Abstract
Objectives
Unintentional weight loss is a prevalent and costly clinical problem among nursing home (NH) residents. One of the most common nutrition interventions for residents at risk for weight loss is oral liquid nutrition supplementation. The purpose of this study was to determine the cost effectiveness of supplements relative to offering residents’ snack foods and fluids between meals to increase caloric intake.
Design
Randomized, controlled trial.
Setting
Three long-term care facilities.
Participants
Sixty-three long-stay residents who had an order for nutrition supplementation.
Intervention
Participants were randomized into one of three groups: (1) usual NH care control; (2) supplement, or (3) between-meal snacks. For groups two and three, trained research staff provided supplements or snacks twice daily between meals, five days per week, for six weeks with assistance and encouragement to promote consumption.
Measurements
Research staff observed residents during and between meals for two days at baseline, weekly, and post six weeks to estimate total daily caloric intake. For both intervention groups, research staff documented residents’ caloric intake between meals from supplements or snack items, refusal rates and the amount of staff time required to provide each intervention.
Results
Both interventions increased between meal caloric intake significantly relative to the control group and required more staff time than usual NH care. The snack intervention was slightly less expensive and more effective than the supplement intervention.
Conclusions
Offering residents a choice among a variety of foods and fluids twice per day may be a more effective nutrition intervention than oral liquid nutrition supplementation.
Keywords: nursing homes, weight loss, intervention, nutrition supplementation
INTRODUCTION
Inadequate food and fluid intake is a common problem among nursing home (NH) residents that can lead to weight loss, hospitalization, and death (1–4). Federal guidelines specify a resident is nutritionally at risk if intake is consistently below 75% of that offered during meals (5), and 64% to 80% of NH residents’ mealtime intake is below this federal criterion (6–8). The use of oral liquid nutrition supplements is a common nutrition intervention in the NH setting; although, there is mixed evidence of efficacy. A 2004 Cochrane review concluded that supplements had only a modest benefit on weight gain and mortality, and additional trial data are needed to determine if supplements are an efficacious intervention for nutritionally at risk elderly people (9).
Supplements are supposed to improve caloric intake and appetite through consistent delivery multiple times per day between meals (10,11). Observational studies have shown that NH residents do not receive supplements consistent with their orders (12,13). Moreover, residents often receive supplements with meals in lieu of adequate feeding assistance to promote meal intake, and residents who do receive supplements between meals do not receive assistance or encouragement to promote consumption (13).
Recent studies have shown that staff assistance and encouragement is necessary to promote adequate food and fluid intake, including supplements, in NH residents with varying levels of cognitive impairment and physical dependency (10, 14,15). Two recent studies showed that approximately 50% of residents with low intake significantly increased their intake during meals in response to a two-day intervention trial that improved the duration and quality of assistance. Most (80%) of the remaining residents, who showed modest to no intake gains in response to mealtime assistance, showed significant intake gains in response to a two-day trial of snacks between meals. The snack intervention included assistance and encouragement to promote consumption and a variety of foods and fluids from which to choose three times per day. Residents responsive to snack delivery consumed an average of 380 additional calories per day outside of meals, which was significantly greater than calories consumed from supplements during usual NH care (< 100 calories/day) (10,15).
Based on research to date, it is unknown if NH residents’ prefer snacks instead of supplements or if it is the consistency of delivery and amount of assistance that represent the critical intervention components. It is also unknown which intervention is more cost-effective in increasing caloric intake among NH residents. The purpose of this study was to evaluate the cost-effectiveness of oral liquid nutrition supplementation and a between-meal snack intervention for improving caloric intake in long-stay NH residents with an existing order for supplementation. This study used a controlled, intervention design to randomize residents into one of three groups following baseline assessments: (1) usual NH care control; (2) supplementation; or, (3) between meal snacks. The following research questions were addressed:
What is the effect of the two nutrition interventions on estimated between-meal and total daily caloric intake compared to usual NH care?
How much staff time is required to implement the two nutrition interventions compared to usual NH care?
Which of the two nutrition interventions is more cost-effective in increasing between-meal and total daily caloric intake?
METHODS
Setting and Recruitment
Participants were recruited from two community NHs and one VA facility housing a total of 588 residents. Staff-to-resident ratios ranged from 7 – 11 residents per nurse aide during the day and evening. A total of 280 (48%) residents met study inclusion criteria which required residents to be long-stay, free of a feeding tube, not receiving hospice care, and have an existing physician or dietitian order for nutrition supplementation.
Written consent was obtained from either the resident or the resident’s responsible party for 86 (31%) of the 280 eligible residents. Recruitment procedures were approved by the university-affiliated institutional review board. Following consent, 23 participants were lost due to prolonged hospitalization or death (n = 9), feeding tube insertion (n = 3), transfer to hospice care (n = 4), consent withdrawal (n = 1), or discontinuation of supplement orders (n = 6). The remaining 63 participants comprised the study sample.
Measures
Demographics, diagnoses, diet and supplement orders, and weight were abstracted from participants’ medical records. Body weight (age, sex, height) was used to calculate Body Mass Index and estimate Resting Energy Expenditure (BMI and REE, Refer to Table 1 footnotes for formulas). A BMI value less than 20 was considered indicative of under-nutrition (16). Residents’ need for eating assistance (section G. physical functioning, item 1h. eating dependency) was retrieved from the most recent Minimum Data Set (MDS) assessment (score range 0, independent, to 4, total dependence) (5). Cognitive status for each participant was assessed by research staff using the standardized Mini-Mental State Examination (MMSE), with a score range from 0 (severe impairment) to 30 (cognitively intact) (17).
Table 1.
Demographic, Medical, and Nutritional Characteristics of Participants (n=63)
| Measure | Percent (n) or Mean (SD)* | |
|---|---|---|
|
| ||
| Demographic Characteristics | ||
| Percent Female | 62 (39) | |
| Percent White | 98 (62) | |
| Age | 86.86 (11.26) | |
| Length of Stay in Years | 3.42 (2.29) | |
| Medical Characteristics | ||
| MMSE Total Score (0–30) † | 14.12 (8.88) | |
| Percent diagnosis of Dementia | 54 (34) | |
| Percent diagnosis of Depression | 68 (43) | |
| Nutritional Characteristics | ||
| Proportion MDS Eating Dependency > 1 | 43 (27) | |
| Percent Special Diet Order‡ | 84 (53) | |
| Percent Body Mass Index (BMI) < 20§ | 24 (15) | |
| Estimated REE needs (daily calories)|| | 1125.67 (221.86) | |
| Proportion with intake below REE needs | 56 (35) | |
SD = Standard Deviation;
MMSE = Mini Mental State Exam
Special diets included any restrictions (no added salt, no concentrated sugars) or altered texture (ground, mechanical soft, puree, thickened liquids).
Body Mass Index (BMI) formula = 0.454 weight in pounds / (0.254 height in inches)2 A BMI below 20 is considered indicative of under-nutrition.
Harris-Benedict Formulas for Resting Energy Expenditure (REE) Needs Estimation Male = 66.5 + 13.75weight in kilograms + 5.0height in centimeters − 6.78age. Female = 655.1 + 9.56weight in kilograms + 1.85height in centimeters − 4.68age.
Oral Food and Fluid Consumption
Food, fluid and supplement intake was measured during and between meals using standardized observation protocols determined in previous studies to be reliable and valid (4,8,13,18). Research staff observed each participant from the time of meal delivery until meal pick up by NH staff for all three scheduled meals and between meals (9–11am, 1–3pm, 6–8pm). Observations were conducted on two consecutive week days at baseline, weekly during intervention, and post six weeks. Previous studies have shown a two-day assessment period is reliable for identifying NH residents with low intake (8,18).
Research staff recorded each food and fluid item offered and the amount consumed using percentage estimates and fluid ounce measures. In addition, a digital camera was used to take photographs of residents’ trays before and after a sample of served meals (1–2 meals per participant at each assessment point) to determine the inter-rater reliability of the percentage estimates. Research staff different from the observer(s) and blind to group assignment estimated intake based on the photographs. Observation and photography methods have been shown to be reliable, valid methods to assess NH residents’ food and fluid intake (8,18). Research staff used a stop watch to record the time staff spent providing assistance to eat (e.g., verbal encouragement, tray set up, physical feeding) to each participant.
Intervention Protocol
Participants who completed baseline assessments were randomized into one of three groups: (1) usual NH care control; (2) supplements; or (3) snacks. Research staff monitored usual care (Group 1) and provided supplements (Group 2) or snacks (Group 3) twice daily between meals (~10am and 2pm), five week days per week for six weeks. For Group 2, research staff offered supplements consistent with each participant’s order in available flavors. For Group 3, research staff offered a variety of foods (e.g., yogurts, puddings, fruits) and fluids (e.g., assorted juices). Snack items were provided consistent with diet specifications in participants’ medical records.
Research staff provided both intervention groups with assistance according to a standardized protocol to enhance eating independence and intake (10,15). Research staff documented each item offered, amount consumed (percent eaten, fluid ounces) and the amount of time (minutes and seconds) spent with the participant during each intervention period (60 total periods per person). Caloric intake was estimated based on the information printed on the item packaging. The following cost-relevant information was collected during the six-week trial: staff time for nutritional care delivery, residents’ refusal rates, and cost of snacks and supplements.
Data Analyses
All baseline characteristics (shown in Table 1) were compared between participants who completed the study (n = 63) and those lost from the study (n = 23) using T-tests for independent samples for continuous variables and chi-square analyses for categorical variables. Baseline intake was compared between groups using T-tests for independent samples. Caloric intake and the amount of staff time spent providing assistance was evaluated from baseline to post intervention by group with independent (between group comparisons) or paired (within group comparisons) samples T-tests and multivariate analysis of variance. Weekly post measures were averaged across intervention weeks. Treatment effects were determined based on the difference in the differences (DD i.e., the difference in caloric intake between groups from baseline to post intervention). This method controls for individual heterogeneity, which might lead to differences in the outcome independent of intervention. Similar analyses were conducted to compare refusal rates and costs between intervention groups. Two participants who completed the study had missing meal intake data because they ate one or more meals outside of the facility on the observation days (see Oral Food and Fluid Intake). Both of these participants were excluded from the caloric intake analyses (n=61); the sample size remained at 63 for all other analyses.
Cost-Effectiveness Analysis
The analysis was framed as a cost-effectiveness analysis, with the measures of effect being between meal and total daily calorie intake. Increasing caloric intake is a therapeutic goal, but the precise relationship between increased calories and better health, or the dollar value of that health, is not known. Thus, different methods of increasing calorie intake were compared and the cost effectiveness analysis addressed the economically most efficient method.
The effectiveness for each intervention was calculated as the DD in average between-meal and total daily caloric intake from baseline to post intervention compared to the control group. Incremental costs were measured as the sum of additional daily food, fluid or supplement spending and labor costs. Labor cost was the product of assistance time (minutes) and the average earning rate of Certified Nursing Assistants (CNAs), who typically provide feeding assistance (19). The most recent Bureau of Labor Statistics estimate for the hourly rate of CNAs at nursing homes was $10.61 (20). Assuming a 10 percent fringe benefit rate, the hourly rate was adjusted up to $11.70, or $0.195 per minute.
The uncertainty in the cost-effectiveness was determined using cost-effectiveness acceptability curves (CEAcc), which is a method that builds on the net benefit (NB) approach (21,22). Given a monetary value (λ) of a one unit gain in caloric intake, the net benefit of the intervention was defined as: NB = λ × E – C where E is the effectiveness (i.e., gain in caloric intake) and C is the total intervention cost. To generate the CEAcc, a distribution of costs and benefits was obtained by bootstrapping the trial data (23). Participants were randomly selected with replacement keeping their own individual costs and caloric gains. A total of 1000 pairs of mean caloric gains and costs were generated using bootstrapping for both intervention groups, then the NB was estimated for each pair as λ ranges from $0 to $0.1 in increments of $0.005. The proportion of bootstrapped pairs with NB greater than zero is the probability the intervention was cost-effective conditional on the assumed monetary value of caloric gain. Those probabilities were subsequently plotted for every value of λ, producing the CEAcc.
RESULTS
Subjects and Setting
Table 1 shows the participant characteristics (n = 63), which were comparable long-stay NH residents in previous studies with similar inclusion criteria (10,13,15). There were no significant differences between participants who completed the study and those lost from the study (n=23) on any of the characteristics shown in Table 1. There also were no significant differences between the three groups at baseline on any of the characteristics shown in Table 1.
Estimated Caloric Intake During and Between Meals
Table 2 shows the estimated daily caloric intake during and between meals by group (control, supplement or snacks) and study phase (baseline, weekly and post). The total sample size for Table 2 is 61 participants due to missing meal intake data for two participants (see Analyses section). The last column in Table 2 shows the mean difference in meal, between meal and total caloric intake from baseline to post intervention for each group. There were no significant differences between groups at baseline for estimated caloric intake.
Table 2.
Estimated Meal, Supplement and Snack Calories by Group Assignment (n = 61)
| Group | Estimated Caloric Intake | Baseline Mean (SD) | Weekly Mean (SD) | Post Mean (SD) | Mean Difference |
|---|---|---|---|---|---|
|
| |||||
| Control (N = 19) | Meals | 1019 (423) | 1023 (418) | 1025 (371) | 5 |
| Between Meals | 130 (129) | 47 (69) | 73 (93) | −70 | |
| Total | 1149 (461) | 1069 (430) | 1098 (415) | −65 | |
| Supplement (N =18) | Meals | 990 (379) | 927 (367) | 805 (326) | −124* |
| Between Meals | 206 (206) | 324 (191) | 391 (339) | 151* | |
| Total | 1196 (427) | 1251 (358) | 1196 (351) | 28 | |
| Snacks (N = 24) | Meals | 1080 (405) | 994 (405) | 975 (407) | −96 |
| Between Meals | 113 (134) | 247 (176) | 304 (245) | 163** | |
| Total | 1193 (419) | 1241 (476) | 1279 (524) | 67 | |
P< .05;
P < .001. Estimated mean caloric intake is reported per person per day based on a 2000 calorie/day diet served by the participating facilities during regularly-scheduled meals and the caloric information printed on the labels for supplements provided by the facility or snack foods and fluids purchased from a local grocery store.
Both interventions resulted in a significant increase in between-meal caloric intake (Table 2. Supplement Group Mean Difference = 151, P < .05; Snack Group Mean Difference = 163, P < .001). The supplement group significantly increased between-meal caloric intake from 206 (± 206) mean calories per person per day at baseline to 391 (± 339) post intervention (DD Treatment Effect = 222, P < .001); while, the snack group significantly increased between-meal caloric intake from 113 (± 134) mean calories at baseline to 304 (± 245) post intervention (DD Treatment Effect = 233, P < .001).
The supplement group showed a significant decline in meal intake from 990 (± 379) at baseline to 805 (± 326) post intervention (Mean Difference = −124, P < .01); and, although the snack group also showed a decline in meal intake, it was not statistically significant. Thus, the treatment effect for the difference in total daily calories was not significant for the supplement group (DD Treatment Effect = 93, P = .14) but approached significance for the snack group (DD Treatment Effect = 132, P = .08). The usual care control group showed no significant changes from baseline to post intervention (Table 2. Control).
Weight Change
There was not a significant effect of either intervention on weight change from baseline to post six weeks according to NH staff recorded weights. The average weight change in the supplement and snack intervention groups was 2.01 (± 5.15) pounds and 0.04 (± 2.75) pounds, respectively; while, the average weight change in the control group was 0.53 (± 4.32) pounds.
Cost-Effectiveness Analyses
The average refusal rate per person (total number of refusals / possible total of 60 offers) across all six weeks was .30 (± .30) for the supplement group (range 0 to .84) and .20 (± .20) for the snack group (range 0 to .75). Both the supplement and the snack interventions required significantly more staff time than usual NH care (1.7 ± 3.2 minutes per person per offer). There was a gradual increase in the amount of staff time required for the supplement intervention over six weeks (mid point average = 9.6 ± 5.5 minutes per participant per offer versus 13.8 ± 12.5 at post 6 weeks). In comparison, the staff time for the snack intervention remained comparable across the six weeks (mid point average = 12.0 ± 5.5 minutes per participant per offer versus 12.3 ± 10.0 at post 6 weeks). The average amount of assistance provided by NH staff to residents during meals remained comparable for all three groups from baseline to post intervention with an average of less than 10 minutes per person per meal at all measurement points and all meals. Inter-rater reliability coefficients between observation and blinded photo estimates of meal intake ranged from .943 to .972 (All P<.001) across baseline, weekly and post assessments.
Table 3 shows the between-meal costs of the two interventions for supplements and snacks provided by research staff relative to the usual care control group. Costs included the cost per serving and the staff time to promote consumption (see Analyses). Across all three groups (n=63) at baseline, participants were offered foods or fluids, including supplements, on average less than once per person per day between meals (mean = .85 ± .72, range 0–3), with little NH staff assistance to promote consumption (mean = 1.77 ± 4.57 minutes per person). Thus, there were significant cost increases for both intervention groups as a result of research staff consistently offering supplements or snacks twice daily, and no change in the control group. The DD for intervention costs were $2.13 (± $0.37) per person per day for the supplement group (mean caloric gain = 221.5 ± 59.9) and $2.09 (± $0.26) for the snack group (mean caloric gain = 232.8 ± 36.1) (both P < .001). The cost-effectiveness ratios for both interventions were comparable (.010 and .011); thus, on average, each intervention costs approximately one cent per calorie gained. The snack intervention resulted in a slightly larger between-meal caloric gain for a lower per person per day cost.
Table 3.
Average Between-Meal Costs Per Person Per Day and Differences by Group (n=63)
| Group | Baseline Mean (SD) | Weekly Mean (SD) | Post Mean (SD) | Mean Difference |
|---|---|---|---|---|
|
| ||||
| Control (N = 20) | $0.23 ($0.28) | $0.19 ($0.25) | $0.21 ($0.51) | $−0.03 |
| Supplement (N =18) | $1.45 ($0.43) | $3.14 ($1.25) | $3.97 ($2.73) | $2.10** |
| Snack (N = 25) | $1.31 ($0.91) | $3.34 ($1.19) | $3.41 ($1.96) | $2.06** |
P < .001
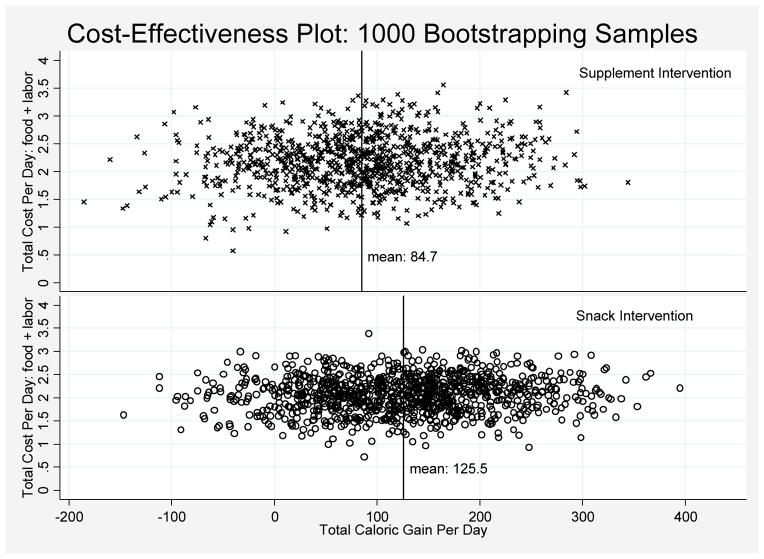
Two effectiveness measures were used in the cost-effectiveness analysis: average between-meal caloric gain and average total caloric gain. The results of the CEA showed the same pattern for both measures, and these results are shown in Figures 1 and 2 for total caloric gain from the two interventions. Figure 1 shows the average (total calories) gained and the average increased cost for each bootstrap replication of each intervention (See Data Analyses). The supplement intervention cases were more concentrated to the left side of the zero line (Figure 1), indicating a higher probability the supplement intervention would result in a negative difference value (or decrease in total daily calories), relative to the snack intervention.
Figure 1.
Cost-effectiveness Analysis for Gain in Total Daily Caloric Intake by Intervention Group: Results of Bootstrap
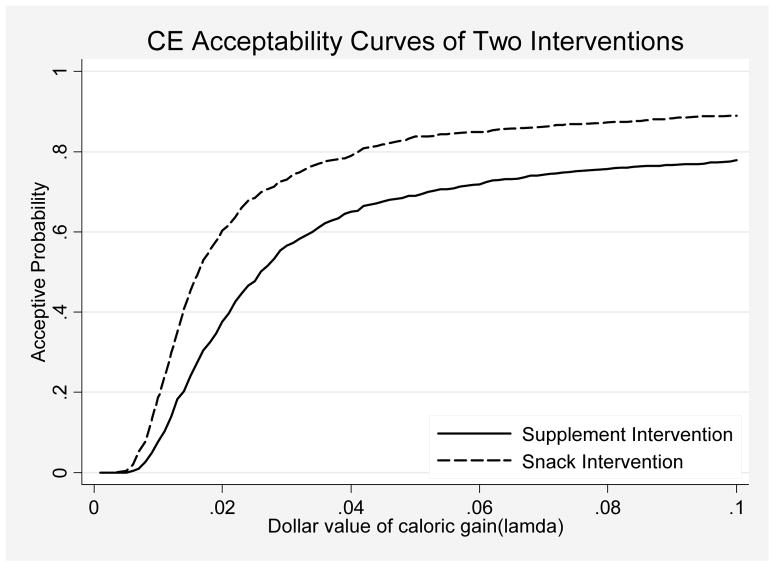
Figure 2.
Cost-effectiveness Analysis for Gain in Total Daily Caloric Intake by Intervention Group: Acceptance Probability Curves
The cost-effectiveness acceptability curves (CEAcc) for the two interventions, which are based on the bootstrap replications, are shown in Figure 2. The CEAcc show the probability that each intervention is worthwhile (i.e. has a “net benefit”) as a function of the dollar value assigned to caloric gain. The “y” axis in Figure 2 begins at “0” probability, indicating that neither intervention is worthwhile if each calorie gained is assigned a low value, and increases to “100%”, indicating that both interventions are worthwhile if each calorie gained is assigned a high value. As the dollar value of caloric gain increases, the number of bootstrapped samples where the value of the gain is greater than the cost (a “net benefit”) also increases. Figure 2 shows that the acceptability curve of the snack intervention consistently exceeds the supplement intervention. For example, given a .04 cents dollar value of one unit caloric gain, the probability of having a net benefit is approximately 80% for the snack intervention versus 65% for the supplement intervention.
DISCUSSION
Supplements are a common treatment for nutritionally at-risk NH residents. This pilot intervention represents a controlled evaluation of supplements versus snack delivery for increasing caloric intake. Results showed that both interventions were efficacious in increasing residents’ daily caloric intake between meals but the supplement intervention also resulted in a significant decline in meal intake. Although the snack intervention group also showed a decline in meal intake, it was not significant. One previous study showed that a brief trial of the snack intervention did not result in a significant decrease in meal intake in NH residents (10). The snack intervention was more cost-effective than the supplement intervention when considering caloric gain, staff time, refusal rates, and costs.
Both interventions required significantly more time than usual care during which NH staff spent little to no time offering assistance to promote consumption of supplements or snacks between meals. Because research staff offered both food and fluid items and choice as part of the snack intervention, one would expect this intervention to require more staff time than offering a supplement alone. However, the research staff time spent providing the supplement intervention showed a gradual increase over the six study weeks whereas the amount of time spent providing the snack intervention remained the same. This trend is likely due to a higher refusal rate in response to the supplement intervention.
Previous studies have shown that the availability of choices is an important nutrition intervention component, especially among less cognitively impaired NH residents (10,14). The sample size in this pilot intervention was too small to determine if the interventions had differential effects on caloric intake and/or refusal rates among participants with varying levels of cognitive impairment, even though the sample sizes were sufficient to detect significant intervention effects on change in caloric intake. Research staff also was not blind to group assignment because the same staff delivered the intervention and documented resident response (refusals, intake); however, there was high agreement between the direct observer and a second, blinded rater for inter-rater reliability estimates of caloric intake, which supports a lack of bias. In addition, the interventions were only six weeks. The availability of choices might be more influential on residents’ refusal rates over a longer intervention period.
The sample sizes within each group were too small to detect significant effects on weight status in the intervention groups. Moreover, it is likely that a 6-week intervention period implemented twice daily during week days only may not be intense enough to have a significant effect on body weight. The results of a recent controlled trial showed that a 24-week intervention provided twice daily during week days only yielded a significant effect of mealtime assistance and between-meal snack delivery on weight outcomes (24). The weight data in the current study also were not independently measured by research staff (25).
There are many costs not measured in this study such as costs associated with food and fluid ordering, storage and inventory. In addition, there might be downstream cost savings of nutrition intervention, such as reduced hospitalization or acute illness rates, not considered in this study. Finally, this intervention did not improve staff assistance during meals, which consistently averaged less than 10 minutes per person per meal. Other studies have shown that residents need at least 20 to 30 minutes of assistance to promote adequate meal intake (10,15,26–28). Other limitations include small sample sizes and low recruitment rates, which were limited by funding constraints for this pilot intervention.
Despite these limitations, this is the first controlled study to evaluate the comparative cost effectiveness of supplements versus an alternative nutrition intervention, and the results suggest the snack intervention is preferable even with modest effects. Residents were not excluded from either intervention due to lack of response, and both intervention groups included residents who were not responsive (e.g., high refusal rate and low caloric gain). The sample sizes were too small to identify differentiating characteristics of participants most responsive to each intervention. Previous studies have shown that a two-day trial of mealtime assistance predicts which residents will be responsive to mealtime assistance over a longer time period (15,24). It is likely that similar two-day trials could be used to determine which residents are appropriate for the supplement and snack interventions to improve cost effectiveness.
It is noteworthy that the snack intervention also is more consistent with regulatory guidelines for NH care to improve residents’ quality of life. These guidelines specify that staff should offer choice during daily nutrition care provision (29). Given the current popularity and significant financial cost of supplements in the NH setting, the results of this pilot study provide preliminary data that physician and dietitian orders for supplements might be more effectively worded as orders to offer the resident a variety of foods and fluids between meals, including supplements, to allow residents’ more choice and improve caloric intake.
Acknowledgments
This research was supported by Grant IIRG-04-1338 from the National Alzheimer’s Association and Grant AG028748 from the National Institute of Aging, UCLA Claude D. Pepper Older Americans Independence Center.
Footnotes
There are no financial conflicts of interest for any of the authors. Each author contributed to study concept, data analyses, interpretation of data, and/or manuscript preparation.
References
- 1.Blaum CS, Fries BE, Fiatarone MA. Factors associated with low body mass index and weight loss in nursing home residents. J Gerontol A:Biol Sci Med Sci. 1995;50A:M162–M168. doi: 10.1093/gerona/50a.3.m162. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson RP, O’Conner P, Crabtree B, et al. Serum albumin and prealbumin as predictors of clinical outcomes of hospitalized elderly nursing home residents. J Am Geriatr Soc. 1993;41:545–549. doi: 10.1111/j.1532-5415.1993.tb01893.x. [DOI] [PubMed] [Google Scholar]
- 3.Gilmore SA, Robinson G, Posthauer ME, et al. Clinical indicators associated with unintentional weight loss and pressure ulcers in elderly residents in nursing facilities. J Am Diet Assoc. 1995;95(9):984–992. doi: 10.1016/S0002-8223(95)00271-5. [DOI] [PubMed] [Google Scholar]
- 4.Simmons SF, Garcia EF, Cadogan MP, et al. The Minimum Data Set weight loss quality indicator: Does it reflect differences in care processes related to weight loss? J Am Geriatr Soc. 2003;51(10):1410–1418. doi: 10.1046/j.1532-5415.2003.51459.x. [DOI] [PubMed] [Google Scholar]
- 5.Minimum Data Set, Version 2: User’s Manual. Health Care Financing Administration. Natick, MA: Eliot Press; Apr, 1999. [Google Scholar]
- 6.Kayser-Jones J, Schell E, Porter C, et al. Reliability of percentage figures used to record the dietary intake of nursing home residents. Nursing Home Medicine. 1997;5(3):69–76. [Google Scholar]
- 7.Pokrywka HS, Koffler KH, Remsburg R, et al. Accuracy of patient care staff in estimating and documenting meal intake of nursing home residents. J Am Geriatr Soc. 1997;45:1223–1227. doi: 10.1111/j.1532-5415.1997.tb03774.x. [DOI] [PubMed] [Google Scholar]
- 8.Simmons SF, Reuben D. Nutritional intake monitoring for nursing home residents: A comparison of staff documentation, direct observation, and photography methods. J Am Geriatr Soc. 2000;48:209–213. doi: 10.1111/j.1532-5415.2000.tb03914.x. [DOI] [PubMed] [Google Scholar]
- 9.Milne AC, Potter J, Avenell A. The Cochrane Collaboration, The Cochrane Library. 1. John Wiley & Sons, Ltd; Publishers: 2008. Protein and energy supplementation in elderly people at risk from malnutrition. [Google Scholar]
- 10.Simmons SF, Schnelle JF. Individualized feeding assistance care for nursing home residents: Staffing requirements to implement two interventions. J Gerontol A:Biol Sci Med Sci. 2004;59A(9):966–973. doi: 10.1093/gerona/59.9.m966. [DOI] [PubMed] [Google Scholar]
- 11.Wilson MG, Purushothaman R, Morley JE. Effect of liquid dietary supplements on energy intake in the elderly. Am J Clin Nutr. 2002;75:944–947. doi: 10.1093/ajcn/75.5.944. [DOI] [PubMed] [Google Scholar]
- 12.Kayser-Jones J, Schell ES, Porter C, et al. A prospective study of the use of liquid oral dietary supplements in nursing homes. J Am Geriatr Soc. 1998;46:1378–1386. doi: 10.1111/j.1532-5415.1998.tb06004.x. [DOI] [PubMed] [Google Scholar]
- 13.Simmons SF, Patel AV. Nursing home staff delivery of oral liquid nutritional supplements to residents at risk for unintentional weight loss. J Am Geriatr Soc. 2006;54(9):1372–1376. doi: 10.1111/j.1532-5415.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 14.Simmons SF, Alessi C, Schnelle JF. An intervention to increase fluid intake in nursing home residents: Prompting and preference compliance. J Am Geriatr Soc. 2001;49(7):926–933. doi: 10.1046/j.1532-5415.2001.49183.x. [DOI] [PubMed] [Google Scholar]
- 15.Simmons SF, Osterweil D, Schnelle JF. Improving food and fluid intake in nursing home residents with feeding assistance: A staffing analysis. J Gerontol A:Biol Sci Med Sci. 2001;56A(12):M790–M794. doi: 10.1093/gerona/56.12.m790. [DOI] [PubMed] [Google Scholar]
- 16.Thomas DR, Ashmen W, Morley JE, et al. Nutritional management in long term care: Development of a clinical guideline. J Gerontol A:Biol Sci Med Sci. 2000;55A(12):M725–M734. doi: 10.1093/gerona/55.12.m725. [DOI] [PubMed] [Google Scholar]
- 17.Molloy DW, Alemayehu E, Roberts R. A standardized Mini-Mental State Examination (SMMSE): Its reliability compared to the traditional Mini-Mental State Examination (MMSE) Am J Psychiatry. 1991;148:102–105. doi: 10.1176/ajp.148.1.102. [DOI] [PubMed] [Google Scholar]
- 18.Simmons SF, Babinou S, Garcia E, et al. Quality assessment in nursing homes by systematic direct observations: Feeding assistance. J Gerontol A:Biol Sci Med Sci. 2002;57A(10):M665–M671. doi: 10.1093/gerona/57.10.m665. [DOI] [PubMed] [Google Scholar]
- 19.Schnelle JF, Cretin S, Saliba D, et al. Health Care Financing Administration. Chapter 14. Vol. 2. Abt Associates, Inc; Cambridge, MA: Summer. 2000. Minimum nurse aide staffing required to implement best practice care in nursing homes. Chapter in report to congress: Appropriateness of Minimum Nurse Staffing Ratios in Nursing Homes; pp. 14.1–14.68. [Google Scholar]
- 20.US Department of Labor National Occupational Employment and Wage Estimates. Bureau of Labor Statistics; May, 2006. [Accessed on 2/01/2008]. http://www.bls.gov/oes/current/oes311012.htm. [Google Scholar]
- 21.Lothgren M, Zethraeus N. Definition, interpretation and calculation of cost-effectiveness acceptability curves. Health Economics. 2000;9:623–630. doi: 10.1002/1099-1050. [DOI] [PubMed] [Google Scholar]
- 22.Stinnett A, Mullahy J. Net Health Benefits: A New Framework for the Analysis of Uncertainty in Cost-Effectiveness Analysis. Medical Decision Making. 1998;18(2):S68–S80. doi: 10.1177/0272989X98018002S09. [DOI] [PubMed] [Google Scholar]
- 23.Efron B, Tibshirani RJ. An introduction to the Bootstrap. Chapman and Hall. 1993:10–28. [Google Scholar]
- 24.Simmons SF, Keeler E, Zhuo X, et al. Prevention of unintentional weight loss in nursing home residents: A controlled trial of feeding assistance. J Am Geriatr Soc. 2008;56:1466–1473. doi: 10.1111/j.1532-5415.2008.01801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmons SF, Peterson E, You C. The accuracy of monthly weight assessments in nursing homes: Implications for the identification of weight loss. J Nutr Health Aging. doi: 10.1007/s12603-009-0074-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu TW, Huang LF, Cartwright WS. Evaluation of the costs for caring for the senile demented elderly: A pilot study. Gerontologist. 1986;26(2):158–163. doi: 10.1093/geront/26.2.158. [DOI] [PubMed] [Google Scholar]
- 27.Simmons SF, Schnelle JF. Feeding assistance needs of long-stay nursing home residents and the staff time to provide care. J Am Geriatr Soc. 2006;54(6):919–924. doi: 10.1111/j.1532-5415.2006.00812.x. [DOI] [PubMed] [Google Scholar]
- 28.Steele CM, Greenwood C, Ens I, et al. Mealtime difficulties in a home for the aged: Not just dysphagia. Dysphagia. 1997;12:43–50. doi: 10.1007/pl00009517. [DOI] [PubMed] [Google Scholar]
- 29.Interpretive Guidelines State Operations Manual. Appendix P- Survey Protocol for Long Term Care Facilities. Part 1. Revision 26, 08-17-07. Retrieved February 22, 2008 from http://www.cms.hhs.gov/manuals/