Abstract
Purpose
To determine the sensitivity to change of the US7 score among RA patients under various therapies and to analyze the effect of each therapeutic option over 1 year. To estimate predictors for development of destructive bone changes.
Methods
Musculoskeletal ultrasound (US7 score), DAS28, CRP and ESR were performed in 432 RA patients at baseline and after 3, 6 and 12 months. The cohort was divided into four sub-groups: first-line DMARDs (Group 1; 27.3%), therapy switch: DMARDs to second DMARDs (Group 2; 25.0%), first-line biologic after DMARDs therapy (Group 3; 35.4%) and therapy change from biologic to second biologic (Group 4; 12.3%).
Results
The US7 synovitis and tenosynovitis sum scores in grey-scale (GSUS) and power Doppler ultrasound (PDUS) as well as ESR, CRP decreased significantly (p<0.05) after 12 months in group 1 to 3. Group 1+2 also illustrated a significant change of DAS28 after 1 year (p<0.001). Only in Group 4, the US7 erosion sum score decreased significantly from 4.3 to 3.6 (p=0.008) after 1 year. Predictors capable of forecasting US erosions after one year were: higher score of US7 synovitis (p<0.001), of US7 erosions in GSUS (p<0.001), as well as of DAS28 (p<0.001) at baseline.
Conclusions
The comparable developments of the US7 score with clinical and laboratory data illustrates its potential to reflect therapeutic response. Therefore, the novel US7 score is sensitive to change. Patients who switched from one biologic to another exhibited a significant decline in erosions after 12 months, while the erosions scores in the other groups were stable.
Keywords: Rheumatoid Arthritis, Ultrasonography, Synovitis, DAS28, TNF-alpha
Introduction
Chronic-inflammatory arthritis such as rheumatoid arthritis (RA) can lead to severe joint damage. Disease progress is determined by the time point of diagnosis, accompanied by early onset of therapy, by disease activity, genetic factors, and autoantibodies positivity.1 In order to prevent structural damage, it is necessary to monitor treatment response in RA patients. Besides clinical and serological assessments, musculoskeletal ultrasound (US) is now an important tool in the diagnosis and monitoring of disease progress. US is suitable to measure both disease activity and structural damage. To justify the use of expensive medication such as biologics, it is essential to monitor treatment response. This can be accomplished by the measurement of disease activity in US, using grey-scale (GS) and power Doppler (PD) mode for synovitis and tenosynovitis. It is also possible to visualise certain bone lesions such as erosions, representing structural damage.
Furthermore, studies established the value of persistent synovitis in grey-scale US (GSUS) and power Doppler US (PDUS) in the prediction of bone erosions.2 A predictive value in the development of erosions in early RA patients is also signified by tenosynovitis of the extensor carpi ulnaris tendon.3
In order to measure RA progress, a semi-quantitative US scoring system ‘US7 score’ was developed. This US score combines soft tissue changes (synovitis and tenosynovitis) and erosive bone lesions in seven preselected joints in one US scoring system. The selected joints give an effective reflection of the overall joint inflammatory activity. The first publication of US7 score data indicated its feasibility in daily rheumatologic practice, while focusing on a small number of joints and showed that this method is more sensitive than the clinical instrument DAS28 in the characterisation of inflammatory joint processes.4
The aim of the present study was to reflect the sensitivity to change of the US7 score among a large cohort of RA patients under various therapies (disease modifying antirheumatic drugs (DMARDs) and/or biologics) and to analyse the effect of each therapeutic option over a period of 1 year. Another purpose of this study was to estimate predictors for the development of erosions after 1 year.
Patients and methods
From 2006 until 2010 a nationwide project recruited 54 centres with a total of 432 patients with RA according to American College of Rheumatology (ACR) criteria 1987.5 All patients agreed by informed consents, and the study was approved by the ethical committee of University of Tubingen. Patients were examined by US (mean amount of patients: 8%) before and after change of treatment because of not achieving DAS28 remission (DAS28<2.6) at four visits (baseline, after 3, 6, and 12 months).
Medication
The cohort was divided into four groups according to therapy: Group 1: first-line DMARD after new initiation (n=118; 27.3%), Group 2: therapy switch from DMARD to a second DMARD (n=108; 25.0%), Group 3: first-line biologic after DMARD therapy (n=153; 35.4%), Group 4: therapy switch from biologic to a second biologic (n=53; 12.3%).
Clinical assessment
The medical examination focused on the disease activity score in the 28 joints assessed to determine the DAS28.
Laboratory parameters
Laboratory parameters were determined at each visit to investigate systemic inflammation. C reactive protein (CRP) level (normal level ≤5 mg/l) and erythrocyte sedimentation rate (ESR; normal level ≤20 mm after 1 hour) were obtained at each visit. IgM-Rheumatoid factor (IgM-RF: normal level <20 U/l) and anti-citrullinated protein antibodies (ACPA: normal level <20 U/l) were assessed baseline.
US7 Score examination
Musculoskeletal ultrasonography of wrist, hands and forefoot was performed with a 10–18 MHz linear scanner and middle class to high-end machine US devices. Settings for GSUS: frequency: 16 MHz, length of scanner: 40–42 mm. The use of GSUS gain depended on joint regions and patients and was average 50%. Settings for PDUS: frequency: 9.1 MHz, pulse repetition frequency: 500–750 Hz (depending on machine setting), PDUS gain depended on joint regions and patients and was average 50%, wall filter was low for example,3, and had to be maintained throughout the study. The PDUS gain was not supposed to change within a joint panel of a patient during the examination. The exact same machine had to be used on every patient during the study time. The hand and forefoot which were more clinically affected by tenderness and/or swelling were chosen for US and were examined at four visits after onset of therapy or switch to actual therapy (DMARDs and/or biologic). This included the joints most likely to be affected by RA: wrist, metacarpophalangeal (MCP) II and III, proximal interphalangeal (PIP) II and III, metatarsophalangeal (MTP) II and V joints. These joints were evaluated for synovitis and tenosynovitis/paratenonitis and superficial bone erosions according to EULAR criteria5 and Outcome measures in Rheumatology (OMERACT) definition6 including GSUS and PDUS (table 1). Synovitis and synovial/tenosynovial vascularity were scored semi-quantitatively (Grades 0–3) by PDUS according to Szkudlarek et al.7 Synovitis (effusion and synovial hypertrophy) in GSUS was classified semi-quantitatively as described by Scheel et al.8 Tenosynovitis/paratenonitis as well as erosions in GSUS were registered as being absent (0) or present (1).
Table 1.
US7 score components
| Wrist | MCP/PIP II+III | MTP II+V | Joint region (range) | |
|---|---|---|---|---|
| US7 synovitis sum score in GSUS (grade 0–3) | Dorso-median Ulnar Palmo-median |
Palmar | Dorsal | 9 (min: 0–max: 27) |
| US7 synonitis sum score in PDUS (grade 0–3) | Dorso-median Ulnar Palmo-median |
Dorsal and palmar | Dorsal | 13 (min: 0–max: 39) |
| US7 tenosynovitis/paratenonitis sum score in GSUS (absent=0 present=1) | Dorso-median Ulnar Palmo-median |
Dorsal and palmar (in level of MCP II+III) | 7 (min: 0–max: 7) | |
| US7 tenosynovitis/paratenonitis sum score in PDUS (grade 0–3) | Dorso-median Ulnar Palmo-median |
Dorsal and palmar (in level of MCP II+III) | 7 (min: 0–max: 21) | |
| US7 erosion sum score in GSUS (absent=0 present=1) | Dorsal and palmar (only radial at MCP II) | Dorsal and plantar (only lateral at MTP V) | 14 (min: 0–max: 14) |
GSUS, grey-scale ultrasound; MCP, metacarpophalangeal; MTP, metatarsophalangeal; PDUS, power Doppler ultrasound; PIP, proximal interphalangeal; US, ultrasound.
Statistical analysis
Statistical analysis was performed with SPSS statistical software, V.18.0 (SPSS, Chicago, Illinois, USA). For quantitative parameters (eg, age of examined patients and disease duration), the mean±SD and range were used. Because of missing normal distribution (tested by Shapiro Wilk-Test) changes were subjected to the 2-sided exact Wilcoxon's test whereas p values below 0.05 were considered as statistically significant. Correlations between changes of the different examination modalities (eg, DAS28 and US) throughout follow-ups were evaluated by 2-sided exact Spearman's correlation coefficients. For the calculation of predictive values, univariate linear regression analysis was performed.
Results
Characteristics of patients
432 patients with RA (81.0% women) and a mean±SD age of 57±12.8 years (range 17–84) and a mean±SD disease duration of 8.3±8.7 years (range 0.08–58.3) were examined at four visits (baseline, after 3, 6, and 12 months). At inclusion, 70.6% were IgM-RF positive and 66.5% were ACPA positive. 73.2% of the 432 RA patients received a prednisolone equivalent with a mean daily dosage of 8.8 mg per day (table 2).
Table 2.
Demographic data, serological and clinical characteristics of the different subgroups dependent on therapy
| Group 1 (n=118) | Group 2 (n=108) | Group 3 (n=153) | Group 4 (n=53) | |
|---|---|---|---|---|
| Female | 77.1% | 79.2% | 80.4% | 88.7% |
| Age (years) | 56.1±13.9 (20–84) | 60.1±11.7 (17–84) | 56.2±12.3 (21–84) | 53.5±13.8 (28–80) |
| Disease duration (years) | 5.3±8.8 (0.08–58.3) | 8.0±8.4 (0.2–37.3) | 9.5±8.3 (0.2–47) | 12.1±6.6 (1.6–31.8) |
| IgM-RF positive | 63.6% | 72.0% | 75.5% | 67.9% |
| ACPA positive | 60% | 72.7% | 69.2% | 64.4% |
| DAS28 (baseline) | 5.0±1.4 (1.4–8.3) | 4.3±1.4 (1.5–7.9) | 5.2±1.3 (1.3–8.2) | 3.8±1.3 (1.4–6.4) |
| ESR, mm/h (baseline) | 31.1±22.3 (2–100) | 25.7±19.4 (2–90) | 33.3±21.7 (1–108) | 26.3±20.8 (2–95) |
| CRP level, mg/l (baseline) | 20.5±28.6 (0–162) | 13.3±23.0 (0–170) | 17.3±22.7 (0–120) | 8.3±11.0 (0–53.6) |
| Prednisolone equivalent (mg/d) (baseline) | 8.8±13.6 (0–90) | 5.3±4.3 (0–20) | 7.0±7.1 (0–50) | 3.6±3.2 (0–12.5) |
*Values are the mean±SD (range).
Group 1: first-line DMARD after new titration, Group 2: therapy switch from DMARD to second DMARD, Group 3: first-line biologic after DMARD therapy, and Group 4: therapy change from biologic to second biologic.
ACPA, anti-cyclic citrullinated peptide antibodies; CRP, C reactive protein; DAS28, Disease Activity Score in 28 joints; ESR, erythrocyte sedimentation rate; IgM-RF, IgM rheumatoid factor.
US, clinical and laboratory parameters under various therapies over 12 months
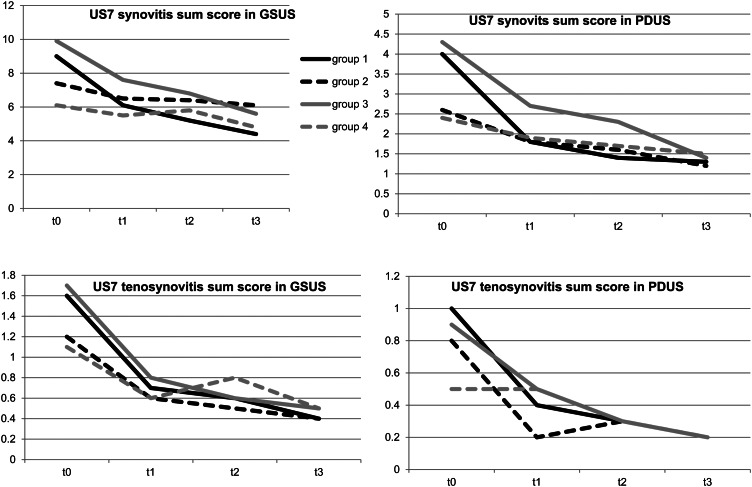
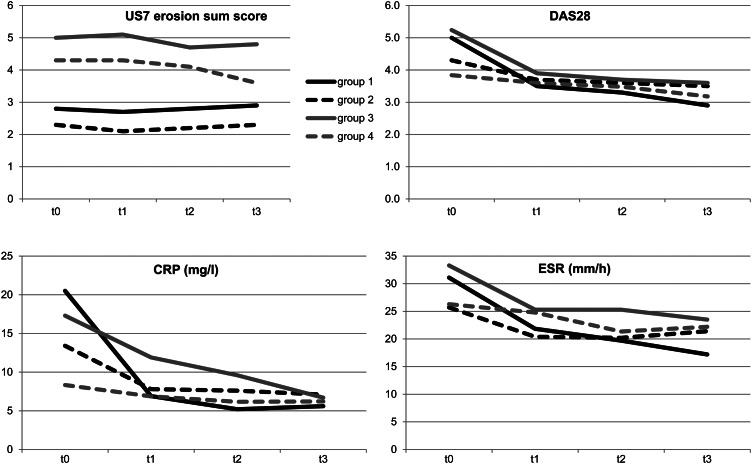
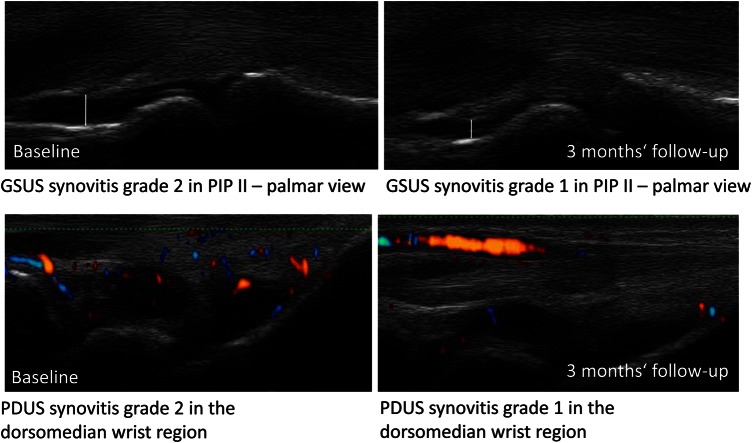
In Group 1, the synovitis (GSUS: 9.0 to 4.4; −51.1%; p<0.001 and PDUS 4.0 to 1.3; −67.5%; p<0.001) and tenosynovitis sum scores (GSUS: 1.6 to 0.4; −75%; p<0.001 and PDUS: 1.0 to 0.2; −80% p<0.001) decreased significantly after 12 months of therapy (figures 1 and 2). One example of the reduction of synovitis by GSUS and PDUS in the wrist and finger joint after 3 months is given in figure 3. The mean DAS28 score declined from 5.0 to 2.9, (−42%) which on average indicates a change from moderate to low disease activity (p<0.001). ESR (31.1 to 17.2; −44.7%) and CRP (20.5 to 5.6; −72.7%) also fell significantly after 1 year of DMARD therapy (p<0.001). The erosion sum score remained almost constant with no further progression after a period of 12 months among first-line DMARD therapy (2.8–2.9; p=0.722) (see supplementary table S1).
Figure 1.
Ultrasound synovitis and tenosynovitis sum score in grey-scale ultrasound and power Doppler ultrasound in comparison of the four treatment groups over 12 months. This figure is only reproduced in colour in the online version.
Figure 2.
Erosion sum score, DAS28, C reactive protein, and erythrocyte sedimentation rate in comparison of the four treatment groups over 12 months. This figure is only reproduced in colour in the online version.
Figure 3.
Grey-scale ultrasound and power Doppler ultrasound synovitis of the hand of a 27 years old active rheumatoid arthritis patient before and after 3 months after initiation of methotrexate. This figure is only reproduced in colour in the online version.
Group 2 exhibited a significant decrease of the US7 synovitis sum score in GSUS from an average of 7.4 to 6.1 (−17.6%; p=0.001), and in PDUS from 2.6 to 1.2 (−53.8%; p<0.001) after 1 year of therapy. A significant reduction of the tenosynovitis sum score in GSUS was observed from a baseline value of 1.2 to 0.4 (−66.7%; p<0.001) after 12 months as well as in PDUS from 0.8 to 0.2 (−75%, p=0.001). Laboratory parameters such as CRP dropped significantly from 13.4 mg/l to 7.1 mg/l (−47%; p=0.001) as well as ESR from 25.7 mm/h to 21.4 mm/h (−16.7%; p=0.014). The DAS28 score decreased significantly from a baseline value of 4.3–3.5 (−18.6%; p<0.001) after 1 year. A significant change of the erosion sum score was not evident (p=0.633) (see supplementary table S2).
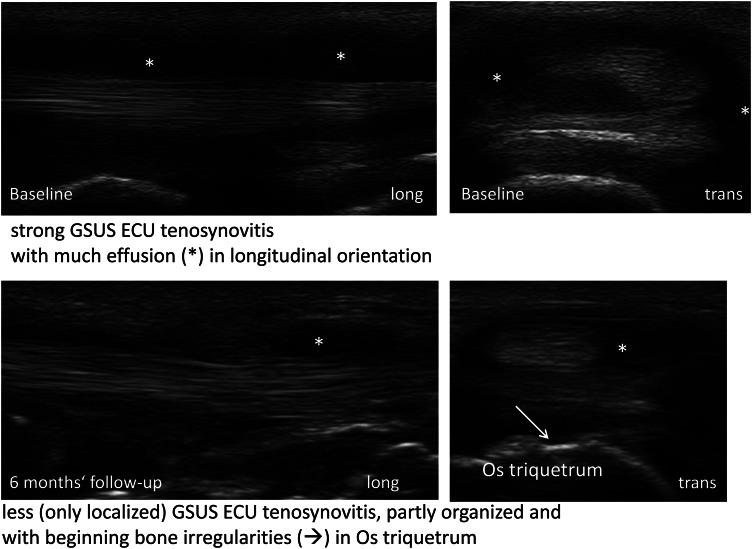
In Group 3, 153 patients with RA had received DMARD therapy before they started a biologic. After change of therapy, US7 synovitis sum score in GSUS dropped significantly from 9.9 to 5.6 (−43.4%; p<0.001) as well as in PDUS from 4.3 to 1.4 (−67.4%; p<0.001) after 12 months. In addition, the US7 tenosynovitis sum scores in GSUS (1.7 to 0.5; −70.6%) and PDUS (0.9 to 0.2; −77.8%) decreased significantly (p<0.001). An example of tenosynovitis reduction after 6 months of biologic therapy is given by figure 4. Initially, the highest erosion sum scores were detected in patients who changed from conventional DMARD to biologic therapy at baseline. A significant change of the erosion sum score was not evident (p=1.0). A significant decrease was seen in the development of the laboratory parameters such as ESR (p<0.001) and CRP (p<0.001) (see supplementary table S3).
Figure 4.
Grey-scale ultrasound tenosynovitis* of a 75 year old active rheumatoid arthritis patient before and after 6 months after initiation of biologic therapy. ECU, extensor carpi ulnaris tendon.
Patients in Group 4 achieved a significant decrease in the US7 synovitis sum score in GSUS from 6.1 to 4.8 (−21.3%; p=0.006) and in PDUS from 2.4 to 1.5 (−37.5%; p=0.005) as well as in the US7 tenosynovitis sum score in GSUS from 1.1 to 0.5 (−54.6%; p=0.003). Additionally, the erosion sum score significantly decreased from 4.3 to 3.6 (−16.3%; p=0.008), when patients received a biologic for more than 12 months. The DAS28 score dropped significantly from moderate to low disease activity (3.8–3.2; p=0.004). No significant reduction was seen in the development of CRP (p=0.186), ESR (p=0.152) and the US7 tenosynovitis sum score in PDUS (p=0.281). This patient group also exhibited the lowest US7 synovitis sum score (GSUS and PDUS) and US7 tenosynovitis/paratenonitis sum score at baseline examination (see supplementary table S4).
Sensitivity to change: longitudinal correlation (1 year observation) between US7 score and clinical/laboratory parameters
Group 1 showed a significant correlation between changes in DAS28 and the synovitis sum score in GSUS (r=0.419; p<0.001), PDUS (r=0.459; p<0.001) as well as in the tenosynovitis sum score in PDUS (r=0.316; p=0.001) after 12 months. In this group, the change in ESR after 1 year correlated significantly with the synovitis sum score in PDUS (r=0.335; p<0.001) after 12 months of DMARDs therapy.
In Group 2, significant correlations were observed between changes in DAS28 and the synovitis sum score in GSUS after 1 year (r=0.257; p=0.008) as well as between ESR and the synovitis sum score in PDUS (r=0.283; p=0.007).
Group 3 presented a significant correlation between changes in ESR and the synovitis sum score in GSUS (r=0.207; p=0.011) and PDUS (r=0.179; p=0.032) after 12 months. A decrease in CRP correlated significantly with changes in the synovitis sum score in PDUS (r=0.312; p<0.001) and the tenosynovitis score in PDUS (r=0.232; p=0.042) after 1 year.
Patients of Group 4 did not show a significant correlation between clinical/laboratory parameters and all components of the US7 score after 1 year.
Predictors for erosions detected by the US7 erosion sum score after 1 year
Predictors of development of erosions detecting with the US7 erosion sum score after 1 year of monitored therapy were US7 synovitis sum score and US7 erosion sum score in GSUS, as well as DAS28 at baseline (table 3). Neither laboratory data (ESR, CRP; IgM-RF; ACPA) nor the US7 synovitis score in PDUS, and US7 tenosynovitis/paratenonitis scores in GSUS, and PDUS were able to significantly reflect the development of erosions in US. Two examples of erosions dependent on disease duration are given by figure 5.
Table 3 .
Prediction of the US7 erosion sum score in GSUS after 12 months
| At baseline | Regressions coefficient ß±SD | 95% CI | p Value |
|---|---|---|---|
| US7 synovitis sum score in GSUS | 0.140±0.038 | 0.066 to 0.215 | p<0.001 |
| US7 synovitis score in PDUS | 0.099±0.051 | −0.001 to 0.200 | p=0.053 |
| US7 tenosynovitis/paratenonitis scores in GSUS | 0.167±0.111 | −0.051 to 0.385 | p=0.132 |
| US7 tenosynovitis/paratenonitis scores in PDUS | 0.068±0.127 | −0.182 to 0.318 | p=0.594 |
| US7 erosion sum score in GSUS | 0.844±0.026 | 0.793 to 0.895 | p<0.001 |
| DAS28 | 0.621±0.141 | 0.345 to 0.898 | p<0.001 |
| ESR | 0.008±0.010 | −0.011 to 0.027 | p=0.399 |
| CRP | 0.001±0.009 | −0.017 to 0.018 | p=0.945 |
| RF | −2.167±0.00 | 0.00 to 0.00 | p=0.631 |
| APCA | 6.392±0.00 | −0.001 to 0.001 | p=0.891 |
ESR, erythrocyte sedimentation rate; GSUS, grey-scale ultrasound; PDUS, power Doppler ultrasound; RF, Rheumatoid factor; US, ultrasound.
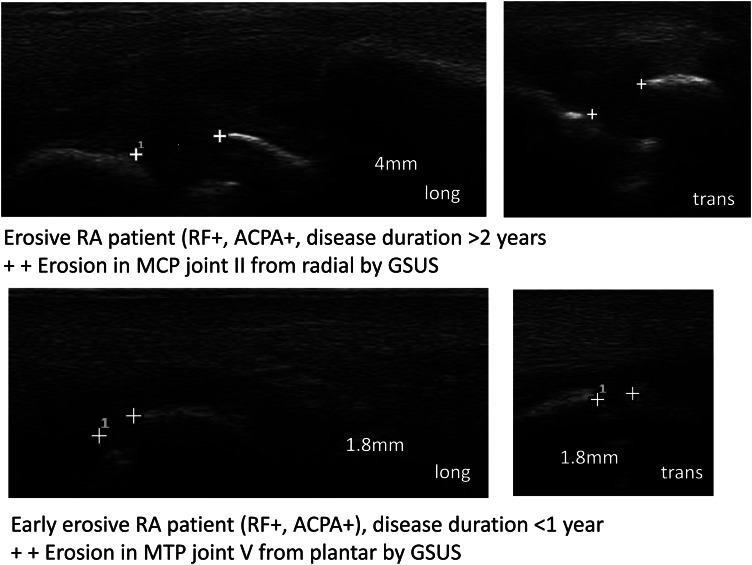
Figure 5.
Bone erosions at 2nd metacarpophalangeal- and 5th metatarsophalangeal-joints.
Discussion
The aim of the present study was to determine the sensitivity to change of the US7 score in a large cohort of patients with RA under various (DMARDs and/or biologic) therapies over a 1 year period. Furthermore, US7 score results were compared with clinical and laboratory parameters at each time of assessment as well as predictors for erosions were estimated.
It presented a unique nationwide project including 54 rheumatologic centres which monitored a large number of RA patients under everyday life conditions which also reflects the feasibility of the US7 score. The correlation between clinical findings (DAS28) and laboratory findings (ESR, CRP) and components of the US7 score shows the ability to reflect disease activity. Not only synovitis, but also tenosynovitis plays an important role in the beginning of the inflammatory process of RA. The highest US7 tenosynovitis score in PDUS was seen in group 1 at baseline, the group with the shortest disease duration of 5.3 years in comparison with the other treatment groups.
Patients in Group 3, who received a biologic for the first time after DMARD therapy, had the highest DAS28 at baseline with the highest proportion of IgM-RF positive patients, indicating the necessity of treatment change. US detected the highest US7 scores for synovitis and tenosynovitis in GSUS and PDUS, which significantly decreased after 12 months, and therefore reflect a good treatment response. The erosion sum score was also stable over this period.
Group 4 was the only one, in which erosions decreased significantly after 1 year. This is a quite remarkable effect, but may lead to controversial discussions. The question is whether massive bone destruction is capable of vanishing or healing. Another indication that there is a kind of ‘healing effect’ has already been discussed for radiographic erosions9 as well as in a recent study conducted by Finzel et al,10 where 30 RA patients were treated with either tumour necrosis factor alpha inhibitors (TNFαi) or methotrexate. The development of bone erosions was observed using micro CT imaging. After 1 year, patients on TNFαi showed partial recovery in terms of a decrease in the mean depth of erosions while the mean width remained the same. In contrast, patients taking only methotrexate demonstrated an increase in the main depth and width of the erosions.10 With micro CT imaging being comparable with high-resolutionUS,11 US is also able to detect limited recovery of erosions in patients who were treated with biologics.
Studies monitoring DMARD versus biologic therapies observed a discrepancy between clinical findings and structural damage. Only within biologic treatment groups, the structural damage stopped significantly in comparison with the DMARD groups.12–14
Patients in Group 4 had the lowest DAS28 score and the lowest synovitis sum scores (GSUS and PDUS) at baseline examination in comparison with the other treatment groups. This might be another reason why only this group showed a significant decrease in erosions. With the decrease of synovitis, a so-called ‘acoustic stand-off’ is missing, which actually would enable a better-visualised bone surface. Therefore, it might be possible to detect less bone erosions, which pretends to be a sort of healing effect of erosions. Nevertheless, larger studies are necessary to prove the matter of bone healing under a long period of biologic treatment in US.
In distinguishing early bone erosions US is comparable with MRI with a proven overall sensitivity of 0.63 and specificity of 0.98, as well as substantial agreement (κ=0.68, p<0.001).15 Therefore, sensitive imaging techniques such as US and MRI are required for the early detection of erosions. Furthermore, what are predictors for the development of bone erosions? High US7 synovitis sum score and US7 erosion sum score in GSUS as well as high DAS28 scores at baseline are predictors of development of erosions seen in US after 12 months. Hypervascular synovitis seen in PDUS is able to reflect radiographic damage as well as further ACR criteria such as DAS28.16 A study by Bukhari et al reported that a high RF titre is an essential variable in forecasting radiographic damage during the first 5 years after presenting with inflammatory polyarthritis.17 This could not be found for US detecting erosions.
Groups 1 and 3 were the groups with the highest disease activity scores at baseline. Patients on first-line DMARDs dropped to mild disease activity after 12 months (DAS28 2.9) while patients with first-line biologics had a moderate disease activity score (DAS28 3.6) after 1 year. However, it is possible that DAS28 is not able to reflect disease activity sufficiently. Subclinical activity might be better detected by US, especially in PD mode. Nevertheless, the question remains whether distinguishing between physiological and pathological vascularity with the help of modern high-definition US imaging techniques is necessary. PDUS is a useful method in differentiating between active and inactive synovitis.18 GSUS can be seen as damage in the US score, while PDUS is able to disclose subclinical activity. Patients with clinical remission with a DAS28<2.6 and a positive PD signal of the wrist (dorsal aspect) were more likely to experience a flare of RA in the following year than patients with a negative PD signal. Therefore, PDUS is able to predict RA relapse within a period of 12 months.19
In order to test the US scoring set for sensitivity to change under different kind of treatments according to the OMERACT filter, correlations were performed between different variables such as CRP, ESR, DAS28 at each visit and each US subdomain (synovitis and tenosynovitis in GSUS and PDUS, and erosions in GSUS). Longitudinal correlations between the US7 score and clinical parameters were detected between DAS28 and synovitis in GSUS in Group 1 and 2 and between DAS28 and synovitis in PDUS in Group 1, comparable with earlier US7 score results.20
Furthermore, ESR correlated with the synovitis sum score in PDUS quite often, that is, there was a significant correlation in Group 1, Group 2, and Group 3. Group 3, which included patients with first-line biologics also showed a significant correlation between ESR and the synovitis sum score in GSUS. Therefore, the US7 synovitis sum score is sensitive to change.
Limitations of the study are the use of different US devices. Group 4 (change to a second-line biologic) was the smallest group and had the lowest DAS28 in comparison with the other groups. This group was the only group with significant reduction of erosion score over 12 months. Another limitation is the missing of x-ray control of hands and feet for detection of erosive progress in this group.
Nevertheless, the feasibility and sensitivity to change of the US7 score under various therapies in a large RA cohort over 12 months period could be well proven.
In conclusion, the novel US7 score provides evidence of therapeutic response. It has already been proven that reduced US scoring systems are able to produce such high correspondence as 78-joint US scores.21 The correlation of the US7 score data with clinical and laboratory data indicates its ability to reflect therapeutic response to different therapeutic regimens (DMARD and/or biologics). Patients who switched from one biologic to another achieved a significant decrease in the US erosion sum score after 12 months, while the erosion scores of the other treatment groups were stable over the 12 months period.
Supplementary Material
Acknowledgments
We thank Abbott GmbH & Co. KG, Max-Planck-Ring 2a, 65 205 Wiesbaden, Germany, for financially supporting this project. We thank Dr Imma Fischer, Biostatistik Tuebingen, for statistical advice.
Footnotes
Contributors: All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. No medical writer was involved in the preparation of the manuscript.
Funding: Abbott GmbH & Co.KG provided financial support for the study procedure. Abbott did not have any influence on the statistical analysis or preparation of the manuscript. This study was also supported by Bundesministerium für Bildung und Forschung (BMBF) project “ArthroMark”, subproject no. 7, “Clinical study on Biomarkers and Imaging”.
Competing interests: None.
Patient consent: Obtained.
Ethics approval: Ethics committee Universitätsklinikum Tübingen.
Provenance and peer review: Not commissioned; externally peer reviewed.
Correction notice: This article has been corrected since it was published online first. The funding statement has been altered.
Open Access: This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/
References
- 1.Vastesaeger N, Xu S, Aletaha D, et al. A pilot risk model for the prediction of rapid radiographic progression in rheumatoid arthritis. Rheumatology (Oxford) 2009;48:1114–21 [DOI] [PubMed] [Google Scholar]
- 2.Fukae J, Isobe M, Kitano A, et al. Radiographic prognosis of finger joint damage predicted by early alteration in synovial vascularity in patients with rheumatoid arthritis: potential utility of power Doppler sonography in clinical practice. Arthritis Care Res (Hoboken) 2011;63:1247–53 [DOI] [PubMed] [Google Scholar]
- 3.Lillegraven S, Bøyesen P, Hammer HB, et al. Tenosynovitis of the extensor carpi ulnaris tendon predicts erosive progression in early rheumatoid arthritis. Ann Rheum Dis 2011;70:2049–50 Epub 2011 Apr 24. No abstract available [DOI] [PubMed] [Google Scholar]
- 4.Backhaus M, Ohrndorf S, Kellner H, et al. Evaluation of a novel 7-joint ultrasound score in daily rheumatologic practice: a pilot project. Arthritis Rheum 2009;61:1194–201 [DOI] [PubMed] [Google Scholar]
- 5.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 Revised Criteria for the Classification of Rheumatoid Arthritis. Arthritis Rheum 1988;31:315–24 [DOI] [PubMed] [Google Scholar]
- 6.Wakefield RJ, Balint PV, Szkudlarek M, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheum 2005;32:2485–7 [PubMed] [Google Scholar]
- 7.Szkudlarek M, Court-Payen M, Jacobsen S, et al. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum 2003;48:955–62 [DOI] [PubMed] [Google Scholar]
- 8.Scheel AK, Hermann KG, Kahler E, et al. A novel ultrasonographic synovitis scoring system suitable for analyzing finger joint inflammation in rheumatoid arthritis. Arthritis Rheum 2005;52:733–43 [DOI] [PubMed] [Google Scholar]
- 9.van der Heijde D, Landewé R, Boonen A, et al. Expert agreement confirms that negative changes in hand and foot radiographs are a surrogate for repair in patients with rheumatoid arthritis. Arthritis Res Ther 2007;9:R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finzel S, Rech J, Schmidt S, et al. Repair of bone erosions in rheumatoid arthritis treated with tumour necrosis factor inhibitors is based on bone apposition at the base of the erosion. Ann Rheum Dis 2011;70:1587–93 [DOI] [PubMed] [Google Scholar]
- 11.Finzel S, Ohrndorf S, Englbrecht M, et al. A detailed comparative study of high-resolution ultrasound and micro-computed tomography for detection of arthritic bone erosions. Arthritis Rheum 2011;63:1231–6 [DOI] [PubMed] [Google Scholar]
- 12.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. ArthritisRheum 2006;54:26–37 [DOI] [PubMed] [Google Scholar]
- 13.van der Heijde D, Klareskog L, Rodriguez-Valverde V, et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. ArthritisRheum 2006;54:1063–74 [DOI] [PubMed] [Google Scholar]
- 14.Smolen JS, Han C, Bala M, et al. Evidence of radiographic benefit of treatment with infliximab plus methotrexate in rheumatoid arthritis patients who had no clinical improvement: a detailed subanalysis of data from the anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study. Arthritis Rheum 2005;52:1020–30 [DOI] [PubMed] [Google Scholar]
- 15.Rahmani M, Chegini H, Najafizadeh SR, et al. Detection of bone erosion in early rheumatoid arthritis: ultrasonography and conventional radiography versus non-contrast magnetic resonance imaging. Clin Rheumatol 2010;29:883–91 [DOI] [PubMed] [Google Scholar]
- 16.Pascual-Ramos V, Contreras-Yañez I, Cabiedes-Contreras J, et al. Hypervascular synovitis and American College of Rheumatology Classification Criteria as predictors of radiographic damage in early rheumatoid arthritis. Ultrasound Q 2009;25:31–8 [DOI] [PubMed] [Google Scholar]
- 17.Bukhari M, Lunt M, Harrison BJ, et al. Rheumatoid factor is the major predictor of increasing severity of radiographic erosions in rheumatoid arthritis: results from the Norfolk Arthritis Register Study, a large inception cohort. Arthritis Rheum 2002;46:906–12 [DOI] [PubMed] [Google Scholar]
- 18.Stone M, Bergin D, Whelan B, et al. Power Doppler ultrasound assessment of rheumatoid hand synovitis. J Rheumatol 2001;28:1979–82 [PubMed] [Google Scholar]
- 19.Peluso G, Michelutti A, Bosello S, et al. Clinical and ultrasonographic remission determines different chances of relapse in early and longstanding rheumatoid arthritis. Ann Rheum Dis 2011;70:172–5 [DOI] [PubMed] [Google Scholar]
- 20.Backhaus M, Burmester GR, Gerber T, et al. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis 2001;60:641–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammer HB, Kvien TK. Comparisons of 7- to 78-joint ultrasonography scores: all different joint combinations show equal response to adalimumab treatment in patients with rheumatoid arthritis. Arthritis Res Ther 2011;13:R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.