Abstract
Background
Although gastroesophageal reflux disease (GERD) is highly prevalent in lung transplantation, the pathophysiology of GERD in these patients is unknown. We hypothesize that the pathophysiology of GERD after lung transplantation differs from that of a control population, and that the 30-d morbidity and mortality of laparoscopic antireflux surgery (LARS) are equivalent in both populations.
Methods
We retrospectively compared the pathophysiology of GERD and the 30-d morbidity and mortality of 29 consecutive lung transplant patients with 23 consecutive patients without lung transplantation (control group), all of whom had LARS for GERD between November 2008 and May 2010.
Results
Both groups had a similar prevalence of endoscopic esophagitis and Barrett’s esophagus, comparable manometric profiles, and similar prevalence of abnormal peristalsis. However, hiatal hernia was more common in controls than in lung transplant patients (57% versus 24%; P = 0.04). Lung transplant patients had a higher prevalence and severity of proximal GERD (65% versus 33%; P = 0.04). The 30-d morbidity and mortality following LARS were similar in both groups regardless of the higher surgical risk of lung transplants (median ASA class: 3 versus 2 for controls, P < 0.001).
Conclusions
These results show that despite similar manometric profiles, lung transplant patients are more prone to proximal reflux than the general population with GERD; the prevalence of endoscopic esophagitis and Barrett’s esophagus is the same in both groups of patients; a hiatal hernia is uncommon after lung transplantation; and the morbidity and mortality of LARS are the same for lung transplant patients as the general population with GERD.
Keywords: gastroesophageal reflux disease (GERD), laparoscopic antireflux surgery, esophageal function testing, lung transplantation
INTRODUCTION
The long-term morbidity and mortality after lung transplantation is largely attributable to bronchiolitis obliterans syndrome (BOS), a form of chronic rejection that occurs after lung transplantation [1, 2]. Evidence suggests that the fibrosing process responsible for BOS may be the result of a nonimmunologic chronic injury, such as that promoted by aspiration of gastroesophageal contents in patients with gastroesophageal reflux disease (GERD) [3]. The reasons why GERD has been given much attention as a risk factor for the development and progression of BOS are 2-fold. First, lung transplant patients have a high prevalence of GERD [4–6]. Second, surgical control of GERD has been shown to stabilize or improve lung function in some patients with BOS [6–9]. GERD therefore may be a modifiable risk factor for the progression of BOS because GERD and aspiration can be stopped by laparoscopic antireflux surgery [LARS]. Collectively, these observations have led to an increased emphasis on the diagnosis and surgical treatment of GERD after lung transplantation. However, information regarding the pathophysiology of GERD and the perioperative outcomes of the surgical treatment in these patients is limited, although initial evidence suggests that LARS may be performed safely in the lung transplant population [10–13]. We hypothesized that lung transplant patients have distinct pathophysiologic characteristics that differentiate them from the patients with GERD, and that despite the higher surgical risk of lung transplant patients, LARS can be performed with equivalent 30-d morbidity and mortality.
PATIENTS AND METHODS
We retrospectively compared the pathophysiologic characteristics and the perioperative outcomes of 29 consecutive patients who had single lung transplantation, double lung transplantation, or re-transplantation with those of 23 consecutive patients without lung disease or lung transplantation (control group) who underwent LARS for GERD between November 2008 and May 2010. We excluded patients with open fundoplication and patients who had a fundoplication for paraesophageal hernia. All patients underwent a symptomatic assessment and a thorough preoperative evaluation, including esophageal function testing (esophageal high-resolution impedance manometry and dual-sensor pH-monitoring), upper endoscopy, and barium swallow. In addition, lung transplant patients with GERD were also evaluated by a gastric emptying study. This study was approved by the Loyola University Medical Center, Institutional Review Board (LU202400).
Symptomatic Assessment and Preoperative Evaluation
All patients underwent a symptom and medication assessment, including the type, dosage, and schedule of antisecretory medications used (proton pump inhibitors or histamine H2-receptor antagonists) as well as a metabolic evaluation, including the calculation of body mass index (kg/m2). A transplant history was also attained from those in the transplant group.
Lung transplant patients were referred for physiologic testing for GERD based on symptoms, objective findings of aspiration at bronchoscopy (or evidence of aspiration on transbronchial biopsy, as defined by the presence of exogenous material with foreign-body giant-cell reaction, large lipid droplets, and/or macrophages with large vacuoles), and an unexplained decrease in pulmonary function. Their candidacy for LARS was then determined by the results of ambulatory pH-monitoring. In one case, we have performed LARS in a patient with direct evidence of aspiration (food in the trachea) and in another patient with typical symptoms of GERD and an unexplained decrease in pulmonary function who refused ambulatory pH-monitoring. Patients in the control group presented with symptoms consistent with GERD, and surgical treatment was considered after a positive 24-h esophageal pH study.
Esophageal High-Resolution Impedance Manometry
After an overnight fast, all patients underwent esophageal high-resolution impedance manometry using an 8-channel solid-state catheter (EFT system with BioVIEW software; Sandhill Scientific Inc., Denver, CO) with four active impedance channels located 5, 10, 15, and 20 cm above the high-pressure zone of the lower esophageal sphincter (LES). The system determined the LES pressure, length, and degree of relaxation (relaxation was determined by a drop of the resting pressure to a residual pressure <8 mm Hg) in addition to the amplitude, duration, and velocity of the peristaltic waves. Peristaltic wave amplitude was calculated for the distal esophagus (distal esophageal amplitude, or DEA) based on data recorded from pressure sensors located 5 and 10 cm above the LES. Esophageal motility was considered normal on manometry if normal peristaltic waves were present in >80% of the swallows with DEA <180 mm Hg. Ineffective esophageal motility (IEM) was defined when DEA was <30 mm Hg or when >30% simultaneous waves were present in the distal esophagus [14, 15].
Ambulatory pH-Monitoring
Proton pump inhibitors were stopped for 14 d and histamine H2-receptor antagonists were stopped for 3 d before pH-monitoring in all patients. A dual-sensor pH catheter (Sleuth system with BioVIEW software; Sandhill Scientific Inc., Denver, CO) was passed through the nose and the two pH sensors were positioned 5 and 20 cm, respectively, from the manometrically-determined upper border of the LES. The DeMeester score was calculated for the distal pH recordings. A score >14.7 was considered abnormal [16]. Proximal reflux was defined as a recording of pH <4 for >1% of total recording time at the proximal sensor [17].
Upper Endoscopy
The endoscopic presence and grading of esophagitis was recorded according to the Los Angeles classification [18]. Biopsies were taken of any abnormal mucosa visualized above the gastroesophageal junction. A definitive confirmation of Barrett’s metaplasia was obtained by pathology. Dysplasia was classified as: negative, indefinite, low-grade dysplasia, or high-grade dysplasia [19].
Barium Swallow
The presence and size of a hiatal hernia was assessed by calculating the axial length of the hernia in the upright position relative to the margin of the diaphragm, on barium esophagogram using eFilm Lite software (Merge Healthcare; Milwaukee, WI). The hiatal hernia was classified as small if its axial length was <3 cm; moderate if its axial length was 3 to 5 cm, and large if its axial length was >5 cm.
Gastric Emptying Scan
Nuclear medicine gastric emptying studies were performed by obtaining dynamic scintigraphic images through the abdomen for 90 min following oral administration of 0.4 mCi 99m Tc-labeled sulfur colloid in ovalbumin. Gastric emptying was considered abnormal if computer analysis demonstrated that <30% of the gastric contents were emptied into the small bowel by 90 min.
Technique of Fundoplication
All patients underwent a strict standardized preoperative anesthesiology assessment previously described [20]. All patients with normal esophageal peristalsis underwent a total (Nissen) fundoplication according to our standardized technique [20]. A partial posterior (Toupet) fundoplication was reserved for patients with aperistalsis. A laparoscopic Heineke–Mikulicz pyloroplasty was performed according to our standardized technique when these criteria were satisfied: a severely delayed gastric emptying (<10%) demonstrated in symptomatic patients who did not benefit from pharmacologic treatment with metoclopramide and who also had evidence of food in their stomach at the time of an upper endoscopy [20].
Postoperative Care and Follow-up
Postoperatively, all lung transplant patients were monitored in the surgical intensive care unit with their care coordinated by a multidisciplinary team of pulmonologists, intensivists, as well as minimally invasive and cardiothoracic surgeons. All other patients were admitted to the regular nursing ward. Patients were kept nil per os overnight and discharged after breakfast in most cases. A barium swallow was performed on the first postoperative day only in those patients who had a pyloroplasty. After a gastric leak was ruled out, these patients were started on a soft mechanical diet prior to discharge. All patients were instructed to follow a soft diet for 10 d and to advance as tolerated, thereafter. Follow-up occurred routinely at 2 and 4 wk, and then at 3 and 6 mo as clinically indicated.
Perioperative Outcome End-points
The demographics, physiologic data, and outcomes were prospectively entered in a dedicated database by one single individual and analyzed retrospectively.
Statistical Analysis
Tests of statistical significance were conducted with SPSS Statistical Software, version 16 (SPSS, Inc., Chicago, IL). Data were analyzed using nonparametric statistical methods. The χ2 test for association was used to compare categorical variables, while the Mann-Whitney U test was used for scaled variables. Results were reported as percentages for categorical variables and as median (with interquartile range) for scaled variables. A difference between observed variables was considered statistically significant when P < 0.05.
RESULTS
Demographics
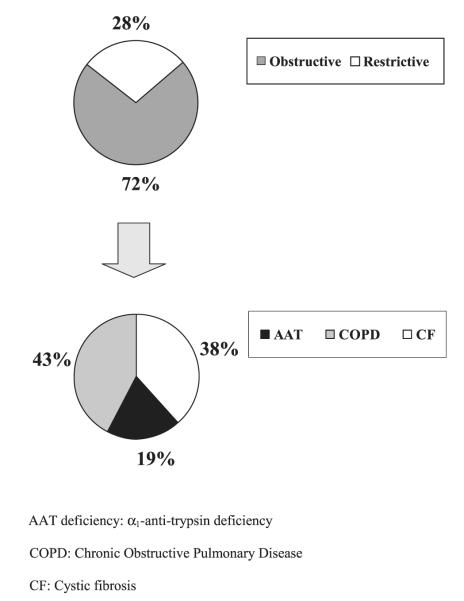
Lung transplant patients and patients in the control group were of comparable age, sex, and race. However, control patients had a larger body habitus (Table 1). The median time between lung transplant and LARS was 41 mo. The distribution of the underlying end-stage lung disease (obstructive or restrictive) in the transplant group is shown in Fig. 1. Specifically, eight patients (28%) had restrictive end-stage lung disease: six had idiopathic pulmonary fibrosis and two had scleroderma. Patients with obstructive end-stage lung disease such as, cystic fibrosis, α1-anti-trypsin deficiency, and chronic obstructive pulmonary disease accounted for 72% of the lung transplant population. In addition, 55% of the patients received a bilateral lung transplant, 24% received a single lung transplant, and 21% were re-transplanted.
TABLE 1.
Demographics of Lung Transplant Patients and Controls
| Lung transplants | Controls | P | |
|---|---|---|---|
| Patients | 29 | 23 | |
| Sex (%) | 0.14 | ||
| Males | 13 (45%) | 5 (22%) | |
| Females | 16 (55%) | 18 (78%) | |
| Age (y) | 54 (44–59) | 49 (37–59) | 0.99 |
| Race | (%) 0.77 | ||
| Caucasian: non Hispanic | 26 (89.5%) | 18 (75%) | |
| Caucasian: Hispanic | 2 (7%) | 3 (12.5%) | |
| African American | 1 (3.5%) | 2 (12.5%) | |
| Time from transplant (mo) | 41 (9–68) | n/a | |
| BMI | 24 (21–29) | 29 (26–33) | 0.01 |
BMI = body mass index expressed in kg/m2.
Data are presented as percentages for categorical variables and as median (with interquartile range) for scaled variables.
FIG. 1.
Underlying end-stage lung diseases in the lung transplant patients
Comparison of Endoscopic and Radiological Characteristics
Endoscopic and radiological characteristics of lung transplant patients and controls are shown in Table 2. The prevalence of erosive esophagitis on endoscopy (Los Angeles class A-D) was 22% (six patients) in lung transplant recipients and 31% (seven patients) in controls (P = 0.52). Severe esophagitis (Los Angeles class C and D) was observed in one patient in each group. The prevalence of biopsy-confirmed Barrett’s esophagus was similar between both groups (26% versus 32%; P = 0.75). No patients in the lung transplant group had dysplasia, whereas three (or 43%) of control patients with Barrett’s esophagus had low-grade dysplasia and one (or 14%) had high-grade dysplasia. In this patient, the dysplastic epithelium was treated first with endoscopic mucosal resection; LARS was then performed to treat the patient’s severe gastroesophageal reflux and her symptomatic hiatal hernia. The prevalence of a hiatal hernia was higher in controls than in lung transplants (57% versus 24%; P = 0.04). However, most hernias were small (<3 cm) in both groups; only one patient in each group had a moderate-size hiatal hernia (3–5 cm). The prevalence of delayed gastric emptying in the lung transplant patients was 27%. Among these patients, four had received bilateral lung transplants, two had been re-transplanted, and one had received a single lung transplant.
TABLE 2.
Endoscopic and Radiological Characteristics of Lung Transplant Patients and Controls
| Lung transplants | Controls | P | |
|---|---|---|---|
| Endoscopic esophagitis (%) | 6/27 (22%) | 7/22 (31%) | 0.52 |
| Grade A | 3/6 (50%) | 3/7 (43%) | |
| Grade B | 2/6 (33%) | 3/7 (43%) | |
| Grade C | 1/6 (17%) | 1/7 (14%) | |
| Grade D | 0 | 0 | |
| Barrett’s esophagus (%) | 7/27 (27%) | 7/22 (32%) | 0.75 |
| Low grade dysplasia | 0 | 3/7 (43%) | |
| High grade dysplasia | 0 | 1/7 (14%) | |
| Hiatal hernia (%) | 6/25 (24%) | 13/23 (57%) | 0.04 |
| Small (<3 cm) | 5/6 (83%) | 12/13 (92%) | |
| Moderate (3–5 cm) | 1 (7%) | 1 (8%) | |
| Large (>5 cm) | 0 | 0 | |
| Delayed gastric emptying (%) | 27% | n/a |
Data are presented as percentages for categorical variables.
Comparison of Manometric and pH-Metric Profile
The manometric profile of the LES and the esophageal body was similar between lung transplant patients and controls (Table 3). Patients in both groups had similar LES resting pressure, as well as total and abdominal LES length. The LES had a normal resting pressure in 79% of lung transplants and 60% of controls (P=0.5). The distal esophageal amplitude (DEA) was comparable in both groups (median DEA: 73 versus 54 mm Hg; P = 0.24) (Table 3). Likewise, delayed peristalsis (IEM) was equally common in both groups (21% versus 16% in controls; P 0.72).
TABLE 3.
Manometric Profile of Lung Transplant Patients and Controls
| Lung transplants | Controls | P | |
|---|---|---|---|
| LES | |||
| LES pressure (mm Hg) | 23 (15–34) | 17 (9–28) | 0.22 |
| LES total length (cm) | 2 (2–3) | 2 (2–3) | 0.53 |
| LES abdominal length (cm) | 1 (1–2) | 1 (1–2) | 0.86 |
| Esophageal body | |||
| DEA (mm Hg) | 73 (46–111) | 54 (38–83) | 0.24 |
LES = lower esophageal sphincter; DEA = distal esophageal amplitude.
Data are presented as median (interquartile range).
The analysis of the reflux profile showed that both groups of patients had similar exposure to distal acid reflux, regardless of patient positioning (upright or supine) (Table 4). Lung transplant patients also had fewer episodes of reflux but a slower total acid clearance (Table 4). Moreover, the analysis of the reflux profile showed a significantly higher prevalence (65% versus 33%; P = 0.04) and severity of proximal acid reflux in the lung transplant group than in the control group (median total time pH <4: 1.6 versus 0.4; P = 0.01;Table 4).
TABLE 4.
Reflux Profile of Lung Transplant Patients and Controls
| Lung transplants | Controls | P | |
|---|---|---|---|
| Total time pH <4 (%) | 11 (9–21) | 11 (6–24) | 0.94 |
| Upright | 8 (6–14) | 13 (8–28) | 0.05 |
| Supine | 12 (5–35) | 6 (2–24) | 0.24 |
| Episodes >5 min | 8 (5–14) | 8 (4–21) | 0.92 |
| Longest episode (min) | 28 (19–62) | 22 (10–33) | 0.11 |
| Total episodes | 65 (50–95) | 104 (73–167) | 0.001 |
| DeMeester score (normal: <14.7) |
36 (27–90) | 41 (21–81) | 0.65 |
| Esophageal clearance (s) | |||
| Total mean acid clearance time |
182 (133–279) | 116 (84–181) | 0.01 |
| Upright | 110 (79–150) | 98 (76–157) | 0.96 |
| Supine | 228 (157–320) | 164 (120–254) | 0.11 |
| Proximal pH sensor data | |||
| Total time pH <4 (normal: <1%) |
1.6 (0.6–3.6) | 0.4 (0–1.1) | 0.01 |
| Upright | 1.5 (0.3–4.6) | 0.6 (0–1.5) | 0.13 |
| Supine | 0.5 (0–1.1) | 0 (0–0.3) | 0.12 |
Data are presented as median (interquartile range).
Comparison of Operative Characteristics, Perioperative Risk, Complications, and Perioperative Outcomes
Comparisons are shown in Table 5. Both groups of patients had previously undergone similar amounts of abdominal surgery. Most patients underwent a total (Nissen) fundoplication (93% versus 96% in controls; P =1.00) (Table 5). One patient in the lung transplant group had a revision of a failed laparoscopic fundoplication performed several years prior to the transplant. The number of additional procedures performed at the time of fundoplication was similar between lung transplant patients and controls (38% versus 17%; P = 0.13). In lung transplant patients, 64% of these procedures were laparoscopic pyloroplasties. In both groups, the estimated blood loss, duration of surgery, and length of hospital stay were similar. There were no conversions to open procedures in all patients. There was no in-hospital or 30-d mortality in either group. Although lung transplant patients had a significantly higher anesthetic and surgical risk profile than controls without lung disease or lung transplantation, there was no difference in complication or readmission rates after LARS between the lung transplant population and the control group. One lung transplant patient presumably had food impaction and was readmitted for upper endoscopy, though at the time of the procedure we believe the impacted bolus had passed already into the stomach, as no foreign body was noted and symptoms resolved. Two patients in the control group had the following complications: one patient with a previous history of deep venous thrombosis developed a pulmonary embolus before discharge despite adherence to a standardized institutional prophylaxis protocol; one patient was readmitted for endoscopic disimpaction of a piece of meat. None required endoscopic dilatation. Overall, the incidence of complications and readmissions were similar between the lung transplant patients and controls (P = 0.57 and P = 1.00, respectively). To date, there have been no reoperations or endoscopic dilatations in either group.
TABLE 5.
Comparison of Operative Characteristics, Perioperative Risk, Complications, and Outcomes Between Lung Transplant Patients and Controls
| Lung transplants | Controls | P | |
|---|---|---|---|
| Previous abdominal surgery (%) |
16 (55%) | 14 (61%) | 0.78 |
| Type of fundoplication (%) | 1.00 | ||
| Total | 27 (93%) | 22 (96%) | |
| Partial | 2 (7%) | 1 (4%) | |
| Additional procedures (%) | 11 (38%) | 4 (17%) | 0.13 |
| Pyloroplasty | 7 (64%) | 0 | |
| Ventral hernia | 1 (11%) | 1 (25%) | |
| Cholecystectomy | 0 | 1 (25%) | |
| Bronchoscopy with biopsy | 3 (33%) | 0 | |
| Urethral sling/cystoscopy | 0 | 1 (25%) | |
| Total hysterectomy (open) | 0 | 1 (25%) | |
| ASA status | 3 | 2 (1–2) | 0.001 |
| Operative time (minutes) | 180 (144–207) | 143 (135–187) | 0.09 |
| Estimated blood loss (cc) | 20 (10–25) | 15 (10–20) | 0.18 |
| Conversions (%) | 0 | 0 | |
| Length of stay (d) | 1 (1–2) | 1 (1–2) | 0.75 |
| 30-d or in-hospital mortality | 0 | 0 |
Data are presented as percentages for categorical variables and as median (with interquartile range) for scaled variables.
ASA = American Society of Anesthesiologists.
DISCUSSION
Recent investigation has revealed that GERD and putative microaspiration of gastroduodenal contents may be a risk factor in the pathogenesis of BOS [3, 21, 22]. Indeed, GERD is highly prevalent after lung transplantation as reported by our group (51%), Young et al. (65%), Hadjiliadis et al. (70%), and Davis et al. (73%) [4–6, 23] Furthermore, surgical correction of GERD has been associated with a delay in the onset or progression of BOS, and in some cases even improvement in lung function [6, 8, 24]. These findings have therefore led to an increased emphasis on the diagnosis and surgical treatment of GERD after lung transplantation. However, the unique pathophysiologic characteristics of GERD have never been directly compared with a control population, and the safety profile of LARS for those with a lung transplant remains unclear. This study was targeted to investigate these concerns.
Pathophysiologic Characteristics
Endoscopic and Radiographic Findings
Endoscopy is often used to detect and stratify any esophageal manifestations of GERD. The absence of endoscopic findings, however, does not exclude the diagnosis of GERD, as many patients presenting with symptoms of GERD have no visible endoscopic findings. Recent reports have shown that up to 85% of patients with GERD may have a normal endoscopic examination [25]. Therefore, the 31% prevalence of erosive esophagitis (Los Angeles grade A–D) observed in the control group of this study is not surprising. Similarly, the equally low 22% prevalence of esophagitis in the lung transplant patients may be explained by the fact that all patients were taking acid suppressive medications at the time of endoscopy to counteract the side-effects of corticosteroids and to prevent the degradation of oral pancreatic enzymes in those with cystic fibrosis. Moreover, the absence of large hiatal hernias and the normal BMI in both groups may further explain the overall low and comparable incidence of erosive esophagitis.
Reports on the prevalence of Barrett’s esophagus specific to the lung transplant population with GERD are sparse, suggesting a frequency of 13% [10]. Contrary to these data, our findings show that 26% of lung transplant patients with GERD had a biopsy proven diagnosis of Barrett’s esophagus. Interestingly, no lung transplant patient with Barrett’s esophagus had dysplasia, whereas 43% of controls with Barrett’s esophagus had low-grade dysplasia, and 14% had high-grade dysplasia. Therefore, the prevalence of Barrett’s esophagus in lung transplant patients is not insignificant and in our report it appears to parallel that of the general population with gastroesophageal reflux. We believe that a strong suspicion of Barrett’s esophagus in lung transplant patients is warranted and that an upper endoscopy should be always performed in these patients for diagnostic, therapeutic, and surveillance purposes.
Another risk factor of GERD is the presence of a hiatal hernia, which has traditionally been considered to contribute to the severity and frequency of reflux in many patients [26–28]. Whether or not a hiatal hernia is a risk factor for GERD in the lung transplant population had not been not fully discerned, and the prevalence of a hiatal hernia in those with a lung allograft has been largely unknown. Few studies report on hiatal hernias in lung transplantation, with frequencies ranging from 10% to 78% [10, 13, 24, 29]. However, these studies do not provide information as to the size of the hiatal hernias and frequently do not discuss the methodology of analysis. In our controlled comparison using barium swallow as an objective measure, we found that a hiatal hernia was significantly more common in controls than in the lung transplant group (57% versus 24%; P = 0.037), though nearly all were less than 3 cm in size and likely not physiologically relevant. As such, a hiatal hernia might be a less significant contributing factor to GERD in lung transplantation, as we have also reported in a comparison of lung transplant patients with and without reflux [23].
The extent to which gastroparesis contributes to GERD after lung transplantation has been debated since Reid et al. highlighted the concerns for aspiration of gastric contents in 1990 [30]. Depending on diagnostic criteria, potential selection bias, and the type of lung transplant, estimates of the prevalence of gastric atony range from 23% to 92%, the latter group being a study of lung transplant patients with GERD [4, 5, 12, 31–34]. Moreover, gastroparesis is a considerable source of morbidity to the lung transplant patient, having been linked to pneumonia, nausea, vomiting, poor nutritional intake, obliterative bronchiolitis, and the need for surgical intervention [32–35]. Our study shows that delayed gastric emptying is indeed common after lung transplantation, with an overall prevalence of 27% as identified by nuclear medicine gastric emptying scan.
Manometry and Ambulatory pH Monitoring
Historically, a hypotensive LES has been regarded as an important risk factor for gastroesophageal reflux, as lower LES pressures correlate with increasing severity of esophagitis [36, 37]. Moreover, the degree of esophagitis has been shown to be predictive of esophageal peristaltic dysfunction [37]. The extent to which this holds true in lung transplant recipients has not yet been validated. Our study demonstrates that our cohort of lung transplant recipients has similar LES pressure and length, and degree of esophageal dysmotility as do patients without lung transplantation. Though the manometric findings are similar, their real value must be viewed in light of a potential detrimental impact on the allograft. First, acid suppression does not prevent against the non-acid components of reflux [38].This has been noted in lung transplantation by Blondeau et al., who showed that the levels of pepsin and proportion of bile acids in bronchoalveolar lavage fluid were similar in lung transplant patients regardless of treatment with proton pump inhibitors [38]. Second, there is burgeoning evidence implicating lung allograft rejection with the aspiration of gastroduodenal contents [38–40]. We have found that although lung transplant patients had fewer reflux episodes, the refluxate required more time to be cleared by peristalsis, a delay of which was noted by Pellegrini et al. to correlate with aspiration in non-lung transplant patients [41]. Furthermore, the lung transplant group had a significantly higher prevalence and severity of proximal reflux than controls. Proximal esophageal reflux is known to be a risk factor for aspiration and has been demonstrated in several studies besides our own to be present after lung transplantation [10, 12, 23, 40–42]. Therefore, given that reflux cannot be managed by acid suppression alone, the increased frequency and severity of proximal reflux in lung transplant patients, and recent evidence suggesting a relation of GERD to lung transplant rejection, we view LARS as a crucial component to the care of the lung transplant recipient which should be performed in the safest manner possible.
Safety of LARS in Lung Transplantation
Several reports have suggested that LARS for GERD in lung transplantation can be performed safely. However, to date, only O’Halloran et al. have employed a control group in their analysis. Their retrospective study demonstrated no difference in operative time, blood loss, or complication rate between those with or without a lung allograft [13]. As opposed to our results, their lung transplant patients did have a longer hospital length of stay than controls (2.89 versus 0.71 d) and higher readmission rate (25% versus 3%), which were attributed to greater operative risk combined with underlying pulmonary pathology. Gasper et al. provided similar evidence for the safety of LARS in lung transplantation, whereby post-operative stay was only 2 d, with a 30-d mortality of 5%, of which the single death was not related to the fundoplication [12]. More recently, in 2009, Burton et al. published their medium-term results of laparoscopic fundoplication after lung transplantation [10]. Of 21 patients studied there were four deaths. One death occurred 17-d post-fundoplication in a patient operated on as a last attempt to control declining lung function with BOS and chronic vascular rejection. The other three deaths were not associated with fundoplication, occurring greater than 250 d from the antireflux procedure. Burton et al. did also note a longer postoperative hospital stay (average: 6.3 d), though many patients apparently lived far from the transplant center and were intentionally observed for a longer period of time.
Our study demonstrates an acceptable safety profile of LARS for GERD in lung transplantation compared with a control series of consecutive cases referred for surgical treatment of GERD. Not only was there no single in-hospital or 30-d mortality for any patient, but all are still alive at the time of submission of this report. We attribute the success of LARS for GERD in lung transplant patients at our institution to a regimented multidisciplinary approach, which involves a strict preoperative anesthesiology assessment and careful postoperative management [20].
CONCLUSIONS
Our report is the first case-control study comparing the pathophysiologic characteristics of GERD in lung transplant patients with a control group, and the second largest institutional series describing LARS for GERD after lung transplantation. This study indicates that lung transplant patients differ from those without a lung transplant or pulmonary disease in that they rarely have a hiatal hernia and have more frequent and severe proximal reflux. Furthermore, we have objectively verified that LARS for GERD in lung transplantation can be performed safely and successfully.
ACKNOWLEDGMENTS
The authors acknowledge Dr. Stephen Sontag from the Department of Gastroenterology, Edward Hines Jr. Veteran Administration Medical Center, Hines IL, for his scientific supervision during the revisions of this manuscript.
This work was supported in part by the Dr. Ralph and Marian C. Falk Medical Research Trust and a grant from the National Institute on Alcohol Abuse and Alcoholism (T32 AA013257).
REFERENCES
- 1.Christie JD, Edwards LB, Aurora P, et al. Registry for the International Society for Heart and Lung Transplantation: Twenty-fifth Official Adult Lung and Heart/Lung Transplantation Report. J Heart Lung Transplant. 2008;27:957. doi: 10.1016/j.healun.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001, an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 3.Li B, Hartwig MG, Appel JZ, et al. Chronic aspiration of gastric fluid induces the development of obliterative bronchiolitis in rat lung transplants. Am J Transplant. 2008;8:1614. doi: 10.1111/j.1600-6143.2008.02298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hadjiliadis D, Davis RD, Steele MP, et al. Gastroesophageal reflux disea se in lung transplant recipients. Clin Transplant. 2003;17:363. doi: 10.1034/j.1399-0012.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- 5.Young LR, Hadjiliadis D, Davis RD, et al. Lung transplantation exacerbates gastroesophageal reflux disease. Chest. 2003;124:1689. doi: 10.1378/chest.124.5.1689. [DOI] [PubMed] [Google Scholar]
- 6.Davis RD, Jr, Lau CL, Eubanks S, et al. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. J Thorac Cardiovasc Surg. 2003;125:533. doi: 10.1067/mtc.2003.166. [DOI] [PubMed] [Google Scholar]
- 7.King BJ, Iyer H, Leidi AA, et al. Gastroesophageal reflux in bronchiolitis obliterans syndrome: A new perspective. J Heart Lung Transplant. 2009;28:870. doi: 10.1016/j.healun.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 8.Palmer SM, Miralles AP, Howell DN, et al. Gastroesophageal reflux as a reversible cause of allograft dysfunction after lung transplantation. Chest. 2000;118:1214. doi: 10.1378/chest.118.4.1214. [DOI] [PubMed] [Google Scholar]
- 9.Parada MT, Alba A, Sepúlveda C. Bronchiolitis obliterans syndrome development in lung transplantation patients. Transplant Proc. 2010;42:331. doi: 10.1016/j.transproceed.2009.11.037. [DOI] [PubMed] [Google Scholar]
- 10.Burton PR, Button B, Brown W, et al. Medium-term outcome of fundoplication after lung transplantation. Dis Esophagus. 2009;22:642. doi: 10.1111/j.1442-2050.2009.00980.x. [DOI] [PubMed] [Google Scholar]
- 11.Lau CL, Palmer SM, Howell DN, et al. Laparoscopic antireflux surgery in the lung transplant population. Surg Endosc. 2002;16:1674. doi: 10.1007/s00464-001-8251-2. [DOI] [PubMed] [Google Scholar]
- 12.Gasper WJ, Sweet MP, Hoopes C, et al. Antireflux surgery for patients with end-stage lung disease before and after lung transplantation. Surg Endosc. 2008;22:495. doi: 10.1007/s00464-007-9494-3. [DOI] [PubMed] [Google Scholar]
- 13.O’Halloran EK, Reynolds JD, Lau CL, et al. Laparoscopic Nissen fundoplication for treating reflux in lung transplant recipients. J Gastrointest Surg. 2004;8:132. doi: 10.1016/j.gassur.2003.10.1013. [DOI] [PubMed] [Google Scholar]
- 14.Spechler SJ, Castell DO. Classification of esophageal motility abnormalities. Gut. 2001;49:145. doi: 10.1136/gut.49.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tutuian R, Vela MF, Balaji NS, et al. Esophageal function testing with combined multichannel intraluminal impedance and manometry: Multicenter study in healthy volunteers. Clin Gastroenterol Hepatol. 2003;1:174. doi: 10.1053/cgh.2003.50026. [DOI] [PubMed] [Google Scholar]
- 16.Streets CG, DeMeester TR. Ambulatory 24-hour esophageal pH monitoring: Why, when, and what to do. J Clin Gastroenterol. 2003;37:14. doi: 10.1097/00004836-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Dobhan R, Castell DO. Normal and abnormal proximal esophageal acid exposure: Results of ambulatory dual-probe pH monitoring. Am J Gastroenterol. 1993;88:25. [PubMed] [Google Scholar]
- 18.Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: Clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reid BJ, Haggitt RC, Rubin CE, et al. Observer variation in the diagnosis of dysplasia in Barrett’s esophagus. Hum Pathol. 1988;19:166. doi: 10.1016/s0046-8177(88)80344-7. [DOI] [PubMed] [Google Scholar]
- 20.Davis CS, Jellish WS, Fisichella PM. Laparoscopic fundoplication with and without pyloroplasty for gastroesophageal reflux disease in the lung transplant population: How I do it. J Gastrointest Surg. 2010;14:1434. doi: 10.1007/s11605-010-1233-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartwig MG, Appel JZ, Li B, et al. Chronic aspiration of gastric fluid accelerates pulmonary allograft dysfunction in a rat model of lung transplantation. J Thorac Cardiovasc Surg. 2006;131:209. doi: 10.1016/j.jtcvs.2005.06.054. [DOI] [PubMed] [Google Scholar]
- 22.Sweet MP, Patti MG, Hoopes C, et al. Gastro-esophageal reflux and aspiration in patients with advanced lung disease. Thorax. 2009;64:167. doi: 10.1136/thx.2007.082719. [DOI] [PubMed] [Google Scholar]
- 23.Davis CS, Shankaran V, Kovacs EJ, et al. Gastroesophageal reflux disease after lung transplantation: Pathophysiology and implications for treatment. Surgery. 2010;148:737. doi: 10.1016/j.surg.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantu E, III, Appel JZ, III, Hartwig MG, et al. Maxwell Chamberlain Memorial Paper. Early fundoplication prevents chronic allograft dysfunction in patients with gastroesophageal reflux disease. Ann Thorac Surg. 2004;78:1142. doi: 10.1016/j.athoracsur.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 25.El-Serag HB. Epidemiology of non-erosive reflux disease. Digestion. 2008;78(Suppl 1):6. doi: 10.1159/000151249. [DOI] [PubMed] [Google Scholar]
- 26.Wright RA, Hurwitz AL. Relationship of hiatal hernia to endoscopically proved reflux esophagitis. Dig Dis Sci. 1979;24:311. doi: 10.1007/BF01296546. [DOI] [PubMed] [Google Scholar]
- 27.Jones MP, Sloan SS, Rabine JC, et al. Hiatal hernia size is the dominant determinant of esophagitis presence and severity in gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:1711. doi: 10.1111/j.1572-0241.2001.03926.x. [DOI] [PubMed] [Google Scholar]
- 28.Patti MG, Goldberg HI, Arcerito M, et al. Hiatal hernia size affects lower esophageal sphincter function, esophageal acid exposure, and the degree of mucosal injury. Am J Surg. 1996;171:182. doi: 10.1016/S0002-9610(99)80096-8. [DOI] [PubMed] [Google Scholar]
- 29.Blondeau K, Mertens V, Vanaudenaerde BA, et al. Nocturnal weakly acidic reflux promotes aspiration of bile acids in lung transplant recipients. J Heart Lung Transplant. 2009;28:141. doi: 10.1016/j.healun.2008.11.906. [DOI] [PubMed] [Google Scholar]
- 30.Reid KR, McKenzie FN, Menkis AH, et al. Importance of chronic aspiration in recipients of heart-lung transplants. Lancet. 1990;336:206. doi: 10.1016/0140-6736(90)91734-r. [DOI] [PubMed] [Google Scholar]
- 31.D’Ovidio F, Mura M, Ridsdale R, et al. The effect of reflux and bile acid aspiration on lung allograft and its surfactant and innate immunity molecules SP-A and SP-D. Am J Transplant. 2006;6:1930. doi: 10.1111/j.1600-6143.2006.01357.x. [DOI] [PubMed] [Google Scholar]
- 32.Au J, Hawkins T, Venables C, et al. Upper gastrointestinal dysmotility in heart-lung transplant recipients. Ann Thorac Surg. 1993;55:94. doi: 10.1016/0003-4975(93)90480-6. [DOI] [PubMed] [Google Scholar]
- 33.Berkowitz N, Schulman LL, McGregor C, et al. Gastroparesis after lung transplantation. Potential role in postoperative respiratory complications. Chest. 1995;108:1602. doi: 10.1378/chest.108.6.1602. [DOI] [PubMed] [Google Scholar]
- 34.Sodhi SS, Guo JP, Maurer AH, et al. Gastroparesis after combined heart and lung transplantation. J Clin Gastroenterol. 2002;34:34. doi: 10.1097/00004836-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Parkman HP, Hasler WL, Fisher RS, American Gastroenterological Association American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592. doi: 10.1053/j.gastro.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 36.Jones MP, Sloan SS, Rabine JC, et al. Hiatal hernia size is the dominant determinant of esophagitis presence and severity in gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:1711. doi: 10.1111/j.1572-0241.2001.03926.x. [DOI] [PubMed] [Google Scholar]
- 37.Kahrilas PJ, Dodds WJ, Hogan WJ, et al. Esophageal peristaltic dysfunction in peptic esophagitis. Gastroenterology. 1986;91:897. doi: 10.1016/0016-5085(86)90692-x. [DOI] [PubMed] [Google Scholar]
- 38.Blondeau K, Mertens V, Vanaudenaerde BA, et al. Gastrooesophageal reflux and gastric aspiration in lung transplant patients with or without chronic rejection. Eur Respir J. 2008;31:707. doi: 10.1183/09031936.00064807. [DOI] [PubMed] [Google Scholar]
- 39.D’Ovidio F, Mura M, Tsang M, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. Thorac Cardiovasc Surg. 2005;129:1144. doi: 10.1016/j.jtcvs.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 40.Stovold R, Forrest IA, Corris PA, et al. Pepsin, a biomarker of gastric aspiration in lung allografts: A putative association with rejection. Am J Respir Crit Care Med. 2007;175:1298. doi: 10.1164/rccm.200610-1485OC. [DOI] [PubMed] [Google Scholar]
- 41.Pellegrini CA, DeMeester TR, Johnson LF, et al. Gastroesophageal reflux and pulmonary aspiration: Incidence, functional abnormality, and results of surgical therapy. Surgery. 1979;86:110. [PubMed] [Google Scholar]
- 42.Patti MG, Debas HT, Pellegrini CA. Clinical and functional characterization of high gastroesophageal reflux. Am J Surg. 1993;165:163. doi: 10.1016/s0002-9610(05)80421-0. [DOI] [PubMed] [Google Scholar]