infectious disease and risk of later celiac disease in childhood. Pediatrics. 2010;125:e530–e536. doi: 10.1542/peds.2009-1200. [DOI] [PubMed] [Google Scholar]
- 31.Stene LC, Honeyman MC, Hoffenberg EJ, et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol. 2006;101:2333–2340. doi: 10.1111/j.1572-0241.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 32.Strzelecki MV. The Pie's the Limit. Snack Food & Wholesale Bakery. 2009;98:38–40. [Google Scholar]
- 33.Ivarsson A, Persson LA, Juto P, et al. High prevalence of undiagnosed coeliac disease in adults: a Swedish population-based study. J Intern Med. 1999;245:63–68. doi: 10.1046/j.1365-2796.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- 34.Ivarsson A, Hernell O, Nystrom L, et al. Children born in the summer have increased risk for coeliac disease. J Epidemiol Community Health. 2003;57:36–39. doi: 10.1136/jech.57.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ludvigsson JF, Montgomery SM, Ekbom A, et al. Small-intestinal histopathology and mortality risk in celiac disease. JAMA. 2009;302:1171–1178. doi: 10.1001/jama.2009.1320. [DOI] [PubMed] [Google Scholar]
- 36.Bai D, Brar P, Holleran S, et al. Effect of gender on the manifestations of celiac disease: evidence for greater malabsorption in men. Scand J Gastroenterol. 2005;40:183–187. doi: 10.1080/00365520510011498. [DOI] [PubMed] [Google Scholar]
- 37.Ciacci C, Cirillo M, Sollazzo R, et al. Gender and clinical presentation in adult celiac disease. Scand J Gastroenterol. 1995;30:1077–1081. doi: 10.3109/00365529509101610. [DOI] [PubMed] [Google Scholar]
- 38.Rampertab SD, Pooran N, Brar P, et al. Trends in the presentation of celiac disease. Am J Med. 2006;119:355, e9–e14. doi: 10.1016/j.amjmed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 39.AGA Institute Medical Position Statement on the Diagnosis and Management of Celiac Disease. Gastroenterology. 2006;131:1977–1980. doi: 10.1053/j.gastro.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen D, Brock-Jacobsen B, Lund E, et al. Clinical benefit of a gluten-free diet in type 1 diabetic children with screening-detected celiac disease: a population-based screening study with 2 years' follow-up. Diabetes Care. 2006;29:2452–2456. doi: 10.2337/dc06-0990. [DOI] [PubMed] [Google Scholar]
- 41.Elfstrom P, Montgomery SM, Kampe O, et al. Risk of thyroid disease in individuals with celiac disease. J Clin Endocrinol Metab. 2008;93:3915–3921. doi: 10.1210/jc.2008-0798. [DOI] [PubMed] [Google Scholar]
- 42.Ludvigsson JF, Michaelsson K, Ekbom A, et al. Coeliac disease and the risk of fractures - a general population-based cohort study. Aliment Pharmacol Ther. 2007;25:273–285. doi: 10.1111/j.1365-2036.2006.03203.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.