Abstract
An efficient synthetic route to a bifunctional chelating agent C-NE3TA-NCS for antibody-targeted radioimmunotherapy (RIT) applications was developed. Various synthetic methods centered on the key reaction steps including bimolecular cyclization, ring opening reactions of aziridine and aziridinium cations, and reductive aminiation were explored to optimize the preparation of a tetraaza-based chelate TANPA and C-NE3TA analogues. Heptadentate C-NE3TA-NCS was conjugated to a tumor targeting antibody and compared to hexadentate C-NOTA-NCS for radiolabeling reaction kinetics with lanthanides for RIT. C-NE3TA-antibody conjugate displayed significantly enhanced complexation kinetics with 90Y as compared to C-NOTA-antibody conjugate. The synthetic methods for TANPA and C-NE3TA-NCS reported herein have broad applications for preparation of bifunctioanl macrocyclic chelating agents.
Keywords: Bifunctional chelating agent, radioimmunotherapy, 90Y, Macrocyclic ligand
Introduction
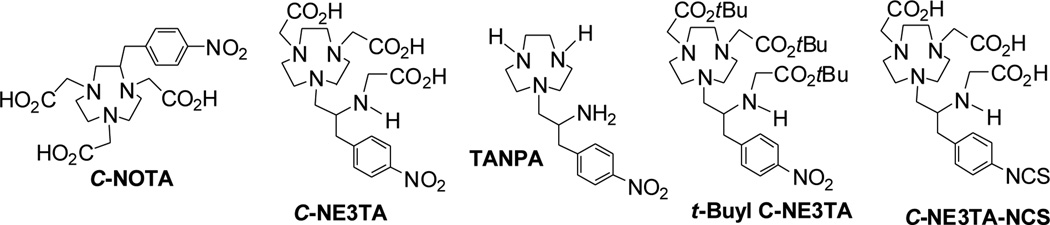
Bifunctional ligand is an essential component for biomedical and radiopharmaceutical applications of biologically active metals as cytotoxic agents or imaging probes.1–3 In particular, receptor-targeted cancer therapy and imaging techniques including radioimmunotherapy (RIT), radioimmunoimaging (RII), and magnetic resonance imaging (MRI) require a bifunctional ligand that can tightly and/or rapidly complex a radioactive or non-radioactive lanthanide such as 90Y (Emax = 2.3 MeV, t1/2 = 64.1 h), 177Lu (Emax = 0.5 MeV, t1/2 = 6.7 d), and Gd(III).2,4,5 Our continued research on chelation chemistry6–12 led to the design and preparation of heptadentate ligand C-NE3TA8 (Figure 1). C-NE3TA contains the macrocyclic component of C-NOTA13 (Figure 1) and an acyclic pendant amino carboxylate donor group appended by an ethylene bridge to the macrocyclic ring. C-NOTA is known to be an inadequate chelate for lanthanides by virtue of its cavity size, insufficient number of donors to saturate the coordination sphere of a lanthanide cation, and inability to assume an optimal geometry for the six donor groups.14,15 It was proposed that the acyclic pendant donor groups in C-NE3TA can rapidly initiate coordination to the metal and thereafter, the macrocyclic component in the chelate can wrap around the metal ion trapped in the acyclic pendant arm to achieve maximum complex stability by saturating the metal coordination sphere as in the case of C-NOTA, while cavity size becomes irrelevant with the metal sitting above the plane of the ring nitrogens. Thus, C-NE3TA is proposed to bind the metal ion more rapidly than C-NOTA while maintaining high complex stability of C-NOTA via dynamic and three-dimensional binding.
Figure 1.
Structure of C-NOTA, C-NE3TA, TANPA, tert-Butyl C-NE3TA, and C-NE3TA-NCS.
C-NE3TA can also form a neutral complex with various lanthanides. Neutral metal complexes do not require counter ions and are known to have lower osmotic activity and reduced protein binding and toxicity as compared to ionic complexes.16,17 The neutral metal complexes are also known to be less sensitive to acid/cation-promoted dissociation as compared to anionic complexes. For example, the neutral Ga(III) complex of NOTA was stable at pH 3.15
We previously evaluated C-NE3TA as a chelator of Y(III), Lu(III), and Gd(III) for RIT and MRI, and 90Y, 177Lu, and 153Gd complexes of C-NE3TA produced excellent in vitro and in vivo complex stability profiles.8 With the potential of C-NE3TA for targeted cancer therapy and imaging, we wanted to develop an efficient synthetic route suitable for scale-up production of C-NE3TA-NCS containing a functional group for conjugation to a tumor targeting antibody or peptide (Figure 1). We herein report an efficient synthesis and evaluation of C-NE3TA-NCS. Various synthetic routes to the key intermediate macrocyclic chelating backbone TANPA (Figure 1) for preparation of C-NE3TA-NCS is also presented. C-NE3TA conjugated to a tumor targeting antibody, trastuzumab, was evaluated for its radiolabeling reaction kinetics with β-emitting radioisotopes, 90Y and 177Lu. For comparison, C-NOTA-Hercpetin conjugate was prepared and evaluated for the same radiolabeling reaction kinetics study.
Results and Discussion
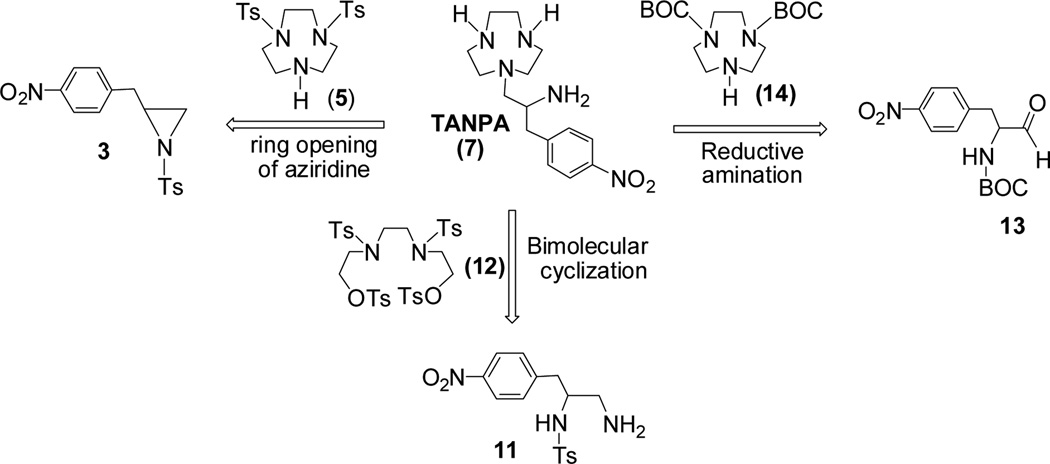
TANPA (7) contains four reactive amines and can be further substituted with potential donor groups. With the potential applications of the macrocyclic chelating backbone 7 for preparation of various chelating systems, we wanted to develop a practical synthetic process for 7. The retrosynthetic routes to TANPA (7)10 are centered on the key reaction steps, ring opening of aziridine, reductive amination, and bimolecular cyclization (Scheme 1).
Scheme 1.
Retrosynthetic routes to the key precursor molecule TANPA (7).
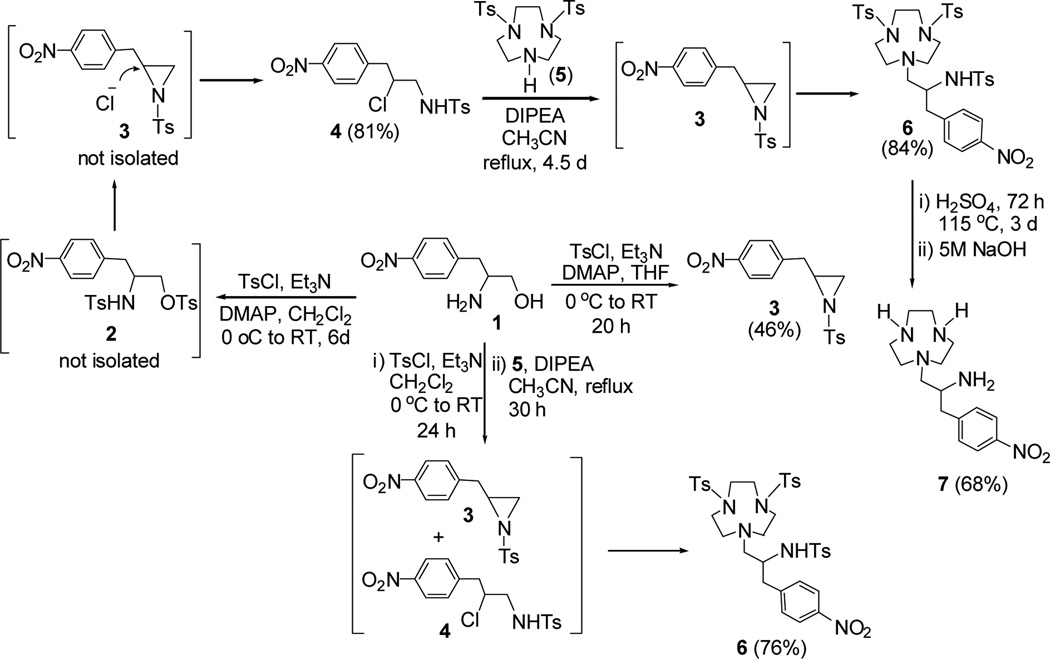
The first synthetic route to 7 includes preparation of the precursor molecule 6 via ring opening of N-Ts protected aziridine 3 by N-tosyl protected TACN 518 (Scheme 2). In our initial approach to prepare 6, we wanted to synthesize compound 2 by reaction of p-nitrophenyl alaniol 110 with TsCl in the presence of triethylamine. However, the reaction afforded compound 4 via formation of compound 5 and subsequent regiospecific ring opening of compound 3 by nucleophilic chloride present in the reaction mixture. Compound 4 was further reacted with 5 to provide 6 in 84% isolated yield. It was reasonably speculated that aziridine 3 was initially formed from intramolecular substitution reaction of 4, and regiospecific ring opening of aziridine 3 at the less hindered carbon by the nucleophilic amine in compound 5 provided 6. The regioisomer of 6 which can be formed from the attack of 5 at the more hindered methine carbon in 3 was not obtained from the reaction. When the reaction was repeated in THF, the aziridine analogue 3 was obtained as the major product (46% isolated yield). To optimize the synthesis of 6, a mixture of 3 and 4 isolated from the reaction of 1 with TsCl was directly reacted with 5. Compound 6 was obtained in an improved isolated yield (76%). The tosyl groups in 6 were removed by treatment of 6 with concentrated sulfuric acid.
Scheme 2.
Synthetic route 1 to TANPA (7): Regiospecific ring opening of N-Ts aziridine 3.
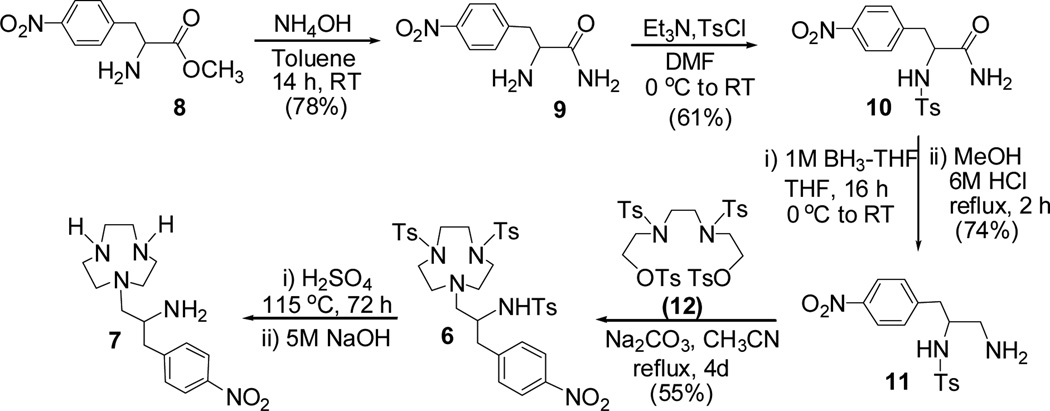
The second synthetic route to 7 includes base-promoted bimolecular cyclization between compound 11 and compound 12 as the key reaction step (Scheme 3). p-nitrophenylalanine methyl ester 8 was converted to 919 in 78% isolated yield. The reaction of compound 9 with TsCl provided N-Ts protected amide 10 which was subsequently reduced to the primary amine 11. The base-promoted reaction of 11 with ditosylate 1220 provided compound 6. The identical 1H and 13C NMR spectral data of compound 6 prepared via the two synthetic routes further confirmed the regiochemistry observed in the ring opening of 3.
Scheme 3.
Synthetic route 2 to TANPA (7): Bimolecular cyclization of 11 and 12.
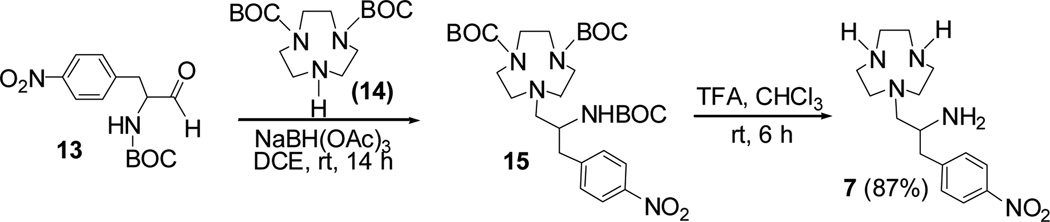
The third synthetic approach is based on reductive amination of 13 with 14 followed by deprotection of BOC groups in 15 (Scheme 4). N-BOC-protected macrocyclic compound 1410 was reacted with 13 in the presence of sodium triacetoxyborohydride to provide 15 which was further reacted with TFA for removal of the BOC groups in 1510. Among the synthetic routes to 7 explored, this chromatography-free route turned out to be the most efficient process for scale-up production of 7. The macrocyclic backbone 7 was obtained in two steps with an overall yield of 87%.
Scheme 4.
Short and chromatography-free synthetic route 3 to TANPA (7): reductive amination of 13 with 14.
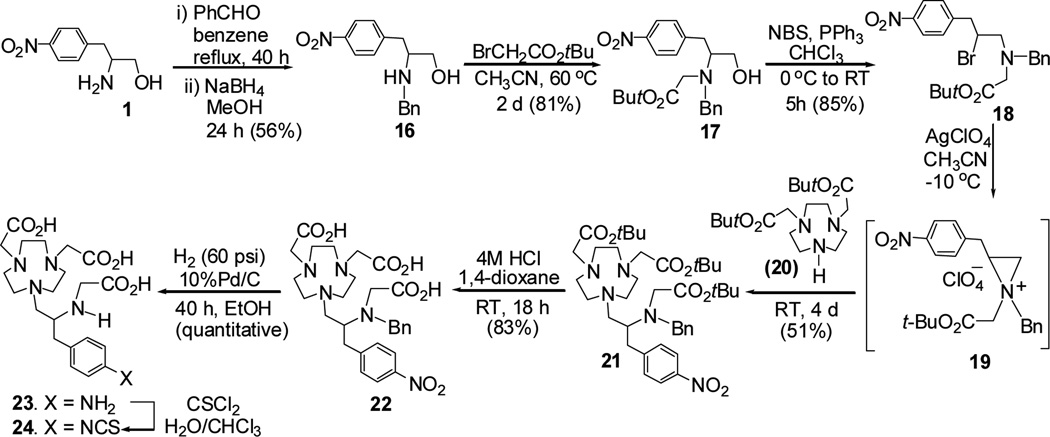
A practical and reproducible synthetic route to C-NE3TA-NCS for RIT applications is shown in Scheme 5. The advantages of the synthetic route include i) formation of the key intermediate compound 21 via efficient ring opening of aziridinium ion; ii) formation of the desired C-NE3TA-NCS in shorter reaction steps with reasonable yields as compared to the original synthetic route to the chelator8; iii) reproducible and relatively convenient chromatographic purification of 21. The original synthetic route8 to C-NE3TA-NCS includes the preparation of tert-butyl C-NE3TA via selective base-promoted alkylation of TANPA (7) using tert-butyl bromoacetate. Although the isololated yield (<65%) of tert-butyl C-NE3TA (Figure 1) was reasonably good, flash silica gel chromatographic purification of the macrocyclic compound is challenging due to its high polarity and unwanted polyalkylated or partially alkylated byproducts present in the reaction mixture. TLC is not a very useful analytical tool to distinguish the desired product and byproducts as both of the compounds appear on TLC as concentration dependent tailing spots. Protection of the secondary amine in tert-butyl C-NE3TA using a benzyl group to enhance liphophilicity of the product was expected to allow for relatively straightforward chromatographic purification process. The ring opening reaction of aziridinium ion 19 by 20 was expected to avoid formation of the byproducts that were produced in the reaction of TANPA (7) with tert-butyl bromoacetate.8 Compound 16 was prepared by reductive amination of 1 and benzaldehyde followed by reduction of the intermediate imine analogue. The amino alcohol 16 was further reacted with tert-butylbromoacetate to provide 17 which was converted to N,N-bisubstituted β-amino bromide 18 via bromination using N-bromosuccinimide (NBS) and triphenylphospine (PPh3). Aziridinium ion 19 was produced by reaction of compound 18 with silver perchlorate and further reacted with bisubstituted TACN 20 to provide the key intermediate compound 21 in 51% isolated yield. tert-Butyl groups in compound 21 was removed by treatment of 21 with 4M HCl (g) in 1,4-dioxane. Compound 22 was subjected to hydrogenolysis to provide compound 23 in a quantitative yield. The amino group in compound 238 was converted to the isothiocyanate group to produce the desired ligand C-NE3TA-NCS (24).8 C-NE3TA-NCS and C-NOTA-NCS were conjugated with a tumor targeting antibody, trastuzumab. The antibody was approved by the FDA for the treatment of metastatic breast cancer and is known to selectively target the HER2 protein overproduced in various tumors including colon and breast cancers.21,22 The corresponding conjugates were evaluated for radiolabeling with β-emitting radionuclides 90Y and 177Lu at room temperature. 90Y and 177Lu have been extensively investigated as potent cancer therapeutic radionuclides. Zevalin™ containing 90Y is the first RIT drug approved for the treatment of non-Hodgkin’s Lukemia.1C-NE3TA-NCS and C-NOTA-NCS was conjugated to trastuzumab, and the concentration of trastuzumab in the corresponding conjugates was quantified by the spectroscopic method as previously described. The Cu(II)-AAIII based UV-Vis spectrophotometric assay was used for the determination of the number of C-NE3TA ligand linked to trastuzumab (L/P ratio). The Cu(II)-AAIII based UV-Vis spectrophotometric assay was used for the determination of the number of C-NOTA ligand linked to trastuzumab (L/P ratio). The respective ligand to protein (L/P) ratio for C-NE3TA-trastuzumab8 and C-NOTA-trastuzumab was measured to be 2.5 and 4.3.
Scheme 5.
Efficient Synthesis of C-NE3TA-NCS for conjugation to antibody.
The purified C-NE3TA-trastuzumab and C-NOTA-trastuzumab conjugates (0.25M NH4OAc, pH 5.5) were labeled with 90Y or 177Lu (60 µCi) at room temperature (RT). During the reaction time (1 h), the components were analyzed using SE-HPLC or ITLC after challenging the reaction mixture with 10 mM DTPA, and the radiolabeling efficiency (%) was determined. The data (Table 1) indicate that C-NE3TA-trastuzumab conjugate displayed relatively rapid radiolabeling with 90Y (5 min, 47%; 1 h 89%). Complexation of C-NE3TA-trastuzumab with 177Lu was slow with radiolabeling efficiency of 38% at 1 h time point. C-NE3TA appears to have insufficient number of donors to rapidly complex a larger metal 177Lu. As expected,14,15 C-NOTA was completely ineffective in binding both 90Y and 177Lu with the respective ionic radius of 104 pm and 100 pm. The macrocyclic cavity of NOTA appears to be too small for binding the lanthanides with high kinetic stability. The results of complexation kinetics indicate that the additional acyclic bidentate donor system was essential for the improved complexation kinetics of C-NE3TA as compared to C-NOTA.
Table 1.
Evaluation of C-NE3TA-trastuzumab conjugate[a] and C-NOTA-trastuzmab Conjguate[b] for Radiolabling efficiency (%) with 90Y and 177Lu (0.25M NH4OAc, pH 5.5, RT) using ITLC or HPLC (Parenthesis).
| 90Y | 177Lu | |||
|---|---|---|---|---|
| Time (min) |
C-NE3TA- trastuzumab |
C-NOTA- trastuzumab |
C-NE3TA- trastuzumab |
C-NOTA- trastuzumab |
| 1 | 23.3 ± 2.7 (22.9 ± 2.0) |
2.9 ± 0.2 | 13.5 ± 0.7 (10.5 ± 0.8) |
1.1 ± 0.2 |
| 5 | 46.6 ± 3.5 (39.3 ± 3.4) |
13.0 ± 8.2 | 21.8 ± 0.6 (18.0 ± 0.2) |
3.7 ± 1.6 |
| 10 | 60.1 ± 0.7 (49.9 ± 1.7) |
10.1 ± 4.8 | 26.8 ± 0.5 (21.7 ± 0.2) |
5.0 ± 1.8 |
| 20 | 73.9 ± 2.6 (61.8 ± 1.6) |
8.6 ± 0.0 | 32.1 ± 1.3 (24.7 ± 0.7) |
9.8 ± 2.1 |
| 30 | 81.6 ± 1.6 (66.1 ± 1.1) |
8.7 ± 2.8 | 33.6 ± 1.7 (26.0 ± 0.4) |
13.3 ± 1.1 |
| 60 | 88.9 ± 1.7 (71.9 ± 1.3) |
16.1 ± 5.4 | 37.7 ± 1.6 (29.0 ± 0.2) |
22.9 ± 4.5 |
Radiolabeling efficiency (mean ± standard deviation %) was measured in triplicate using HPLC and ITLC.
Radiolabeling efficiency (mean ± standard deviation %) was measured in duplicate using ITLC.
Conclusion
We presented the efficient synthetic route to C-NE3TA-NCS containing a functional group for conjugation to antibody. Bimolecular cyclization and ring opening reactions of aziridine and aziridinium ions were explored for improved and efficient synthesis of TANPA as the useful macrocyclic chelating backbone and the key precursor molecule to the C-NE3TA analogue. The synthetic methods reported herein can be applied for preparation of various macrocyclic ligand systems. C-NE3TA-trastuzumab conjugate exhibited significantly enhanced complexation kinetics with 90Y relative to C-NOTA-trastuzumab conjugate. C-NE3TA can be further evaluated for RIT application using 90Y and is a potential bifunctional chelator of other small divalent and trivalent cations such as 64Cu and 68Ga for radioimmunoimaging.
Experimental Section
Instruments and Methods
1H, 13C, and DEPT NMR spectra were obtained using a Bruker 300 instrument and chemical shifts are reported in parts per million on the δ scale relative to TMS. Elemental microanalyses were performed by Galbraith Laboratories, Knoxville, TN. Fast atom bombardment (FAB) high-resolution mass spectra (HRMS) were obtained on JEOL double sector JMS-AX505HA mass spectrometer (University of Notre Dame, South Bend, IN). The analytical HPLC was performed on an Agilent 1200 equipped with a diode array detector (λ = 254 and 280 nm), a themostat set at 35 °C, and a Zorbax Eclipse XDB-C18 column (4.6 × 150 mm, 80Å). The mobile phase of a binary gradient (0–100% B/40 min; solvent A, 0.05M AcOH/Et3N, pH 6.0; solvent B, CH3CN for method 1 or 0–50% B/30 min, 50–100% B/31min, 100%B/40min; solvent A, 0.05M AcOH/Et3N, pH 6.0; solvent B, CH3OH for method 2) at a flow rate of 1 mL/min was used. Size-exclusion HPLC (SE-HPLC) chromatograms were obtained on an Agilent 1200 equipped with an in-line IN/US γ-Ram Model 2 radiodetector (Tampa, FL), fitted with a TSKgel G3000PW column (Tosoh Biosep, Montgomeryville, PA). The isocratic mobile phase (0.05M NaSO4, 0.02M NaH2PO4, 0.05% NaN3, pH 6.8) at a flow rate of 1 mL/min was used for SE-HPLC. 90Y and 177Lu in the chloride form was obtained from NEN Perkin-Elmer.
Caution: 90Y (t1/2 = 72 h) and 177Lu (t1/2 = 6.7 d) are β and/or γ-emitting radionuclides. Appropriate shielding and handling protocols should be in place when using the isotope.
1-[(4-Methylbenzene)sulfonyl]-2-[(4-nitrophenyl)methyl] aziridine (3)
To a solution of 1 (1.0 g, 5.1 mmol) in THF (15 mL) at 0 °C was added TsCl (2.14 g, 11.2 mmol), Et3N (3.7 g, 36.7 mmol) and DMAP (61 mg, 0.50 mmol). The resulting mixture was allowed to stir at room temperature for 20 h, at which time the reaction mixture was evaporated. The residue was purified via column chromatography on silica gel (60–230 mesh) eluting with 20% ethyl acetate/hexane to provide pure 4 (780 mg, 46%) as a light yellow solid. 1H NMR (CDCl3, 300 MHz) δ 2.22 (d, J = 4.2 Hz, 1H), 2.38 (s, 3H), 2.53 (dd, J = 13.8 Hz, J = 4.0 Hz, 1H), 2.80 (d, J = 4.2 Hz, 1H), 2.83-2.95 (m, 1H), 3.03-3.12 (m, 1H), 7.12-7.22 (m, 4H), 7.59 (d, J = 8.1 Hz, 2H), 7.93 (d, J = 8.1 Hz, 2H); 13C NMR (CDCl3, 300 MHz) δ 21.5 (q), 32.7 (t), 37.3 (t), 40.7 (d), 123.5 (d), 127.9 (d), 129.5 (d), 134.5 (s), 144.9 (s), 145.0 (s), 146.6 (s). HRMS (Positive ion ESI) Calcd for C16H17N2O4S [M + H]+ m/z 333.0904. Found: [M + H]+ m/z 333.0901.
N-[2-chloro-3-(4-nitrophenyl)propyl]-4-methylbenzene-1-sulfonamide (4)
To a solution of 1 (50 mg, 0.255 mmol), Et3N (77.2 mg, 1.28 mmol) and DMAP (3.1 mg, 0.0255 mmol) in CH2Cl2 (1.0 mL) at 0 °C was added portionwise TsCl (243 mg, 1.28 mmol). The resulting mixture was stirred at room temperature for 6 days, at which time the reaction mixture was evaporated. The residue was purified via column chromatography on silica gel (60-230 mesh) eluting with 18% ethyl acetate/hexane to provide pure 4 (76 mg, 81%) as a light yellow solid. 1H NMR (CDCl3, 300 MHz) δ 2.39 (s, 3H), 2.83 (dd, J = 13.8 Hz, J = 4.2 Hz, 1H), 3.04 (dd, J = 13.8 Hz; J = 3.0 Hz, 1H), 3.55-3.58 (m, 2H), 3.68-3.78 (m, 1H), 5.41-5.55 (d, J = 8.9 Hz, 1H) 7.12-7.22 (m, 4H), 7.53 (d, J = 8.2 Hz, 2H), 7.96 (d, J = 8.2 Hz, 2H); 13C NMR (CDCl3, 300 MHz) δ 21.4 (q), 38.2 (t), 47.6 (t), 55.2 (d), 123.7 (d), 126.8 (d), 129.7 (d), 130.1 (d), 136.9 (s), 144.0 (s), 144.2 (s), 146.8 (s). HRMS (Positive ion ESI) Calcd for C16H17N2O4SClNa [M + H + Na]+ m/z 391.0490. Found: [M + H +Na]+ m/z 391.0506.
N-(1-{4,7-bis[(4-methylbenzene)sulfonyl]-1,4,7-triazonan-1-yl}-3-(4-nitrophenyl) propan-2-yl)-4-methylbenzene-1-sulfonamide (6)
Preparation from the reaction of compound 4 with 5
To a solution of 4 (50 mg, 0.136 mmol) and 518 (59.4 mg, 0.136 mmol) in CH3CN (3 mL) was added DIPEA (19.4 mg, 0.150 mmol). The resulting mixture was refluxed for 4.5 days after which time the reaction mixture was cooled to room temperature and evaporated. The residue was purified via column chromatography on silica gel (60–230 mesh) eluting with 3% ethyl acetate/DCM to provide pure 6 (87 mg, 84%) as a light yellow solid. 1H NMR (CDCl3, 300 MHz) δ 2.17 (s, 3H), 2.43 (s, 6H), 2.70-3.60 (m, 17H), 7.10 (d, J = 8.0 Hz, 2H), 7.28-7.34 (m, 6H), 7.63-7.69 (m, 6H), 7.99 (d, J = 8.3 Hz, 2H); 13C NMR (CDCl3, 300 MHz) δ 21.1 (q), 21.5 (q), 40.0 (t), 52.4 (t), 53.4 (t), 53.7 (d), 55.6 (t), 60.4 (t), 123.4 (d), 127.0 (d), 127.1 (d), 129.4 (d), 129.9 (d), 130.4 (d), 135.0 (s), 137.8 (s), 142.9 (s), 143.8 (s), 145.8 (s), 146.6 (s). HRMS (Positive ion ESI) Calcd for C36H44N5O8S3 [M + H]+ m/z 770.2347. Found: [M + H]+ m/z 770.2311.
Preparation from the stepwise reaction starting from compound 1
To a solution of 1 (200 mg, 1.02 mmol), Et3N (515 mg, 5.10 mmol) and DMAP (12.5 mg, 0.102 mmol) in DCM (3.0 mL) at 0 °C was added portionwise TsCl (972 mg, 5.10 mmol). The resulting mixture was allowed to stir at room temperature for 1 day, at which time the reaction mixture was evaporated. The residue was purified via column chromatography on silica gel (60–230 mesh) eluting with 20% ethyl acetate/hexane to provide a mixture of 3 and 4 (332 mg) as a light yellow solid. The mixture was directly used for the next step without further purification. To a solution of mixture (3 and 4) (50 mg) and 5 (66.9 mg, 0.153 mmol) in CH3CN (3 mL) was added DIPEA (21.7 mg, 0.168 mmol). The resulting mixture was refluxed for 30 h after which time the reaction mixture was cooled to room temperature, filtered and evaporated. The residue was purified via column chromatography on silica gel (60–230 mesh) eluting with 3% ethyl acetate/DCM to provide pure 6 (89 mg, yield of two steps is 76%) as a light yellow solid. 1H and 13C NMR data of 6 obtained in this reaction is essentially same as those of 6 described above.
1-(4-Nitrophenyl)-3-(1,4,7-triazonan-1-yl)propan-2-amine (7)
To compound 6 (705 mg, 0.92 mmol) was added Conc. H2SO4 (10 mL). The resulting mixture was heated to 115 °C and stirred for 3 days at which time the reaction mixture was cooled to room temperature. The reaction mixture was added dropwise into ethyl ether (65 mL) at −60 °C over 1 h. The resulting mixture was stirred for 20 min, filtered, and washed with cold ether. The residue was quickly dissolved with water (30 mL), and the resulting aqueous solution was extracted with CHCl3 (30 mL). The aqueous solution was adjusted to pH 7 using 5M NaOH (aq) and extracted with CHCl3 (2 × 50 mL). The aqueous solution was further adjusted to pH 10 and pH 13. At each pH, the aqueous solution was extracted with CHCl3 (3 × 50 mL). The organic layers extracted at pH 10 and pH 13 were combined, dried over MgSO4, filtered, and concentrated to provide pure 7 (191 mg, 68%) as a light yellowed oil. 1H NMR (CDCl3, 300 MHz) δ 2.25-2.42 (m, 5H), 2.50-2.89 (m, 15H), 3.10-3.18 (m, 1H), 7.35 (d, J = 8.5 Hz, 2H), 8.12 (d, J = 8.5 Hz, 2H); 13C NMR (CDCl3, 300 MHz) δ 42.1 (t), 46.1 (t), 46.5 (t), 51.1 (d), 53.2 (t), 64.8 (t), 123.6 (d), 129.9 (d), 146.4 (s), 147.6 (s).
2-Amino-3-(4-nitrophenyl)propanamide (9).19
To a solution of 8 (7.15 g, 27.8 mmol) in toluene (50 mL) at room temperature was added NH4OH (50 mL). The resulting mixture was stirred at room temperature overnight. The reaction mixture was filtered and washed with toluene to provide pure 9 (4.46 g, 78%) as a light yellow solid. 1H NMR (d6-DMSO, 300 MHz) δ 2.42-3.10 (m, 4H), 3.42 (t, J = 3.0 Hz, 1H), 7.32-7.04 (s, 1H), 7.30-7.53 (m, 3H), 8.12 (d, J = 8.5 Hz, 2H); 13C NMR (d6-DMSO, 300 MHz) 41.1 (t), 56.2 (d), 123.5 (d), 131.1 (d), 146.5 (s), 148.1 (s), 176.6 (s).
2-[(4-Methylbenzene)sulfonamido]-3-(4-nitrophenyl)propan-amide (10)
To a solution of 9 (500 mg, 2.39 mmol) and Et3N (869 mg, 8.61 mmol) in DMF (10 mL) at 0 °C was added dropwise a solution of TsCl (1.09 g, 5.74 mmol) in DMF (5 mL) over 30 min. The resulting mixture was warmed to room temperature and stirred for 3 days at which time the reaction mixture was evaporated. The residue was washed with CH2Cl2 to provide pure 10 (526 mg, 61%) as a colorless solid. 1H NMR (d6-DMSO, 300 MHz) δ 2.25 (s, 3H), 2.64-2.79 (m, 1H), 2.92-3.04 (m, 1H), 3.90 (m, 1H), 7.02-7.13 (m, 3H), 7.30-7.36 (m, 4H), 7.44 (s, 1H), 7.94 (d, J = 8.2 Hz, 2H), 8.06 (d, J = 8.5 Hz, 1H); 13C NMR (d6-DMSO, 300 MHz) 21.2 (q), 38.5 (t), 57.7 (d), 123.4 (d), 126.6 (d), 129.5 (d), 131.0 (d), 138.6 (s), 142.5 (s), 146.2 (s), 146.5 (s), 172.5 (s).
N-[1-amino-3-(4-nitrophenyl)propan-2-yl]-4-methylbenzene-1-sulfonamide (11)
To a solution of 10 (573.2 mg, 1.58 mmol) in THF (10 mL) at 0 °C was added dropwise 1M BH3 in THF (9.5 mL) for 30 min. The resulting mixture was warmed to room temperature and stirred for 2 h and then heated to reflux overnight. The reaction mixture was concentrated, and the residue was treated with MeOH (10 mL) and concentrated to the dryness. This quenching process was repeated twice. The residue was dried in vacuo and treated with 6M HCl (10mL). The resulting mixture was stirred at room temperature for 1 h and then heated to reflux for 2 h. The aqueous solution was cooled to room temperature and was adjusted to pH 7 using 2M NaOH and then extracted with CHCl3 (2 × 30 mL). The aqueous layer was further adjusted to pH 10 and pH 13. At each pH, the aqueous solution was extracted with CHCl3 (2 × 30 mL). The organic layers extracted at pH 7 and 10 were combined, dried over MgSO4, filtered, and concentrated in vacuo to provide free amine 11 (408 mg, 74%) as a light yellow solid. 1H NMR (d6-DMSO, 300 MHz) δ 2.26 (s, 3H), 2.40-2.62 (m, 2H), 3.16-3.80 (m, 5H), 7.12 (d, J = 8.2 Hz, 2H), 7.28 (d, J = 8.2 Hz, 2H), 7.37 (d, J = 8.4 Hz, 2H), 7.92 (d, J = 8.4 Hz, 2H); 13C NMR (d6-DMSO, 300 MHz) 21.2 (q), 37.9 (t), 46.9 (t), 58.4 (d), 123.4 (d), 126.5 (d), 129.6 (d), 130.8 (d), 139.1 (s), 142.4 (s), 146.2 (s), 147.7 (s).
N-(1-{4,7-bis[(4-methylbenzene)sulfonyl]-1,4,7-triazonan-1-yl}-3-(4-nitrophenyl)propan-2-yl)-4-methylbenzene-1-sulfonamide (6)
To a solution of 11 (146mg, 0.42 mmol) and 1220 (319.8 mg, 0.42 mmol) in CH3CN (8 mL) was added Na2CO3 (445.2 mg, 4.2 mmol). The resulting mixture was refluxed for 4 days after which time the reaction mixture was cooled to room temperature, filtered, and evaporated. The residue was purified via column chromatography on silica gel (60–230 mesh) eluting with 3% ethyl acetate/DCM to provide pure 6 (176 mg, 55%) as a white solid. 1H and 13C NMR data of 6 obtained in this reaction is essentially same as those of 6 described above.
1,4-Di-tert-butyl 7-(2-{[(tert-butoxy)carbonyl]amino}-3-(4-nitrophenyl)propyl)-1,4,7-triazonane-1,4-dicarboxylate (15).10
To a solution of 13 (2.00 g, 6.80 mmol) in 1,2-dichloroethane (50 mL) at 0 °C was added 14 (2.24 g, 6.80 mmol). The resulted solution was stirred for 10 min, and sodium triacetoxyborohydride (2.02 g, 9.52 mmol) was added portionwise to the solution over 30 min. The mixture was stirred at room temperature overnight. The reaction mixture was concentrated, treated with saturated NaHCO3 (50 mL), extracted, and washed with ethyl acetate (3 ×50 mL). The combined organic layers were dried over MgSO4, filtered, and concentrated in vacuo to provide 15 (4.08 g, 99%) as a yellowish solid. 1H NMR and 13C NMR data of compound 15 were the same as those that was previously reported. Compound 15 was directly used for the next step without further purification.
1-(4-Nitrophenyl)-3-(1,4,7-triazonan-1-yl)propan-2-amine (7)
To a solution of 10 (70 mg, 0.115 mmol) in CHCl3 (3 mL) at 0–5 °C was added dropwise TFA (3 mL) over 10 min. The resulting mixture was warmed to room temperature. After 6 h, the reaction mixture was concentrated, and deionized water (5 mL) was added to the residue. The aqueous layer was adjusted to pH 7 using 2M NaOH (aq) and then extracted with CHCl3 (3 × 5 mL). The aqueous layer was further adjusted to pH 10 and pH 13. At each step, the aqueous layer was extracted with CHCl3 (3 × 5 mL). The organic layers obtained from extraction of the aqueous solution (pH 10 and pH 13) were combined and dried over MgSO4, filtered, and concentrated in vacuo to provide free amine 7 (31 mg, 88%) as a yellow oil. 1H and 13C NMR data of 7 obtained in this reaction is essentially same as those of 7 described above.
2-(benzylamino)-3(4-nitrophenyl)propan-1-ol (16)
A stirred solution of 1 (1.0 g, 5.10 mmol) and benzaldehyde (568 mg, 5.35 mmol) in benzene (20 mL) was refluxed for 40 h by using Dean-Stark trap (5 mL). The solvent was evaporated in vacuo, and MeOH (20 mL) was added to the crude imine residue. The resulting mixture was cooled to 0°C, and NaBH4 (482 mg, 12.7 mmol) was added portionwise over 15 min. The reaction mixture was stirred for 24 h and was concentrated to the dryness in vacuo. The residue was quenched with H2O (20 mL) and extracted with ethyl acetate (3 × 20 mL). The organic layer was dried over MgSO4, filtered, and concentrated in vacuo. The crude product was dissolved in saturated citric acid (20 mL) and extracted with ethyl acetate (3 × 20 mL). pH of aqueous layer was adjusted to 12 by using 25% NaOH (aq) and extracted with ethyl acetate (3 × 20 mL). The organic layer was dried over MgSO4, filtered and concentrated in vacuo to afford pure 16 (810 mg, 56%). The product was directly used for the next step without further purification. 1H NMR (CDCl3, 300 MHz) δ 1.65-2.20 (broad, 2H), 2.80-3.06 (m, 3H), 3.30-3.41 (m, 1H), 3.59-3.68 (m, 1H), 3.74-3.85 (m, 1H), 7.14-7.34 (m, 7H), 8.15 (d, J = 8.4 Hz, 2H); 13C NMR (CDCl3, 300 MHz) δ 38.0 (t), 51.1 (t), 59.0 (d), 62.2 (t), 123.8 (d), 127.3 (d), 128.0 (d), 128.6 (d), 130.1 (d), 139.7 (s), 146.6 (s).
tert-Butyl 2-{benzyl[1-hydroxy-3-(4-nitrophenyl)propan-2-yl] amino}acetate (17)
To a stirred solution of 16 (400 mg, 1.40 mmol) in CH3CN (2 mL) at 0 °C was added K2CO3 (212 mg, 1.54 mmol). A solution of tert-butyl bromoacetate (301 mg, 1.54 mmol) in CH3CN (1 mL) was added dropwise to the resulting mixture over 10 min. The reaction mixture was heated to 60°C and stirred for 2 days while the reaction progress was continuously monitored using TLC. The reaction mixture was cooled to room temperature and filtered. The solvent was evaporated in vacuo to provide the residue which was purified via column chromatography on silica gel (60–230 mesh) eluted with 20% ethyl acetate/hexane. Pure 17 (451 mg, 81%) was obtained as a light yellow oil. 1H NMR (CDCl3, 300 MHz) δ 1.40 (s, 9H), 2.52-2.66 (m, 1H), 2.92-3.04 (m, 1H), 3.11-3.52 (m, 5H), 3.71-3.98 (m, 2H), 7.16-7.33 (m, 7H), 8.15 (d, J = 8.4 Hz, 2H); 13C NMR (CDCl3, 300 MHz) δ 27.9 (q), 33.9 (t), 51.5 (t), 56.1 (t), 61.9 (t), 64.9(d), 81.8 (s), 123.8 (d), 127.4 (d), 128.4 (d), 128.9 (d), 129.9 (d), 138.3 (s), 146.6 (s), 147.3 (s), 172.3 (s).
tert-Butyl 2-{benzyl[2-bromo-3-(4-nitrophenyl)propyl]amino} acetate (18)
To a solution of 17 (395 mg, 0.986 mmol) and PPh3 (310 mg, 1.18 mmol) in CHCl3 (6 mL) at 0 °C was added portionwise NBS (211 mg, 1.18 mmol) over 5 min. The resulting mixture was stirred for 4 h while being maintained at 0 °C. The ice bath was removed, and the reaction mixture was warmed to room temperature and stirred for 1 h. The solvent was evaporated, and the residue was purified by silica gel column chromatography eluted with 5 % EtOAc in hexanes to afford 18 (390 mg, 85%) as a yellow oil. 1H NMR (CDCl3, 300 MHz) δ 1.48 (s, 9H), 2.84-2.96 (m,1H), 2.99-3.14 (m,1H), 3.24-3.34 (m, 1H), 3.36 (s, 2H), 3.61-3.73 (m, 1H), 3.84-3.96 (m, 2H), 3.96-4.09 (m, 1H), 7.21-7.42 (m, 7H), 8.13 (d, J = 8.4 Hz, 2H); 13C NMR (CDCl3, 300 MHz) δ 28.2 (q), 42.0 (t), 53.8 (d), 56.6 (t), 59.3 (t), 61.9(t), 81.4 (s), 123.5 (d), 127.5 (d), 128.6 (d), 128.9 (d), 130.1 (d), 138.8 (s), 146.6 (s), 146.8 (s), 170.6 (s). HRMS (Positive ion ESI) Calcd for C22H28N2O4Br [M + H]+ m/z 463.1227. Found: [M + H]+ m/z 463.1233.
tert-Butyl 2-[benzyl(1-{4,7-bis[2-(tert-butoxy)-2-oxoethyl]-1,4,7-triazonan-1-yl}-3-(4-nitrophenyl)propan-2-yl)amino]acetate (21)
To a solution of 18 (100 mg, 0.216 mmol) in CH3CN (1 mL) at −5 °C was added AgClO4 (44.8 mg, 0.216 mmol). The resulting mixture was stirred for 10 min at the same temperature. 207 (77.2 mg, 0.216 mmol) and DIPEA (83.7 mg, 0.648 mmol) in CH3CN (1 mL) was sequentially added to the reaction mixture at −5 °C. The resulting mixture was gradually warmed to room temperature and stirred for 4.5 days. The reaction mixture was filtered and concentrated to the dryness. 0.1M HCl solution (10 mL) was added to the residue, and the resulting mixture was extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were dried over MgSO4, filtered, and concentrated in vacuo to the dryness. The residue was purified via column chromatography on silica gel (220–440 mesh) eluting with 2% CH3OH in CH2Cl2 to provide pure product 21 (81 mg, 51%) as a yellowish oil. 1H NMR (CDCl3, 300 MHz) δ 1.31-1.45 (m, 27H), 2.68 (s, 4H), 2.76-3.95 (m, 21H), 7.18-7.34 (m, 5H), 7.52 (d, J = 8.6 Hz, 2H), 8.14 (d, J = 8.6 Hz, 2H); 13C NMR (CDCl3, 300 MHz) 28.0 (q), 28.0 (q), 28.1 (q), 33.3 (t), 49.8 (t), 51.1 (t), 51.6 (t), 53.5 (t), 53.6 (t), 56.3 (t), 57.9 (d), 58.2 (t), 58.4 (t), 81.6 (s), 81.7 (s), 82.6 (s), 124.1 (d), 128.0 (d), 128.8 (d), 129.4 (d), 129.9 (d), 130.5 (d), 137.2 (s), 146.0 (s), 146.8 (s), 170.4 (s). 172.7 (s). HRMS (Positive ion ESI) Calcd for C40H62N5O8 [M + H]+ m/z 740.4593. Found: [M + H]+ m/z 740.4579.
2-[Benzyl({1-[4,7-bis(carboxymethyl)-1,4,7-triazonan-1-yl]-3-(4-nitrophenyl)propan-2-yl})amino]acetic acid (22)
To a round bottom flask containing compound 21 (33.0 mg, 0.0446 mmol) at 0–5 °C was added dropwise 4M HCl (g) in 1,4-dioxane (3 mL) over 10 min. The resulting mixture was allowed to warm to room temperature. After 18 h, ethyl ether (20 mL) was added, and the resulting mixture was stirred for 10 min and capped and placed in the freezer for 1 h. The resulting precipitate was filtered, washed with ether, and quickly dissolved in deionized water. The aqueous solution was concentrated in vacuo to provide 22 (26.5 mg, 83%) as a light yellow solid. 1H NMR (D2O, pH 14, 300 MHz) δ 1.50-3.14 (m, 22H), 3.31-3.50 (m, 2H), 3.61-3.75 (m, 1H), 7.07-7.31 (m, 7H), 7.88 (d, J = 8.4 Hz, 2H); 13C NMR (D2O + NaOD, 300 MHz) δ 31.6 (t), 51.4 (t), 53.3 (t), 56.2 (t), 56.3 (d), 57.8 (t), 61.0 (t), 123.6 (d), 127.8 (d), 128.7 (d), 130.0 (d), 137.7 (s), 145.8 (s), 149.2 (s), 179.6 (s), 179.8 (s). HRMS (Positive ion ESI) Calcd for C28H38N5O8Na2 [M + 2Na − H]+ m/z 616.2354. Found: [M + 2Na − H]+ m/z 616.2322.
2-{[1-(4-Aminophenyl)-3-[4,7-bis(carboxymethyl)-1,4,7-triazo-nan-1-yl]propan-2-yl]amino}acetic acid (23).8
To a solution of 22 (13.2 mg, 0.0184 mmol) in ethanol (5 mL) at room temperature was added wet 10% Pd/C (8.1 mg) under Ar (g). The reaction mixture was placed under hydrogenation apparatus set at the pressure of 60 psi for 40 h. The resulting mixture was filtered via celite bed and washed thoroughly with ethanol. The filtrate was concentrated to provide 23 (11.7 mg, 100%) as a yellowish solid. 1H and 13C NMR data of 23 were essentially identical to those of 23 as previously reported.
Conjugation of C-NE3TA-NCS and C-NOTA-NCS to trastuzumab
C-NOTA and C-NOTA-NCS were purchased from Macrocylics (Dallas, TX). Trastuzumab was provided as a gift by Dr. Martin Brechbiel (NCI, NIH). All absorbance measurements were obtained on an Agilent 8453 diode array spectrophotometer equipped with a 8-cell transport system (designed for 1 cm cells). Metal-free stock solutions of all buffers were prepared using Chelex®-100 resin (200–400 mesh, Bio-Rad Lab, Hercules, CA, Cat# 142–2842 ). Chelex resin (1g/100mL) was added into the buffer solution and the mixture was shaken overnight in a shaker and filtered through a Corning filter system (Cat# 430513, pore size 0.2 µm). Disposable PD-10 columns (Sephadex™, G-25M, GE Healthcare, #17-0851-01) were rinsed with the appropriate buffer solution (25 mL) prior to addition of antibody or its ligand conjugates. Amicon centricon C-50 (50,000 MWCO) centrifugal filter devices (Millipore, Cat# UFC805008) were used for purification of trastuzumab conjugate. The initial concentration of trastuzumab was determined by UV spectroscopic method. Phosphate buffered saline (PBS, 1×, 11.9 mM Phosphates, 137 mM NaCl, and 2.7 mM KCl, pH 7.4) was purchased from Fisher and used as received. Conjugation buffer (50 mM HEPES, 150 mM NaCl, pH 8.6) were prepared as 1× solutions, chelexed, and filtered through the Corning filter. C-NE3TA-NCS was conjugated to trastuzumab as previously reported.8 The ratio of ligand to protein (L/P) for the C-NE3TA-trastuzumab conjugate was measured using Cu(II)-AAIII based spectrometric method and found to be 2.5 to 1. Trastuzumab (6.7 mg for C-NOTA) was diluted to 2.5 mL using conjugation buffer and the resulting solution was added to a PD-10 column. Conjugation buffer (6.0 mL) was added to the PD-10 column to exchange the buffer solution of the antibody and collected in a sterile test tube and checked for the presence of trastuzumab via analysis of the UV spectrum at 280 nm. To a sterile test tube containing the recovered trastuzumab (6.1 mg for C-NOTA) was added a 10-fold excess of C-NOTA-NCS (39.6 µL, 10 mM). The resulting solution was gently agitated for 16 h at room temperature and placed on a Centricon C-50 membrane and spun down to reduce volume. PBS (3 × 2 mL) was added to the remaining solution of C-NOTA-trastuzumab conjugate, followed by centrifugation in order to remove unreacted ligand. The volume of the purified antibody conjugate solution was brought to 1.0 mL. To measure [Trastuzumab] in the conjugates, a UV/Vis spectrometer was zeroed against a cuvette filled with 2.0 mL of PBS with a window open from 190 nm to 1100 nm. A 50 µL portion of PBS was removed and discarded, 50 µl of C-NOTA-trastuzumab conjugate solution was added, and absorbance at 280 nm was noted. Beer’s Law was used to calculate [Trastuzumab] in the conjugate with molar absorptivity of 1.42. After centrifugation, 2.7 mg (1.83×10-5 M, 44.0%) of Trastuzumab remained.
The L/P of C-NOTA-trastuzumab conjugate was measured using Cu(II)-AAIII based spectrometric method. A stock solution of Cu(II)-AAIII reagent was prepared in 0.15 M NH4OAc, pH 7.0 by adding an aliquot of copper (1.55×10-2 M) atomic absorption solution into a 10 µM solution of AAIII to afford a 5 µM solution of Cu(II). This solution was stored in the dark to avoid degradation over time. A UV/Vis spectrometer was zeroed against well-dried blank 8 cuvettes with a window open from 190 nm to 1100 nm. A cuvette was filled with AAIII solution (2 mL), and the other seven cuvettes with Cu(II)-AAIII solution (2 mL). Cu(II)-AAIII solution (50 µL) in the seven cuvettes was removed and discarded. Milli-Q water (50 µL) was added to the second cuvette, and one to five 10 µL additions of C-NOTA (0.1mM) were added to the five cuvettes to give a series of five different concentrations (2 mL total volume). The solutions in the third to the seventh cuvette were diluted to 2.0 mL by adding an aliquot of milli-Q water. C-NOTA-trastuzumab conjugate (50 µL) was added to the eighth cuvette containing Cu(II)-AAIII reagent (1950 µL). After addition of the conjugate to Cu(II)-AAIII solution, the resulting solution was equilibrated for 10 min. The absorbance of the resulting solution at 610 nm (for Cu(II)-AAIII) was monitored every 30 seconds over 6 min. The average of the absorbance of each solution was calculated, and the absorbance data from Cu(II)-AAIII solutions containing six different concentrations were used to construct a calibration plot of A610nm versus [C-NOTA] by the equation, Y = 0.0596-(8.125 × 103)[C-NOTA] (R2 = 0.9938), wherein Y = A610nm. The concentration of C-NOTA in C-NOTA-trastuzumab conjugate was calculated (7.79×10-5 M). The L/P ratio of C-NOTA-trastuzumab conjugate ([7.79×10-5 M]/[1.83×10-5 ]) was measured to be 4.3.
Radiolabeling reaction kinetics of C-NE3TA-Hercpetin and C-NOTA-Trastuzumab conjugates with 90Y and 177Lu
All HCl solutions were prepared from ultra-pure HCl (Fisher, Cat# A466-500). For metal-free radiolabeling, plasticware including pipette tips, tubes, and caps was soaked in 0.1M HCl overnight, washed thoroughly with Milli-Q (18.2 MΩ) water, and air-dried overnight. Ultra pure NH4OAc (Aldrich, #372331) was purchased from Aldrich and used to prepare all NH4OAc buffer solutions (0.25 M, pH 5.5). The buffer solutions were treated with Chelex-100 resin (Biorad, #142-2842, 1g/100 ml buffer solution), shaken overnight at room temperature, and filtered through 0.22 µm filter (Corning, #430320) prior to use. To a buffer solution (30 µL, pH 5.5, 0.25M NH4OAc) in a capped microcentrifuge tube (1.5 mL, Fisher Scientific #05-408-129) was sequentially added a solution of C-NE3TA-trastuzumab or C-NOTA-trastuzumab conjugate (30 µg) in PBS (4.5 µL) and 177Lu (0.05 M HCl, 60 µCi, 2.4 µL). To a solution of C-NE3TA-trastuzumab or C-NOTA-trastuzumab conjugate (30 µg) in PBS (4.5 µL) in a microcentrifuge tube containing a buffer solution (30 µL, pH 5.5, 0.25M NH4OAc) was sequentially added a solution of 0.05 M HCl (1.6~2.9 µL) and 90Y (0.05 M HCl, 60 µCi, 2~3 µL). The final volume of the resulting solution was around 40 µL, and the pH of the resulting reaction mixture was 5.5. The reaction mixture was agitated on the thermomixer (Eppendorf, #022670549) set at 1,000 rpm at room temperature for 1 h. The labeling efficiency was determined by SE-HPLC (Phenomenex, BioSep-SEC S 3000, 7.8 × 30 cm, eluent: 0.05M NaSO4/0.02M NaH2PO4/0.05% NaN3, pH 6.8) and ITLC (eluent: 20mM EDTA/0.15M NH4OAc). A solution of radiolabeled mixture (6 µL) was withdrawn at the designated time points (1 min, 5 min, 10 min, 20 min, 30 min, and 60 min), and DTPA solution (10 mM or 5 mM, 0.6 µL) was added to the mixture, and the resulting mixture was left for at least 20 min at RT. In ITLC analysis, peaks for bound and unbound radioisotope appeared around 30 mm and 50mm from the origin, respectively. Bound and unbound radioisotope peaks appeared around 9.0 min and 12.0 min as shown by SE-HPLC (the supporting information).
Supplementary Material
Acknowledgments
We acknowledge financial support from the National Institutes of Health (R01CA112503) and thank Dr. Mamta Dadwal for the preparation of compound 3.
Footnotes
Supporting information for this article is available on the WWW under http://www.eurjoc.org/ or from the author.
Supporting Information: HPLC and ITLC chromatograms for radiolabeling reaction kinetics of C-NE3TA-Hercpetin and C-NOTA-trastuzumab conjugates.
References
- 1.Milenic DE, Brad ED, Brechbiel MW. Nature Rev. 2004;3:488–498. doi: 10.1038/nrd1413. [DOI] [PubMed] [Google Scholar]
- 2.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem. Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 3.Parker D. Chem. Soc. Rev. 1990:271–291. [Google Scholar]
- 4.Harrison A, Walker CA, Parker D, Jankowski KJ, Cox JP. Nucl. Med. Biol. 1991;18:469–476. doi: 10.1016/0883-2897(91)90107-v. [DOI] [PubMed] [Google Scholar]
- 5.Chappell LL, Ma D, Milenic DE, Garmasteni K, Venditto V, Beitzel MP, Brechbiel MW. Nucl. Med. Biol. 2003;30:581–595. doi: 10.1016/s0969-8051(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 6.Chong HS, Garmestani K, Millenic DE, Brechbiel MW. J. Med. Chem. 2002;45:3458–3464. doi: 10.1021/jm0200759. [DOI] [PubMed] [Google Scholar]
- 7.Chong HS, Song HA, Birch N, Le T, Lim SY, Ma X. Bioorg. Med. Chem. Lett. 2008;18:3436–3439. doi: 10.1016/j.bmcl.2008.03.084. [DOI] [PubMed] [Google Scholar]
- 8.Chong HS, Song HA, Ma X, Milenic DE, Brady ED, Lim S, Lee H, Baidoo K, Cheng D, Brechbiel MW. Bioconjugate Chem. 2008;19:1439–1447. doi: 10.1021/bc800050x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song HA, Kang CS, Baidoo K, Milenic DE, Chen Y, Dai A, Brechbiel MW, Chong HS. Bioconjugate Chem. 2011;22:1128–1135. doi: 10.1021/bc100586y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chong HS, Ma X, Le T, Baidoo K, Milenic DE, Song HA, Brady ED, Brechbiel MW. J. Med. Chem. 2008;51:118–125. doi: 10.1021/jm070401q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chong HS, Song HA, Kang CS, Le T, Sun X, Dadwal M, Lee HB, Lan X, Chen Y, Dai A. Chem. Commun. 2011;47:5584–5586. doi: 10.1039/c0cc05707j. [DOI] [PubMed] [Google Scholar]
- 12.Dadwal M, Kang CS, Song HA, Sun X, Dai A, Baidoo K, Brechbiel MW, Chong HS. Bioorg. Med. Chem. Lett. 2011 doi: 10.1016/j.bmcl.2011.06.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Studer M, Meares CF. Bioconjugate Chem. 1992;3:337–341. doi: 10.1021/bc00016a013. [DOI] [PubMed] [Google Scholar]
- 14.Brucher E, Sherry AD. Inorg. Chem. 1990;29:1555–1559. [Google Scholar]
- 15.Clarke ET, Martell AE. Inorg. Chim. Acta. 1991;181:273–280. [Google Scholar]
- 16.Barrett BJ, Parfrey PS, McDonald JR, Hefferton DM, Reddy ER, McManamon PJ. Radiology. 1992;183:105–110. doi: 10.1148/radiology.183.1.1549654. [DOI] [PubMed] [Google Scholar]
- 17.Vogler H, Platzek J, Schuhmann-Giampieri GS, Frenzel T, Weinmann H-J, Radűchel B, Press W-R. Eur J Radiol. 1995;21:1–10. doi: 10.1016/0720-048x(95)00679-k. [DOI] [PubMed] [Google Scholar]
- 18.Pulacchini S, Watkinson M. Eur J. Org. Chem. 2001:4233–4238. [Google Scholar]
- 19.Anil MK, Panwar P, Chopra M, Sharma RK, Chatal J-F. New J. Chem. 2003;27:1054–1058. [Google Scholar]
- 20.Nicolas C, Borel M, Maurizis J-C, Gallais N, Rapp M, Ollier M, Verny M, Madelmont J-C. J. Labelled Compd. Radiopharm. 2000;43:585–594. [Google Scholar]
- 21.Agus DB, Bunn PA, Jr, Franklin W, Garcia M, Ozols RF. Semin. Oncol. 2000;27:53–63. [PubMed] [Google Scholar]
- 22.Baselga J. Ann. Oncol. 2001;12:S49–S55. doi: 10.1093/annonc/12.suppl_1.s49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.