Abstract
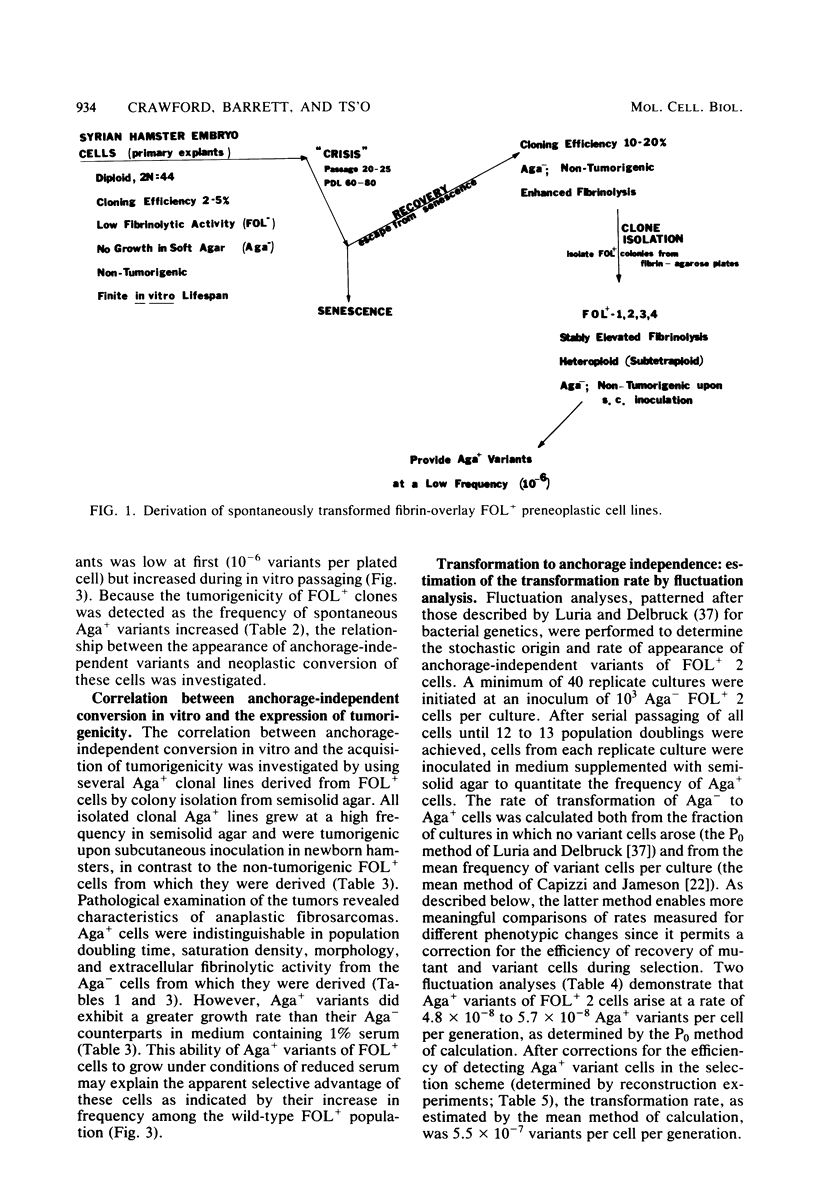
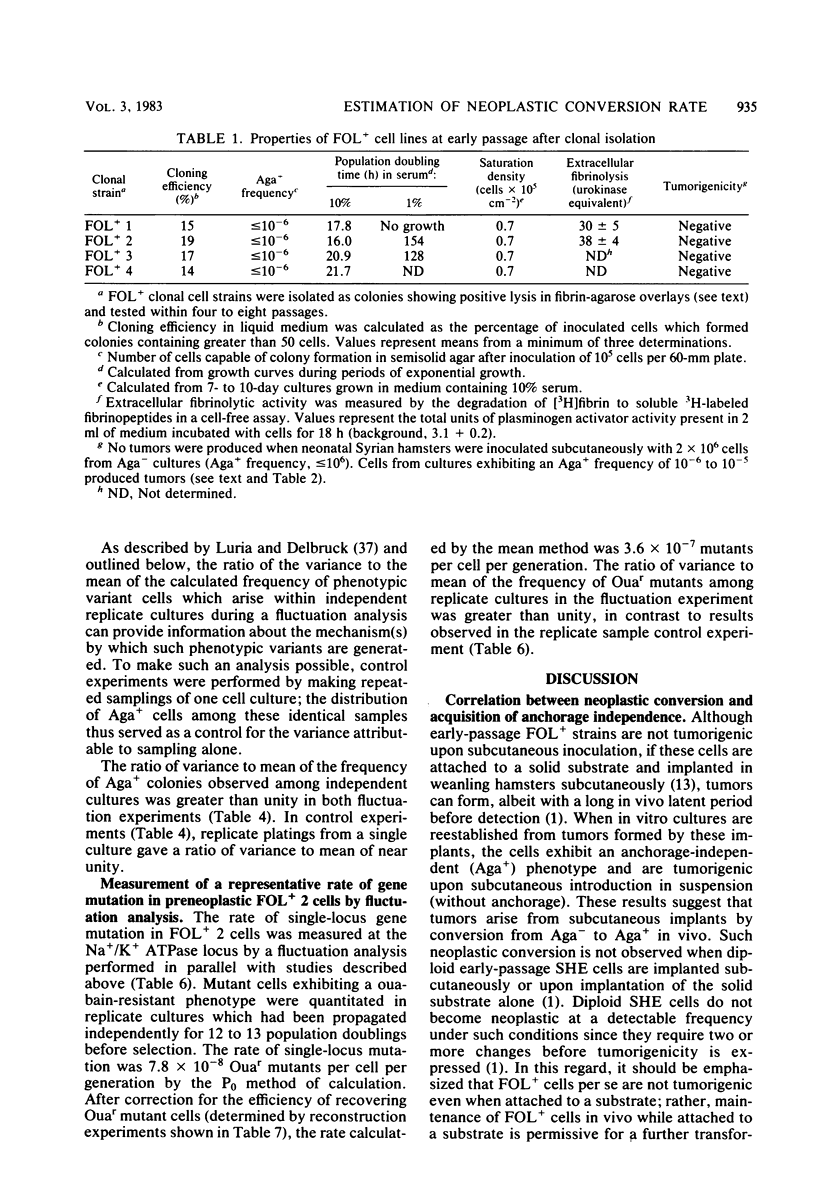
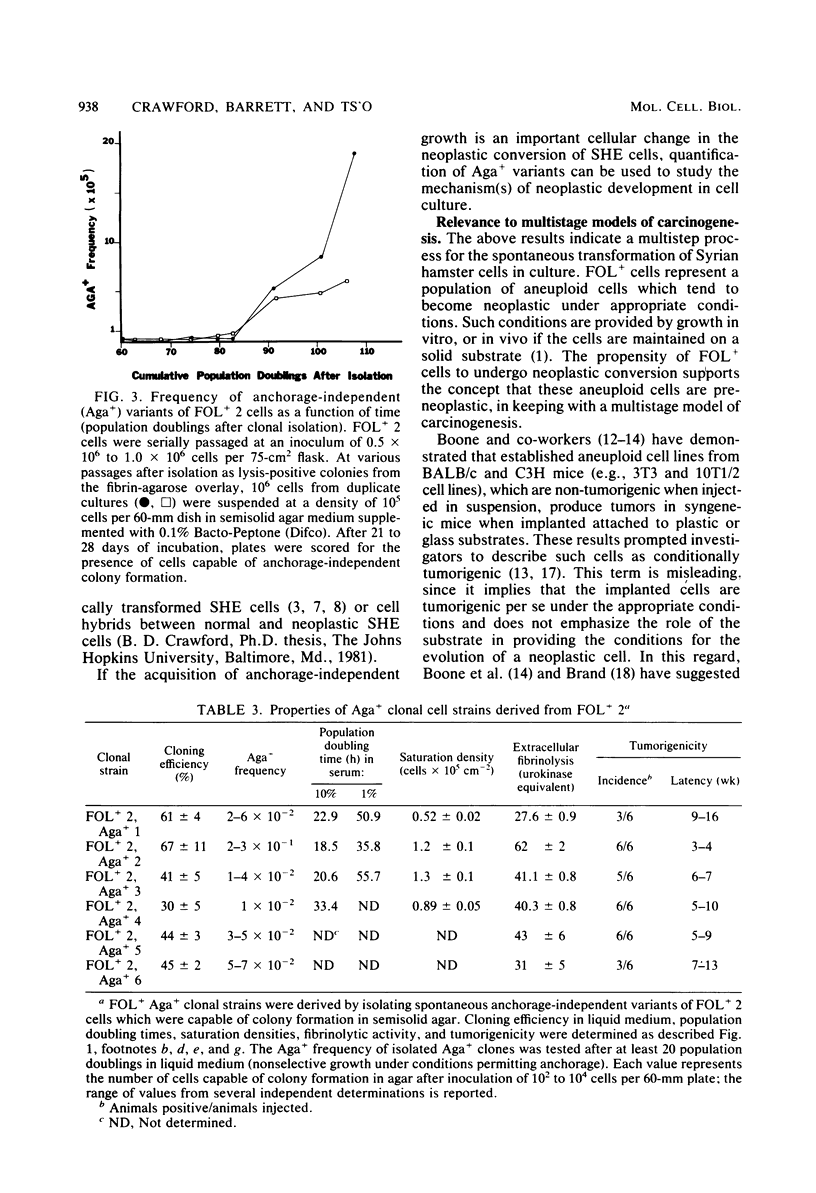
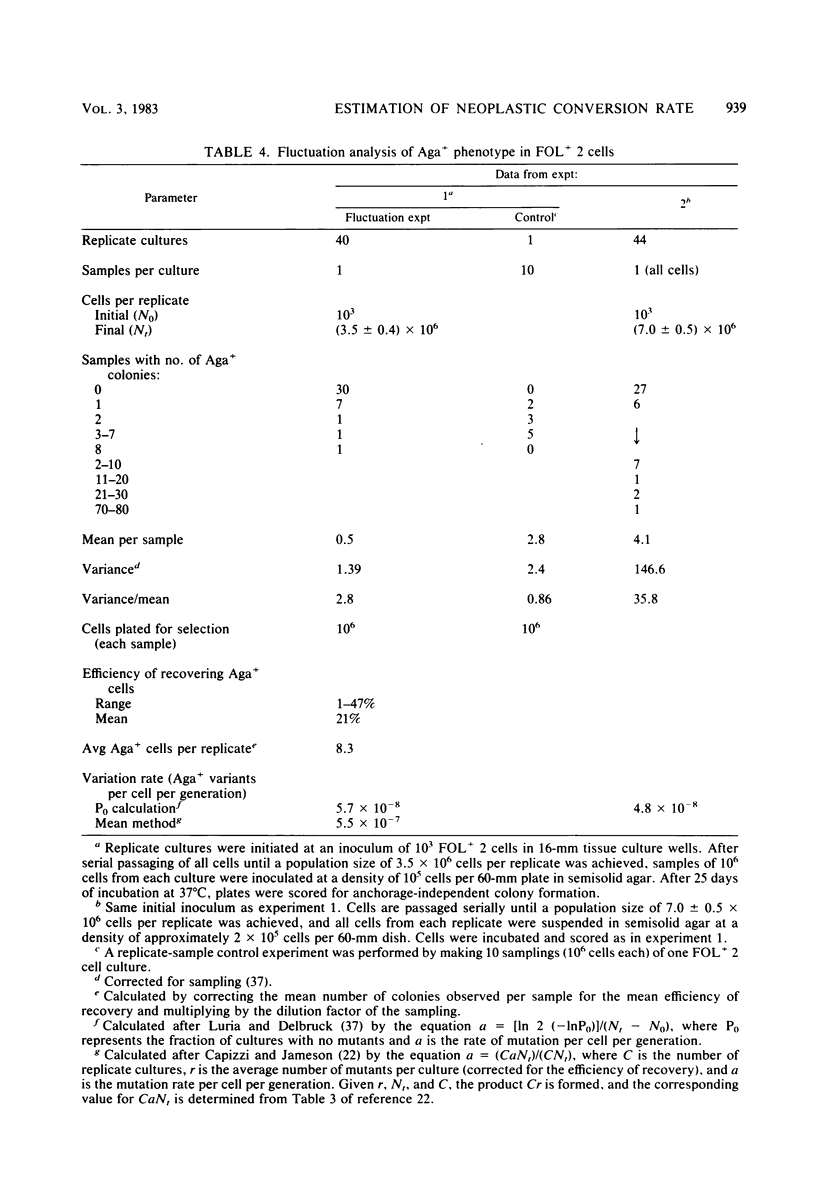
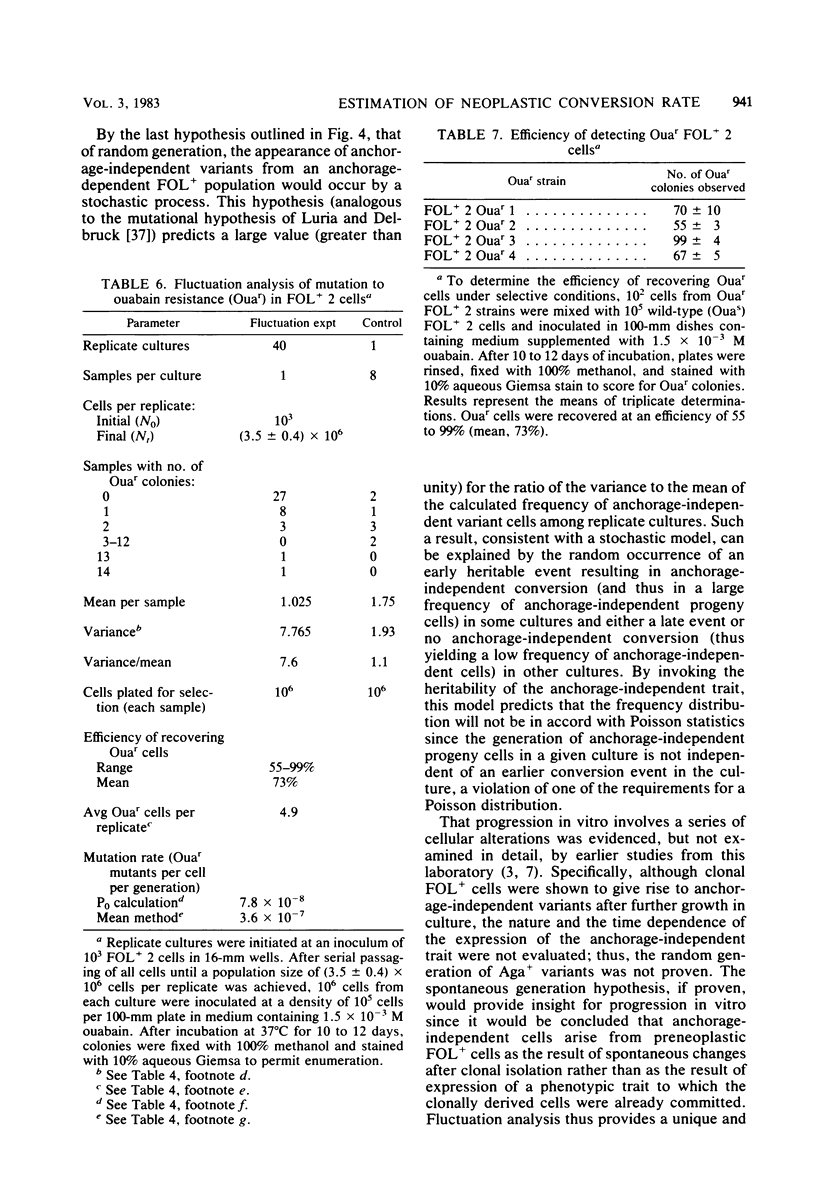
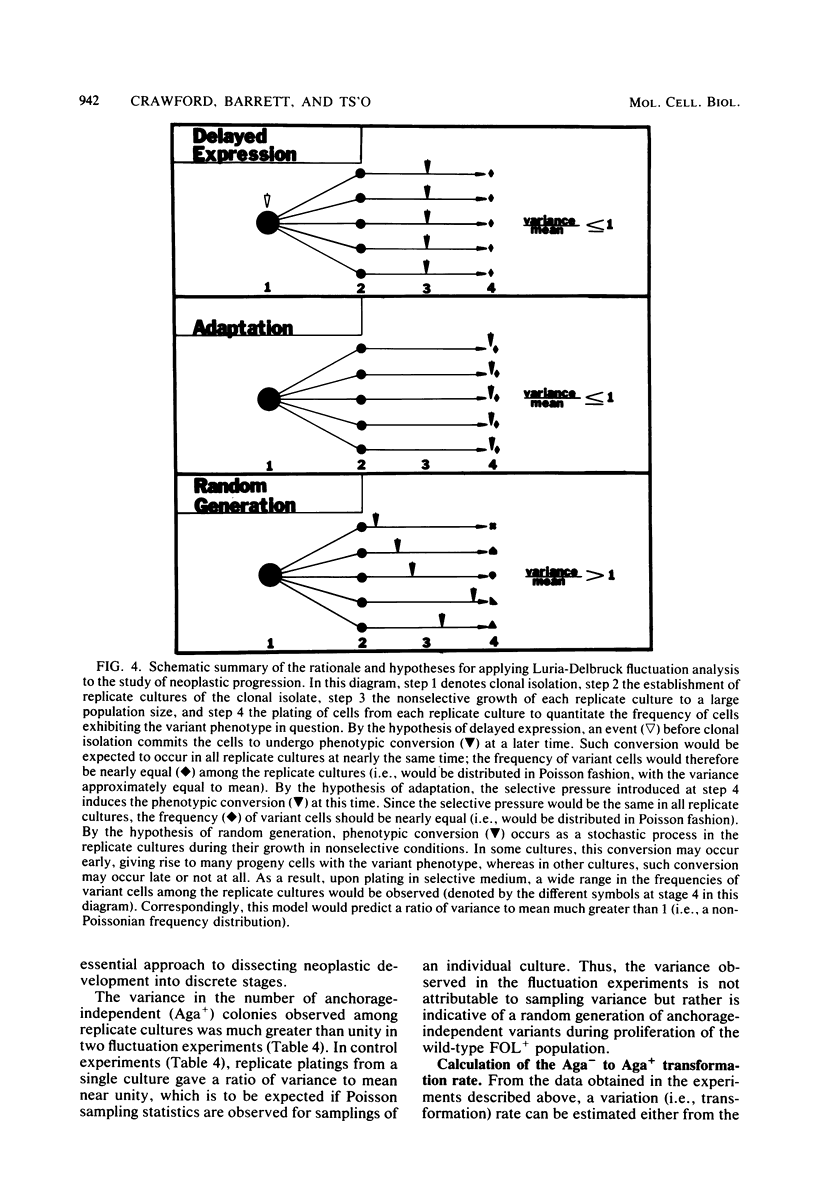
Analysis of the role of gene mutations in the multistep process of neoplastic transformation requires that the discrete steps in carcinogenesis first be dissected. Toward this end, we have isolated and characterized preneoplastic Syrian hamster cells which exhibit in vitro a trait highly correlated with neoplastic conversion in vivo. Previous findings (J. C. Barrett, Cancer Res. 40:91-94, 1980) indicate that spontaneous neoplastic transformation of Syrian hamster cells occurs in at least two steps. An intermediate stage, characterized by an aneuploid established cell line which has a propensity to become neoplastic spontaneously upon further growth in vitro, has been described. These preneoplastic cells differ from diploid early-passage Syrian hamster cells in becoming capable of anchorage-independent growth in semisolid agar, as well as becoming neoplastic in vivo when attached to a solid substrate. Evidence presented here demonstrates that anchorage-independent conversion in vitro is a reliable marker for neoplastic conversion in this cell system. Fluctuation analyses, patterned after those described by Luria and Delbruck for microbial genetics, demonstrate that anchorage-independent variants are generated randomly from clonally derived preneoplastic cells at the rate of 10(-8) to 10(-7) variants per cell per generation. These results establish a multistep stochastic process for transformation in vitro and indicate that conversion to anchorage independence may be necessary for Syrian hamster cells to become tumorigenic. The possible role of gene mutation in this step during neoplastic progression is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. C. A preneoplastic stage in the spontaneous neoplastic transformation of syrian hamster embryo cells in culture. Cancer Res. 1980 Jan;40(1):91–94. [PubMed] [Google Scholar]
- Barrett J. C., Bias N. E., Ts'o P. O. A mammalian cellular system for the concomitant study of neoplastic transformation and somatic mutation. Mutat Res. 1978 Apr;50(1):121–136. doi: 10.1016/0027-5107(78)90067-2. [DOI] [PubMed] [Google Scholar]
- Barrett J. C., Crawford B. D., Grady D. L., Hester L. D., Jones P. A., Benedict W. F., Ts'o P. O. Temporal acquistion of enhanced fibrinolytic activity by syrian hamster embryo cells following treatment with benzo(a)pyrene. Cancer Res. 1977 Oct;37(10):3815–3823. [PubMed] [Google Scholar]
- Barrett J. C., Crawford B. D., Mixter L. O., Schechtman L. M., Ts'o P. O., Pollack R. Correlation of in vitro growth properties and tumorigenicity of Syrian hamster cell lines. Cancer Res. 1979 May;39(5):1504–1510. [PubMed] [Google Scholar]
- Barrett J. C., Crawford B. D., Ts'o P. O. Quantitation of fibrinolytic activity of Syrian hamster fibroblasts using 3H-labeled fibrinogen prepared by reductive alkylation. Cancer Res. 1977 Apr;37(4):1182–1185. [PubMed] [Google Scholar]
- Barrett J. C., Ts'o P. O. Evidence for the progressive nature of neoplastic transformation in vitro. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3761–3765. doi: 10.1073/pnas.75.8.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. C., Ts'o P. O. Relationship between somatic mutation and neoplastic transformation. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3297–3301. doi: 10.1073/pnas.75.7.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. C., Tsutsui T., Ts'o P. O. Neoplastic transformation induced by a direct perturbation of DNA. Nature. 1978 Jul 20;274(5668):229–232. doi: 10.1038/274229a0. [DOI] [PubMed] [Google Scholar]
- Bellett A. J., Younghusband H. B. Spontaneous, mutagen-induced and adenovirus-induced anchorage independent tumorigenic variants of mouse cells. J Cell Physiol. 1979 Oct;101(1):33–47. doi: 10.1002/jcp.1041010106. [DOI] [PubMed] [Google Scholar]
- Boone C. W., Jacobs J. B. Sarcomas routinely produced from putatively nontumorigenic Balb/3T3 and C3H/10T1/2 cells by subcutaneous inoculation attached to plastic platelets. J Supramol Struct. 1976;5(2):131–137. doi: 10.1002/jss.400050204. [DOI] [PubMed] [Google Scholar]
- Boone C. W. Malignant hemangioendotheliomas produced by subcutaneous inoculation of Balb/3T3 cells attached to glass beads. Science. 1975 Apr 4;188(4183):68–70. doi: 10.1126/science.1114343. [DOI] [PubMed] [Google Scholar]
- Boone C. W., Takeichi N., Eaton S. D., Paranjpe M. "Sontaneous" neoplastic transformation in vitro: a form of foreign body (smooth surface) tumorigenesis. Science. 1979 Apr 13;204(4389):177–179. doi: 10.1126/science.373119. [DOI] [PubMed] [Google Scholar]
- Bouck N., di Mayorca G. Somatic mutation as the basis for malignant transformation of BHK cells by chemical carcinogens. Nature. 1976 Dec 23;264(5588):722–727. doi: 10.1038/264722a0. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F. Different calcium requirements for proliferation of conditionally and unconditionally tumorigenic mouse cells. Proc Natl Acad Sci U S A. 1976 May;73(5):1651–1654. doi: 10.1073/pnas.73.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand K. G., Buoen L. C., Johnson K. H., Brand I. Etiological factors, stages, and the role of the foreign body in foreign body tumorigenesis: a review. Cancer Res. 1975 Feb;35(2):279–286. [PubMed] [Google Scholar]
- Brand K. G. Diversity and complexity of carcinogenic processes: conceptual inferences from foreign-body tumorigenesis. J Natl Cancer Inst. 1976 Nov;57(5):973–976. doi: 10.1093/jnci/57.5.973. [DOI] [PubMed] [Google Scholar]
- Brand K. G., Johnson K. H., Buoen L. C. Foreign body tumorigenesis. CRC Crit Rev Toxicol. 1976 Oct;4(4):353–394. doi: 10.1080/10408447609164018. [DOI] [PubMed] [Google Scholar]
- Capizzi R. L., Jameson J. W. A table for the estimation of the spontaneous mutation rate of cells in culture. Mutat Res. 1973 Jan;17(1):147–148. doi: 10.1016/0027-5107(73)90265-0. [DOI] [PubMed] [Google Scholar]
- Cifone M. A., Fidler I. J. Correlation of patterns of anchorage-independent growth with in vivo behavior of cells from a murine fibrosarcoma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1039–1043. doi: 10.1073/pnas.77.2.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn N. H., Bruegge W. F., Bates J. R., Gray R. H., Rossen J. D., Kelsey W. H., Shimada T. Correlation of anchorage-independent growth with tumorigenicity of chemically transformed mouse epidermal cells. Cancer Res. 1978 Mar;38(3):624–634. [PubMed] [Google Scholar]
- FOULDS L. The experimental study of tumor progression: a review. Cancer Res. 1954 Jun;14(5):327–339. [PubMed] [Google Scholar]
- Fidler I. J. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 1978 Sep;38(9):2651–2660. [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Freedman V. H., Shin S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974 Dec;3(4):355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H., McDougall J. K., Chen L. B. In vitro traits of adenovirus-transformed cell lines and their relevance to tumorigenicity in nude mice. Cell. 1977 Apr;10(4):669–678. doi: 10.1016/0092-8674(77)90100-3. [DOI] [PubMed] [Google Scholar]
- Ishii Y., Elliott J. A., Mishra N. K., Lieberman M. W. Quantitative studies of transformation by chemical carcinogens and ultraviolet radiation using a subclone of BHK21 clone 13 Syrian hamster cells. Cancer Res. 1977 Jul;37(7 Pt 1):2023–2029. [PubMed] [Google Scholar]
- Jones P. A., Laug W. E., Gardner A., Nye C. A., Fink L. M., Benedict W. F. In vitro correlates of transformation in C3H/10T1/2 clone 8 mouse cells. Cancer Res. 1976 Aug;36(8):2863–2867. [PubMed] [Google Scholar]
- Kahn P., Shin S. I. Cellular tumorigenicity in nude mice. Test of associations among loss of cell-surface fibronectin, anchorage independence, and tumor-forming ability. J Cell Biol. 1979 Jul;82(1):1–16. doi: 10.1083/jcb.82.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakunaga T., Kamahora J. Properties of hamster embryonic cells transformed by 4-nitroquinoline-1-oxide in vitro and their correlations with the malignant properties of the cells. Biken J. 1968 Dec;11(4):313–332. [PubMed] [Google Scholar]
- Leavitt J. C., Crawford B. D., Barrett J. C., Ts'o P. O. Regulation of requirements for anchorage-independent growth of Syrian hamster fibroblasts by somatic mutation. Nature. 1977 Sep 1;269(5623):63–65. doi: 10.1038/269063a0. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Miyashita K., Kakunaga T. Isolation of heat- and cold-sensitive mutants of chinese hamster lung cells affected in their ability to express the transformed state. Cell. 1975 Jun;5(2):131–138. doi: 10.1016/0092-8674(75)90021-5. [DOI] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Peehl D. M., Stanbridge E. J. Anchorage-independent growth of normal human fibroblasts. Proc Natl Acad Sci U S A. 1981 May;78(5):3053–3057. doi: 10.1073/pnas.78.5.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Sanford K. K. Biologic manifestations of oncogenesis in vitro: a critique. J Natl Cancer Inst. 1974 Nov;53(5):1481–1485. doi: 10.1093/jnci/53.5.1481. [DOI] [PubMed] [Google Scholar]
- Shapiro N. I., Varshaver N. B. Mutagenesis in cultured mammalian cells. Methods Cell Biol. 1975;10:209–234. doi: 10.1016/s0091-679x(08)60739-6. [DOI] [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinskas K. C., Kateley S. A., Tower J. E., Maher V. M., McCormick J. J. Induction of anchorage-independent growth in human fibroblasts by propane sultone. Cancer Res. 1981 May;41(5):1620–1627. [PubMed] [Google Scholar]
- Stanbridge E. J., Wilkinson J. Analysis of malignancy in human cells: malignant and transformed phenotypes are under separate genetic control. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1466–1469. doi: 10.1073/pnas.75.3.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanbridge E. J., Wilkinson J. Dissociation of anchorage independence form tumorigenicity in human cell hybrids. Int J Cancer. 1980 Jul 15;26(1):1–8. doi: 10.1002/ijc.2910260102. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Desmond W., Chuman L. M., Sato G., Saier M. H., Jr Relationship of cell growth behavior in vitro to tumorigenicity in athymic nude mice. Cancer Res. 1976 Sep;36(9 PT1):3300–3305. [PubMed] [Google Scholar]
- Tsutsui T., Barrett J. C., Ts'o P. O. Morphological transformation, DNA damage, and chromosomal aberrations induced by a direct DNA perturbation of synchronized Syrian hamster embryo cells. Cancer Res. 1979 Jul;39(7 Pt 1):2356–2365. [PubMed] [Google Scholar]
- Tsutsui T., Crawford B. D., Ts'o P. O., Barrett J. C. Comparison between mutagenesis in normal and transformed Syrian hamster fibroblasts: difference in the temporal order of HPRT gene replication. Mutat Res. 1981 Feb;80(2):357–371. doi: 10.1016/0027-5107(81)90108-1. [DOI] [PubMed] [Google Scholar]
- Tucker R. W., Sanford K. K., Handleman S. L., Jones G. M. Colony morphology and growth in agarose as tests for spontaneous neoplastic transformation in vitro. Cancer Res. 1977 May;37(5):1571–1579. [PubMed] [Google Scholar]





