Abstract
Uncontrolled and controlled clinical trials with different compounds and procedures are reviewed to define the risk-benefit profiles for therapeutic options in pulmonary arterial hypertension (PAH). A grading system for the level of evidence of treatments based on the controlled clinical trials performed with each compound is used to propose an evidence-based treatment algorithm. The algorithm includes drugs approved by regulatory agencies for the treatment of PAH and/or drugs available for other indications. The different treatments have been evaluated mainly in idiopathic PAH, heritable PAH, and in PAH associated with the scleroderma spectrum of diseases or with anorexigen use. Extrapolation of these recommendations to other PAH subgroups should be done with caution. Oral anticoagulation is proposed for most patients; diuretic treatment and supplemental oxygen are indicated in cases of fluid retention and hypoxemia, respectively. High doses of calcium channel blockers are indicated only in the minority of patients who respond to acute vasoreactivity testing. Nonresponders to acute vasoreactivity testing, or responders who remain in World Health Organization (WHO) functional class III, should be considered candidates for treatment with either an oral phosphodiesterase-5 inhibitor or an oral endothelin-receptor antagonist. Continuous intravenous administration of epoprostenol remains the treatment of choice in WHO functional class IV patients. Combination therapy is recommended for patients treated with PAH monotherapy who remain in New York Heart Association functional class III. Atrial septostomy and lung transplantation are indicated for refractory patients or where medical treatment is unavailable.
Keywords: Pulmonary Arterial Hypertension, Algorithm, Evidence-Based Treatment
Introduction
In 1891, Ernst von Romberg, a German physician, described an autopsy subject as having “pulmonary vascular sclerosis”; however, it is only since 1995 with the introduction of intravenous epoprostenol that disease-specific targeted medical therapies for pulmonary arterial hypertension (PAH) have become available. Furthermore, significant advances in the treatment of PAH have occurred during the past 15 years. Currently nine medical therapies have either received regulatory approval or are under regulatory review. These agents target the prostacyclin pathway, the nitric oxide pathway, and the endothelin pathway. Combination trials have demonstrated additive or synergistic benefit by targeting two or all three of these pathways.
Until the 1980s, attempts to reduce pulmonary arterial pressure were performed with nonselective (pulmonary and systemic) vasodilators. Favorable and sustained results were convincingly shown only with the use of high doses of calcium-channel blockers (CCBs) and only in the minority of patients who responded to acute vasoreactivity testing (1-6). In addition, oral anticoagulant treatment was considered effective based on retrospective or uncontrolled studies (1,7-9). In the 1990s, treatment with continuous intravenous (IV) administration of epoprostenol was shown in three nonblinded randomized clinical trials (RCTs) to improve symptoms, exercise capacity, and hemodynamics in PAH, and to improve survival in idiopathic pulmonary arterial hypertension (IPAH)/heritable PAH (HPAH) (10-12). During that period, favorable results of several uncontrolled series of PAH patients who underwent atrial septostomy or lung transplantation were also reported (13-16).
Twenty RCTs with nine new compounds as monotherapy have been completed in PAH patients (10-12,17-31). In addition, six RCTs testing combinations of agents, eg, endothelin-receptor antagonists (ERA) and phosphodiesterase-5 (PDE-5) inhibitors, or prostanoid and ERA or PDE-5 inhibitors, have been completed (32-37). Approximately 5,000 patients have participated in these studies aimed at developing effective treatments for PAH.
The conclusions derived from clinical trials over the past 15 years have provided us with an evidence-based treatment strategy. The purpose of the present report is to review the RCTs performed in PAH and to propose an evidence-based updated treatment algorithm that incorporates currently available therapies. This algorithm can be used worldwide, subject to the availability of specific drug therapies.
Uncontrolled Clinical Studies in PAH
Anticoagulants
The evidence for favorable effects of oral anticoagulant treatment in patients with IPAH, HPAH, or PAH associated with anorexigens is based on retrospective analyses from seven studies, of which five were positive and two were negative (1,7-9). The survival of anticoagulated patients, selected on the basis of clinical judgment, was improved, as compared with a concurrent population that was not treated with oral anticoagulants. Three-year survival improved from 21% to 49% in the series reported by Fuster et al (7); and the 3- and 5-year survival rates increased from 31% to 47% and from 31% to 62%, respectively in the series reported by Rich et al (1). These studies were not randomized, and one can argue that the lower survival of the control groups could be related to comorbidity that precluded the use of anticoagulation in the untreated patients. In addition, only IPAH, HPAH, and anorexigen-related PAH patients were included in the studies. In recent RCTs, ~70% of patients were treated with oral anticoagulants (10-12, 17-37). Interestingly, the highest prevalence of oral anticoagulant treatment was seen in the trials involving mainly IPAH and HPAH patients in World Health Organization (WHO) functional class III and IV, whereas the lowest prevalence was observed in a trial of patients with scleroderma. It should be emphasized that there is no evidence of any difference in the efficacy of oral anticoagulant therapy based on functional class severity.
Diuretics, digoxin, and oxygen
The symptomatic and clinical benefits of diuretic treatment in right heart failure preclude the need for controlled trials to demonstrate efficacy in PAH. In recent RCTs with new treatments, ~50%–70% of patients were treated with diuretics (38,39). However, the lack of trials with specific classes of diuretics in PAH and individual variability in responses leave the choice of the type and dose of drug to be used in individual cases to the experience of the physician.
Short-term IV administration of digoxin in IPAH produces a modest increase in cardiac output and a significant reduction in circulating norepinephrine (40); however, no data are available on the effects of long-term treatment. Accordingly, the use of digitalis in PAH patients is based primarily on the judgment of the physician rather than on scientific evidence of efficacy. Digoxin was administered to ~25%–50% of patients in recent RCTs in PAH (38).
No consistent data are currently available on the effects of long-term oxygen treatment in PAH. Although improvement in pulmonary hypertension with low-flow supplemental oxygen has been reported in some PAH patients (41), this has not been confirmed in controlled trials. In a controlled study in patients with Eisenmenger syndrome, nocturnal oxygen therapy had no effect on hematologic variables, quality of life, or survival (42); in contrast, a previous study suggested increased survival (43).
Calcium channel blockers
Favorable clinical and prognostic effects of high doses of oral calcium channel blocker (CCB) drugs in acutely vasoreactive patients with IPAH have been shown in single-center, nonrandomized, uncontrolled studies (1-6). In these studies, the control group consisted of nonresponders, who may have a poorer prognosis, as compared with acutely vasoreactive individuals (3). Furthermore, the demonstration of a consistent reduction of pulmonary artery pressure by acute pharmacologic testing in vasoreactive patients raises ethical questions concerning the appropriateness of performing placebo-controlled clinical trials in these patients.
A definition of “a positive acute vasoreactive response” to predict long-term response with high-dose oral CCB was proposed at the third World Symposium on Pulmonary Hypertension in 2003 (5). Using this definition—reduction of mean pulmonary arterial pressure ≥10 mm Hg to reach a mean pulmonary arterial pressure ≤40 mm Hg with a normalized or increased cardiac output with acute pulmonary vasodilator challenge using either inhaled nitric oxide or intravenous epoprostenol—<10% of IPAH patients have a positive acute vasoactive response.
Favorable results of long-term administration of high doses of oral CCBs have also been shown in children with IPAH (4,6). In contrast, the effects of high-dose CCBs on associated forms of PAH have not yet been clearly demonstrated (41). However, acute vasodilator testing is recommended for all PAH patients, even though patients with IPAH and anorexigen-induced PAH are more likely to respond. Furthermore, although functional class IV patients are less likely to respond than functional class II and III patients, some functional class IV patients may respond favorably to acute vasodilator testing and may benefit from CCBs; however, it is recommended that these patients be evaluated in a specialized PH center. Empirical treatment with CCBs without a positive response with acute vasodilator testing using either inhaled nitric oxide or IV epoprostenol is contraindicated (41).
Surgical and interventional procedures
Lung transplantation or atrial septostomy may be indicated in select patients who progress despite optimal medical therapy or for whom medical therapy is not available. Lung transplantation and atrial septostomy are discussed in detail in another article in this supplement (44).
Controlled Clinical Trials in PAH
Synthetic prostacyclin and prostacyclin analogues
The efficacy of continuous IV administration of epoprostenol (synthetic prostacyclin) has been evaluated in three unblinded, controlled clinical trials: two in IPAH/HPAH (10,11), and one in PAH associated with the scleroderma spectrum of diseases (12). Although IV epoprostenol improves symptoms, exercise capacity, and hemodynamics in both clinical conditions, survival was increased only in IPAH and HPAH.
Five RCTs with three prostacyclin analogues as monotherapy have been performed in PAH patients. (19,45). The effects of continuous subcutaneous administration of treprostinil were assessed in a pilot RCT in which the improvement in exercise capacity was not statistically significant (45). In the two pivotal RCTs, improvements were reported in symptoms, exercise capacity, and hemodynamics (19) Continuous IV administration of treprostinil appears to be safe and effective based on two small, open-label, uncontrolled studies in patients with PAH (46,47).
The orally active prostacyclin analogue beraprost was evaluated in PAH patients in two RCTs, one in Europe (20) and one in the United States (23) In the first study, an increase in exercise capacity was seen after three months. In the second, which lasted 12 months, improvement in exercise capacity was observed at three and six months but not thereafter (23). No hemodynamic improvements were observed in the 12-month study, and clinical events were reduced only at the six-month evaluation.
Inhaled iloprost as monotherapy was evaluated in one RCT that enrolled patients with both PAH and chronic thromboembolic pulmonary hypertension (21). Overall, this study showed an increase in exercise capacity and improvement in symptoms, pulmonary vascular resistance, and clinical events in PAH patients. Continuous IV administration of iloprost was shown to be effective in a small, open-label, uncontrolled series of patients with PAH and chronic thromboembolic pulmonary hypertension (48).
Endothelin-1 receptor antagonists
Nine RCTs using one of three ERAs as monotherapy have been performed in PAH patients. The orally active endothelin A and B (ETA/ETB) ERA bosentan was evaluated in four RCTs in PAH patients (17,22,27,30,49), including one RCT performed in a cohort of patients with the Eisenmenger syndrome (27), and one RCT performed in a cohort of patients with only mildly symptomatic PAH (30). Overall, bosentan improved exercise capacity, functional class, hemodynamics, echocardiographic and Doppler variables, and time to clinical worsening (17,22,27,31,49). Small increases in the dose of warfarin may be required to maintain therapeutic international normalized ratio (INR) when bosentan is coadministered with warfarin.
Sitaxsentan, an orally active ETA selective ERA, has been assessed in PAH patients in two RCTs, both of which demonstrated improvement in exercise capacity (assessed by the 6-minute walk test) and hemodynamics (25,28,50). However, in one of the two studies (25), the primary endpoint (peak O2 consumption as assessed by cardiopulmonary exercise testing) was not statistically significant. Coadministration of sitaxsentan and warfarin requires the reduction of the warfarin dose up to 80% to maintain a therapeutic INR, due to a drug-drug interaction.
Ambrisentan, an orally active ETA selective ERA, has been evaluated in three RCTs (29,51,52). Results showed improvements in exercise capacity and clinical events that appear similar to the results observed with other ERAs.
Based on the results of RCTs using ERAs, the incidence of elevated hepatic transaminases ≥3 times the upper limit of normal appears to be ~10% with bosentan, ~4 % with sitaxsentan, and ~2 % with ambrisentan. However, the patient populations in the various RCTs differed, and these numbers should be considered only as approximations.
Phosphodiesterase-5 inhibitors
Two RCTs with two different PDE-5 inhibitors have been performed in PAH patients (26,31). Used as monotherapy, both sildenafil and tadalafil improved exercise capacity and hemodynamics in ~50% of enrolled patients; tadalafil also improved clinical events (31).
The optimal agent for PAH monotherapy remains unclear.
Combination therapy
More recently, combination treatment has been evaluated to address the multiple pathobiologic mechanisms present in PAH. The combination of oral bosentan and IV epoprostenol was investigated in one small study, with inconclusive results (32). Five additional RCTs have evaluated combination therapy in PAH. The addition of inhaled iloprost to background oral bosentan demonstrated improved hemodynamics and clinical events in one RCT (35); however, these results were not confirmed in an open trial (34). In another study, the addition of oral sildenafil to background IV epoprostenol demonstrated improved exercise capacity, hemodynamics, and clinical events; furthermore, in post hoc analysis, the addition of oral sildenafil to background IV epoprostenol increased survival vs IV epoprostenol alone (37). In the pivotal tadalafil RCT, ~50% of the patients had oral tadalafil added to background oral bosentan; in that study overall, tadalafil improved exercise capacity, hemodynamics, and clinical events (31). Inhaled treprostinil has also been studied as add-on therapy to either background bosentan or background sildenafil; in both combinations, the addition of inhaled treprostinil improved exercise capacity (36). These studies support the efficacy of combination treatment in patients who remain symptomatic on monotherapy. The optimal combination based on overall risk-benefit considerations remains unknown.
Although there appears to be an interaction between sildenafil and bosentan (increased bosentan and decreased sildenafil levels) (53), the clinical relevance of this is unclear. Similarly, although the interaction between tadalafil and bosentan is less than that between sildenafil and bosentan—ie, tadalafil exposure decreased with minimal changes in bosentan exposure (54)—the clinical relevance is also unknown. Tadalafil has also been evaluated in the presence of ambrisentan, with no clinically relevant pharmacokinetic interactions reported (55). There is no clinically relevant pharmacokinetic interaction between ambrisentan and sildenafil (56), with no dose adjustment of ambrisentan or sildenafil recommended compared with administration of either drug alone. There is a minimal interaction reported between sitaxsentan and sildenafil, with no changes in sitaxsentan plasma concentrations in the presence of sildenafil and only modest increases in sildenafil plasma concentrations (57). Overall, no dose adjustments have been recommended for patients treated with one of the above-mentioned ERAs in combination with either sildenafil or tadalafil.
Early intervention
For functional class II or III patients, the role of early aggressive intervention, ie, IV epoprostenol as first-line treatment, either as monotherapy or in conjunction with either a PDE-5 inhibitor and/or an ERA, remains unknown. Although the first RCTs in PAH focused primarily on functional class III and IV patients, results from a more recent RCT evaluating the efficacy of bosentan in only mildly symptomatic PAH patients support early intervention (30). In addition, prespecified subgroup analyses of the sildenafil, tadalafil, and ambrisentan RCTs did not show any significant differences in the therapeutic efficacy of these drugs between patients in WHO functional classes II and III (30). The apparent lack of “catch-up” in placebo-treated patients supports early intervention in PAH (41). Future studies appear warranted.
General comments on controlled clinical trials
Although these studies have similar designs, treatment duration, and end points, analyses of baseline WHO functional class and etiology profiles show substantial differences. Accordingly, comparisons may be misleading. Improvement of exercise capacity as assessed by the 6-minute walk test has been observed in all of these studies, albeit to different degrees. In evaluating the clinical relevance of exercise capacity improvements, additional elements, such as baseline functional class, effects on combined clinical events (eg, hospitalizations, mortality, rescue therapies), and hemodynamic effects, should be considered. As mentioned previously, a survival benefit has been demonstrated in only 1 controlled, unblinded study of IV epoprostenol in patients with severe IPAH/HPAH (11). Because, based on these results, IV epoprostenol is considered rescue therapy, subsequent RCTs assessing mortality as an end point could not ethically be performed. Furthermore, severely ill subjects requiring IV epoprostenol treatment were excluded in recent RCTs, resulting in a low mortality in these study populations. A recent meta-analysis performed on all RCTs in PAH patients published through October 2008 reports a 43% decrease in mortality and a 61% reduction in hospitalizations in patients treated with targeted therapies versus patients randomized to placebo (39). These results, achieved after an average treatment period of 14.3 weeks, support the efficacy of the currently approved PAH treatments.
Evidence-Based Treatment Algorithm
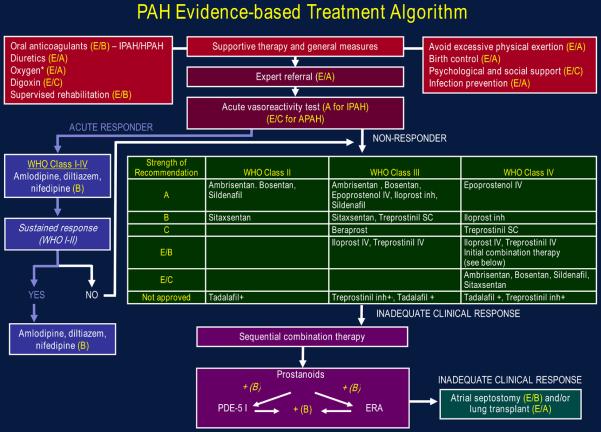
A treatment algorithm based on a consensus of the PH community evaluating the clinical trials presented in this review is presented in Figure. The recommendations in this guideline are based on a grading system in which the strength of the recommendation results from the interaction of two components: the quality of the evidence, and the net benefit of the therapy (Tables 1 and 2). Because treatments have been evaluated primarily in IPAH, HPAH, and PAH associated with scleroderma or anorexigen use, extrapolation of these recommendations to other PAH subgroups should be done with caution.
Figure. PAH evidence-based treatment algorithm.
Drugs within the same grade of evidence are listed in alphabetical order, not order of preference. Not all agents listed are approved or available for use in all countries. Strengths of recommendations are defined in Table 1. *To maintain O2 at 92%.+Investigational, under regulatory review.
Table 1.
Quality of evidence, net benefit, and strength of recommendation.
| Variables | Description |
|---|---|
| Quality of the Evidence |
|
| Good | Evidence is based on good randomized controlled trials or meta-analyses. |
| Fair | Evidence is based on other controlled trials or randomized controlled trials with minor flaws. |
| Low | Evidence is based on nonrandomized, case-control, or other observational studies. |
| Expert opinion | Evidence is based on the consensus of the carefully selected panel of experts in the topic field. |
| There are no studies that meet the criteria for inclusion in the literature review. |
|
| Net benefit | |
| Substantial | |
| Intermediate | |
| Small/weak | |
| None | |
| Conflicting | |
| Negative | |
| Strength of recommendation | |
| A | Strong recommendation |
| B | Moderate recommendation |
| C | Weak recommendation |
| D | Negative recommendation |
| I | No recommendation possible (inconclusive) |
| E/A | Strong recommendation based on expert opinion only |
| E/B | Moderate recommendation based on expert opinion only |
| E/C | Weak recommendation based on expert opinion only |
| E/D | Negative recommendation based on expert opinion only |
Table 2.
Relationship of strength of the recommendations scale to quality of evidence and net benefits.*
| Net Benefit |
||||||
|---|---|---|---|---|---|---|
| Quality of Evidence |
Substantial | Intermediate | Small/Weak | None | Conflicting | Negative |
| Good | A | A | B | D | I | D |
| Fair | A | B | C | D | I | D |
| Low | B | C | C | I | I | D |
| Expert Opinion | E/A | E/B | E/C | I | I | E/D |
See Table 1 for definition of designations.
Conclusions
The suggested initial approach after the diagnosis of PAH is to treat patients with oral anticoagulant drugs if no contraindication exists, diuretics in cases of fluid retention, and supplemental oxygen in cases of hypoxemia, even though RCTs with these compounds are lacking. Patients should be referred without delay to centers experienced in acute vasoreactivity testing and the treatment of pulmonary vascular diseases. Acute vasoreactivity testing should be performed in all patients with PAH, although patients with IPAH, HPAH, and PAH associated with anorexigen use are the most likely to exhibit a positive response. Vasoreactive patients, as defined above, should be treated with optimally tolerated doses of CCBs; maintenance of response, defined as WHO functional class I or II with near-normal hemodynamics, should be confirmed by repeat right heart catheterization and clinical assessment after three to six months of treatment. Nonresponders to acute vasoreactivity testing, or responders who remain in WHO functional class III, should be considered candidates for treatment with either a PDE-5 inhibitor or an ERA. Among prostanoids, treprostinil is administered subcutaneously, intravenously, or by inhalation; iloprost can be given intravenously or by inhalation; beraprost is administered orally, and epoprostenol is administered intravenously.
The choice of drug is dependent on a variety of factors, including the approval status, route of administration, side-effect profile, patient preference, and the physician's experience and clinical judgment. Continuous IV epoprostenol remains first-line therapy for PAH patients in WHO functional class IV because of its demonstrated survival benefit in IPAH/HPAH, with extrapolation to associated PAH patients in WHO functional class IV. Combination therapy should be considered for patients who fail to show improvement or who deteriorate with monotherapy. The goal in treating PAH patients is to improve WHO functional class III and IV patients to functional class I or II, and to improve all functional class II patients to functional class I, or at least to maintain functional class II in patients presenting in that functional class. Finally, both atrial septostomy and lung transplantation are indicated in carefully selected patients for refractory PAH or in cases where medical treatments are unavailable. These procedures should be performed only in experienced centers.
Major therapeutic advances for PAH patients have been achieved in the last decade; however, none of the currently approved therapies represents a cure for this progressive disease. The search for such treatments continues, with promising new concepts arising from a better understanding of the pathobiology of pulmonary vascular diseases. Patients and physicians should be encouraged to foster such research by participating in RCTs conducted at specialized PH centers.
Abbreviations and Acronyms
- CCB
calcium channel blocker
- ERA
endothelin-1 receptor antagonist
- ETA
endothelin receptor A
- HPAH
heritable pulmonary arterial hypertension
- INR
international normalized ratio
- IPAH
idiopathic pulmonary arterial hypertension
- IV
intravenous
- PAH
pulmonary arterial hypertension
- PDE-5
phosphodiesterase type 5
- RCT
randomized controlled trial
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Dr. Barst has received honoraria for serving as a consultant, advisory board member, and/or speaker from Actelion Pharmaceuticals, Eli Lilly, GlaxoSmithKline, Gilead Sciences, Novartis, and Pfizer.
Dr. Galiè has served on advisory boards for Actelion Pharmaceuticals, Eli Lilly, Encysive Pharmaceuticals, Gilead Sciences (Myogen), GlaxoSmithKline, mondoBIOTECH, Pfizer, and Schering; and has received lecture fees from Actelion and Schering. His Institute has received grant support from Actelion, Eli Lilly, Encysive, Gilead (Myogen), MondoBIOTECH, Pfizer, Schering, and United Therapeutics.
Dr. Ghofrani has received honoraria and research funds from Actelion Pharmaceuticals, Bayer Schering, Encysive Pharmaceuticals, ErgoNex Pharma, GlaxoSmithKline, Novartis, and Pfizer.
Dr. Gibbs has received honoraria for advisory boards and/or lecturing from Actelion Pharmaceuticals, Encysive Pharmaceuticals, GlaxoSmithKline, Lung Rx, Pfizer, Schering, and United Therapeutics.
Dr. Hoeper has received pharmaceutical grants from Actelion Pharmaceuticals, Bayer Schering, and Encysive Pharmaceuticals; travel accommodations and speaker's honoraria from Actelion, Encysive, GlaxoSmithKline, LungRx, Pfizer, and Schering; and has served as a consultant to Actelion, Bayer Schering, Encysive, GlaxoSmithKline, and LungRx.
Dr. McLaughlin has received honoraria and/or consulting fees from Actelion Pharmaceuticals, Gilead Sciences, MondoBIOTECH, and United Therapeutics.
The University of Michigan has received research grants from Actelion, Pfizer, and United Therapeutics.
Dr. Rubin has received research grants from Actelion Pharmaceuticals, Gilead Sciences, the National Heart, Lung and Blood Institute, Pfizer, and United Therapeutics; and has served on advisory committees for Actelion, Gilead, and Pfizer; and as a consultant for Actelion, Aires Pharmaceuticals, Bayer Schering Pharma, Cerulean Biosciences, Gilead, mondoBIOTECH, the National Heart, Lung and Blood Institute, Onyx Pharmaceuticals, Pfizer, Solvay Pharmaceuticals, and United Therapeutics. He owns stock in United Therapeutics.
Dr. Sitbon TK
Dr. Tapson has received research grants from Actelion Pharmaceuticals, Gilead Sciences, GlaxoSmithKline, Pfizer, and United Therapeutics; and has served as a consultant and on advisory boards for Actelion, Gilead, and United Therapeutics.
References
- 1.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992;327:76–81. doi: 10.1056/NEJM199207093270203. [DOI] [PubMed] [Google Scholar]
- 2.Rich S, Brundage BH. High-dose calcium channel-blocking therapy for primary pulmonary hypertension: evidence for long-term reduction in pulmonary arterial pressure and regression of right ventricular hypertrophy. Circulation. 1987;76:135–41. doi: 10.1161/01.cir.76.1.135. [DOI] [PubMed] [Google Scholar]
- 3.Raffy O, Azarian R, Brenot F, et al. Clinical significance of the pulmonary vasodilator response during short-term infusion of prostacyclin in primary pulmonary hypertension. Circulation. 1996;93:484–8. doi: 10.1161/01.cir.93.3.484. [DOI] [PubMed] [Google Scholar]
- 4.Barst RJ, Maislin G, Fishman AP. Vasodilator therapy for primary pulmonary hypertension in children. Circulation. 1999;99:1197–208. doi: 10.1161/01.cir.99.9.1197. [DOI] [PubMed] [Google Scholar]
- 5.Sitbon O, Humbert M, Jaïs X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–11. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 6.Yung D, Widlitz AC, Rosenzweig EB, Kerstein D, Maislin G, Barst RJ. Outcomes in children with idiopathic pulmonary arterial hypertension. Circulation. 2004;110:660–5. doi: 10.1161/01.CIR.0000138104.83366.E9. [DOI] [PubMed] [Google Scholar]
- 7.Fuster V, Steele PM, Edwards WD, Gersh BJ, McGoon MD, Frye RL. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation. 1984;70:580–7. doi: 10.1161/01.cir.70.4.580. [DOI] [PubMed] [Google Scholar]
- 8.Frank H, Mlczoch J, Huber K, Schuster E, Gurtner HP, Kneussl M. The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest. 1997;112:714–21. doi: 10.1378/chest.112.3.714. [DOI] [PubMed] [Google Scholar]
- 9.Johnson SR, Mehta S, Granton JT. Anticoagulation in pulmonary arterial hypertension: a qualitative systematic review. Eur Respir J. 2006;28:999–1004. doi: 10.1183/09031936.06.00015206. [DOI] [PubMed] [Google Scholar]
- 10.Rubin LJ, Mendoza J, Hood M, et al. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol): results of a randomized trial. Ann Intern Med. 1990;112:485–91. doi: 10.7326/0003-4819-112-7-485. [DOI] [PubMed] [Google Scholar]
- 11.Barst RJ, Rubin LJ, Long WA, et al. Primary Pulmonary Hypertension Study Group A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334:296–301. doi: 10.1056/NEJM199602013340504. for the. [DOI] [PubMed] [Google Scholar]
- 12.Badesch DB, Tapson VF, McGoon MD, et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease: a randomized, controlled trial. Ann Intern Med. 2000;132:425–34. doi: 10.7326/0003-4819-132-6-200003210-00002. [DOI] [PubMed] [Google Scholar]
- 13.Kerstein D, Levy PS, Hsu DT, Hordof AJ, Gersony WM, Barst RJ. Blade balloon atrial septostomy in patients with severe primary pulmonary hypertension. Circulation. 1995;91:2028–35. doi: 10.1161/01.cir.91.7.2028. [DOI] [PubMed] [Google Scholar]
- 14.Sandoval J, Rothman A, Pulido T. Atrial septostomy for pulmonary hypertension. Clin Chest Med. 2001;22:547–60. doi: 10.1016/s0272-5231(05)70291-4. [DOI] [PubMed] [Google Scholar]
- 15.Klepetko W, Mayer E, Sandoval J, et al. Interventional and surgical modalities of treatment for pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:73S–80S. doi: 10.1016/j.jacc.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 16.Sandoval J, Gaspar J, Pulido T, et al. Graded balloon dilation atrial septostomy in severe primary pulmonary hypertension: a therapeutic alternative for patients nonresponsive to vasodilator treatment. J Am Coll Cardiol. 1998;32:297–304. doi: 10.1016/s0735-1097(98)00238-1. [DOI] [PubMed] [Google Scholar]
- 17.Channick RN, Simonneau G, Sitbon O, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119–23. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 18.Langleben D, Christman BW, Barst RJ, et al. Effects of the thromboxane synthetase inhibitor and receptor antagonist terbogrel in patients with primary pulmonary hypertension. Am Heart J. 2002;143:E4. doi: 10.1067/mhj.2002.121806. [DOI] [PubMed] [Google Scholar]
- 19.Simonneau G, Barst RJ, Galiè N, et al. Treprostinil Study Group Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:800–4. doi: 10.1164/ajrccm.165.6.2106079. for the. [DOI] [PubMed] [Google Scholar]
- 20.Galiè N, Humbert M, Vachiéry J-L, et al. Arterial Pulmonary Hypertension and Beraprost European Trial (ALPHABET) Study Group Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol. 2002;39:1496–1502. doi: 10.1016/s0735-1097(02)01786-2. the. [DOI] [PubMed] [Google Scholar]
- 21.Olschewski H, Simonneau G, Galiè N, et al. Aerosolized Iloprost Randomized Study Group Inhaled iloprost in severe pulmonary hypertension. N Engl J Med. 2002;347:322–9. doi: 10.1056/NEJMoa020204. for the. [DOI] [PubMed] [Google Scholar]
- 22.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan Randomized Trial of Endothelin Antagonist Therapy Study Group Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. for the. [DOI] [PubMed] [Google Scholar]
- 23.Barst RJ, McGoon M, McLaughlin VV, et al. Beraprost Study Group Beraprost therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2003;41:2119–25. doi: 10.1016/s0735-1097(03)00463-7. for the. [DOI] [PubMed] [Google Scholar]
- 24.Sastry BK, Narasimhan C, Reddy NK, Raju BS. Clinical efficacy of sildenafil in primary pulmonary hypertension: a randomized, placebo-controlled, double-blind, crossover study. J Am Coll Cardiol. 2004;43:1149–53. doi: 10.1016/j.jacc.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 25.Barst RJ, Langleben D, Frost A, et al. STRIDE-1 Study Group Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;169:441–7. doi: 10.1164/rccm.200307-957OC. the. [DOI] [PubMed] [Google Scholar]
- 26.Galiè N, Ghofrani HA, Torbicki A, et al. Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–57. doi: 10.1056/NEJMoa050010. for the. [DOI] [PubMed] [Google Scholar]
- 27.Galiè N, Beghetti M, Gatzoulis MA, et al. Bosentan Randomized Trial of Endothelin Antagonist Therapy-5 (BREATHE-5) Investigators Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation. 2006;114:48–54. doi: 10.1161/CIRCULATIONAHA.106.630715. for the. [DOI] [PubMed] [Google Scholar]
- 28.Barst RJ, Langleben D, Badesch D, et al. STRIDE-2 Study Group Treatment of pulmonary arterial hypertension with the selective endothelin-A receptor antagonist sitaxsentan. J Am Coll Cardiol. 2006;47:2049–56. doi: 10.1016/j.jacc.2006.01.057. on behalf of the. [DOI] [PubMed] [Google Scholar]
- 29.Galiè N, Olschewski H, Oudiz RJ, et al. Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy Studies (ARIES) Group Ambrisentan for the treatment of pulmonary arterial hypertension: results of the Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy (ARIES) Study 1 and 2. Circulation. 2008;117:3010–9. doi: 10.1161/CIRCULATIONAHA.107.742510. for the. [DOI] [PubMed] [Google Scholar]
- 30.Galiè N, Rubin LJ, Hoeper M, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet. 2008;371:2093–100. doi: 10.1016/S0140-6736(08)60919-8. [DOI] [PubMed] [Google Scholar]
- 31.Galiè N, Brundage BH, Ghofrani A, et al. Tadalafil therapy in pulmonary arterial hypertension: results of a randomized, double-blind, placebo-controlled, phase III study. Eur Heart J. 2008;29:519. Abstract. [Google Scholar]
- 32.Humbert M, Barst RJ, Robbins IM, et al. Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. Eur Respir J. 2004;24:353–9. doi: 10.1183/09031936.04.00028404. [DOI] [PubMed] [Google Scholar]
- 33.Hoeper MM, Markevych I, Spiekerkoetter E, Welte T, Niedermeyer J. Goal-oriented treatment and combination therapy for pulmonary hypertension. Eur Respir J. 2005;26:858–63. doi: 10.1183/09031936.05.00075305. [DOI] [PubMed] [Google Scholar]
- 34.Hoeper MM, Leuchte H, Halank M, et al. Combining inhaled iloprost with bosentan in patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2006;28:691–4. doi: 10.1183/09031936.06.00057906. [DOI] [PubMed] [Google Scholar]
- 35.McLaughlin VV, Oudiz RJ, Frost A, et al. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;174:1257–63. doi: 10.1164/rccm.200603-358OC. [DOI] [PubMed] [Google Scholar]
- 36.McLaughlin V, Rubin L, Benza R, et al. TRIUMPH I: Efficacy and safety of inhaled treprostinil sodium in patients with pulmonary arterial hypertension (PAH) Am J Respir Crit Care Med. 2008;177:A965. Abstract. [Google Scholar]
- 37.Simonneau G, Rubin LJ, Galiè N, et al. PACES Study Group Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med. 2008;149:521–30. doi: 10.7326/0003-4819-149-8-200810210-00004. for the. [DOI] [PubMed] [Google Scholar]
- 38.Galiè N, Torbicki A, Barst R, et al. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Eur Heart J. 2004;25:2243–78. doi: 10.1016/j.ehj.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Galiè N, Manes A, Negro L, et al. A meta-analysis of randomized controlled trials in pulmonary arterial hypertension. Eur Heart J. 2009;30:394–403. doi: 10.1093/eurheartj/ehp022. Epub 2009 Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rich S, Seidlitz M, Dodin E, et al. The short-term effects of digoxin in patients with right ventricular dysfunction from pulmonary hypertension. Chest. 1998;114:787–92. doi: 10.1378/chest.114.3.787. [DOI] [PubMed] [Google Scholar]
- 41.McLaughlin VM, Archer SA, Badesch DB, et al. ACCF/AHA Clinical Expert Consensus Document on Pulmonary Hypertension. Circulation. 2009 J Am Coll Cardiol 2009. (In press) [Google Scholar]
- 42.Sandoval J, Aguirre JS, Pulido T, et al. Nocturnal oxygen therapy in patients with the Eisenmenger syndrome. Am J Respir Crit Care Med. 2001;164:1682–7. doi: 10.1164/ajrccm.164.9.2106076. [DOI] [PubMed] [Google Scholar]
- 43.Bowyer JJ, Busst CM, Denison DM, Shinebourne EA. Effect of long term oxygen treatment at home in children with pulmonary vascular disease. Br Heart J. 1986;55:385–90. doi: 10.1136/hrt.55.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Keogh this supplement [citation TK once assigned by JACC.]
- 45.McLaughlin VV, Gaine SP, Barst RJ, et al. Treprostinil Study Group Efficacy and safety of treprostinil: an epoprostenol analog for primary pulmonary hypertension. J Cardiovasc Pharmacol. 2003;41:293–9. doi: 10.1097/00005344-200302000-00019. on behalf of the. [DOI] [PubMed] [Google Scholar]
- 46.Tapson VF, Gomberg-Maitland M, McLaughlin VV, et al. Safety and efficacy of IV treprostinil for pulmonary arterial hypertension: a prospective, multicenter, open-label, 12-week trial. Chest. 2006;129:683–8. doi: 10.1378/chest.129.3.683. [DOI] [PubMed] [Google Scholar]
- 47.Gomberg-Maitland M, Tapson VF, Benza RL, et al. Transition from intravenous epoprostenol to intravenous treprostinil in pulmonary hypertension. Am J Respir Crit Care Med. 2005;172:1586–9. doi: 10.1164/rccm.200505-766OC. [DOI] [PubMed] [Google Scholar]
- 48.Higenbottam TW, Butt AY, Dinh-Xaun AT, Takao M, Cremona G, Akamine S. Treatment of pulmonary hypertension with the continuous infusion of a prostacyclin analogue, iloprost. Heart. 1998;79:175–9. doi: 10.1136/hrt.79.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galiè N, Hinderliter AL, Torbicki A, et al. Effects of the oral endothelin-receptor antagonist bosentan on echocardiographic and Doppler measures in patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2003;41:1380–6. doi: 10.1016/s0735-1097(03)00121-9. [DOI] [PubMed] [Google Scholar]
- 50.Barst RJ. Sitaxsentan: a selective endothelin-A receptor antagonist, for the treatment of pulmonary arterial hypertension. Expert Opin Pharmacother. 2007;8:95–109. doi: 10.1517/14656566.8.1.95. [DOI] [PubMed] [Google Scholar]
- 51.Galiè N, Badesch D, Oudiz R, et al. Ambrisentan therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2005;46:529–35. doi: 10.1016/j.jacc.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 52.McLaughlin V. Long-term ambrisentan therapy for pulmonary arterial hypertension in patients who previously received placebo in ARIES-1 or ARIES-2. Am J Respir Crit Care Med. 2008;177:A697. Abstract. [Google Scholar]
- 53.Burgess G, Hoogkamer H, Collings L, Dingemanse J. Mutual pharmacokinetic interactions between steady-state bosentan and sildenafil. Eur J Clin Pharmacol. 2008;64:43–50. doi: 10.1007/s00228-007-0408-z. [DOI] [PubMed] [Google Scholar]
- 54.Wrishko RE, Dingemanse J, Yu A, Darstein C, Phillips DL, Mitchell MI. Pharmacokinetic interaction between tadalafil and bosentan in healthy male subjects. J Clin Pharm. 2008;48:610–8. doi: 10.1177/0091270008315315. [DOI] [PubMed] [Google Scholar]
- 55.Spence R, Harrison B, Mandagere A, Dufton C. No clinically relevant pharmacokinetic interactions between ambrisentan and tadalafil; Presented at Chest; Philadelphia, PA. 2008, October 25-30; 2008. Abstract. [Google Scholar]
- 56.Spence R, Mandagere A, Dufton C, Venitz J. Pharmacokinetics and safety of ambrisentan in combination with sildenafil in healthy volunteers. J Clin Pharmacol. 2008;48:1451–9. doi: 10.1177/0091270008324180. [DOI] [PubMed] [Google Scholar]
- 57.Coyne TC, Garces PC, Kramer W. No clinical interactions between sitaxsentan and sildenafil; Presented at the annual meeting of the American College of Rheumatology; San Diego, California. November 2005; Abstract. [Google Scholar]