Abstract
Background Context
Few studies have directly evaluated the association of lumbar lordosis and segmental wedging of the vertebral bodies and intervertebral disks with prevalence of spinal degenerative features.
Purpose
To evaluate the association of CT-evaluated lumbar lordosis, segmental wedging of the vertebral bodies and that of the intervertebral disks with various spinal degeneration features.
Study design
This cross-sectional study was a nested project to the Framingham Heart Study.
Sample
A random consecutive subset of 191 participants chosen from the 3590 participants enrolled in the Framingham Heart Study who underwent multi-detector CT to assess aortic calcification.
Outcome Measures
Physiologic Measures
Dichotomous variables indicating the presence of intervertebral disc narrowing, facet joint osteoarthritis, spondylolysis, spondylolisthesis and spinal stenosis and density (in Hounsfield units) of multifidus and erector spinae muscles were evaluated on supine CT, as well as the lordosis angle (LA) and the wedging of the vertebral bodies and intervertebral disks. Sum of vertebral bodies wedging (ΣB) and sum of intervertebral discs wedging (ΣD) were used in analyses.
Methods
Mean values (±SD) of LA, ΣB and ΣD were calculated in males and females and compared using the t-test. Mean values (±SD) of LA, ΣB and ΣD in 4 age groups: <40, 40–49, 50–59 and 60+ years were calculated. We tested the linear relationship between LA, ΣB and ΣD and age groups. We evaluated the association between each spinal degeneration feature and LA, ΣB and ΣD using multiple logistic regression analysis where studied degeneration features were the dependent variable and all LA, ΣB and ΣD (separately) as well as age, sex, and BMI were independent predictors.
Results
LA was slightly lower than the normal range for standing individuals, and no difference was found between males and females (p=0.4107). However, the sex differences in sum of vertebral bodies wedging (ΣB) and sum of intervertebral discs wedging (ΣD) were statistically significant (0.0001 and 0.001, respectively). Females exhibit more dorsal wedging of the vertebral bodies and less dorsal wedging of the intervertebral discs than do males. All these parameters showed no association (p>0.05) with increasing age.
LA showed statistically significant association with presence of spondylolysis (OR(95%CI): 1.08(1.02–1.14)) and with density of multifidus (1.06 (1.01–1.11). as well as a marginally significant association with isthmic spondylolisthesis (1.07(1.00–1.14). ΣB showed a positive association with degenerative spondylolisthesis and disc narrowing ((1.14(1.06–1.23) and 1.04 (1.00–1.08), correspondingly), whereas ΣD showed negative one (0.93(0.87–0.98) and (0.93(0.89–0.97), correspondingly).
Conclusions
Significant associations were found between lumbar lordosis evaluated in supine position and segmental wedging of the vertebral bodies and intervertebral disks and prevalence of spondylolysis and spondylolisthesis. Additional studies are needed, to evaluate the association between spondylolysis, isthmic and degenerative spondylolisthesis and vertebral and disc wedging at segmental level.
Keywords: computer tomography, spine, degeneration, lordosis, segmental wedging angle, vertebral body, intervertebral disc
Introduction
There is increasing recognition of the functional and clinical importance of the lumbar lordosis [1–5]. Lumbar lordosis is formed by the wedging of the lumbar vertebral bodies and of the intervertebral disks [6, 7]. Lordotic or dorsal wedging (ventral height greater than dorsal height) of the vertebral bodies and the intervertebral disks will increase the lordosis angle (LA), while kyphotic or ventral wedging will decrease it.
Very few studies have directly evaluated the association of lumbar LA and segmental wedging of the vertebral bodies and intervertebral disks with prevalence of spinal degenerative features. For example, one recent study [5] found that there are two independent predictors of degenerative spondylolisthesis, the higher kyphotic wedging of vertebral bodies and decreased anterior disc height.
The etiology of spinal degeneration is not fully understood, nor is the broad morphological changes that might result from degeneration process. Finding an association between morphological parameters and degeneration of the spine could be a first step to understanding the etiology of lumbar spine degeneration. Understanding the etiology of spinal pathology, in turn, can help to identify subjects at risk, to develop better treatments, or to develop prevention strategies.
The aim of this study was to examine the associations of CT-evaluated lumbar lordosis and segmental wedging of vertebral bodies and of intervertebral disks with various spinal degeneration features.
Methods
Study design
Cross-sectional community-based study that was a nested project to the Framingham Heart Study.
Sample
This project was a nested project to the Framingham Heart Study. The Framingham Heart Study began in 1948 as a longitudinal population-based cohort study of the causes of heart disease. Initially, 5209 men and women between the ages of 30 and 60 years living in Framingham, Massachusetts were enrolled. All subjects underwent biennial examinations. In 1971, 5,124 offspring (and their spouses) of the original cohort were entered into the Offspring cohort. In 2002, 4095 men and women who were children of the Offspring cohort were enrolled in the Third Generation cohort. A description of the Offspring and Third Generation cohorts has been previously reported [8, 9]. 3590 participants of the Framingham study (participants in both the Offspring and Third Generation cohorts) aged 40–80 years underwent abdominal and chest multi-detector computed tomography (CT) scanning to assess coronary and aortic calcification. The recruitment and conduct of CT scanning have been previously reported [10, 11]. During the CT study, 191 participants were consecutively enrolled in this nested study to assess the different aspects of spinal degeneration. The sample is representative of the larger study sample all of whom also had CT scans acquired but did not have a self-reported assessment of spinal symptoms.
Imaging parameters
Study participants were imaged with an eight-slice multi-detector CT scanner (Lightspeed Ultra, GE, Milwaukee, WI, USA). Each subject underwent unenhanced abdominal multi-detector CT performed with patients lying in a supine position with extended legs, using a sequential scan protocol with a slice collimation of 8 mm × 2.5 mm (120 KVp, 320/400 mA for 220 lbs body weight, respectively) during a single end-inspiratory breath hold. For the abdominal scan, thirty contiguous 5 mm thick slices of the abdomen were acquired covering 150 mm above the level of S1.
The evaluation of all spinal degeneration parameters in this study was performed using eFilm Workstation (Version 2.0.0) software.
Measurements of lumbar lordosis and wedging angle of vertebral bodies and intervertebral discs
For each of the five lumbar vertebrae, two lines were drawn (Figure 1A): along the superior endplate of the vertebral body (also on the first sacral vertebra) and along the inferior endplate of the vertebral body. These lines were used to measure three angles: the LA between the superior endplate of L1 and the superior endplate of S1; the body wedge angle (B) between the superior and inferior endplates of a single vertebra; and the intervertebral disk angle (D) between the inferior endplate of one vertebra and the superior endplate of the successive vertebra. Measurements B and D were taken for each of the five lumbar segments. All measurements were taken by the same investigator (EB) that was not aware of readings of degenerative features.
Figure 1.
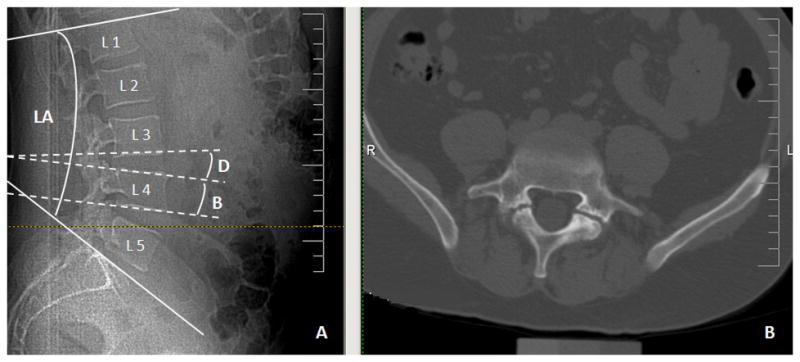
Example of evaluated CT:
A. Measurements of lumbar angle (LA), vertebral body angle (B) and intervertebral disc angle (D).
B. Bilateral spondylolysis of L5 is shown on axial views image.
Measurements B and D were used to calculate ΣB, the sum of the lumbar L1-L5 body wedge angles; and ΣD, the sum of the lumbar L1-L5 intervertebral disk angles.
Evaluation of spinal degeneration features
For CT reading we used transverse plane images as well as sagittal and coronal reconstructions, where needed. A reading protocol for evaluation of spinal degeneration features was developed. Using this protocol, the intra- and inter-rater reliability was calculated for two readers. One investigator, blinded to patient identifiers, read all of the CTs. All spinal degeneration features were evaluated between L2 and S1 spinal levels. To evaluate reader-drift, we re-assessed intra-rater reliability by inserting one original reliability scan for every 10 new scans. Before analyzing each new set of CT scans, 5 previously analyzed CTs were reevaluated to “recalibrate” the readings to a standard.
Intervertebral disc narrowing
Disc narrowing was estimated on a sagittal reconstruction image using the 4- grade scale by Videman et al. [12]: 0–normal, disc height greater than the cephalad disc, except for the L5-S1 disc; 1-slight, disc height equal to the cephalad disc if it is normal; 2-moderate, disc height less than cephalad disc if it is normal; and 3- severe, endplates almost in contact. For this study, this scale was collapsed to two grades: 1–normal, included grades 0 and 1; 2–affected, included grades 2 and 3. Subject with at least one affected level was considered as having intervertebral disc narrowing.
Facet joint OA
Four grades (0-normal, 1-mild, 2-moderate and 3-severe degeneration) of facet joint OA were defined using criteria that were described in Kalichman et al. [13] and similar to those published by Pathria et al. [14] and Weishaupt et al. [15]. This semi-quantitative score accounts for such changes as joint space narrowing, osteophytes, hypertrophy of the articular process, subarticular sclerosis, subchondral cysts and vacuum phenomenon. Lumbar facet joints were graded on both sides at L2-L3, L3-L4, L4-L5, and L5-S1 levels. For this study this index was dichotomized based on the presence or absence of facet joint OA (≥grade 2) on any side at any level.
Spondylolysis and Spondylolisthesis
The lumbar spine was reviewed for each case, using bone windows. Spondylolysis and spondylolisthesis were defined as present or absent (dichotomous indices) for each subject.
Spinal stenosis
Bone and soft tissue windows were used. For measurements of congenital spinal stenosis, the midsagittal diameter of the spinal canal was measured at the level of the middle of the vertebra using a CT bone window. Acquired spinal stenosis was measured as a midsagittal diameter of the spinal canal at the level of the intervertebral disc (the effective canal diameter was determined between the margin of the intervertebral disc anteriorly and the junction of bilateral ligamenta flava posteriorly) using a CT soft tissue window. Spinal stenosis was defined as diameter <10 mm in accordance with previous studies [16–20]. A dichotomous index was obtained based on the presence or absence of lumbar spinal stenosis of any type and at any level.
Paraspinal muscles density evaluation
The density of multifidus and erector spinae muscles was measured in Hounsfield units (HU) on both the left and right side at the level of the upper endplates of L3, L4 and L5 vertebrae. Mean density was measured by a 6 mm circle in the center of muscle mass in the region of interest. A similar method was previously used in Keller et al. study of paraspinal muscle density [21].
To avoid the problem of multiple comparisons and because there were no significant differences between right and left sides and between spinal levels, we performed principal component analyses for each muscle separately, using the original density measurements. The first principal components derived from these analyses were dichotomized according the following rule: lowest 33.3% of the distribution=1, other=0. Resulting dichotomous indices were used in the further analyses.
Body mass index (BMI)
BMI was computed as the ratio of the body mass in kg divided by the height in meters squared.
Statistical analysis
Because the prevalence of various spinal degeneration features and mean density of multifidus and erector spinae muscles was previously described [22] we did not provide this data in the present paper.
First, we calculated the mean values (±SD) of LA, ΣB and ΣD in males, females and in total sample. To compare between males and females an unpaired t-test was used. Second, we calculated the mean values (±SD) of LA, ΣB and ΣD in 4 age groups: <40, 40–49, 50–59 and 60+ years. We tested the linear relationship between LA, ΣB and ΣD and age groups. Third, we evaluated the association between each spinal degeneration feature and LA, ΣB and ΣD using multiple logistic regression analysis where studied degeneration features were the dependent variable and all LA, ΣB and ΣD (separately) as well as age, sex, and BMI were independent predictors.
Results
Results of reliability tests (kappa statistics) were as follows: The intra-observer reliability for disc narrowing varied at different spinal levels between 0.84 and 0.90. The inter-observer reliability for disc narrowing ranged from 0.78 to 0.88. The intra-observer reliability for grading different facet joint OA indices varied between 0.64 and 0.91. The inter-observer reliability ranged from 0.59 to 0.94. The intra-observer reliability for identification of spondylolysis was 1.00. The inter-observer reliability was 0.98. For spondylolisthesis, the intra-observer reliability varied at different levels between 0.95 and 1.00, and the inter-observer reliability ranged from 0.75 to 0.98. For spinal stenosis, the intra-observer reliability varied at different spinal levels between 0.95 and 0.98 for congenital stenosis and between 0.92 and 0.98 for acquired stenosis. The inter-observer reliability ranged from 0.80 to 0.92 and from 0.86 to 0.96, respectively. The intra-observer reliability for multifidus and erector spinae at different levels varied between 0.94 and 0.99. The inter-observer reliability ranged from 0.70 to 0.97, respectively. The intra-observer reliability for measurements of LA was 0.93, for B measurements ranged from 0.80 to 0.95 and for D measurements ranged from 0.85–0.96). This range of kappa statistics represents good to excellent reproducibility.
The study sample included 191 study participants, 104 (55.6%) males and 87 (44.4%) females. This subsample was representative of the whole group of individuals that underwent multi-detector CT scanning (N=3590). The comparison tests showed that there was no difference between the whole sample and the sub-sample studied here in age (p=0.95), BMI (p=0.92) or prevalence of males (p=0.31).
Table 1 shows the descriptive statistics (mean±SD) of LA, ΣB and ΣD in males, females and in total sample, as well as the results of comparison between sexes. No difference in LA was found between males and females (p=0.4107). However, the sex differences in ΣB and ΣD were statistically significant (0.0001 and 0.001, respectively). Females exhibit more dorsal wedging of the vertebral bodies (ΣB) and less dorsal wedging of the intervertebral discs (ΣD) than do males.
Table 1.
Descriptive statistics and comparison between sexes.
| Frequencies | Males | Females | Total | Comparison (p-values)* |
|---|---|---|---|---|
| N | 89 | 65 | 154 | |
| LA (mean±SD) | 46.2 (10.1) | 47.6 (10.5) | 46.8 (10.2) | 0.4107 |
| ΣB (mean±SD) | 2.2 (9.9) | 8.6 (10.1) | 4.9 (10.4) | 0.0001 |
| ΣD (mean±SD) | 42.9 (10.0) | 37.4 (10.2) | 40.6 (10.4) | 0.0010 |
Results of t-test for continuous variables. LA – lordosis angle, ΣB -sum of 5 lumbar vertebral bodies wedging, ΣD- sum of 5 lumbar intervertebral discs wedging. Statistically significant differences at p≤0.05 level are highlighted bold.
Mean values (±SD) of LA, ΣB and ΣD, in different age groups as well as estimation of linear trend probability are presented in Table 2. All these parameters showed no association (p>0.05) with increasing age.
Table 2.
Age-specific means of studied parameters and estimation of association with age.
| <40 | 40–49 | 50–59 | 60+ | P for trend | |
|---|---|---|---|---|---|
|
| |||||
| N | 23 | 40 | 55 | 36 | |
| LA (mean±SD) | 45.9 (10.2) | 46.4 (10.1) | 47.4 (10.7) | 46.8 (10.0) | 0.2800 |
| ΣB (mean±SD) | 6.1 (8.6) | 6.7 (8.3) | 4.0 (11.6) | 3.6 (11.7) | 0.6400 |
| ΣD (mean±SD) | 38.9 (10.0) | 39.0 (10.4) | 42.2 (9.6) | 41.0 (11.8) | 0.9554 |
The p-values in the above table are the result of linear relationship test between each outcome and age groups.
Table 3 shows the association between indices of lordosis and spinal degeneration features, adjusting age, sex and BMI. LA showed statistically significant association with presence of spondylolysis (OR(95%CI): 1.08(1.02–1.14)) and with density of multifidus (1.06 (1.01–1.11). as well as marginally significant association isthmic spondylolisthesis (1.07(1.00–1.14). ΣB and ΣD, both showed significant association with degenerative spondylolisthesis and disc narrowing. ΣB showed positive association ((1.14(1.06–1.23) for degenerative spondylolisthesis and (1.04(1.00–1.08) for disc narrowing), whereas ΣD showed a negative association ((0.93(0.87–0.98) for degenerative spondylolisthesis and (0.93(0.89–0.97) for disc narrowing), meaning lumbar spines with more dorsal wedging of their vertebral bodies and less dorsal wedging of their intervertebral disks have higher prevalence of degenerative spondylolisthesis and of disc narrowing.
Table 3.
Association (OR(95%CI) between spinal degeneration features (outcomes) and indices of lordosis (predictors), adjusted for age, sex and BMI.
| Facet Joints OA | Spondylolysis | Isthmic Spond. | Degenerative Spond. | Spinal Stenosis | Disc narrowing | Density of Multifidus | Density of Erector Spinae | |
|---|---|---|---|---|---|---|---|---|
| LA | 1.03 (0.99–1.07) | 1.08 (1.02–1.14) | 1.07 (1.00–1.14) | 1.04 (0.98–1.10) | 1.02 (0.95–1.09) | 0.97 (0.94–1.01) | 1.06 (1.01–1.11) | 1.03 (0.99–1.07) |
| ΣB | 1.02 (0.98–1.06) | 1.05 (0.99–1.12) | 1.02 (0.96–1.10) | 1.14 (1.06–1.23) | 1.04 (0.97–1.12) | 1.04 (1.00–1.08) | 1.02 (0.97–1.06) | 1.02 (0.98–1.06) |
| ΣD | 1.02 (0.98–1.05) | 1.02 (0.96–1.07) | 1.05 (0.98–1.12) | 0.93 (0.87–0.98) | 0.97 (0.90–1.03) | 0.93 (0.89–0.97) | 1.04 (0.99–1.08) | 1.02 (0.98–1.05) |
Significant associations (p<0.05) are highlighted bold. Spond. - spondylolisthesis.
Discussion
This is the first cross-sectional study to describe simultaneously the associations of the most common CT-evaluated spinal degeneration features with lumbar lordosis and segmental wedging of vertebral bodies and of intervertebral disks.
In the present study lordosis angle (L1-S1) was measured on CT. Study participants underwent CT lying in supine position with straight legs (not in psoas-relaxed position). Recently several studies [23–25] have found that lumbar lordosis measured on horizontal MRI when patient lie supine with straight legs was comparable to one measured on vertical MRI, when the patient is standing. The results are in agreement with earlier report by Schmid et al [26] who studied 12 young volunteers with positional MRI. The authors concluded that the supine extended position was a functionally relevant position and suggested that it could replace examination in the upright extended position.
The mean lordosis angle was 46.2±10.1 for males and 47.6±10.5 for females. In other studies, depending on type of imaging used, position of subjects during imaging, and the lumbar spinal level measured, the lumbar lordosis extensively varied: 45±12 degrees for Taiwan sample [27], 46.5 degrees in young French volunteers [6], 51.3± 10.7 degrees in Israeli sample [28], 52.72 (95%CI: 55.16–50.28) degrees in Greek females [29], and 60.0±10.0 degrees in healthy French volunteers [7]. Lumbar lordosis found in our study is in a normal range found by other studies.
The results of our study showed that the lumbar lordosis angle is not different between sexes. Similar results were previously found in population based Taiwan sample [27], as well as in sample of spondylolysis patients from Japan [30]. However, one MRI study [31] and one x-ray study [32], both measured lumbar lordosis at L2-S1 level, found that women have a greater lordosis than men.
There were significant sex differences in wedging of vertebral bodies and intervertebral discs. Males have smaller mean ΣB (less lordotic vertebral bodies), whereas females have smaller ΣD (less lordotic intervertebral disks). These findings are in accord with previously published data [33].
We found no association between age and lordosis, as well as between age and wedging of vertebral bodies and intervertebral discs. Similar results were shown in previous studies [31, 34, 35]. It contradicts the commonly hold opinion that the lumbar lordosis ‘flattens’ out with spinal problems and subsequent age-related degenerative changes [31]. However, there were studies that claimed that lumbar lordosis increases with age [36] or that it decreases after the sixth decade [37]. Based on previous conflicting evidence in the literature we believe that additional, large-scale studies need to be performed to clarify the association between lumbar lordosis and age.
Results of our study show that lumbar lordosis angle is positively and significantly associated with spondylolysis and isthmic spondylolisthesis, as well as with density of multifidus muscle. In addition, we found that ΣB is positively and ΣD is negatively associated with degenerative spondylolisthesis. Few previous studies evaluated the association between lumbar lordosis and spinal degeneration features [1, 5, 27, 29, 38–41]. In the present study, no association was found between spinal stenosis or density of erector spinae and lumbar lordosis angle or its components. We do not know about previous studies that evaluated such associations.
We found no differences in lumbar lordosis angle, as well as sum of wedging angles of vertebral bodies and of intervertebral discs (ΣB and ΣD) in individuals with and without facet joint OA. Similar results were obtained in Greek [29] and Chinese [27] individuals. It is suggested that lumbar lordosis or its components are neither an outcome nor a contributing factor of facet joint OA.
In our study, intervertebral disc narrowing was not associated with lordosis angle. Previously, Lebkowski et al [39] also did not find diminished lordosis in patients with lumbar degenerative disk disease. On the other hand, negative association was found between wedging of the intervertebral disks (ΣD) and prevalence of disc narrowing; i.e. disk narrowing associates with a decrease in disk wedging. Similar finding was recently found by Chen and Wei [5] in lower lumbar vertebrae. Additional study is needed to evaluate these association at all lumbar segments. If the same type of association will be found, it will mean that disc degeneration process manifests not only in disc narrowing, but also in change in disc wedging. This can have potential implication in diagnosis of disc pathology and in disc replacement surgery.
This is a first study that found significant association between low density of multifidus muscles and higher lumbar lordosis angle. This association was found after adjustment for age, sex and BMI. Muscle density is an expression of degeneration of the muscles and reflects the number of muscle fibers, the area of the individual muscle fiber, and the packing of the contractile material [42]. If we assume, that the density of multifidus is an indication of muscle fitness, these results show that individuals with weaker multifidus have a more prominent lumbar lordosis. This association needs to be confirmed in other samples, and the mechanism of this association need to be studied.
The results of our study suggest that lumbar spines with more dorsal wedging of their vertebral bodies and less dorsal wedging of their intervertebral disks have higher prevalence of degenerative spondylolisthesis. No association was found between lumbar lordosis angle and degenerative spondylolisthesis. Spondylolysis and isthmic spondylolisthesis, on the other hand, showed positive significant association with lumbar lordosis angle but no association with the wedging of the vertebral bodies and intervertebral disks (ΣB and ΣD). Chen and Wei [5] report lower anterior disk height in patients with degenerative spondylolisthesis, similar to the smaller wedging in the intervertebral disks of patients with degenerative spondylolisthesis demonstrated in the current study. Several studies analyzed the lumbar index (that reflects the degree of wedge deformity of vertebrae, similarly to the wedging of the vertebral bodies, in our study) of vertebrae adjacent to the slipped vertebra in spondylolisthesis. Most found lower lumbar index in spondylolysis patients (more lordotic wedging of the vertebral bodies) [43]. Rosenberg [38] reported that anterior vertical height is, on average, greater than the posterior vertical height of L5 by 2 mm in patients with L5 on S1 spondylolisthesis. Similar results were presented by Saraste et al. [44, 45]. A recent study showed conflicting results; the greater lumbar index (less lordotic wedging) of the vertebra below the slipped level in women with degenerative spondylolisthesis than in normal controls [5]. The possible explanation to the differences in results is that most previous studies studied the spondylolisthesis at L5-S1 level [38, 43–45], but in last study of degenerative spondylolisthesis [5] most of vertebral slipping was at L4-L5 level (63.64%). In our sample only 44% of degenerative spondylolisthesis occurred at L4-L5 level [46]. Additional studies are needed, to evaluate the association between spondylolysis, isthmic and degenerative spondylolisthesis and vertebral and disc wedging at segmental level.
There are several limitations of the present study. First, the cross-sectional design of this study does not allow us to establish the causal relationships between lumbar lordosis parameters and spinal degenerative features. Second limitation is the relatively small sample size. Third, the lumbar lordosis evaluation in our study was performed using abdominal CTs of subjects lying in supine position with straight legs. Additional studies including in standing position need to be done to evaluate the influence of subjects’ position on lumbar lordosis measurements. The results of this study need to be replicated in different, larger sample, and preferably using longitudinal design.
Conclusions
This study adds another component to our understanding of spinal degeneration. The results of our study showed that the lumbar lordosis angle, measured in supine position, is not different between sexes, however, males in average, have less lordotic vertebral bodies, whereas females have less lordotic intervertebral disks. We found no association between age and lordosis, as well as between age and wedging of vertebral bodies and intervertebral discs.
Significant positive associations of lumbar lordosis angle were found with spondylolysis and isthmic spondylolisthesis, as well as with density of multifidus muscle. Negative association was found between wedging of the intervertebral disks and prevalence of disc narrowing. In addition, we found that sum of wedging angles of vertebral bodies is positively and sum of wedging angles of intervertebral discs is negatively associated with degenerative spondylolisthesis. Additional studies are needed, to evaluate the causal relationships between spondylolysis, isthmic and degenerative spondylolisthesis and vertebral and disc wedging at segmental level.
Acknowledgments
From the Framingham Heart Study of the National Heart Lung and Blood Institute of the National Institutes of Health and Boston University School of Medicine. This work was supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study contract (No. N01-HC-25195) for the recruitment, enrollment, and examination of the Offspring and Third Generation Cohort and the imaging by computed tomography scan.
Footnotes
Conflicts of interest statement: None of the authors have any conflict of interest regarding the contents of this article.
References
- 1.Berlemann U, Jeszenszky DJ, Buhler DW, Harms J. The role of lumbar lordosis, vertebral end-plate inclination, disc height, and facet orientation in degenerative spondylolisthesis. J Spinal Disord. 1999;12:68–73. [PubMed] [Google Scholar]
- 2.Booth KC, Bridwell KH, Lenke LG, Baldus CR, Blanke KM. Complications and predictive factors for the successful treatment of flatback deformity (fixed sagittal imbalance) Spine (Phila Pa 1976) 1999;24:1712–1720. doi: 10.1097/00007632-199908150-00013. [DOI] [PubMed] [Google Scholar]
- 3.Adams MA, Mannion AF, Dolan P. Personal risk factors for first-time low back pain. Spine (Phila Pa 1976) 1999;24:2497–2505. doi: 10.1097/00007632-199912010-00012. [DOI] [PubMed] [Google Scholar]
- 4.Jang JS, Lee SH, Min JH, Maeng DH. Influence of lumbar lordosis restoration on thoracic curve and sagittal position in lumbar degenerative kyphosis patients. Spine (Phila Pa 1976) 2009;34:280–284. doi: 10.1097/BRS.0b013e318191e792. [DOI] [PubMed] [Google Scholar]
- 5.Chen IR, Wei TS. Disc height and lumbar index as independent predictors of degenerative spondylolisthesis in middle-aged women with low back pain. Spine (Phila Pa 1976) 2009;34:1402–1409. doi: 10.1097/BRS.0b013e31817b8fbd. [DOI] [PubMed] [Google Scholar]
- 6.Vaz G, Roussouly P, Berthonnaud E, Dimnet J. Sagittal morphology and equilibrium of pelvis and spine. Eur Spine J. 2002;11:80–87. doi: 10.1007/s005860000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vialle R, Levassor N, Rillardon L, Templier A, Skalli W, Guigui P. Radiographic analysis of the sagittal alignment and balance of the spine in asymptomatic subjects. J Bone Joint Surg Am. 2005;87:260–267. doi: 10.2106/JBJS.D.02043. [DOI] [PubMed] [Google Scholar]
- 8.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study Design and preliminary data. Prev Med. 1975;4:518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 9.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann U, Siebert U, Bull-Stewart A, Achenbach S, Ferencik M, Moselewski F, et al. Evidence for lower variability of coronary artery calcium mineral mass measurements by multi-detector computed tomography in a community-based cohort--consequences for progression studies. Eur J Radiol. 2006;57:396–402. doi: 10.1016/j.ejrad.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Parikh NI, Hwang SJ, Larson MG, Cupples LA, Fox CS, Manders ES, et al. Parental occurrence of premature cardiovascular disease predicts increased coronary artery and abdominal aortic calcification in the Framingham Offspring and Third Generation cohorts. Circulation. 2007;116:1473–1481. doi: 10.1161/CIRCULATIONAHA.107.705202. [DOI] [PubMed] [Google Scholar]
- 12.Videman T, Battie MC, Ripatti S, Gill K, Manninen H, Kaprio J. Determinants of the progression in lumbar degeneration: a 5-year follow-up study of adult male monozygotic twins. Spine. 2006;31:671–678. doi: 10.1097/01.brs.0000202558.86309.ea. [DOI] [PubMed] [Google Scholar]
- 13.Kalichman L, Li L, Kim D, Guermazi A, Berkin V, O’Donnell C, et al. Facet joint osteoarthritis and low back pain in the community-based population. Spine. 2008;33:2560–2565. doi: 10.1097/BRS.0b013e318184ef95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pathria M, Sartoris DJ, Resnick D. Osteoarthritis of the facet joints: accuracy of oblique radiographic assessment. Radiology. 1987;164:227–230. doi: 10.1148/radiology.164.1.3588910. [DOI] [PubMed] [Google Scholar]
- 15.Weishaupt D, Zanetti M, Boos N, Hodler J. MR imaging and CT in osteoarthritis of the lumbar facet joints. Skeletal Radiol. 1999;28:215–219. doi: 10.1007/s002560050503. [DOI] [PubMed] [Google Scholar]
- 16.Verbiest H. Pathomorphologic aspects of developmental lumbar stenosis. Orthop Clin North Am. 1975;6:177–196. [PubMed] [Google Scholar]
- 17.Verbiest H. Results of surgical treatment of idiopathic developmental stenosis of the lumbar vertebral canal. A review of twenty-seven years’ experience. J Bone Joint Surg Br. 1977;59:181–188. doi: 10.1302/0301-620X.59B2.141452. [DOI] [PubMed] [Google Scholar]
- 18.Bolender NF, Schonstrom NS, Spengler DM. Role of computed tomography and myelography in the diagnosis of central spinal stenosis. J Bone Joint Surg Am. 1985;67:240–246. [PubMed] [Google Scholar]
- 19.Sortland O, Magnaes B, Hauge T. Functional myelography with metrizamide in the diagnosis of lumbar spinal stenosis. Acta Radiol Suppl. 1977;355:42–54. [PubMed] [Google Scholar]
- 20.Verbiest H. The significance and principles of computerized axial tomography in idiopathic developmental stenosis of the bony lumbar vertebral canal. Spine. 1979;4:369–378. doi: 10.1097/00007632-197907000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Keller A, Gunderson R, Reikeras O, Brox JI. Reliability of computed tomography measurements of paraspinal muscle cross-sectional area and density in patients with chronic low back pain. Spine. 2003;28:1455–1460. doi: 10.1097/01.BRS.0000067094.55003.AD. [DOI] [PubMed] [Google Scholar]
- 22.Kalichman L, Kim DH, Li L, Guermazi A, Hunter DJ. Computed tomography-evaluated features of spinal degeneration: prevalence, intercorrelation, and association with self-reported low back pain. Spine J. 2010;10:200–208. doi: 10.1016/j.spinee.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andreasen ML, Langhoff L, Jensen TS, Albert HB. Reproduction of the lumbar lordosis: a comparison of standing radiographs versus supine magnetic resonance imaging obtained with straightened lower extremities. J Manipulative Physiol Ther. 2007;30:26–30. doi: 10.1016/j.jmpt.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Hirasawa Y, Bashir WA, Smith FW, Magnusson ML, Pope MH, Takahashi K. Postural changes of the dural sac in the lumbar spines of asymptomatic individuals using positional stand-up magnetic resonance imaging. Spine (Phila Pa 1976) 2007;32:E136–140. doi: 10.1097/01.brs.0000255202.94153.ca. [DOI] [PubMed] [Google Scholar]
- 25.Madsen R, Jensen TS, Pope M, Sorensen JS, Bendix T. The effect of body position and axial load on spinal canal morphology: an MRI study of central spinal stenosis. Spine (Phila Pa 1976) 2008;33:61–67. doi: 10.1097/BRS.0b013e31815e395f. [DOI] [PubMed] [Google Scholar]
- 26.Schmid MR, Stucki G, Duewell S, Wildermuth S, Romanowski B, Hodler J. Changes in cross-sectional measurements of the spinal canal and intervertebral foramina as a function of body position: in vivo studies on an open-configuration MR system. AJR Am J Roentgenol. 1999;172:1095–1102. doi: 10.2214/ajr.172.4.10587155. [DOI] [PubMed] [Google Scholar]
- 27.Lin RM, Jou IM, Yu CY. Lumbar lordosis: normal adults. J Formos Med Assoc. 1992;91:329–333. [PubMed] [Google Scholar]
- 28.Been E, Barash A, Pessah H, Peleg S. A new look at the geometry of the lumbar spine. Spine (Phila Pa 1976) 2010;35:E1014–1017. doi: 10.1097/BRS.0b013e3181ddd433. [DOI] [PubMed] [Google Scholar]
- 29.Papadakis M, Papadokostakis G, Kampanis N, Sapkas G, Papadakis SA, Katonis P. The association of spinal osteoarthritis with lumbar lordosis. BMC Musculoskelet Disord. 2010;11:1. doi: 10.1186/1471-2474-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takao S, Sakai T, Sairyo K, Kondo T, Ueno J, Yasui N, et al. Radiographic comparison between male and female patients with lumbar spondylolysis. J Med Invest. 57:133–137. doi: 10.2152/jmi.57.133. [DOI] [PubMed] [Google Scholar]
- 31.Murrie VL, Dixon AK, Hollingworth W, Wilson H, Doyle TA. Lumbar lordosis: study of patients with and without low back pain. Clin Anat. 2003;16:144–147. doi: 10.1002/ca.10114. [DOI] [PubMed] [Google Scholar]
- 32.Fernand R, Fox DE. Evaluation of lumbar lordosis. A prospective and retrospective study. Spine (Phila Pa 1976) 1985;10:799–803. doi: 10.1097/00007632-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Whitcome KK, Shapiro LJ, Lieberman DE. Fetal load and the evolution of lumbar lordosis in bipedal hominins. Nature. 2007;450:1075–1078. doi: 10.1038/nature06342. [DOI] [PubMed] [Google Scholar]
- 34.Youdas JW, Garrett TR, Egan KS, Therneau TM. Lumbar lordosis and pelvic inclination in adults with chronic low back pain. Phys Ther. 2000;80:261–275. [PubMed] [Google Scholar]
- 35.Youdas JW, Garrett TR, Harmsen S, Suman VJ, Carey JR. Lumbar lordosis and pelvic inclination of asymptomatic adults. Phys Ther. 1996;76:1066–1081. doi: 10.1093/ptj/76.10.1066. [DOI] [PubMed] [Google Scholar]
- 36.Tuzun C, Yorulmaz I, Cindas A, Vatan S. Low back pain and posture. Clin Rheumatol. 1999;18:308–312. doi: 10.1007/s100670050107. [DOI] [PubMed] [Google Scholar]
- 37.Amonoo-Kuofi HS. Changes in the lumbosacral angle, sacral inclination and the curvature of the lumbar spine during aging. Acta Anat (Basel) 1992;145:373–377. doi: 10.1159/000147392. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg NJ. Degenerative spondylolisthesis. Predisposing factors. J Bone Joint Surg Am. 1975;57:467–474. [PubMed] [Google Scholar]
- 39.Lebkowski WJ, Lebkowska U, Niedzwiecka M, Dzieciol J. The radiological symptoms of lumbar disc herniation and degenerative changes of the lumbar intervertebral discs. Med Sci Monit. 2004;10 (Suppl 3):112–114. [PubMed] [Google Scholar]
- 40.Harrison DD, Cailliet R, Janik TJ, Troyanovich SJ, Harrison DE, Holland B. Elliptical modeling of the sagittal lumbar lordosis and segmental rotation angles as a method to discriminate between normal and low back pain subjects. J Spinal Disord. 1998;11:430–439. [PubMed] [Google Scholar]
- 41.Schuller S, Charles YP, Steib JP. Sagittal spinopelvic alignment and body mass index in patients with degenerative spondylolisthesis. Eur Spine J. 2010 doi: 10.1007/s00586-010-1640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones DA, Rutherford OM, Parker DF. Physiological changes in skeletal muscle as a result of strength training. Q J Exp Physiol. 1989;74:233–256. doi: 10.1113/expphysiol.1989.sp003268. [DOI] [PubMed] [Google Scholar]
- 43.Hefti F, Brunazzi M, Morscher E. Natural course in spondylolysis and spondylolisthesis. Orthopade. 1994;23:220–227. [PubMed] [Google Scholar]
- 44.Saraste H, Brostrom LA, Aparisi T. Prognostic radiographic aspects of spondylolisthesis. Acta Radiol Diagn (Stockh) 1984;25:427–432. doi: 10.1177/028418518402500515. [DOI] [PubMed] [Google Scholar]
- 45.Saraste H, Brostrom LA, Aparisi T. Radiographic assessment of anatomic deviations in lumbar spondylolysis. Acta Radiol Diagn (Stockh) 1984;25:317–323. doi: 10.1177/028418518402500412. [DOI] [PubMed] [Google Scholar]
- 46.Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine (Phila Pa 1976) 2009;34:199–205. doi: 10.1097/BRS.0b013e31818edcfd. [DOI] [PMC free article] [PubMed] [Google Scholar]


