Abstract
The conventional view of AD (Alzheimer's disease) is that much of the pathology is driven by an increased load of β-amyloid in the brain of AD patients (the ‘Amyloid Hypothesis’). Yet, many therapeutic strategies based on lowering β-amyloid have so far failed in clinical trials. This failure of β-amyloid-lowering agents has caused many to question the Amyloid Hypothesis itself. However, AD is likely to be a complex disease driven by multiple factors. In addition, it is increasingly clear that β-amyloid processing involves many enzymes and signalling pathways that play a role in a diverse array of cellular processes. Thus the clinical failure of β-amyloid-lowering agents does not mean that the hypothesis itself is incorrect; it may simply mean that manipulating β-amyloid directly is an unrealistic strategy for therapeutic intervention, given the complex role of β-amyloid in neuronal physiology. Another possible problem may be that toxic β-amyloid levels have already caused irreversible damage to downstream cellular pathways by the time dementia sets in. We argue in the present review that a more direct (and possibly simpler) approach to AD therapeutics is to rescue synaptic dysfunction directly, by focusing on the mechanisms by which elevated levels of β-amyloid disrupt synaptic physiology.
Keywords: Alzheimer's disease, β-amyloid, hippocampus, neuropathology, synaptic
THE HISTORY OF THE AMYLOID HYPOTHESIS
Alois Alzheimer gave a lecture in 1906 that detailed the cognitive decline and resulting neuropathology in a 51-year-old woman named Auguste D [1]. In his subsequent papers [2,3], he described the extracellular plaques which have come to be seen as a histological hallmark of AD (Alzheimer's disease). The extracellular plaques were subsequently found to have β-amyloid protein as their major component [4,5], and in the ensuing decades a massive research effort has focused on understanding the physiology of this putative disease-causing protein. Understanding the pathogenesis of AD has become particularly urgent because of the rapidly aging world population and the realization that AD causes the overwhelming majority of dementia cases in the elderly [6,7].
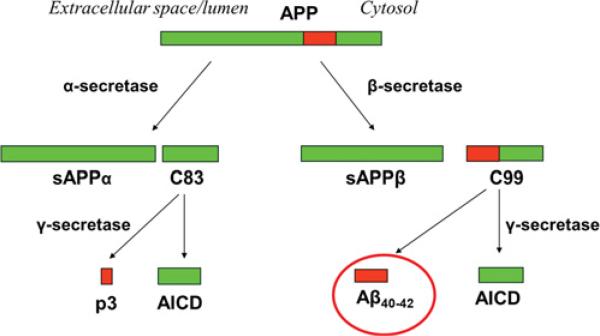
β-Amyloid protein is derived from APP (amyloid precursor protein). Initial work found that APP is capable of being processed by two different enzymes; α-secretase and BACE (β-secretase) (Figure 1; see [8] for a thorough review). The N-terminal fragments generated from this initial cleavage are called sAPP-α and sAPP-β respectively, and the C-terminal fragments generated by α-secretase and BACE cleavage are called C83 and C99 respectively. In both cases, the C-terminal fragment undergoes a subsequent additional cleavage event by an enzyme complex called γ -secretase [made up of PS (presenilin) 1 or 2, nicastrin, PS enhancer 2 and anterior pharynx defective 1]. This second cleavage results in a fragment called the AICD (APP intracellular domain), and either the p3 protein in the case of the α-secretase pathway or the β-amyloid protein in the case of the BACE pathway. Thus β-amyloid protein results from a proteolytic cascade involving multiple enzymes and associated by-products.
Figure 1. Amyloid β-protein generation by normal proteolytic processing of β-APP.
APP is shown at the top, with the β-amyloid region in red. APP can be processed through two mutually exclusive pathways. The α-secretase pathway involves an initial cleavage by α-secretase which goes through the β-amyloid region, precluding further processing that can yield β-amyloid. This initial cleavage event releases sAPP-α into the cytosol, and leaves behind the membrane-bound C83 protein. Processing of C83 by γ -secretase yields the p3 fragment and the AICD. β-Secretase cleavage of APP yields sAPP-β and C99, and subsequent processing of C99 yields β-amyloid and the AICD fragment.
Around the same time as the discovery that β-amyloid was the major component of the amyloid plaques in AD, a search began to find a genetic cause of the disease. After several decades of research, it has become increasingly clear that AD can be broken down into two types. On the one hand are cases caused by mutations that are passed down in a Mendelian fashion. These cases usually occur before the age of 65 and constitute 1–6% of all AD cases [9,10]. On the other hand are the remaining 94–99% of all AD cases, which generally occur after the age of 65 and have a weak association with a wide range of genes, most notably APOE (apolipoprotein E) [11]. Despite the fact that only 1–6% of AD cases are caused by a Mendelian mutation, the rare cases where this was true generated great excitement, as well as hope that studying the unfortunate families with these mutations would shed light on the pathogenesis of the disease in all cases. After a great deal of effort many disease-causing mutations have been found, and virtually every mutation that causes AD involves APP itself or subsequent APP processing [9]. The three main genes involved in familial AD cases are APP and the two γ -secretase components PS1 and PS2. These mutations affect APP processing in a way that either increases the overall amount of β-amyloid production or increases the relative fraction of the 42-amino-acid variant of β-amyloid protein (β-amyloid42 is the primary component of the β-amyloid plaques in AD) [9,10,12]. These discoveries fuelled the Amyloid Hypothesis, which states that β-amyloid deposition is a central event in the aetiology of AD [13]. This hypothesis (as it was originally stated), also suggests that β-amyloid deposition leads to tau pathology and cell death, and that understanding this cascade could facilitate rational drug design [13]. This world view is not only consistent with the known familial cases of AD, but also meshes nicely with the observation that patients with Down's Syndrome (trisomy 21) who live long enough will usually develop AD [14]. Since the APP gene is located on chromosome 21 (and AD is allegedly caused by increased β-amyloid levels), a gene-dosing effect could explain the phenomenon.
Subsequent studies demonstrated that the levels of β-amyloid found in AD are in fact toxic to neurons [15–17], confirming the genetic evidence. The mounting evidence in favour of the Amyloid Hypothesis also had the effect of somewhat marginalizing the tau-centric view of AD. This view focuses on neurofibrillary tangles as the aetiological agent in AD. Neurofibrillary tangles are the other pathological hallmark of AD described by Alois Alzheimer; they are intracellular (intraneuronal) flame-shaped inclusions that are relatively insoluble and are positive on silver staining. These tangles were subsequently found to be composed predominantly of tau protein [18], which normally binds to and stabilizes microtubules. The so-called ‘Tauists’ were long at odds with the ‘Baptists’ who believed in a β-amyloid-centric view of AD [19]. One of the main arrows in the Tauist quiver has always been that the pathological progression of tau-containing neurofibrillary tangles in the brain correlates with the progression of dementia much better than the relative deposition of β-amyloid plaques [20]. However, the genetic evidence from familial cases of AD is overwhelmingly in favour of the Amyloid Hypothesis. Furthermore, disease-causing mutations in tau were eventually found that cause neurodegeneration, but they do not cause AD. That is to say, mutations in tau can cause neurodegeneration with neurofibrillary tangles, but no β-amyloid plaques [21]. However, mutations that effect β-amyloid processing cause neurodegeneration with both β-amyloid plaques and tau-containing neurofibrillary tangles, identical with sporadic AD found in the general population [9]. Thus it was concluded that AD is caused by β-amyloid deposits, which cause damage upstream of tau-positive neurofibrillary tangle deposition [13].
Despite the evidence in support of the Amyloid Hypothesis, drugs that are based on this hypothesis have had a poor track record [22]. One popular strategy has been to develop compounds that modulate or inhibit γ -secretase; the idea is to inhibit the production of β-amyloid at the final cleavage step (see Figure 1). This strategy has gained particular popularity given the historical difficulty in developing good BACE inhibitors [23]. Semagacestat is so far the most well-known γ -secretase inhibitor to have been studied extensively in AD trials [24]. Semagacestat can reduce CNS (central nervous system) β-amyloid production in healthy volunteers in a dose-dependent manner [25]. However, two large Phase III trials with over 2600 participants were recently discontinued after failure to demonstrate efficacy. Compared with placebo, patients receiving semagacestat actually did worse both in cognition and in daily function, and were at a higher risk of developing skin cancer [26].
Another strategy using the Amyloid Hypothesis has focused on inhibiting oligomer formation. Recent evidence suggests that the neurotoxic form of β-amyloid is soluble oligomers of β-amyloid, rather than either monomers or the fibrillar β-amyloid found in plaques [27–29]. Thus compounds have been explored that inhibit or destabilize oligomer formation. In pre-clinical studies, tramiprosate reduced plaque levels in the mouse brain and plasma by preventing the β-amyloid conformational changes necessary for oligomer and fibril production [30]. However, in a Phase III study, 1052 AD patients showed no overall significant differences on the ADAS-cog (AD assessment scale-cognitive), although there was some improvement on individual cognitive measures [31]. Other compounds are currently being developed that inhibit β-amyloid aggregation, notably scyllo-inositol. This compound reduces brain β-amyloid concentrations and plaque burden, preserves synaptic density, and improves learning deficits in transgenic animals [32,33], but a recent Phase II study revealed no significant differences between the treatment groups and the control group on two measures of cognitive function [34]. However, another aggregation inhibitor (PBT2) has also completed a recent Phase II trial, and patients taking this compound did show significant improvements in two measures of executive function [35]. Thus the anti-aggregation strategy may still hold promise.
Finally, clearance of β-amyloid via the immune system is currently a popular strategy. There are two ways to do this; active immunization using an epitope that generates an immune response to β-amyloid, or passive immunization using direct administration of anti-β-amyloid antibodies. The active immunization approach has lost some popularity after an early-stage clinical trial found meningoencephalitis in the vaccinated patients [36]. There are two ongoing passive immunization trials in the late stage of Phase III testing, Bapineuzumab and Solanezumab; both studies have Phase III results that can be expected at some point in the next year [37–39]. Solanezumab has so far shown some toxicity advantages to Bapineuzumab, in so far as Bapineuzumab has caused cerebral oedema and micro-haemorrhages in some patients, whereas these toxic effects are not seen in Solanezumab. This difference may be due to the fact that Solanezumab preferentially binds soluble β-amyloid, whereas Bapinezumab also binds fibrillar β-amyloid. This predilection of Bapinezumab for fibrillar β-amyloid may cause bleeding because the antibodies will not only react to β-amyloid plaques, but also to cerebral vessels affected by cerebral amyloid angiopathy (a common vasculopathy in AD patients where β-amyloid is deposited in cerebral blood vessels). In any case, in terms of therapeutic efficacy, the jury is still out on both therapies, as neither one has shown efficacy in Phase II trials. In a Phase II trial of Bapinezumab, no significant differences were found in the cognitive status of the treatment compared with the control group, although exploratory analyses showed a potential effect on cognitive and functional endpoints in study ‘completers’ and APOEε4 non-carriers [37]. Solanezumab has also shown no significant cognitive change compared with the controls in a Phase II trial, although this trial may not have been sufficiently powered for efficacy [40]. In any case, the Phase III trial data will be in soon for both compounds.
A CLOSER LOOK AT β-AMYLOID
When do β-amyloid levels increase in AD patients?
Is β-amyloid accumulating in the brain during a prodromal (asymptomatic) early phase of AD? This question is particularly relevant for therapies that target β-amyloid, as these therapies may fail because they are given too late in the disease course. However, they may be quite effective early on when β-amyloid is accumulating and initiating damage in the nervous system. One of the initial roadblocks with answering this question is defining what ‘early AD’ is. Of course, to answer this question, one must first define what AD itself is. Although this may seem relatively straightforward, the task is made difficult by the fact that memory can decline for a variety of reasons during aging, and that elderly people can have β-amyloid pathology in the absence of any cognitive impairment [41]. These facts have long confounded the Alzheimer's field, and made it difficult to draw a clear line between ‘normal aging’ and ‘AD’. Nevertheless, in 1984, clinical guidelines for AD were established by a joint commission of the NINCDS [National Institute of Neurological and Communicative Disorders and Stroke; now NINDS (National Institute of Neurological Disorders and Stroke)] and the ADRDA (Alzheimer's Disease and Related Disorders Association; now the Alzheimer's Association) [42]. These guidelines state that for a diagnosis of ‘probable’ AD, one must have a clinical diagnosis of dementia, with deficits in two or more areas, an onset after the age of 40, a gradual decline in memory and no alteration in consciousness or evidence of some other medical condition that could cause the observed deficits in cognition. The term ‘definite’ AD was reserved for people with autopsy-confirmed plaques and tangles, so that ‘AD’ was defined as a clinical-pathological entity (i.e. both dementia in life and the characteristic pathology at death is required for the diagnosis). The clinical criteria to diagnose probable AD continued to be refined over the years by additional expert panels, including an early panel organized by the NIA (National Institute on Aging) [43], a panel organized by the CRAD (Consortium to Establish a Registry for AD) [44], and a panel organized by the NIA and the Alzheimer's Association (the NIA-Reagan Institute criteria) [45]. The original 1984 guidelines are largely still used, with a recent update that incorporates research into AD biomarkers [46]. Despite the difficulty of clinically diagnosing AD, the 1984 clinical guidelines correlate well with post-mortem Alzheimer's pathology (plaques and tangles), with sensitivity and specificity both approaching 90%, depending on the threshold of pathology and the strictness of exclusion criteria for vascular pathology [47]. However, this still provides a substantial 10% window for patients to be falsely labelled (or not labelled) with AD. Furthermore, the diagnostic success implied by these figures should be tempered by the fact that up to 70% of all dementia in the elderly is thought to be related to AD pathology [7].
If defining AD is difficult, defining early (or non-demented) AD is even more so. It has long been recognized that elderly people can develop cognitive impairment that does not meet the formal definition of dementia, and the concept of MCI (‘mild cognitive impairment’) was originally developed to describe this phenomenon [48,49]. Patients with the ‘amnestic’ variant of MCI (where memory is affected) progress to AD at a rate of 10–15% per year [50,51], so the concept does have some clinical relevance. Nevertheless, the diagnosis of MCI is somewhat subjective, as it relies heavily on self-reporting by the patient (or reporting by a relative/family member). In addition, MCI may be diagnosed by a clinician if subtle cognitive impairment is found in an elderly patient that is more than would be expected given the patient's age and education level. However, strict criteria for diagnosing MCI by a clinician remain to be formally established {although a recent NIA-AA (Alzhemier's Association) workshop has made substantial progress on this issue [52]}.
In any case, given our current understanding of AD and MCI, is there evidence of early β-amyloid accumulation before cognitive symptoms develop? Up until very recently it has been difficult to directly address this issue, as the only way to assess β-amyloid content in the brain has been at autopsy. Although it has long been known that β-amyloid pathology can exist in non-demented people, one would ideally like to show the initial development of β-amyloid pathology followed by the development of dementia in the same person (i.e. β-amyloid pathology is predictive of dementia). In lieu of a brain autopsy, living AD patients in the past have had CSF (cerebrospinal fluid) drawn and levels of β-amyloid and tau measured. In patients already suffering from AD, measured CSF levels of β-amyloid42 are actually lower than normal using a variety of methods [53], for reasons that are unclear. Traditionally, the decrease in β-amyloid42 levels has been thought to be due to the fact that β-amyloid is forming fibrils and depositing in plaques, thus ‘removing’ β-amyloid from the CSF [53]. This can't be the whole story, however, because in AD, brain tissue has increased levels of both soluble and insoluble levels of β-amyloid (including β-amyloid42) [54]. In any case, CSF tau levels increase in AD [55]. The increase in tau is not very specific for AD, as CSF tau levels also increase in other neurodegenerative diseases {such as CJD (Creutzfeldt–Jakob disease) [56]} as well as in stroke [57]. Thus increased CSF tau is generally regarded as a marker of neuronal injury in a variety of diseases. More specific for AD are the CSF levels of phosphorylated tau, which are also increased in AD, but not in stroke or CJD [53,57,58].
What about MCI? These patients also show low levels of β-amyloid, and recent evidence has shown that low β-amyloid CSF levels not only predict the development of AD, but also may precede the increase in CSF phosphorylated tau levels [53,59–62]. This supports the Amyloid Hypothesis world-view that β-amyloid pathology is an upstream event, which subsequently leads to tau hyperphosphorylation and neurodegeneration.
Recently radiological compounds have been developed that allow one to visualize fibrillar β-amyloid in the brain, most notably PiB (Pittsburg compound B) [63]. This technology has shown the early development of β-amyloid pathology, both in MCI as well as in healthy controls [64–66]. Interestingly, radiological evidence of β-amyloid pathology is significantly higher in patients with amnestic MCI than in patients with nonamnestic MCI (i.e. cognitive impairment not related to episodic memory), consistent with the idea that amnestic MCI is a prodromal stage of AD [64,65,67] (remember that patients with the ‘amnestic’ variant of MCI progress to AD at a rate of 10–15% per year [50,51]). In addition, the degree of radiological β-amyloid pathology correlates with the chance of progressing from normal cognition to MCI, or from MCI to AD [64–66], despite the fact that fluctuations in radiological β-amyloid pathology do not correlate very well with cognitive status [68]. This is consistent with the view that elevations in β-amyloid are an early event in the pathogenesis of this disorder, and that subsequent changes in β-amyloid levels do not affect cognition, especially late in the disease.
In summary, studies with CSF and β-amyloid-binding radiological compounds largely support the Amyloid Hypothesis. However, they suggest that β-amyloid may play an important role early in the disease, before the development of dementia. This has important implications for therapeutics based on the Amyloid Hypothesis, as these drugs may have their maximal effect if administered at these early stages. To do this effectively will require reliable ways to diagnose this ‘early’ form of AD (before dementia sets in). Such a goal is being pursued, but has not yet been achieved. One should also keep in mind that even if β-amyloid has an ‘early’ role in AD from a clinical perspective, it is not necessarily the ultimate ‘cause’ of the disease. Indeed, there could be many additional factors upstream of β-amyloid that are influencing the ultimate course of the disease. For example, we should consider the fact that there could be factors that are playing a role decades before the development of symptoms, similarly to the role of trauma in the development of chronic traumatic encephalopathy [69].
An additional problem one must consider with β-amyloid-lowering drugs is that β-amyloid results from a proteolytic cascade involving multiple enzymes and associated by-products, which in turn affect a multitude of cellular processes. Thus any therapeutic intervention that affects β-amyloid levels may have an undesirable side effect as a result of affecting one of these processes. To give a sense of the complexity of this situation, we will now briefly summarize the role of a selection of the enzymes and by-products involved in the generation of β-amyloid which may have a role in complicating β-amyloid-based therapeutic strategies.
γ -Secretase
The γ -secretase inhibitors have figured prominently in drug development, and as such, it is worthwhile taking a moment to review γ -secretase physiology. The vast literature on γ -secretase will not be fully discussed in the present review for the sake of brevity (see [70] for a more extensive review), but a couple of key features are worth noting. First of all, the γ -secretase complex is involved in processing a wide range of substrates other than APP; at least 90 different substrates are currently known, including APP-like proteins 1 and 2, as well as E-cadherin, neuregulin-1 and 2, Notch, RAGE (receptor for advanced glycation end-products), and ApoER2 (ApoE receptor 2) [71]. Not surprisingly, a vast range of cellular processes are affected by the substrates of γ -secretase, including functions regulating cell fate, adhesion, migration, neurite outgrowth and synaptogenesis. Despite this diversity, the targets of γ -secretase often have several things in common. First, most are single-pass transmembrane proteins, with a large ectodomain and a cytoplasmic C-terminus that often plays a role in intracellular signalling. Secondly, the targets of γ -secretase themselves are often involved in intracellular signalling in a wide range of events, including events related to neurite outgrowth and synapse formation that are disrupted in neurodegeneration in AD [71–73]. Finally, γ -secretase often targets membrane-bound proteins after cleavage and release of the ectodomain fragment by a prior enzyme [71,74].
Perhaps the most well-known (and problematic) alternate substrate of γ -secretase is Notch. Notch is a single-pass transmembrane protein found in a variety of tissues [75,76]. The extracellular domain on Notch binds to a Notch ligand (which is usually on another cell), and this triggers cleavage of Notch by ADAM10 (a disintegrin and metalloprotease 10) and shedding of the Notch ectodomain into the extracellular space. This is then followed by γ -secretase cleavage of the remaining membrane-bound protein and release of the NICD (Notch intracellular domain) into the cytoplasm, where it translocates to the nucleus and influences gene transcription (note the similarity to APP processing). Notch has been found to be involved in a wide range of different processes involving cell proliferation, cell death, and acquisition of specific cell fates [75,77,78]. Notch has also been tied to a wide range of disorders, such as developmental disorders [76,79,80], vascular disease {CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) [81]} and cancer [82]. Thus it is perhaps not surprising that γ -secretase inhibition may have many side effects just through affecting Notch function alone. One possible strategy may be to develop Notch-sparing inhibitors of γ -secretase that preferentially inhibit the ability of γ -secretase to produce β-amyloid without affecting Notch cleavage [70]. Recently, a novel γ -secretase-activating protein has been discovered that increases β-amyloid production through interaction with both γ -secretase and its substrate [83]. Notably, this γ -secretase-activating protein does not affect the interaction of γ -secretase with Notch. Thus this raises the possibility that one may develop a Notch-sparing γ -secretase inhibitor by inhibiting this activating protein. The same paper demonstrated that the drug imatinib was capable of lowering β-amyloid levels specifically through inhibiting the interaction of this γ -secretase activating protein with its substrate. Thus this research presents one possible way to develop a Notch-sparing γ -secretase inhibitor. It is important to keep in mind, however, that despite the focus of the drug-discovery community on Notch-sparing γ -secretase inhibitors, any γ -secretase inhibitor could potentially have adverse side effects due to the involvement of γ -secretase with any of the other 90 substrates it interacts with.
AICD fragment
The AICD has been linked to a number of cellular processes [84,85]. These include the modulation of intracellular calcium and ATP [86], as well as the regulation of cytoskeletal dynamics and intracellular trafficking [87]. AICD has also been linked to the regulation of cell-death pathways [88–90], notably through activation of FADD (Fas-associated death domain protein) [91]. In addition, AICD mediates cell death through the p53 pathway [92,93]. Notably, AICD has been shown to directly increase transcription and production of p53 [93], and AICD-mediated cell death has been shown to be impaired by p53 deficiency [92]. The AICD fragment has also been implicated in the regulation of β-amyloid, primarily through the regulation of neprilysin, one of the main β-amyloid-degrading enzymes [94,95]. Recent evidence suggests that the AICD fragment binds to the promoter region of neprilysin, and cells depleted of APP or γ -secretase components PS or nicastrin show a depleted neprilysin mRNA and protein levels that is reversible upon complementation by PS, nicastrin or AICD [96,97]. Thus inhibiting γ -secretase may have side effects from disrupting the normal physiology of the AICD fragment, one of which is (ironically) the down-regulation of one of the prime pathways for β-amyloid clearance.
BACE
Although no BACE inhibitors have made it to late-stage clinical trials yet for a variety of reasons, BACE presents an obvious target to inhibit the production of β-amyloid, and several ongoing early-stage clinical trials are employing this strategy [23]. As such, it is worth reviewing the fact that ongoing research suggests that BACE may have a large number of substrates other than APP. A screen for novel substrates of BACE1 (the predominant BACE isoform in the brain) identified 68 putative BACE substrates [98]. Most of the potential targets identified by this screen are type I transmembrane proteins, and most are involved in contact-dependent intercellular communication. Although the majority of these targets have not yet been validated (as noted in [99]), a few have been experimentally tested, including APLP (amyloid β precursor-like protein) 1 and APLP2 [100–102], low-density lipoprotein receptor-related protein [103] and the voltage-gated sodium channel β-subunits [104,105]. BACE1 has also been shown to be involved in the regulation of myelination via cleavage of neuregulin-1 [106,107] and neuregulin-3 [108]. Thus putative BACE inhibitors may run into many of the same challenges that have plagued γ -secretase inhibitors, although compounds inhibiting BACE are in a far earlier stage of development. However, despite some initial setbacks, the limited available human data from clinical trials on BACE inhibitors suggests that this strategy may present an exciting additional pathway to treat AD [109].
sAPPβ
The sAPPβ fragment has not been particularly well studied and, as such, not as much is known about it [110]. However, BACE inhibitors would obviously decrease sAPPβ levels, so it is worth briefly considering what is known about this protein. So far, sAPPβ has been shown to promote axonal outgrowth [111]. In addition, sAPPβ has been shown to robustly drive neuronal differentiation of human embryonic stem cells [112]. Thus sAPPβ may be important for neural growth and differentiation. More work is needed to understand how inhibiting the production of sAPPβ might affect patients using BACE inhibitors.
β-Amyloid
Finally, what is the normal physiological role of β-amyloid? Would depleting the brain of β-amyloid below a certain level have a detrimental effect in any way? This is an often overlooked problem, as the Amyloid Hypothesis often assumes that β-amyloid is a toxic protein that needs to be cleared. However, β-amyloid is produced in the brain throughout life, and the normal in vivo concentration in the rodent brain has been estimated to be in the picomolar range [113]. Therefore the normal physiological role of β-amyloid should be understood if β-amyloid-lowering therapy is going to be clinically pursued. Unfortunately, studies regarding the physiological function of β-amyloid peptides have been limited. For many years, most researchers assumed that β-amyloid peptides had no function, a sort of ‘waste’ byproduct of the APP processing with the exception of a few studies. Picomolar levels of exogenously applied β-amyloid40 have been found to play a neurotrophic role in cell cultures [15,114], and treatment of hippocampal neural stem cell progeny with β-amyloid42 induces an increase in the number of newly generated neurons [115]. β-Amyloid levels are likely to be regulated by synaptic activity [116–119]. In systems overexpressing familial AD mutant APP, this release of β-amyloid has the capacity to depress synaptic function [118]. However, in non-transgenic systems, β-amyloid release may up-regulate synaptic activity [119–121]. Moreover, the brain interstitial fluid concentration of β-amyloid seems to be correlated with neurological status, with β-amyloid concentrations increasing as neurological status improves and vice versa [122]. Previously, we have demonstrated that low picomolar amounts of exogenously applied β-amyloid42 enhance synaptic plasticity and memory [121]. Specifically, we have shown that low picomolar concentrations of β-amyloid42 (similar to the concentrations normally found in the brain) cause a marked increase in hippocampal long-term potentiation, whereas high nanomolar concentrations lead to the well-established reduction of synaptic plasticity. In addition, we have shown that picomolar levels of β-amyloid42 produce a pronounced enhancement of both reference and contextual fear memory. In subsequent studies, we have demonstrated the role of endogenous β-amyloid by showing that both anti-rodent β-amyloid antibody and siRNA (small interfering RNA) against murine APP reduced LTP (long-term potentiation) as well as contextual fear memory and reference memory, and that these effects are rescued by the addition of human β-amyloid42. This work has been extended by Puzzo et al. [123] who have demonstrated that β-amyloid42 acts on hippocampal LTP and reference memory in a biphasic dose–response curve. In other words, low doses enhance synaptic plasticity and memory, and high doses inhibit plasticity and memory, similar to many other hormones that affect memory. Collectively, these findings strongly support a model for β-amyloid in which low concentrations play a positive modulatory role on neurotransmission and memory, whereas high concentrations play the well-known detrimental effect culminating in dementia. This positive role for β-amyloid may complicate therapeutic strategies that reduce β-amyloid if the normal physiological function of this protein is disturbed.
AMYLOID TOXICITY AT THE SYNAPSE: A SHORTER ROAD TO VICTORY?
Given that high concentrations of β-amyloid have been shown to cause synaptic and memory dysfunction [27], and given that dementia is ultimately the key clinical impairment in patients with AD, one might ask whether addressing synaptic dysfunction directly might not be the best therapeutic approach. This is the approach that our laboratory has taken over the past decade. It is a relatively simple straightforward solution. First, we identify molecular pathways involved in synaptic plasticity that are disrupted by high concentrations of β-amyloid, and then we intervene to rescue these pathways. Although this approach is consistent with the Amyloid Hypothesis, it is ultimately agnostic on whether high β-amyloid levels actually cause AD, or whether high β-amyloid levels are simply a component of AD that contribute to dysfunction. Our approach also side-steps the issue of how to distinguish AD from normal aging (see above). Any brain that has memory impairment caused by high levels of β-amyloid will benefit from our approach, regardless of whether or not the patient formally has AD. On the downside, this approach may not produce a total ‘cure’ of the disease, as rescuing synaptic function will probably not completely halt the progression of degeneration. However, there is mounting evidence that early synaptic dysfunction may contribute to degeneration [124–127], so rescuing synaptic dysfunction may not only produce symptomatic relief, but may also slow the course of the disease. Furthermore, although there is a gradual physical loss of synapses in AD [128], recent studies on animal models have highlighted a discrepancy between behavioural deficits and neuropathological findings. Specifically, electrophysiological studies on mice that overexpress β-amyloid show impairment of LTP that does not correlate with the extent of synaptic loss, amyloid deposition or cell death [129–131]. In addition, animals without amyloid plaque deposition have been reported to have behavioural deficits [132,133]. This is consistent with the idea that β-amyloid itself is disruptive to synaptic physiology even in physically intact synapses, and gives further support to our approach. In any case, a complete cure will require a full understanding of the pathogenesis of AD, and this goal has yet to be achieved. Furthermore, even if β-amyloid turns out to be the causative agent in AD, developing drugs that lower β-amyloid load in the brain may be difficult to develop due to side effect issues, and as we have already argued, they will probably need to be given early in the course of the disease to be effective. For all of these reasons, we believe that rescuing synaptic dysfunction is a viable and straightforward approach to developing therapies for AD where clinical success may be easier than strategies based on lowering β-amyloid levels. We will now describe some of our efforts along these lines. The results of these studies are summarized in Figure 2.
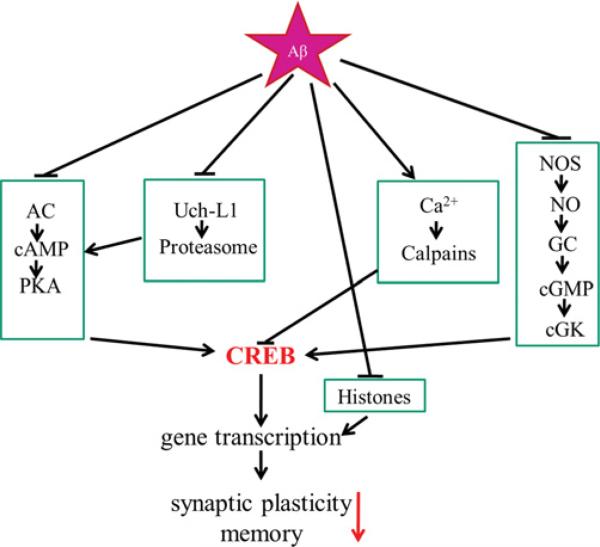
Figure 2. β-Amyloid can inhibit synaptic plasticity through a variety of mechanisms.
CREB phosphorylation may be inhibited through inhibition of the PKA pathway, the Uch-L1 proteasomal system, aberrant calpain activity, or inhibition of the cGK pathway. In addition, high β-amyloid levels may reduce histone acetylation, which can impair gene expression necessary for learning and memory. See the text for details.
cAMP/PKA (protein kinase A)/CREB (cAMP-response-element-binding protein) cascade
LTP is a form of synaptic plasticity that is traditionally broken down into several components, where the early component is independent of protein synthesis, and the late component (long-term maintenance) is dependent on gene expression and new protein synthesis [134]. The details of the biochemical pathway mediating the switch from early to late LTP have been worked out in aplysia and mice [135], and depend on the activation of the transcription factor CREB by phosphorylation by PKA. This pathway is well preserved across species, functioning in olfactory memory in Drosophila as well as hippocampus-dependent memory in mice [136,137]. We have found that nanomolar concentrations of β-amyloid42 (a concentration well below that required to induce cell death) causes a rapid and sustained decrease in the activity of PKA in cultured hippocampal neurons and a rapid inhibition of CREB phosphorylation in response to glutamate stimulation [138]. Furthermore, rolipram and forskolin, agents that increase the intracellular levels of cAMP, reverse this inhibition, most probably by favouring the dissociation of regulatory and catalytic subunits of PKA and the restoration of PKA activity. This reversal is blocked by H89, an inhibitor of PKA.
On the basis of these findings, we asked whether rolipram could exert beneficial effects in the brains of mice carrying both the mutant APP (K670N,M671L) and PS1 (M146L) transgenes (APP/PS1 mice), an animal model of amyloid deposition that partially reproduces the cognitive deficits that occur in AD patients [139,140]. These mice display impaired LTP, spatial working memory and contextual learning as early as 3–4 months of age, and they show deficits in basal synaptic transmission and spatial reference memory after 5–6 months of age [141]. We found that the impairment in LTP in APP/PS1 slices was rescued by a 20 min rolipram perfusion prior to LTP induction. We also found that rolipram injections improve contextual fear conditioning (a form of hippocampal-dependent fear memory) and spatial working memory in 3-month-old APP/PS1 mice. In addition, daily injections of rolipram for 3 weeks in 3-month-old APP/PS1 mice continue to show benefits as the mice age; for example, improvement in the radial-arm water maze and Morris water maze performance (measurements of hippocampal-dependent spatial memory) that are still sustainable at 6–7 months. Furthermore, LTP impairments in hippocampal slices also remain rescued at 7–8 months of age. These findings are particularly exciting, as they suggest that the beneficial effect of rolipram administration can be extended beyond the duration of the administration, possibly due to stabilization of synaptic circuitry via alterations in gene expression and activation of the cAMP-dependent protein kinase (PKA)/CREB signalling pathway. Consistent with this hypothesis, daily injections of rolipram for 3 weeks in 3-month-old APP/PS1 mice reverse the decrease in CREB phosphorylation both in hippocampal extracts and in hippocampal slices from the same mice at 7–8 months of age.
Is there any downside to chronically increasing CREB phosphorylation? It is important to note that memory can actually be impaired if CREB activity is increased too much [142]. Furthermore, CREB itself is involved in a wide range of other physiological processes [143] and CREB overexpression has been linked to leukaemia [144]. This has led some to question a therapeutic strategy based on elevating CREB activity [143]. These are important points. However, they are unlikely to contradict the strategy we described in the present review. This is because our goal is not to elevate CREB activity to abnormally high levels, but rather to return it to its normal level of activity (i.e. to rescue the decrease in CREB activity seen in AD).
NO/sGC (soluble guanylate cyclase)/cGMP/cGK (cGMP-dependent protein kinase)/CREB cascade
Hippocampal and cortical LTP have both been shown to involve nitric oxide (NO) [145,146]. NO is a membrane-permeable gas that may be involved in CREB phosphorylation [147–149]. NO is synthesized from L-arginine by the enzyme NOS (NO synthase) and induces cGMP production through the activation of the enzyme sGC [150]. cGMP, in turn, activates cGKs, a family of signal transduction mediators [151], leading to an increase in CREB phosphorylation during LTP [149]. To determine whether NO plays a beneficial effect on β-amyloid-induced reduction of LTP, we used the NO donor DEA/NO (diethylamine NO) and found that it rescues LTP impairment in hippocampal slices perfused with β-amyloid42 [152]. We then explored several steps downstream of NO production. The following compounds all rescued LTP impairment in hippocampal slices perfused with β-amyloid42: BAY41–2272 (a sGC stimulator), and both 8-Br-cGMP and 8-pCPT-cGMP (cGMP analogues). Another important finding of our experiments was that the increase in cGMP levels, normally occurring immediately after tetanic stimulation [153], is blocked in β-amyloid42-treated slices. This finding indicates that β-amyloid42 directly interferes with the NO/cGMP/cGK/CREB pathway to suppress LTP.
We also showed that β-amyloid42 blocks an increase in CREB phosphorylation during LTP, and that application of an NO donor, an sGC stimulator, or cGMP analogues re-establishes normal levels of phospho-CREB during potentiation in a high β-amyloid environment. Most importantly, the effect of cGMP analogues on CREB phosphorylation and LTP was specifically mediated through cGK, because treatment with the cAK (cAMP-dependent protein kinase) inhibitor KT57230 did not suppress the beneficial effect of cGMP analogues, as occurred with the cGK inhibitor KT5823. Our study suggests that intervention using NO donors or drugs enhancing cGMP levels may ameliorate cognitive deficits in patients affected by AD and other neurodegenerative diseases characterized by abnormal production of β-amyloid42.
The PDE5 (phosphodiesterase 5) inhibitor sildenafil (popularly known as Viagra) enhances phosphorylation of CREB through elevation of cGMP levels. We were curious to see whether this drug could rescue synaptic plasticity and memory in APP/PS1 mice [154]. We found that: (i) a 10 min perfusion of 3-month-old APP/PS1 mouse hippocampal slices with sildenafil reverses CA (cornu ammonis) 1 LTP impairment; (ii) a single intraperitoneal injection of sildenafil in APP/PS1 mice can fully rescue associative memory and partially rescue spatial working memory; and (iii) daily injections of sildenafil for 3 weeks in 3-month-old APP/PS1 mice improve both associative and spatial memory (both short- and long-term) in 6–8-month-old APP/PS1 mice, and also rescues LTP in hippocampal slices from these same mice. Thus the therapeutic effects of sildenafil (like rolipram) persist long after the drug has been given. We also found that sildenafil re-establishes the normal increase in CREB phosphorylation after tetanic stimulation in APP/PS1 mice, and also reduces β-amyloid levels in these same mice. Sildenafil is currently on the market (Viagra), and its relatively mild side-effect profile is well known. Thus this compound or, even better, other compounds with PDE5-inhibitory activity and improved toxicity profile and pharmacokinetic characteristics are excellent candidates for therapeutic intervention in AD. Our laboratory is currently developing novel PDE5 inhibitors that are highly blood–brain barrier permeant and are tailored for the chronic administration to a senile population.
Calpains
At least 14 mammalian calpains, cytosolic calcium-activated cysteine proteases, have been identified [155]. Two major forms, calpain 1 and calpain 2, also known as μ-calpain and m-calpain, have been linked to AD [156]. Calpain 1 is the form most concentrated in synapses [157] and is abnormally activated in AD brain [158]. Calpastatin, the endogenous inhibitor of calpains, is significantly decreased in AD as well [156]. The activated form of calpain 2 is increased in neurites of neurons at risk for developing neurofibrillary pathology and is extensively bound to neurofibrillary tangles in brains of AD patients [159]. Calpain overactivation is triggered by abnormally high calcium levels; this and calpastatin depletion leads to limited cleavage or degradation of key neuronal proteins in AD [156,160]. Indeed, calpains modulate the trafficking and, indirectly, the proteolytic processing of the APP [161–165]. Moreover, calpains influence the phosphorylation and proteolysis of tau (the major component of neurofibrillary tangles) [155,160]. Other calpain substrates affected in AD include CaMK-IIα (CaM-kinase IIα) and PKC (protein kinase C) [166–168], second-messenger-related enzymes such as phospholipase C-1, -2 and -β3 [169], and Cdk-5 (cyclin-dependent kinase 5) [170], transcription factors such as c-Jun, c-Fos and IκB (inhibitor of nuclear factor κB) [171,172], and CREB [138,152,173]. Calpains also regulate cytoskeletal proteins such as spectrin [174] and MAP2 [175] and, through direct proteolytic actions and indirect modulatory effects on several protein kinases [PKC, ERK1/2 (extracellular-signal-regulated kinase 1/2), CaMK-II and Cdk-5/p35], play a key role in regulating the dynamic behaviour and turnover of cytoskeletal proteins, especially those in synapses where calpain concentrations are high [157]. Recently, calpain actions on the GluR1 subunit of AMPA receptors [176], amphiphysin I [177] and suprachiasmatic nucleus circadian oscillatory protein [178], have been shown to modulate synaptic activity and memory formation.
Given the role of deranged calpain activity in synaptic dysfunction in AD, we investigated the role of calpain inhibition on synaptic function and memory in APP/PS1 mice [179]. We found that the cysteine protease inhibitor E64 and the more specific calpain inhibitor BDA-410 re-established normal synaptic function [mEPSC (miniature excitatory postsynaptic current) frequency] in APP/PS1 mouse hippocampal cultures, rescued LTP in APP/PS1 hippocampal slices, and rescued associative memory, spatial working memory and long-term spatial memory in APP/PS1 mice. Calpain inhibition also re-established the increase in CREB phosphorylation during synaptic plasticity in APP/PS1 mice and produced a normal distribution of the synaptic protein synapsin I. Thus calpain inhibition is another method of therapeutic intervention that restores synaptic function in AD. To this end, our laboratory is synthesizing specific calpain inhibitors tailored to CNS diseases and chronic administration to a senile population.
Other targets
Uch-L1 (ubiquitin C-terminal esterase L1)
The brains of AD patients show an accumulation of ubiquinated proteins [180], suggesting inhibition of the protein degradation machinery. Interestingly, Uch-L1, a neuron- and testis-specific enzyme, is down-regulated in AD brains. AD brains show prominent Uch-L1 immunostaining associated with neurofibrillary tangles, and levels of soluble Uch-L1 are inversely proportional to the number of tangles [181]. A possible link between Uch enzymes, memory and AD is suggested by the requirement for Uch in long-term facilitation in Aplysia [182], by reports of decreased levels of Uch-L1 in post-mortem AD brain [181], and by β-amyloid aggregates and intraneuronal ubiquitin deposits in gad (gracile axonal dystrophy) mice that lack Uch-L1 activity [183,184]. We have investigated the role of Uch-L1 reduction in synaptic dysfunction in AD [185]. We have shown: (i) a reduction of soluble Uch-L1 protein levels in the hippocampus of APP/PS1 mice; (ii) that inhibition of Uch-L1 activity leads to the inhibition of hippocampal LTP; and (iii) that transduction of Uch-L1 protein restores LTP in APP/PS1 mice and also reestablishes normal Uch activity, basal neurotransmission and synaptic plasticity, and improves associative memory in mice. Uch-L1 has also been shown to be associated with Parkinson's disease. Lansbury and colleagues have shown that C-terminal farnesylation promotes the association of Uch-L1 with cellular membranes, such as the endoplasmic reticulum, and that the levels of this membrane-associated Uch-L1 correlate with α-synuclein pathology in transfected cells [186]. Furthermore, a reduction in membrane-associated Uch-L1 levels with a farnesyltransferase inhibitor reduces α-synuclein levels and increases cell viability in cell culture, suggesting that this may be a successful therapeutic approach in Parkinson's disease and other synucleinopathies. It is unclear exactly how membrane-associated Uch-L1 promotes α-synuclein pathology, although one hypothesis may be that membrane association reduces the pool of soluble Uch-L1 that is involved in α-synuclein degradation.
We have previously proposed a model where a fall in cAMP shifts the equilibrium of the PKA complex towards the inactive form [bound to the PKA-R (PKA regulatory subunit) protein], resulting in decreased CREB phosphorylation and transcription [138]. We asked whether the effect of Uch-L1 on β-amyloid-induced synaptic dysfunction is associated with this PKA complex equilibrium. Our hypothesis is that Uch-L1 degrades the PKA-R protein, freeing up PKA to activate CREB. Therefore, when Uch-L1 levels fall, PKA-R is not degraded, PKA is sequestered and CREB phosphorylation is impaired. We first treated hippocampal slices with 200 nM β-amyloid for 20 min and found PKA activity reduced by 30%. Pre-treatment with 20 nM Uch-L1 for 1 h before applying β-amyloid blocked the reduction. Furthermore, PKA-RIIa subunit levels in hippocampal slices were increased after exposure to β-amyloid, and this increase was blocked by pretreatment with Uch-L1. Finally, treatment with β-amyloid blocks the increase in phosphorylated CREB induced by theta burst in hippocampal slices; this inhibition is reversed by Uch-L1 pretreatment. Later work has demonstrated that Uch-L1 treatment can restore spine density to near control conditions in mouse models of AD, and that this effect is seen even in elderly mice [187]. Thus up-regulating Uch-L1 activity is another possible therapeutic strategy for rescuing synaptic function, both in AD and in other neurodegenerative diseases [188]. Although the above discussion focuses on Uch-L1, the ubiquitin–proteasome system is widely studied as a target for therapeutic intervention in AD, such as recent work by Pasinetti and colleagues showing that this system may regulate levels of BACE in AD [189].
Epigenetic mechanisms
The use of HDAC (histone deacetylase) inhibitors in rodents has demonstrated the role of chromatin modification in the transcriptional regulation of processes underlying memory. A cursory review of the literature reveals that HDAC inhibition can increase both learning and hippocampal LTP in association with a selective increase in histone acetylation [190–193]. Also, in neuropathological models, a role for the epigenetic modulation of memory and learning has been proposed [194]. Specifically, levels of CBP (CREB-binding protein; a histone acetyltransferase driven by CREB that is involved in learning and memory) are reduced in mice lacking functional PS1 and PS2 [195]. Moreover, wild-type PS1 stimulates CBP transcriptional ability, whereas a PS1 mutant does not have this effect [196]. Additionally, CBP overexpression ameliorates memory deficits in a mouse model of AD [197]. Most importantly, we have found that Aβ reduces hippocampal levels of CBP and PCAF (p300/CBP-associated factor), two HATs (histone acetyltransferases) that are relevant to memory formation [191,198,199].
We have shown that a brief HDAC inhibition enables the recovery of impaired memory in APP/PS1 mice through histone epigenetic modification [200]. Specifically, we observed that APP/PS1 mice display a reduced endogenous level of H4 acetylation after a learning task. Nevertheless, the acute exposure to a class I/II HDAC inhibitor, TSA, rescued contextual spatial learning and hippocampal CA3–CA1 LTP, as well as H4 acetylation in APP/PS1 mice. Our results suggest that these effects from TSA are likely to be mediated, at least in part, by the re-elevation in H4 acetylation (hyperacetylation), which might be low as a consequence of either reduced endogenous HAT activity or increased endogenous HDAC activity following overexpression of the APP and PS1 transgenes. In addition, inhibition of HDACs, particularly HDAC2, also reinstates learning and long-term memory in other mouse models of neurodegeneration [201–203]. Thus these findings demonstrate a possible epigenetic component to synaptic dysfunction in AD.
CONCLUSION
The Amyloid Hypothesis of AD is supported not only by genetic evidence in familial cases, but is also supported by our increasingly sophisticated understanding of the pathogenesis of AD in non-familial cases. Although it is true that amyloid plaques do not correlate well with the course of the disease at later stages, various biomarkers do suggest that amyloid pathology correlates with progression at earlier stages of the disease. This is in keeping with our understanding of the role of β-amyloid in disease pathogenesis. Nevertheless, therapies based on the Amyloid Hypothesis have a poor track record to date. We believe that several impediments need to be overcome before β-amyloid-lowering therapies will be effective (namely, identifying patients at the early stage of the disease and achieving lower β-amyloid levels without deleterious side effects). In addition, we believe that further study is warranted on whether β-amyloid is the true ultimate ‘cause’ of AD or whether there are other factors that are up-stream of β-amyloid. We believe that an alternative strategy to lowering β-amyloid levels is to rescue synaptic dysfunction that results from a high β-amyloid environment. This alternative strategy is agnostic on whether β-amyloid is the ultimate ‘cause’ of AD or whether β-amyloid is simply a toxic by-product of an upstream event. In either case, our goal is simply to rescue synaptic dysfunction that results from high β-amyloid levels. This goal can be achieved using a number of different strategies (see Figure 2), and some of the compounds we and others are currently developing have well-established tolerable side effect profiles. While an absolute cure for AD should be our ultimate goal, we believe that a synaptic rescue strategy can bring more immediate relief to Alzheimer's patients, before the pathogenesis of this terrible disease is completely understood. Although this approach may not produce a total ‘cure’ of the disease, there is mounting evidence that early synaptic dysfunction may contribute to degeneration [124–127], so rescuing synaptic dysfunction may not only produce symptomatic relief, but may also slow the course of the disease. In addition, we believe that a synaptic rescue strategy is entirely consistent with the Amyloid Hypothesis if one looks at this hypothesis from a broader perspective. Just because β-amyloid is central to AD does not necessarily mean that decreasing β-amyloid levels is the most effective way to combat the disease. Rather, going ‘around’ β-amyloid and alleviating its toxic effects may actually be the best strategy. Thus we believe that the Amyloid Hypothesis should be viewed more broadly, and that the AD field should consider alternative ways of treating AD other than manipulating β-amyloid itself.
Acknowledgments
FUNDING
This work was supported by the National Institutes of Health [grant numbers R01-AG034248, R01-NS049442, U01-AG032973 and U01-AG028713 (to O.A.)], the Alzheimer's Association [grant numbers IIRG-09-133001 (to O.A.) and NIRG-11-203583 (to A.F.T)], the Thome Memorial Foundation and the Louis V. Gerstner, Jr. Scholars Program.
Abbreviations used
- AD
Alzheimer's disease
- AICD
amyloid precursor protein intracellular domain
- APLP
amyloid β precursor-like protein
- APOE
apolipoprotein E
- APP
amyloid precursor protein
- BACE
β-secretase
- CA
cornu ammonis
- CaMK-IIα
CaM-kinase IIα
- CBP
cAMP-response-element-binding protein-binding protein
- Cdk-5
cyclin-dependent kinase 5
- cGK
cGMP-dependent protein kinase
- CJD
Creutzfeldt–Jakob disease
- CNS
central nervous system
- CREB
cAMP-response-element-binding protein
- CSF
cerebrospinal fluid
- HAT
histone acetyltransferase
- HDAC
histone deacetylase
- LTP
long-term potentiation
- MCI
mild cognitive impairment
- NIA
National Institute on Aging
- PDE5
phosphodiesterase 5
- PKA
protein kinase A
- PKA-R
PKA regulatory subunit
- PKC
protein kinase C
- PS
presenilin
- sGC
soluble guanylate cyclase
- Uch-L1
ubiquitin C-terminal esterase L1
REFERENCES
- 1.Maurer K, Volk S, Gerbaldo H. Auguste D and Alzheimer's disease. Lancet. 1997;349:1546–1549. doi: 10.1016/S0140-6736(96)10203-8. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. Allg. Z. Psychiatr. Psych. Gerichtl. Med. 1907;64:146–148. [Google Scholar]
- 3.Alzheimer A. Über eigenartige Krankheitsfälle des späteren Alters. Z. Gesamte Neurol. Psychiatr. 1911;4:356–385. [Google Scholar]
- 4.Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 5.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. U.S.A. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 7.Simmons BB, Hartmann B, Dejoseph D. Evaluation of suspected dementia. Am. Fam. Physician. 2011;84:895–902. [PubMed] [Google Scholar]
- 8.Chow VW, Mattson MP, Wong PC, Gleichmann M. An overview of APP processing enzymes and products. NeuroMol. Med. 2010;12:1–12. doi: 10.1007/s12017-009-8104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bekris LM, Yu CE, Bird TD, Tsuang DW. Genetics of Alzheimer disease. J. Geriatr. Psychiatry Neurol. 2010;23:213–227. doi: 10.1177/0891988710383571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanzi RE, Kovacs DM, Kim TW, Moir RD, Guenette SY, Wasco W. The gene defects responsible for familial Alzheimer's disease. Neurobiol. Dis. 1996;3:159–168. doi: 10.1006/nbdi.1996.0016. [DOI] [PubMed] [Google Scholar]
- 11.Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Goate A, Hardy J. Twenty years of Alzheimer's disease-causing mutations. J. Neurochem. 2012;120:3–8. doi: 10.1111/j.1471-4159.2011.07575.x. [DOI] [PubMed] [Google Scholar]
- 13.Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol. Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- 14.Glenner GG, Wong CW. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem. Biophys. Res. Commun. 1984;122:1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- 15.Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid β protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- 16.Frautschy SA, Baird A, Cole GM. Effects of injected Alzheimer β-amyloid cores in rat brain. Proc. Natl. Acad. Sci. U.S.A. 1991;88:8362–8366. doi: 10.1073/pnas.88.19.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. In vitro aging of β-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 1991;563:311–314. doi: 10.1016/0006-8993(91)91553-d. [DOI] [PubMed] [Google Scholar]
- 18.Goedert M, Wischik CM, Crowther RA, Walker JE, Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc. Natl. Acad. Sci. U.S.A. 1988;85:4051–4055. doi: 10.1073/pnas.85.11.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mudher A, Lovestone S. Alzheimer's disease: do tauists and baptists finally shake hands? Trends Neurosci. 2002;25:22–26. doi: 10.1016/s0166-2236(00)02031-2. [DOI] [PubMed] [Google Scholar]
- 20.Nagy Z, Esiri MM, Jobst KA, Morris JH, King EM, McDonald B, Litchfield S, Smith A, Barnetson L, Smith AD. Relative roles of plaques and tangles in the dementia of Alzheimer's disease: correlations using three sets of neuropathological criteria. Dementia. 1995;6:21–31. doi: 10.1159/000106918. [DOI] [PubMed] [Google Scholar]
- 21.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 22.Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discovery. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh AK, Brindisi M, Tang J. Developing β-secretase inhibitors for treatment of Alzheimer's disease. J. Neurochem. 2012;120:71–83. doi: 10.1111/j.1471-4159.2011.07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrmann N, Chau SA, Kircanski I, Lanctot KL. Current and emerging drug treatment options for Alzheimer's disease: a systematic review. Drugs. 2011;71:2031–2065. doi: 10.2165/11595870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Bateman RJ, Siemers ER, Mawuenyega KG, Wen G, Browning KR, Sigurdson WC, Yarasheski KE, Friedrich SW, Demattos RB, May PC, et al. A γ-secretase inhibitor decreases amyloid-β production in the central nervous system. Ann. Neurol. 2009;66:48–54. doi: 10.1002/ana.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lilly Eli. Lilly halts development of Semagacestat for Alzheimer's disease based on preliminary results of phase III clinical trials. 2010 http://newsroom.lilly.com/releasedetail.cfm?releaseid=499794.
- 27.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 28.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat. Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, Krafft GA, Klein WL. Alzheimer's disease-affected brain: presence of oligomeric A β ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gervais F, Paquette J, Morissette C, Krzywkowski P, Yu M, Azzi M, Lacombe D, Kong X, Aman A, Laurin J, et al. Targeting soluble Aβ peptide with Tramiprosate for the treatment of brain amyloidosis. Neurobiol. Aging. 2007;28:537–547. doi: 10.1016/j.neurobiolaging.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Saumier D, Duong A, Haine D, Garceau D, Sampalis J. Domain-specific cognitive effects of tramiprosate in patients with mild to moderate Alzheimer's disease: ADAS-cog subscale results from the Alphase Study. J Nutr. Health Aging. 2009;13:808–812. doi: 10.1007/s12603-009-0217-4. [DOI] [PubMed] [Google Scholar]
- 32.Fenili D, Brown M, Rappaport R, McLaurin J. Properties of scyllo-inositol as a therapeutic treatment of AD-like pathology. J. Mol. Med. 2007;85:603–611. doi: 10.1007/s00109-007-0156-7. [DOI] [PubMed] [Google Scholar]
- 33.McLaurin J, Kierstead ME, Brown ME, Hawkes CA, Lambermon MH, Phinney AL, Darabie AA, Cousins JE, French JE, Lan MF, et al. Cyclohexanehexol inhibitors of Aβ aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat. Med. 2006;12:801–808. doi: 10.1038/nm1423. [DOI] [PubMed] [Google Scholar]
- 34.Salloway S, Sperling R, Keren R, Porsteinsson AP, van Dyck CH, Tariot PN, Gilman S, Arnold D, Abushakra S, Hernandez C, et al. A phase 2 randomized trial of ELND005, scyllo-inositol, in mild to moderate Alzheimer disease. Neurology. 2011;77:1253–1262. doi: 10.1212/WNL.0b013e3182309fa5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J, Masters CL, Targum S, Bush AI, Murdoch R, et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Aβ as a modifying therapy for Alzheimer's disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008;7:779–786. doi: 10.1016/S1474-4422(08)70167-4. [DOI] [PubMed] [Google Scholar]
- 36.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, et al. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 37.Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, Sabbagh M, Honig LS, Doody R, van Dyck CH, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siemers ER, Friedrich S, Dean RA, Gonzales CR, Farlow MR, Paul SM, Demattos RB. Safety and changes in plasma and cerebrospinal fluid amyloid beta after a single administration of an amyloid β monoclonal antibody in subjects with Alzheimer disease. Clin. Neuropharmacol. 2010;33:67–73. doi: 10.1097/WNF.0b013e3181cb577a. [DOI] [PubMed] [Google Scholar]
- 39.Delrieu J, Ousset PJ, Caillaud C, Vellas B. ‘Clinical trials in Alzheimer's disease’: immunotherapy approaches. J. Neurochem. 120:186–193. doi: 10.1111/j.1471-4159.2011.07458.x. [DOI] [PubMed] [Google Scholar]
- 40.Imbimbo BP, Ottonello S, Frisardi V, Solfrizzi V, Greco A, Seripa D, Pilotto A, Panza F. Solanezumab for the treatment of mild-to-moderate Alzheimer's disease. Expert Rev. Clin. Immunol. 2012;8:135–149. doi: 10.1586/eci.11.93. [DOI] [PubMed] [Google Scholar]
- 41.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 43.Khachaturian ZS. Diagnosis of Alzheimer's disease. Arch. Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 44.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 45.Hyman BT. The neuropathological diagnosis of Alzheimer's disease: clinical-pathological studies. Neurobiol. Aging. 1997;18:S27–S32. doi: 10.1016/s0197-4580(97)00066-3. [DOI] [PubMed] [Google Scholar]
- 46.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tierney MC, Fisher RH, Lewis AJ, Zorzitto ML, Snow WG, Reid DW, Nieuwstraten P. The NINCDS-ADRDA Work Group criteria for the clinical diagnosis of probable Alzheimer's disease: a clinicopathologic study of 57 cases. Neurology. 1988;38:359–364. doi: 10.1212/wnl.38.3.359. [DOI] [PubMed] [Google Scholar]
- 48.Flicker C, Ferris SH, Reisberg B. A two-year longitudinal study of cognitive function in normal aging and Alzheimer's disease. J. Geriatr. Psychiatry Neurol. 1993;6:84–96. doi: 10.1177/089198879300600205. [DOI] [PubMed] [Google Scholar]
- 49.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 50.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 51.Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, Belleville S, Brodaty H, Bennett D, Chertkow H, et al. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 52.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer's disease. NeuroRx. 2004;1:213–225. doi: 10.1602/neurorx.1.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Dickson DW, Trojanowski JQ, Lee VM. The levels of soluble versus insoluble brain Aβ distinguish Alzheimer's disease from normal and pathologic aging. Exp. Neurol. 1999;158:328–337. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- 55.Blennow K, Vanmechelen E, Hampel H. CSF total tau, Aβ42 and phosphorylated tau protein as biomarkers for Alzheimer's disease. Mol. Neurobiol. 2001;24:87–97. doi: 10.1385/MN:24:1-3:087. [DOI] [PubMed] [Google Scholar]
- 56.Otto M, Wiltfang J, Tumani H, Zerr I, Lantsch M, Kornhuber J, Weber T, Kretzschmar HA, Poser S. Elevated levels of tau-protein in cerebrospinal fluid of patients with Creutzfeldt–Jakob disease. Neurosci. Lett. 1997;225:210–212. doi: 10.1016/s0304-3940(97)00215-2. [DOI] [PubMed] [Google Scholar]
- 57.Hesse C, Rosengren L, Vanmechelen E, Vanderstichele H, Jensen C, Davidsson P, Blennow K. Cerebrospinal fluid markers for Alzheimer's disease evaluated after acute ischemic stroke. J. Alzheimer's Dis. 2000;2:199–206. doi: 10.3233/jad-2000-23-402. [DOI] [PubMed] [Google Scholar]
- 58.Riemenschneider M, Wagenpfeil S, Vanderstichele H, Otto M, Wiltfang J, Kretzschmar H, Vanmechelen E, Forstl H, Kurz A. Phospho-tau/total tau ratio in cerebrospinal fluid discriminates Creutzfeldt–Jakob disease from other dementias. Mol. Psychiatry. 2003;8:343–347. doi: 10.1038/sj.mp.4001220. [DOI] [PubMed] [Google Scholar]
- 59.De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP, Coart E, Hansson O, Minthon L, Zetterberg H, et al. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch. Neurol. 2010;67:949–956. doi: 10.1001/archneurol.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stomrud E, Hansson O, Blennow K, Minthon L, Londos E. Cerebrospinal fluid biomarkers predict decline in subjective cognitive function over 3 years in healthy elderly. Dementia Geriatr. Cognit. Disord. 2007;24:118–124. doi: 10.1159/000105017. [DOI] [PubMed] [Google Scholar]
- 61.Gustafson DR, Skoog I, Rosengren L, Zetterberg H, Blennow K. Cerebrospinal fluid β-amyloid 1-42 concentration may predict cognitive decline in older women. J. Neurol. Neurosurg. Psychiatry. 2007;78:461–464. doi: 10.1136/jnnp.2006.100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skoog I, Davidsson P, Aevarsson O, Vanderstichele H, Vanmechelen E, Blennow K. Cerebrospinal fluid β-amyloid 42 is reduced before the onset of sporadic dementia: a population-based study in 85-year-olds. Dementia Geriatr. Cognit. Disord. 2003;15:169–176. doi: 10.1159/000068478. [DOI] [PubMed] [Google Scholar]
- 63.Mathis CA, Bacskai BJ, Kajdasz ST, McLellan ME, Frosch MP, Hyman BT, Holt DP, Wang Y, Huang GF, Debnath ML, et al. A lipophilic thioflavin-T derivative for positron emission tomography (PET) imaging of amyloid in brain. Bioorg. Med. Chem. Lett. 2002;12:295–298. doi: 10.1016/s0960-894x(01)00734-x. [DOI] [PubMed] [Google Scholar]
- 64.Villemagne VL, Rowe CC. Amyloid imaging. Int. Psychogeriatr. 2011;23:S41–S49. doi: 10.1017/S1041610211000895. [DOI] [PubMed] [Google Scholar]
- 65.Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Mathis CA, Klunk WE, Masters CL, Rowe CC. β-Amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 66.Villemagne VL, Fodero-Tavoletti MT, Pike KE, Cappai R, Masters CL, Rowe CC. The ART of loss: Aβ imaging in the evaluation of Alzheimer's disease and other dementias. Mol. Neurobiol. 2008;38:1–15. doi: 10.1007/s12035-008-8019-y. [DOI] [PubMed] [Google Scholar]
- 67.Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, Ackermann U, Jones G, Szoeke C, Salvado O, et al. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann. Neurol. 2011;69:181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O'Keefe G, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol. Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 69.Stern RA, Riley DO, Daneshvar DH, Nowinski CJ, Cantu RC, McKee AC. Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PM&R. 2011;3:S460–S467. doi: 10.1016/j.pmrj.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 70.Wolfe MS. γ-Secretase inhibitors and modulators for Alzheimer's disease. J. Neurochem. 2012;120:89–98. doi: 10.1111/j.1471-4159.2011.07501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haapasalo A, Kovacs DM. The many substrates of presenilin/γ-secretase. J. Alzheimer's Dis. 2011;25:3–28. doi: 10.3233/JAD-2011-101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bossy-Wetzel E, Schwarzenbacher R, Lipton SA. Molecular pathways to neurodegeneration. Nat. Med. 2004;10:S2–S9. doi: 10.1038/nm1067. [DOI] [PubMed] [Google Scholar]
- 73.Querfurth HW, LaFerla FM. Alzheimer's disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 74.Struhl G, Adachi A. Requirements for presenilin-dependent cleavage of notch and other transmembrane proteins. Mol. Cell. 2000;6:625–636. doi: 10.1016/s1097-2765(00)00061-7. [DOI] [PubMed] [Google Scholar]
- 75.Artavanis-Tsakonas S, Muskavitch MA. Notch: the past, the present, and the future. Curr. Top. Dev. Biol. 2010;92:1–29. doi: 10.1016/S0070-2153(10)92001-2. [DOI] [PubMed] [Google Scholar]
- 76.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klusza S, Deng WM. At the crossroads of differentiation and proliferation: precise control of cell-cycle changes by multiple signaling pathways in Drosophila follicle cells. BioEssays. 2011;33:124–134. doi: 10.1002/bies.201000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Udolph G. Notch signaling and the generation of cell diversity in Drosophila neuroblast lineages. Adv. Exp. Med. Biol. 2012;727:47–60. doi: 10.1007/978-1-4614-0899-4_4. [DOI] [PubMed] [Google Scholar]
- 79.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 80.Gridley T. Notch signaling and inherited disease syndromes. Hum. Mol. Genet. 2003;12:R9–R13. doi: 10.1093/hmg/ddg052. [DOI] [PubMed] [Google Scholar]
- 81.Louvi A, Arboleda-Velasquez JF, Artavanis-Tsakonas S. CADASIL: a critical look at a Notch disease. Dev. Neurosci. 2006;28:5–12. doi: 10.1159/000090748. [DOI] [PubMed] [Google Scholar]
- 82.Lobry C, Oh P, Aifantis I. Oncogenic and tumor suppressor functions of Notch in cancer: it's NOTCH what you think. J. Exp. Med. 208:1931–1935. doi: 10.1084/jem.20111855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.He G, Luo W, Li P, Remmers C, Netzer WJ, Hendrick J, Bettayeb K, Flajolet M, Gorelick F, Wennogle LP, et al. γ-Secretase activating protein is a therapeutic target for Alzheimer's disease. Nature. 2010;467:95–98. doi: 10.1038/nature09325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chang KA, Suh YH. Possible roles of amyloid intracellular domain of amyloid precursor protein. BMB Rep. 2010;43:656–663. doi: 10.5483/BMBRep.2010.43.10.656. [DOI] [PubMed] [Google Scholar]
- 85.Pardossi-Piquard R, Checler F. The physiology of the β-amyloid precursor protein intracellular domain AICD. J. Neurochem. 2012;120:109–124. doi: 10.1111/j.1471-4159.2011.07475.x. [DOI] [PubMed] [Google Scholar]
- 86.Hamid R, Kilger E, Willem M, Vassallo N, Kostka M, Bornhovd C, Reichert AS, Kretzschmar HA, Haass C, Herms J. Amyloid precursor protein intracellular domain modulates cellular calcium homeostasis and ATP content. J. Neurochem. 2007;102:1264–1275. doi: 10.1111/j.1471-4159.2007.04627.x. [DOI] [PubMed] [Google Scholar]
- 87.Ghosal K, Vogt DL, Liang M, Shen Y, Lamb BT, Pimplikar SW. Alzheimer's disease-like pathological features in transgenic mice expressing the APP intracellular domain. Proc. Natl. Acad. Sci. U.S.A. 2009;106:18367–18372. doi: 10.1073/pnas.0907652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakayama K, Ohkawara T, Hiratochi M, Koh CS, Nagase H. The intracellular domain of amyloid precursor protein induces neuron-specific apoptosis. Neurosci. Lett. 2008;444:127–131. doi: 10.1016/j.neulet.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 89.Vazquez MC, Vargas LM, Inestrosa NC, Alvarez AR. c-Abl modulates AICD dependent cellular responses: transcriptional induction and apoptosis. J. Cell. Physiol. 2009;220:136–143. doi: 10.1002/jcp.21743. [DOI] [PubMed] [Google Scholar]
- 90.Kinoshita A, Whelan CM, Berezovska O, Hyman BT. The γ secretase-generated carboxyl-terminal domain of the amyloid precursor protein induces apoptosis via Tip60 in H4 cells. J. Biol. Chem. 2002;277:28530–28536. doi: 10.1074/jbc.M203372200. [DOI] [PubMed] [Google Scholar]
- 91.Passer B, Pellegrini L, Russo C, Siegel RM, Lenardo MJ, Schettini G, Bachmann M, Tabaton M, D'Adamio L. Generation of an apoptotic intracellular peptide by γ-secretase cleavage of Alzheimer's amyloid β protein precursor. J. Alzheimer's Dis. 2000;2:289–301. doi: 10.3233/jad-2000-23-408. [DOI] [PubMed] [Google Scholar]
- 92.Ozaki T, Li Y, Kikuchi H, Tomita T, Iwatsubo T, Nakagawara A. The intracellular domain of the amyloid precursor protein (AICD) enhances the p53-mediated apoptosis. Biochem. Biophys. Res. Commun. 2006;351:57–63. doi: 10.1016/j.bbrc.2006.09.162. [DOI] [PubMed] [Google Scholar]
- 93.Alves da Costa C, Sunyach C, Pardossi-Piquard R, Sevalle J, Vincent B, Boyer N, Kawarai T, Girardot N, St George-Hyslop P, Checler F. Presenilin-dependent γ-secretase-mediated control of p53-associated cell death in Alzheimer's disease. J. Neurosci. 2006;26:6377–6385. doi: 10.1523/JNEUROSCI.0651-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang S, Wang R, Chen L, Bennett DA, Dickson DW, Wang DS. Expression and functional profiling of neprilysin, insulin-degrading enzyme, and endothelin-converting enzyme in prospectively studied elderly and Alzheimer's brain. J. Neurochem. 2010;115:47–57. doi: 10.1111/j.1471-4159.2010.06899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nalivaeva NN, Beckett C, Belyaev ND, Turner AJ. Are amyloid-degrading enzymes viable therapeutic targets in Alzheimer's disease? J. Neurochem. 2012;120:167–185. doi: 10.1111/j.1471-4159.2011.07510.x. [DOI] [PubMed] [Google Scholar]
- 96.Pardossi-Piquard R, Petit A, Kawarai T, Sunyach C, Alves da Costa C, Vincent B, Ring S, D'Adamio L, Shen J, Muller U, et al. Presenilin-dependent transcriptional control of the Aβ-degrading enzyme neprilysin by intracellular domains of βAPP and APLP. Neuron. 2005;46:541–554. doi: 10.1016/j.neuron.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 97.Pardossi-Piquard R, Dunys J, Yu G, St George-Hyslop P, Alves da Costa C, Checler F. Neprilysin activity and expression are controlled by nicastrin. J. Neurochem. 2006;97:1052–1056. doi: 10.1111/j.1471-4159.2006.03822.x. [DOI] [PubMed] [Google Scholar]
- 98.Hemming ML, Elias JE, Gygi SP, Selkoe DJ. Identification of β-secretase (BACE1) substrates using quantitative proteomics. PLoS ONE. 2009;4:e8477. doi: 10.1371/journal.pone.0008477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kandalepas PC, Vassar R. Identification and biology of β-secretase. J. Neurochem. 2012;120:55–61. doi: 10.1111/j.1471-4159.2011.07512.x. [DOI] [PubMed] [Google Scholar]
- 100.Eggert S, Paliga K, Soba P, Evin G, Masters CL, Weidemann A, Beyreuther K. The proteolytic processing of the amyloid precursor protein gene family members APLP-1 and APLP-2 involves α-, β-, γ-, and ε-like cleavages: modulation of APLP-1 processing by n-glycosylation. J. Biol. Chem. 2004;279:18146–18156. doi: 10.1074/jbc.M311601200. [DOI] [PubMed] [Google Scholar]
- 101.Li Q, Sudhof TC. Cleavage of amyloid-β precursor protein and amyloid-β precursor-like protein by BACE 1. J. Biol. Chem. 2004;279:10542–10550. doi: 10.1074/jbc.M310001200. [DOI] [PubMed] [Google Scholar]
- 102.Pastorino L, Ikin AF, Lamprianou S, Vacaresse N, Revelli JP, Platt K, Paganetti P, Mathews PM, Harroch S, Buxbaum JD. BACE (β-secretase) modulates the processing of APLP2 in vivo. Mol. Cell. Neurosci. 2004;25:642–649. doi: 10.1016/j.mcn.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 103.von Arnim CA, Kinoshita A, Peltan ID, Tangredi MM, Herl L, Lee BM, Spoelgen R, Hshieh TT, Ranganathan S, Battey FD, et al. The low density lipoprotein receptor-related protein (LRP) is a novel β-secretase (BACE1) substrate. J. Biol. Chem. 2005;280:17777–17785. doi: 10.1074/jbc.M414248200. [DOI] [PubMed] [Google Scholar]
- 104.Kim DY, Ingano LA, Carey BW, Pettingell WH, Kovacs DM. Presenilin/γ-secretase-mediated cleavage of the voltage-gated sodium channel β2-subunit regulates cell adhesion and migration. J. Biol. Chem. 2005;280:23251–23261. doi: 10.1074/jbc.M412938200. [DOI] [PubMed] [Google Scholar]
- 105.Wong HK, Sakurai T, Oyama F, Kaneko K, Wada K, Miyazaki H, Kurosawa M, De Strooper B, Saftig P, Nukina N. β Subunits of voltage-gated sodium channels are novel substrates of β-site amyloid precursor protein-cleaving enzyme (BACE1) and γ-secretase. J. Biol. Chem. 2005;280:23009–23017. doi: 10.1074/jbc.M414648200. [DOI] [PubMed] [Google Scholar]
- 106.Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, Yan R. Bace1 modulates myelination in the central and peripheral nervous system. Nat. Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 107.Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C. Control of peripheral nerve myelination by the β-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 108.Hu X, He W, Diaconu C, Tang X, Kidd GJ, Macklin WB, Trapp BD, Yan R. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J. 2008;22:2970–2980. doi: 10.1096/fj.08-106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.May PC, Dean RA, Lowe SL, Martenyi F, Sheehan SM, Boggs LN, Monk SA, Mathes BM, Mergott DJ, Watson BM, et al. Robust central reduction of amyloid-β in humans with an orally available, non-peptidic β-secretase inhibitor. J. Neurosci. 2011;31:16507–16516. doi: 10.1523/JNEUROSCI.3647-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chasseigneaux S, Allinquant B. Functions of Aβ, sAPPα and sAPPβ: similarities and differences. J. Neurochem. 2012;120:99–108. doi: 10.1111/j.1471-4159.2011.07584.x. [DOI] [PubMed] [Google Scholar]
- 111.Chasseigneaux S, Dinc L, Rose C, Chabret C, Coulpier F, Topilko P, Mauger G, Allinquant B. Secreted amyloid precursor protein β and secreted amyloid precursor protein α induce axon outgrowth in vitro through Egr1 signaling pathway. PLoS ONE. 2011;6:e16301. doi: 10.1371/journal.pone.0016301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Freude KK, Penjwini M, Davis JL, LaFerla FM, Blurton-Jones M. Soluble amyloid precursor protein induces rapid neural differentiation of human embryonic stem cells. J. Biol. Chem. 2011;286:24264–24274. doi: 10.1074/jbc.M111.227421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cirrito JR, May PC, O'Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, et al. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-β metabolism and half-life. J. Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Plant LD, Boyle JP, Smith IF, Peers C, Pearson HA. The production of amyloid β peptide is a critical requirement for the viability of central neurons. J. Neurosci. 2003;23:5531–5535. doi: 10.1523/JNEUROSCI.23-13-05531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lopez-Toledano MA, Shelanski ML. Neurogenic effect of β-amyloid peptide in the development of neural stem cells. J. Neurosci. 2004;24:5439–5444. doi: 10.1523/JNEUROSCI.0974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 117.Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-β in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 119.Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I. Amyloid-β as a positive endogenous regulator of release probability at hippocampal synapses. Nat. Neurosci. 2009;12:1567–1576. doi: 10.1038/nn.2433. [DOI] [PubMed] [Google Scholar]
- 120.Puzzo D, Privitera L, Fa’ M, Staniszewski A, Hashimoto G, Aziz F, Sakurai M, Ribe EM, Troy CM, Mercken M, et al. Endogenous amyloid-β is necessary for hippocampal synaptic plasticity and memory. Ann. Neurol. 2011;69:819–830. doi: 10.1002/ana.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Puzzo D, Privitera L, Leznik E, Fa M, Staniszewski A, Palmeri A, Arancio O. Picomolar amyloid-β positively modulates synaptic plasticity and memory in hippocampus. J. Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brody DL, Magnoni S, Schwetye KE, Spinner ML, Esparza TJ, Stocchetti N, Zipfel GJ, Holtzman DM. Amyloid-β dynamics correlate with neurological status in the injured human brain. Science. 2008;321:1221–1224. doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Puzzo D, Privitera L, Palmeri A. Hormetic effect of amyloid-β peptide in synaptic plasticity and memory. Neurobiol. Aging. 2012;1484:e15–e24. doi: 10.1016/j.neurobiolaging.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 124.Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, et al. Synaptic targeting by Alzheimer's-related amyloid β oligomers. J. Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Aβ oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J. Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mukaetova-Ladinska EB, Garcia-Siera F, Hurt J, Gertz HJ, Xuereb JH, Hills R, Brayne C, Huppert FA, Paykel ES, McGee M, et al. Staging of cytoskeletal and β-amyloid changes in human isocortex reveals biphasic synaptic protein response during progression of Alzheimer's disease. Am. J. Pathol. 2000;157:623–636. doi: 10.1016/s0002-9440(10)64573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Scheff SW, Price DA, Schmitt FA, Scheff MA, Mufson EJ. Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer's disease. J. Alzheimer's Dis. 2011;24:547–557. doi: 10.3233/JAD-2011-101782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat. Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 130.Giacchino J, Criado JR, Games D, Henriksen S. In vivo synaptic transmission in young and aged amyloid precursor protein transgenic mice. Brain Res. 2000;876:185–190. doi: 10.1016/s0006-8993(00)02615-9. [DOI] [PubMed] [Google Scholar]
- 131.Larson J, Lynch G, Games D, Seubert P. Alterations in synaptic transmission and long-term potentiation in hippocampal slices from young and aged PDAPP mice. Brain Res. 1999;840:23–35. doi: 10.1016/s0006-8993(99)01698-4. [DOI] [PubMed] [Google Scholar]
- 132.D'Hooge R, Nagels G, Westland CE, Mucke L, De Deyn PP. Spatial learning deficit in mice expressing human 751-amino acid β-amyloid precursor protein. NeuroReport. 1996;7:2807–2811. doi: 10.1097/00001756-199611040-00080. [DOI] [PubMed] [Google Scholar]
- 133.Yamaguchi F, Richards SJ, Beyreuther K, Salbaum M, Carlson GA, Dunnett SB. Transgenic mice for the amyloid precursor protein 695 isoform have impaired spatial memory. NeuroReport. 1991;2:781–784. doi: 10.1097/00001756-199112000-00013. [DOI] [PubMed] [Google Scholar]
- 134.Bramham CR. Local protein synthesis, actin dynamics, and LTP consolidation. Curr. Opin. Neurobiol. 2008;18:524–531. doi: 10.1016/j.conb.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 135.Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- 136.Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu. Rev. Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- 138.Vitolo OV, Sant'Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid β-peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Arendash GW, King DL, Gordon MN, Morgan D, Hatcher JM, Hope CE, Diamond DM. Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res. 2001;891:42–53. doi: 10.1016/s0006-8993(00)03186-3. [DOI] [PubMed] [Google Scholar]
- 140.Battaglia F, Trinchese F, Liu S, Walter S, Nixon RA, Arancio O. Calpain inhibitors, a treatment for Alzheimer's disease: position paper. J. Mol. Neurosci. 2003;20:357–362. doi: 10.1385/JMN:20:3:357. [DOI] [PubMed] [Google Scholar]
- 141.Trinchese F, Liu S, Battaglia F, Walter S, Mathews PM, Arancio O. Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann. Neurol. 2004;55:801–814. doi: 10.1002/ana.20101. [DOI] [PubMed] [Google Scholar]
- 142.Viosca J, Malleret G, Bourtchouladze R, Benito E, Vronskava S, Kandel ER, Barco A. Chronic enhancement of CREB activity in the hippocampus interferes with the retrieval of spatial information. Learn. Mem. 2009;16:198–209. doi: 10.1101/lm.1220309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barco A, Pittenger C, Kandel ER. CREB, memory enhancement and the treatment of memory disorders: promises, pitfalls and prospects. Expert Opin. Ther. Targets. 2003;7:101–114. doi: 10.1517/14728222.7.1.101. [DOI] [PubMed] [Google Scholar]
- 144.Cho EC, Mitton B, Sakamoto KM. CREB and leukemogenesis. Crit. Rev. Oncog. 2011;16:37–46. doi: 10.1615/critrevoncog.v16.i1-2.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Arancio O, Kiebler M, Lee CJ, Lev-Ram V, Tsien RY, Kandel ER, Hawkins RD. Nitric oxide acts directly in the presynaptic neuron to produce long-term potentiation in cultured hippocampal neurons. Cell. 1996;87:1025–1035. doi: 10.1016/s0092-8674(00)81797-3. [DOI] [PubMed] [Google Scholar]
- 146.Bon CL, Garthwaite J. On the role of nitric oxide in hippocampal long-term potentiation. J. Neurosci. 2003;23:1941–1948. doi: 10.1523/JNEUROSCI.23-05-01941.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ohki K, Yoshida K, Hagiwara M, Harada T, Takamura M, Ohashi T, Matsuda H, Imaki J. Nitric oxide induces c-fos gene expression via cyclic AMP response element binding protein (CREB) phosphorylation in rat retinal pigment epithelium. Brain Res. 1995;696:140–144. doi: 10.1016/0006-8993(95)00914-c. [DOI] [PubMed] [Google Scholar]
- 148.Gudi T, Huvar I, Meinecke M, Lohmann SM, Boss GR, Pilz RB. Regulation of gene expression by cGMP-dependent protein kinase. Transactivation of the c-fos promoter. J. Biol. Chem. 1996;271:4597–4600. doi: 10.1074/jbc.271.9.4597. [DOI] [PubMed] [Google Scholar]
- 149.Lu YF, Kandel ER, Hawkins RD. Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus. J. Neurosci. 1999;19:10250–10261. doi: 10.1523/JNEUROSCI.19-23-10250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Garthwaite J, Boulton CL. Nitric oxide signaling in the central nervous system. Annu. Rev. Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- 151.Lohmann SM, Vaandrager AB, Smolenski A, Walter U, De Jonge HR. Distinct and specific functions of cGMP-dependent protein kinases. Trends Biochem. Sci. 1997;22:307–312. doi: 10.1016/s0968-0004(97)01086-4. [DOI] [PubMed] [Google Scholar]
- 152.Puzzo D, Vitolo O, Trinchese F, Jacob JP, Palmeri A, Arancio O. Amyloid-β peptide inhibits activation of the nitric oxide/cGMP/cAMP-responsive element-binding protein pathway during hippocampal synaptic plasticity. J. Neurosci. 2005;25:6887–6897. doi: 10.1523/JNEUROSCI.5291-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Monfort P, Munoz MD, Kosenko E, Felipo V. Long-term potentiation in hippocampus involves sequential activation of soluble guanylate cyclase, cGMP-dependent protein kinase, and cGMP-degrading phosphodiesterase. J. Neurosci. 2002;22:10116–10122. doi: 10.1523/JNEUROSCI.22-23-10116.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Puzzo D, Staniszewski A, Deng SX, Privitera L, Leznik E, Liu S, Zhang H, Feng Y, Palmeri A, Landry DW, et al. Phosphodiesterase 5 inhibition improves synaptic function, memory, and amyloid-β load in an Alzheimer's disease mouse model. J. Neurosci. 2009;29:8075–8086. doi: 10.1523/JNEUROSCI.0864-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Huang Y, Wang KK. The calpain family and human disease. Trends Mol. Med. 2001;7:355–362. doi: 10.1016/s1471-4914(01)02049-4. [DOI] [PubMed] [Google Scholar]
- 156.Nixon RA. The calpains in aging and aging-related diseases. Ageing Res. Rev. 2003;2:407–418. doi: 10.1016/s1568-1637(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 157.Perlmutter LS, Siman R, Gall C, Seubert P, Baudry M, Lynch G. The ultrastructural localization of calcium-activated protease ‘calpain’ in rat brain. Synapse. 1988;2:79–88. doi: 10.1002/syn.890020111. [DOI] [PubMed] [Google Scholar]
- 158.Saito K, Elce JS, Hamos JE, Nixon RA. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc. Natl. Acad. Sci. U.S.A. 1993;90:2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Grynspan F, Griffin WR, Cataldo A, Katayama S, Nixon RA. Active site-directed antibodies identify calpain II as an early-appearing and pervasive component of neurofibrillary pathology in Alzheimer's disease. Brain Res. 1997;763:145–158. doi: 10.1016/s0006-8993(97)00384-3. [DOI] [PubMed] [Google Scholar]
- 160.Wang KK. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01536-2. [DOI] [PubMed] [Google Scholar]
- 161.Siman R, Card JP, Davis LG. Proteolytic processing of β-amyloid precursor by calpain I. J. Neurosci. 1990;10:2400–2411. doi: 10.1523/JNEUROSCI.10-07-02400.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen M, Durr J, Fernandez HL. Possible role of calpain in normal processing of β-amyloid precursor protein in human platelets. Biochem. Biophys. Res. Commun. 2000;273:170–175. doi: 10.1006/bbrc.2000.2919. [DOI] [PubMed] [Google Scholar]
- 163.Mathews PM, Jiang Y, Schmidt SD, Grbovic OM, Mercken M, Nixon RA. Calpain activity regulates the cell surface distribution of amyloid precursor protein. Inhibition of clapains enhances endosomal generation of β-cleaved C-terminal APP fragments. J. Biol. Chem. 2002;277:36415–36424. doi: 10.1074/jbc.M205208200. [DOI] [PubMed] [Google Scholar]
- 164.Steiner H, Capell A, Pesold B, Citron M, Kloetzel PM, Selkoe DJ, Romig H, Mendla K, Haass C. Expression of Alzheimer's disease-associated presenilin-1 is controlled by proteolytic degradation and complex formation. J. Biol. Chem. 1998;273:32322–32331. doi: 10.1074/jbc.273.48.32322. [DOI] [PubMed] [Google Scholar]
- 165.Zhang L, Song L, Parker EM. Calpain inhibitor I increases β-amyloid peptide production by inhibiting the degradation of the substrate of γ-secretase. Evidence that substrate availability limits beta-amyloid peptide production. J. Biol. Chem. 1999;274:8966–8972. doi: 10.1074/jbc.274.13.8966. [DOI] [PubMed] [Google Scholar]
- 166.Pontremoli S, Melloni E, Michetti M, Sparatore B, Salamino F, Sacco O, Horecker BL. Phosphorylation and proteolytic modification of specific cytoskeletal proteins in human neutrophils stimulated by phorbol 12-myristate 13-acetate. Proc. Natl. Acad. Sci. U.S.A. 1987;84:3604–3608. doi: 10.1073/pnas.84.11.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gandy S, Czernik AJ, Greengard P. Phosphorylation of Alzheimer disease amyloid precursor peptide by protein kinase C and Ca2+/calmodulin-dependent protein kinase II. Proc. Natl. Acad. Sci. U.S.A. 1988;85:6218–6221. doi: 10.1073/pnas.85.16.6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang KK, Yuen PW. Development and therapeutic potential of calpain inhibitors. Adv. Pharmacol. 1997;37:117–152. doi: 10.1016/s1054-3589(08)60949-7. [DOI] [PubMed] [Google Scholar]
- 169.Banno Y, Nakashima S, Hachiya T, Nozawa Y. Endogenous cleavage of phospholipase C-β3 by agonist-induced activation of calpain in human platelets. J. Biol. Chem. 1995;270:4318–4324. doi: 10.1074/jbc.270.9.4318. [DOI] [PubMed] [Google Scholar]
- 170.Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- 171.Carillo S, Pariat M, Steff AM, Roux P, Etienne-Julan M, Lorca T, Piechaczyk M. Differential sensitivity of FOS and JUN family members to calpains. Oncogene. 1994;9:1679–1689. [PubMed] [Google Scholar]
- 172.Lin YC, Brown K, Siebenlist U. Activation of NF-κB requires proteolysis of the inhibitor IκB-α: signal-induced phosphorylation of IκB-α alone does not release active NF-κB. Proc. Natl. Acad. Sci. U.S.A. 1995;92:552–556. doi: 10.1073/pnas.92.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mbebi C, See V, Mercken L, Pradier L, Muller U, Loeffler JP. Amyloid precursor protein family-induced neuronal death is mediated by impairment of the neuroprotective calcium/calmodulin protein kinase IV-dependent signaling pathway. J. Biol. Chem. 2002;277:20979–20990. doi: 10.1074/jbc.M107948200. [DOI] [PubMed] [Google Scholar]
- 174.Siman R, Baudry M, Lynch G. Brain fodrin: substrate for calpain I, an endogenous calcium-activated protease. Proc. Natl. Acad. Sci. U.S.A. 1984;81:3572–3576. doi: 10.1073/pnas.81.11.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Johnson GV, Litersky JM, Jope RS. Degradation of microtubule-associated protein 2 and brain spectrin by calpain: a comparative study. J. Neurochem. 1991;56:1630–1638. doi: 10.1111/j.1471-4159.1991.tb02061.x. [DOI] [PubMed] [Google Scholar]
- 176.Yuen EY, Liu W, Yan Z. The phosphorylation state of GluR1 subunits determines the susceptibility of AMPA receptors to calpain cleavage. J. Biol. Chem. 2007;282:16434–16440. doi: 10.1074/jbc.M701283200. [DOI] [PubMed] [Google Scholar]
- 177.Wu Y, Liang S, Oda Y, Ohmori I, Nishiki T, Takei K, Matsui H, Tomizawa K. Truncations of amphiphysin I by calpain inhibit vesicle endocytosis during neural hyperexcitation. EMBO J. 2007;26:2981–2990. doi: 10.1038/sj.emboj.7601741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shimizu K, Phan T, Mansuy IM, Storm DR. Proteolytic degradation of SCOP in the hippocampus contributes to activation of MAP kinase and memory. Cell. 2007;128:1219–1229. doi: 10.1016/j.cell.2006.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Trinchese F, Fa M, Liu S, Zhang H, Hidalgo A, Schmidt SD, Yamaguchi H, Yoshii N, Mathews PM, Nixon RA, et al. Inhibition of calpains improves memory and synaptic transmission in a mouse model of Alzheimer disease. J. Clin. Invest. 2008;118:2796–2807. doi: 10.1172/JCI34254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.de Vrij FM, Fischer DF, van Leeuwen FW, Hol EM. Protein quality control in Alzheimer's disease by the ubiquitin proteasome system. Prog. Neurobiol. 2004;74:249–270. doi: 10.1016/j.pneurobio.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 181.Choi J, Levey AI, Weintraub ST, Rees HD, Gearing M, Chin LS, Li L. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson's and Alzheimer's diseases. J. Biol. Chem. 2004;279:13256–13264. doi: 10.1074/jbc.M314124200. [DOI] [PubMed] [Google Scholar]
- 182.Chain DG, Casadio A, Schacher S, Hegde AN, Valbrun M, Yamamoto N, Goldberg AL, Bartsch D, Kandel ER, Schwartz JH. Mechanisms for generating the autonomous cAMP-dependent protein kinase required for long-term facilitation in Aplysia. Neuron. 1999;22:147–156. doi: 10.1016/s0896-6273(00)80686-8. [DOI] [PubMed] [Google Scholar]
- 183.Ichihara N, Wu J, Chui DH, Yamazaki K, Wakabayashi T, Kikuchi T. Axonal degeneration promotes abnormal accumulation of amyloid β-protein in ascending gracile tract of gracile axonal dystrophy (GAD) mouse. Brain Res. 1995;695:173–178. doi: 10.1016/0006-8993(95)00729-a. [DOI] [PubMed] [Google Scholar]
- 184.Saigoh K, Wang YL, Suh JG, Yamanishi T, Sakai Y, Kiyosawa H, Harada T, Ichihara N, Wakana S, Kikuchi T, et al. Intragenic deletion in the gene encoding ubiquitin carboxy-terminal hydrolase in gad mice. Nat. Genet. 1999;23:47–51. doi: 10.1038/12647. [DOI] [PubMed] [Google Scholar]
- 185.Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, Moolman D, Zhang H, Shelanski M, Arancio O. Ubiquitin hydrolase Uch-L1 rescues β-amyloid-induced decreases in synaptic function and contextual memory. Cell. 2006;126:775–788. doi: 10.1016/j.cell.2006.06.046. [DOI] [PubMed] [Google Scholar]
- 186.Liu Z, Meray RK, Grammatopoulos TN, Fredenburg RA, Cookson MR, Liu Y, Logan T, Lansbury PT., Jr Membrane-associated farnesylated UCH-L1 promotes alpha-synuclein neurotoxicity and is a therapeutic target for Parkinson's disease. Proc. Natl. Acad. Sci. U.S.A. 2009;106:4635–4640. doi: 10.1073/pnas.0806474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Smith DL, Pozueta J, Gong B, Arancio O, Shelanski M. Reversal of long-term dendritic spine alterations in Alzheimer disease models. Proc. Natl. Acad. Sci. U.S.A. 2009;106:16877–16882. doi: 10.1073/pnas.0908706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gong B, Leznik E. The role of ubiquitin C-terminal hydrolase L1 in neurodegenerative disorders. Drug News Perspect. 2007;20:365–370. doi: 10.1358/dnp.2007.20.6.1138160. [DOI] [PubMed] [Google Scholar]
- 189.Gong B, Chen F, Pan Y, Arrieta-Cruz I, Yoshida Y, Haroutunian V, Pasinetti GM. SCFFbx2-E3-ligase-mediated degradation of BACE1 attenuates Alzheimer's disease amyloidosis and improves synaptic function. Aging Cell. 2010;9:1018–1031. doi: 10.1111/j.1474-9726.2010.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB: CBP-dependent transcriptional activation. J. Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein–Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 192.Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J. Biol. Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 194.Abel T, Zukin RS. Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr. Opin. Pharmacol. 2008;8:57–64. doi: 10.1016/j.coph.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang D, Shankaranarayana Rao BS, Chattarji S, Kelleher RJ, III, Kandel ER, Duff K, et al. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- 196.Francis YI, Stephanou A, Latchman DS. CREB-binding protein activation by presenilin 1 but not by its M146L mutant. NeuroReport. 2006;17:917–921. doi: 10.1097/01.wnr.0000220137.06542.a0. [DOI] [PubMed] [Google Scholar]
- 197.Caccamo A, Maldonado MA, Bokov AF, Majumder S, Oddo S. CBP gene transfer increases BDNF levels and ameliorates learning and memory deficits in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U.S.A. 2010;107:22687–22692. doi: 10.1073/pnas.1012851108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Roelfsema JH, Peters DJ. Rubinstein–Taybi syndrome: clinical and molecular overview. Expert Rev. Mol. Med. 2007;9:1–16. doi: 10.1017/S1462399407000415. [DOI] [PubMed] [Google Scholar]
- 199.Maurice T, Duclot F, Meunier J, Naert G, Givalois L, Meffre J, Celerier A, Jacquet C, Copois V, Mechti N, et al. Altered memory capacities and response to stress in p300/CBP-associated factor (PCAF) histone acetylase knockout mice. Neuropsychopharmacology. 2008;33:1584–1602. doi: 10.1038/sj.npp.1301551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Francis IY, Fa’ M, Ashraf H, Zhang H, Staniszewski A, Latchman D, Arancio O. Dysregulation of histone acetylation in the APP/PS1 mouse model of Alzheimer's disease. J. Alzheimer's Dis. 2009;18:131–139. doi: 10.3233/JAD-2009-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 202.Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Graff J, Rei D, Guan JS, Wang WY, Seo J, Hennig KM, Nieland TJ, Fass DM, Kao PF, Kahn M, et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483:222–226. doi: 10.1038/nature10849. [DOI] [PMC free article] [PubMed] [Google Scholar]