Abstract
Attention-Deficit/Hyperactivity Disorder (ADHD) and Bipolar Disorder (BPD) co-occur frequently and represent a particularly morbid clinical form of both disorders, however underlying neural circuitry contributing to the comorbidity remain understudied. Our aim was to investigate functional brain circuitry during working memory in a group of participants who meet criteria for both disorders (ADHD+BPD), and to explore the relationship of symptoms of each disorder to brain function. We used fMRI to image brain activity in 18 male adults with both ADHD and BPD, and 18 healthy control participants matched one-to-one on age, sex, and handedness, while they performed a sequential letter n-back task. We investigated differences in activation between these groups, and also correlations of brain activity during the task to symptoms of ADHD and BPD independently. We found significant hypoactivity in the subjects with ADHD+BPD vs. controls across frontal and parietal regions, and further, found that BPD and ADHD symptoms related to activity in anatomically distinct regions that were respectively characterized by activation and suppression during task. We conclude that comorbid ADHD+BPD is associated with alterations across anterior and posterior nodes of the working memory network, and symptoms of each disorder are related to anatomically and functionally distinct brain regions.
Keywords: fMRI, Bipolar Disorder, ADHD, Working Memory, Executive Function, Default Mode Network
Objectives of the Study
Attention-Deficit/Hyperactivity Disorder (ADHD) and Bipolar Disorder (BPD) are estimated to affect up to 4.4% and 2.6% of adults, respectively (Kessler et al., 2005; 2006). These conditions frequently co-occur, with up to ~30% of adults with BPD meeting lifetime criteria for ADHD (Kessler et al., 2006; Tamam et al., 2008), and up to ~20% of adults diagnosed with ADHD meeting criteria for BPD (Kessler et al., 2006). When comorbid with ADHD, BPD is associated with a lower age of onset, more frequent manic episodes, mixed presentations, and poorer functional outcomes (Nierenberg et al., 2005). While a large (and growing) body of research exists on neurobiological and neuropsychological alterations in each of these disorders separately, neuroimaging studies of comorbid patients are relatively few, and therefore there is little understanding of the underlying neural substrates in patients with both disorders.
Our review of the literature reveals only one published neurocognitive (Rucklidge, 2006) and 4 neuroimaging papers (Adler et al., 2005; Biederman et al., 2008; Leibenluft et al., 2007; Lopez-Larson et al., 2009) specifically addressing the ADHD+BPD comorbidity. Rucklidge et al (2006) found that a group of ADHD+BPD adolescents were impaired in processing, naming speed, working memory, and response inhibition, more impaired than the BPD-alone group. The neuroimaging studies have been somewhat inconsistent in terms of study design and results: 3 studies included child participants (Adler et al., 2005; Leibenluft et al., 2007; Lopez-Larson et al., 2009) while one included adults (Biederman et al., 2008); 2 used fMRI (Adler et al., 2005; Leibenluft et al., 2007) and 2 used structural MRI (Biederman et al., 2008; Lopez-Larson et al., 2009). Across these neuroimaging studies, the ADHD+BPD groups were found to have abnormal activity and volumes in basal ganglia, limbic system and prefrontal cortex, although results vary widely from paper to paper, likely due to heterogeneity of methods and regions of interest investigated.
Uncertainties remain as to whether ADHD+BPD comorbidity represents two separable disease entities, a single clinical entity, or represents a subtype of either ADHD or BPD. Wingo & Ghaemi (2007) review the literature on the subject and conclude that the topic has been insufficiently studied, particularly using brain imaging techniques. Since this review, two neuroimaging studies investigating ADHD+BPD groups tested the diagnostic validity question (Biederman et al., 2008; Lopez-Larson et al., 2009). These studies compared brain measures in comorbid subjects to those diagnosed with ADHD-alone, BPD-alone, and healthy controls, therefore directly assessing the contributions of each disorder to subjects who met criteria for both. Lopez-Larson et al (2009) found that volumetric abnormalities in the comorbid group overlapped anatomically with those regions altered in children with BPD-alone but not ADHD-alone, supporting the idea that ADHD+BPD may actually be a subtype of bipolar disorder. However, in a group of adults, Biederman et al (2008) found an additive effect of disorder-specific alterations, with the profile of abnormalities in the ADHD+BPD group encompassing those found independently in the BPD- and ADHD-only groups. These latter results, therefore, support separable underlying neural correlates of the two syndromes even in the comorbid subjects. These results are consistent with family studies documenting co-segregation between BPD and ADHD (Faraone et al., 1997; 2001).
Our primary goal was to use functional neuroimaging of working memory (WM) to further investigate the underlying brain alterations associated with comorbid ADHD+BPD, and secondarily, to test the specific contributions of symptoms within each disorder to these alterations. We chose to test the integrity of brain networks supporting WM because of the alterations in WM performance (Robinson et al., 2006; Thompson et al., 2005) and in underlying neural circuitry in adults with BPD (Robinson et al., 2006; Thermenos et al., 2010; Townsend et al., 2010) and those with ADHD (Hervey et al., 2004; Paloyelis et al., 2007).
Our primary hypothesis was that ADHD+BPD adults would show alterations in widespread regions supporting WM, including fronto-cerebellar regions previously associated with ADHD (Valera et al., 2005) and BPD (Townsend et al., 2010), and limbic regions previously associated with BPD (Thermenos et al., 2010). Given preliminary structural neuroimaging work from our group (Biederman et al., 2008) supporting the independence of neural correlates associated with each disorder within an ADHD+BPD group, we additionally hypothesized that bipolar symptoms and ADHD symptoms would relate to different nodes of the WM network.
Materials and Methods
This investigation was carried out in accordance with the Partners Healthcare System Human Research Committee Institutional Review Board and with the latest version of the Declaration of Helsinki. Informed consent of all participants was obtained after the nature of the procedures had been fully explained.
Participants
Participants were culled from a larger NIH-funded study of ADHD (Brown et al., 2010; Brown et al., 2011; MH062152; see Seidman et al., 2011 for details; Valera et al., 2010). Exclusion criteria for this study were an estimated full scale IQ <80; recent alcohol or substance dependence or abuse; inadequate command of the English language; sensorimotor handicaps. ADHD+BPD participants were recruited through referrals to the Adult ADHD program at Massachusetts General Hospital (MGH) and advertisements in the greater Boston area, and healthy control subjects were recruited through the same advertisements.
For the current report we’ve included all male subjects from this pool who meet criteria for both ADHD and BPD and who had N-back fMRI data. Because we only had data on five females with ADHD+BPD, not a large enough N for a meaningful random effects analysis (Thirion et al., 2007), we chose to study a male-only sample. Previous reports of significant sex differences in WM circuitry in controls (Goldstein et al., 2005), ADHD (Valera et al., 2010), and psychosis (Elsabagh et al., 2009), support this decision.
Our resulting sample included 18 ADHD+BPD male subjects. From a pool of 68 male adult controls that took part in the same study and met our inclusion criteria, we constructed a comparison group by matching one-to-one on age, ethnicity, and handedness (see Table 1). fMRI data from subjects in our healthy control sample has been reported in previous studies (Valera et al., 2005; 2010), but not for the ADHD+BPD group. Data from 15 of the 18 of the ADHD+BPD subjects as well as 7 of the 18 healthy control subjects was included in a report of brain volumes (Biederman et al., 2008).
Table 1.
Demographics and N-back performance data
| Controls (N=18) | ADHD+BPD (N=18) | Test statistic (p-value) | |||
|---|---|---|---|---|---|
|
| |||||
| Mean | SD | Mean | SD | ||
| Demographics | |||||
| Age | 32.9 | 10.4 | 33.8 | 12.3 | t(35) = .219 (0.828) |
| Caucasian, n (%) | 15 | 83.3 | 17 | 94.4 | χ2(1) = 1.13 (0.603) |
| Left-Handed, n (%) | 1 | 5.6 | 3 | 16.7 | χ2(1) = 1.13 (0.603) |
| Cognitive | |||||
| IQ | 113.3 | 12.4 | 107.3 | 11.1 | t(35) = 1.68 (0.103) |
| WRAT-Reading | 109.2 | 6.9 | 105.1 | 9.7 | t(35) = 1.54 (0.134) |
| WRAT-Arithmetic | 105.1 | 12.2 | 90.8 | 13.5 | t(35) = 3.40 (0.002) |
| Mood State | |||||
| POMS-Tension/Anxiety | 33.2 | 6.6 | 40.7 | 9.0 | t(35) = 2.83 (0.008) |
| POMS-Depression | 37.4 | 2.7 | 44.3 | 9.3 | t(35) = 2.98 (0.007) |
| POMS-Anger/Hostility | 40.7 | 5.1 | 49.2 | 8.7 | t(35) = 3.57 (0.001) |
| N Back Performance Data | |||||
| X Task % Correct | 98.0 | 1.7 | 95.6 | 4.4 | t(35) = 2.13 (0.045) |
| X Task RT | 552.4 | 80.3 | 549.9 | 109.2 | t(35) = 0.08 (0.938) |
| 2 Back % Correct | 87.9 | 7.5 | 81.8 | 10.0 | t(35) = 2.07 (0.047) |
| 2 Back RT | 811.1 | 111.6 | 813.5 | 218.3 | t(35) = 0.04 (0.967) |
POMS: Profile of Mood States (McNair et al., 1992)
All subjects received identical assessments (consistent with previous studies from this laboratory) which included a Structured Clinical Interview for DSM-IV (SCID-I; First et al., 1997) to assess psychopathology, and modules derived from the Schedule of Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version (K-SADS-E; Orvaschel & Puig-Antich, 1987) to assess ADHD. Previous work has shown that retrospective childhood diagnoses of ADHD can be made in a reliable and valid manner using this method (Biederman et al., 1990; Faraone et al., 2000). A comorbid ADHD+BPD diagnosis was made if DSM-IV criteria for ADHD were met in childhood and persisted into adulthood, and if DSM criteria were met for Bipolar I (American Psychiatric Association, 1994). All ADHD+BPD subjects reported both their ADHD and manic symptoms as either moderately or severely impairing to their overall functioning (see Table 2).
Table 2.
Descriptive clinical data for comorbid ADHD+BPD sample.
| Mean | Range | |
|---|---|---|
| ADHD – Hyperactive/Impulsive Symptoms | 6.54 | 3–9 |
| ADHD – Inattentive Symptoms | 7.00 | 1–9 |
| ADHD – Total Symptoms | 13.65 | 6–18 |
| ADHD Age of Onset | 6.24 | 1–13 |
| ADHD Impairment | 2.53 | 2–3 |
| Bipolar - Mania Symptoms Endorsed | 5.35 | 3–7 |
| Bipolar - Age of Mania Onset | 14.06 | 1–20 |
| Bipolar Impairment | 2.56 | 2–3 |
ADHD and BPD Impairment scores are self-report of impact of disorder-specific symptoms on daily functioning: 1 = minimally impairing, 2 = moderately impairing, and 3 = severely impairing.
We administered the Profile of Mood States (POMS; McNair et al., 1992) to all participants just prior to MRI scanning (within an hour) to assess current mood state at the time of the scan. From this measure, we derived current scores of depressive mood, tension/anxiety, and anger/hostility.
fMRI paradigm
We used a variant of the sequential letter visual “N-back” task as described in previous publications from our group (Thermenos et al., 2011; Valera et al., 2005; 2010; Whitfield-Gabrieli et al., 2009). The task contained intermittent blocks of the 2-back working memory condition, the 0-back vigilance condition, and a baseline fixation condition. Stimuli were sequences of white capital letters on a black background, presented centrally (200 ms duration, 1800 ms inter-stimulus interval) in pseudo-random order. Participants were instructed to respond to every stimulus using a response box, pressing one button to signal targets and another to signal non-targets. In the 0-back condition, the target was the letter “X” (23% of trials), and all other stimuli were non-targets. In the 2-back condition, the target was any letter identical to the letter that preceded it two trials back, “2-back” (26% of trials). Participants were administered three runs of the task, each lasting 5.6 minutes. Each run of trials incorporated a block design with 12 epochs: 1) three 36-second epochs of 0-back; 2) three 36-second epochs of 2-back; and 3) six 20-second epochs of “fixation”. Condition order was randomized across runs and subjects. Percent of correct responses (accuracy) and mean RT for correct responses (speed) were used as performance measures.
fMRI acquisition and analysis
Imaging data was collected using a Siemens Sonata 1.5T full-body scanner at the Athinoula A. Martinos Center for Biomedical Imaging in Charlestown, MA. fMRI was performed with an echo-planar imaging pulse sequence (21 axial slices, TR=2000ms, 5mm thickness, 1mm interslice interval, TE=40ms, flip angle=90°, 168 volumes/run).
fMRI data were analyzed with SPM2 (Wellcome Department of Cognitive Neurology, London). Preprocessing included correction for head motion, spatial normalization, and spatial smoothing with a Gaussian filter (8-mm full width at half maximum). We dropped any single runs that contained more than 3mm of scan-to-scan head motion, stimulus correlated motion of r ≥ 0.5, or accuracy of less than 68% on the WM task. This resulted in dropping single runs from 7 controls and 3 participants with ADHD+BPD, and 2 runs from 1 ADHD+BPD participant. However, because all participants were administered 3 runs, we were able to retain these subjects by including remaining runs in our analysis.
After preprocessing, statistical analysis was performed at the single-subject level. Each epoch of trials was modeled using a boxcar function convolved with a canonical hemodynamic response function. Low-frequency components of the blood-oxygen-level-dependent (BOLD) signal were modeled as confounding covariates. Our contrast of interest was the activation associated with the 2-back condition using the 0-back condition as a control baseline, as in previous studies (Cohen et al., 1994; Valera et al., 2005; 2010). Individual contrast maps were submitted to a second-level analysis in which subjects were treated as random effects. A voxel-level threshold of p < 0.005 uncorrected with an extent threshold of K > 5 voxels was used to define clusters of activation across the entire brain. Only clusters that exceed a p < 0.05 threshold after correction for multiple comparisons are reported. For multiple comparison correction we used Gaussian Random Field theory, which takes into account both extent and height thresholds of the cluster, and the size of the search map. This conjoint thresholding provides p-values that are corrected for the entire volume, has been validated for use in the SPM2 package, and is sensitive to avoiding both Type 1 and Type 2 errors (Friston et al., 2007). All p-values reported therefore describe the significance level of each cluster, corrected for the entire brain (except where noted).
Tests of hypothesis
In order to test the hypothesis that ADHD+BPD adults have alterations in the neural circuitry supporting working memory, we ran a two group t-test comparing the activation in the entire brain in the ADHD+BPD vs. healthy control group, using the 2back>0back contrast. Confirmatory two-sample analyses of covariance (ANCOVAs) were used to control for POMS scores in the individual mood domains to assess if differences between the groups could be explained by current mood state at the time of scan.
To test the relationship between brain response during working memory and ADHD symptoms or BPD symptoms, for each diagnosis we ran a correlation between number of symptoms endorsed in the structured interview, and activity in each brain voxel during the working memory task in the affected subjects only. For the ADHD symptoms we used total number of ADHD symptoms endorsed (out of 18), and for BPD we used total number of symptoms of mania endorsed (out of 7). In our sample, the range of ADHD symptoms was 6–18, and range of BPD symptoms was 3–7. Due to an internal error, one subject was missing his symptom scores, and was therefore left out of the correlational analyses. Only clusters with p-values corrected for the entire search volume (whole brain) are reported. R-values reported represent the Pearson correlation coefficient for the association of number of symptoms to mean contrast values across the cluster during the 2-back condition as identified in our whole brain correlation.
Results
Demographic, clinical, and working memory task data
By design, the participant groups did not differ on age, handedness or ethnicity. Groups were not statistically different on IQ or reading ability. ADHD+BPD subjects performed significantly more poorly on the WRAT-arithmetic subtest (see Table 1). The ADHD+BPD group reported more agitation, depression, and anxiety on the day of the scan, congruent with clinical expectations. Performance on the working memory fMRI task was also worse in the affected group than in controls, with accuracy scores lower on both the vigilance and the working memory tasks, although reaction time was not different between the groups.
Medications
Ten of the 18 ADHD+BPD subjects had taken psychotropic medications within 24 hours of the scan. Three had taken mood stabilizers alone, 6 had taken mood stabilizers in conjunction with other medications (plus a beta-blocker [n = 2], plus an SSRI [n = 1], plus an atypical antipsychotic [n = 1], plus a benzodiazepine [n = 1], plus a tricyclic and an SSRI [n = 1]), and one subject had taken an SSRI and a benzodiazepine. Two subjects were additionally prescribed stimulants for ADHD, but had not taken them within 24 hours of the scan. No healthy control subjects were prescribed psychotropic medications at the time of scan.
fMRI Results
Group differences in working memory-related brain activity
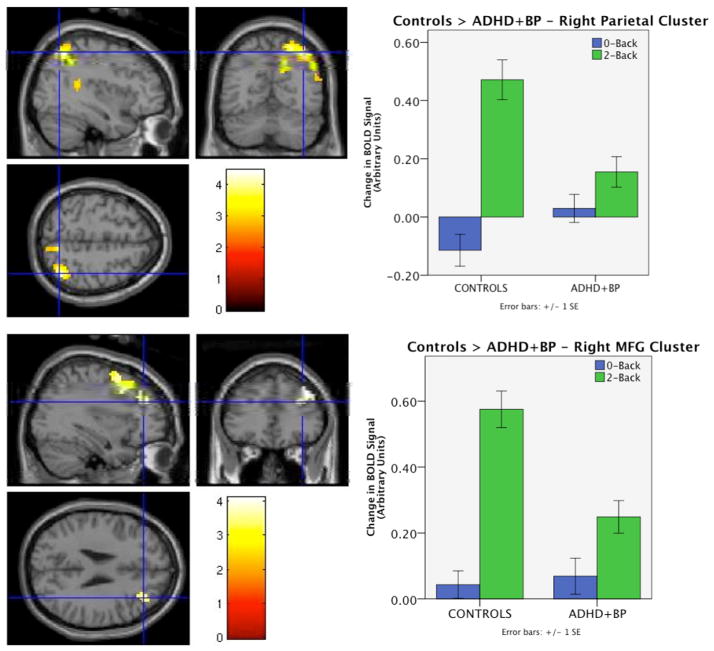
Activity in a cluster in the right parietal lobe (including superior and inferior parietal lobule and angular gyrus: peak voxel = [39 −63 54], K = 706, corrected p-value < 0.0001), and in the right middle frontal gyrus (peak voxel = [30 12 42], K = 351, corrected p-value = 0.002) was significantly greater in the healthy control group than in the ADHD+BPD group. Two additional clusters emerged with greater activation in the healthy control vs. ADHD+BPD group, but the cluster-level p-values did not survive correction for multiple comparisons. These were in the left middle frontal gyrus (peak voxel = [−42 30 30], K = 89, uncorrected p-value = 0.027), and in the right frontal pole stretching 126mm anterior-posterior through BA10 (peak voxel = [27 60 27], K = 107, uncorrected p-value = 0.017). No regions were more active in the ADHD+BPD group than in the healthy control group. See Figure 1 for significant diagnosis effects and Supplementary Figure 1 for marginal diagnosis effects.
Figure 1. Main effect of diagnosis in 2-Back > 0-Back contrast.
Left panels show statistical t-maps displaying clusters significantly more active in healthy controls than ADHD+BPD including right lateral parietal lobe (top: peak voxel = [39 −63 54], K=706, corrected p-value < 0.0001) and right lateral prefrontal cortex (bottom: peak voxel = [30 12 42], K=351, corrected p-value = 0.002). Right panels show magnitude of BOLD signal change in each condition in each task compared to fixation baseline for the mean activation over the significant parietal (top) and frontal clusters (bottom). No brain regions were significantly more active the comorbid vs. control group.
Effect of mood state on group differences
None of the POMS subscale scores (depression, anxiety/tension, or anger/hostility) explained the group differences in a meaningful way. ANCOVAs revealed that the right parietal cluster remained significant after controlling for depression and anger/hostility, as did the right frontal cluster, which further, became contiguous with the R frontal pole previously identified as a trend. Controlling for tension/anxiety scores resulted in a similar map with the right frontal cluster remaining significant, whereas some voxels in the right parietal cluster did not pass the significance threshold and so the cluster became non-contiguous, resulting in 3 smaller but spatially proximal clusters that were significant before but not after correcting for voxels across the entire brain. Overall, the maps were nearly identical suggesting that current mood state does not explain the differences found in brain activation.
Brain-symptom correlations
Activity in a cluster in the posterior cingulate (PCC) was significantly negatively associated with number of ADHD symptoms (cluster r = −0.865, max vox = [−9 −33 42], K = 245, cluster p-corrected = 0.002). Examining the mean signal change values shows that this association emerged because ADHD+BPD participants with a greater number of ADHD symptoms evinced more suppression of this region during task (see Figure 2). Incidentally, two other regions emerged that were suggestive of a negative association (corrected p-values were at p<0.1), and notably these regions were also more active at rest than during task. They included inferior temporal gyrus (ITG; cluster r = −0.879, max vox = [51 −15 −30], K = 110, cluster p-corrected = 0.097) and subgenual anterior cingulate (sACC; cluster r = −0.870, max vox = [−6 51 −12], K = 112, cluster p-corrected = 0.090). See Supplementary Figure 2.
Figure 2. Brain region significantly correlated with ADHD symptoms during working memory task.
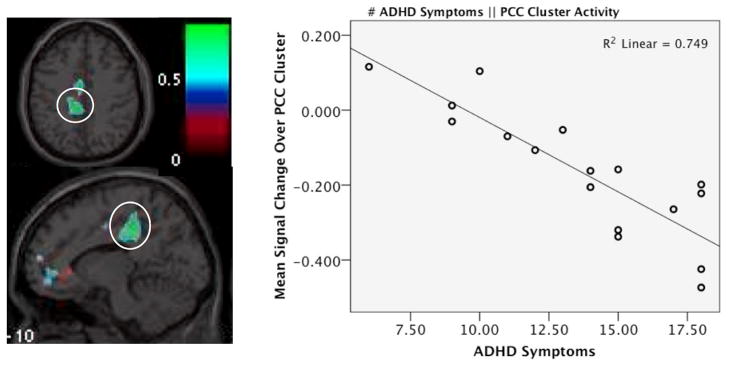
Left panel shows statistical r-map for correlation of activity during working memory task to number of ADHD symptoms endorsed. Posterior cingulate cluster (circled) emerged as significantly correlated (cluster r = −0.865, max vox = [−9 −33 42], K = 245, corrected p-value = 0.002, and right panel shows the scatterplot of this relationship, displaying correlation of mean signal change in this cluster during working memory task to number of ADHD symptoms endorsed.
Activity in the left dorsal anterior cingulate cortex (dACC; cluster r = −0.810, max vox = [−3 45 18], K = 151, cluster p-corrected = 0.029) was found to be significantly negatively associated with number of mania symptoms. Examining the mean signal change values shows that this association emerged because ADHD+BPD participants with a greater number of symptoms of mania had less activity in this region during task, with the subjects with at least 6 BPD symptoms evincing less activity during working memory even than during fixation (see Figure 3). A cluster in the left superior and middle frontal gyri also emerged in the whole-brain analysis, but was only at a trend level after correction for multiple comparisons (cluster r = −0.837, max vox = [−33 60 6], K = 120, cluster p-corrected = 0.076). This association suggested that in this area more symptoms also predicted less activation during the working memory condition. See Supplementary Figure 3.
Figure 3. Brain region significantly correlated with BPD symptoms during working memory task.
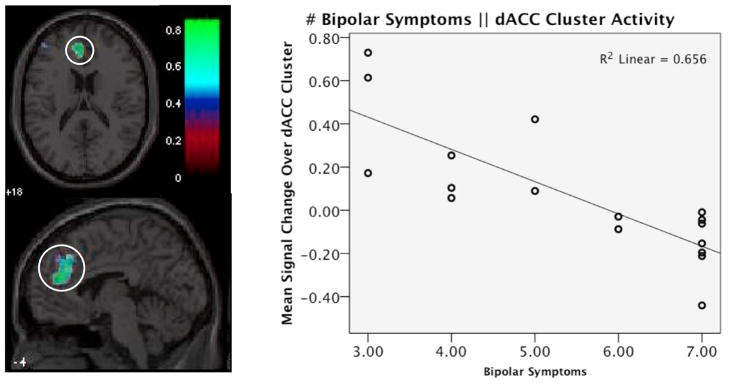
Left panel shows statistical r-map for correlation of activity during working memory task to number of symptoms endorsed on the mania module. Dorsal anterior cingulate cortex cluster (circled) emerged as significantly correlated (cluster r = −0.810, max vox = [−3 45 18], K = 151, corrected p-value = 0.029), and right panel shows the scatterplot of this relationship, displaying correlation of mean signal change in this cluster during working memory task to number of BPD symptoms endorsed.
Discussion
In this first functional MRI study of adults who meet criteria for both ADHD and Bipolar Disorder, we found alterations across several fronto-parietal regions. Specifically, regions in right lateral PFC and the right parietal lobe showed significantly less activity during working memory. We additionally found that activation in a region in the left lateral PFC and the right frontal pole were marginally less active in the affected group, although these clusters did not pass our statistical threshold. These findings implicate widespread alterations across the anterior and posterior dorsal executive control system in ADHD+BPD participants. Results could not be explained by current mood state at the time of scan.
Previous neuroimaging literature in ADHD+BPD subjects is limited, with only two structural studies (one with adults and one with children) and one functional study (with children) comparing brain measures to controls. Our results are not directly comparable to these former studies in which regions of interest were limited to areas outside the DLPFC, posterior parietal cortex, and frontal pole. The neuroimaging literature in BPD alone is quite mixed. For instance, in functional neuroimaging studies of working memory, regions found to be differentially activated in BPD vs. healthy control subjects include all lobes of the brain, cerebellum, and subcortical regions, and these findings are quite heterogeneous across studies both in terms of anatomical location and direction of activation (Adler et al., 2004; Deckersbach et al., 2008; Drapier et al., 2008; Frangou et al., 2008; Gruber et al., 2010; Lagopoulos et al., 2007; Monks et al., 2004; Robinson et al., 2009; Silverstone et al., 2005; Thermenos et al., 2011; Townsend et al., 2010).
The ADHD literature on working memory networks is somewhat more consistent. Generally the findings show hypoactivation in fronto-parietal-cerebellar ROIs (Bayerl et al., 2010; Hale et al., 2007; Sheridan et al., 2007; Valera et al., 2005; 2010), as well as in subcortical regions when they are studied (Vance et al., 2007; Wolf et al., 2009). Neither the BPD nor ADHD functional imaging literature however, takes comorbidity for the other explicitly into account, which as we previously noted is common in both disorders. Lack of attention to comorbidity may explain some of the heterogeneity in the findings. This may particularly be the case in BPD studies, because ADHD studies may incidentally exclude the more severe BPD patients through the common exclusion criteria of psychotropic medications other than stimulants.
Our study investigated the separate contributions of ADHD and BPD by testing the statistical association of symptoms of each disorder to brain activity within the same set of subjects. In these analyses we found that BPD symptoms were associated with dACC and marginally associated with DLPFC, regions of the classic dorsal executive network important for supporting working memory and related processes. Because we found hypoactivation in the comorbid subjects in the fronto-parietal system, it is not surprising that more symptoms would predict less activation in these related regions. In contrast, we found that ADHD symptoms were significantly negatively associated with PCC and marginally with sACC and IFG, regions of the “task-negative” default mode network (DMN) found to be more active during rest than during task. The relationship is not entirely unexpected given the growing volume of literature showing a relationship between DMN dysregulation and ADHD, both in functional connectivity (Castellanos et al., 2008; Uddin et al., 2008), and in suppression during task (Fassbender et al., 2009; Peterson et al., 2009). However, whereas several previous studies have found that decreased DMN suppression is associated with the diagnosis of ADHD and more inattention to task (Eichele et al., 2008; Hampson et al., 2006; Kelly et al., 2008; Weissman et al., 2006), we found the opposite relationship. It should be noted however that there was no relationship between ADHD symptoms and performance on the task (all r’s < 0.26, all p’s > 0.34), suggesting that subjects with a greater number of ADHD symptoms needed more suppression of DMN to perform as well as those with fewer symptoms. Further, our finding is consistent in direction with an earlier study from our group, where we found that increased DMN suppression predicted a greater number of ADHD symptoms in an independent ADHD-only sample (Brown et al., 2011).
Our correlational findings are particularly interesting as they relate to the discussion of syndromatic independence of ADHD and BPD in comorbid subjects (Wingo & Ghaemi, 2007). As noted above, the regions associated with BPD symptoms were in the classic “task-positive” dorsal executive working memory network, while regions associated with ADHD symptoms were part of the DMN, brain networks that work in opposition to each other in order for efficient cognitive processes to occur. It may be that in comorbid subjects, alterations in DMN represent an intermediate phenotype associated with the expression of ADHD symptoms, while functional alterations in the dorsal executive system are more related to expression of BPD in these subjects. Whether or not alterations in the working memory network leading to these syndromes is different in subjects with only one of the disorders alone, has yet to be tested. Our findings do suggest however, that there may be different neural substrates underlying symptoms of BPD and those of ADHD in patients who meet criteria for both disorders. Hence, these data are consistent with previous brain volume findings (Biederman et al., 2008), which together support a model of syndromatic independence of the disorders in individuals comorbid for both ADHD and BPD.
Limitations of this study include limited generalizability to females because all subjects included were males. In addition, 56% (10 of 18) of the comorbid group was medicated at the time of scan, and in fact many were receiving multiple medications. However, it would not have been ethical to withdraw BPD patients from their treatments for the purpose of scanning. Thus, while it is certainly possible that our results are confounded by medication, previous studies have shown that alterations in frontal and parietal regions supporting working memory are associated with ADHD in non-medicated subjects (Bayerl et al., 2010; Wolf et al., 2009) and in medication-free BPD relatives (Thermenos et al., 2010). These findings suggesting that the brain alterations found in ADHD+BPD are likely not entirely accounted for by medication.
Overall, our data provides compelling evidence for widespread alterations in working memory circuitry in comorbid subjects with both ADHD+BPD, and complements our earlier work suggesting syndromatic independence of the constituent disorders in individuals with the comorbidity. Future studies should investigate working memory and default mode networks in comorbid subjects comparing them to well matched groups of subjects with ADHD-alone, BPD-alone, as well as controls, in order to more fully understand the contributions of the two disorders to subjects who meet criteria for both.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler CM, Delbello MP, Mills NP, Schmithorst V, Holland S, Strakowski SM. Comorbid ADHD is associated with altered patterns of neuronal activation in adolescents with bipolar disorder performing a simple attention task. Bipolar Disord. 2005;7:577–88. doi: 10.1111/j.1399-5618.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- Adler CM, Holland SK, Schmithorst V, Tuchfarber MJ, Strakowski SM. Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord. 2004;6:540–9. doi: 10.1111/j.1399-5618.2004.00117.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Bayerl M, Dielentheis TF, Vucurevic G, Gesierich T, Vogel F, Fehr C, Stoeter P, Huss M, Konrad A. Disturbed brain activation during a working memory task in drug-naive adult patients with ADHD. Neuroreport. 2010;21:442–446. doi: 10.1097/WNR.0b013e328338b9be. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Knee D, Munir K. Retrospective assessment of DSM-III attention deficit disorder in nonreferred individuals. J Clin Psychiatry. 1990;51:102–6. [PubMed] [Google Scholar]
- Biederman J, Makris N, Valera EM, Monuteaux MC, Goldstein JM, Buka S, Boriel DL, Bandyopadhyay S, Kennedy DN, Caviness VS, Bush G, Aleardi M, Hammerness P, Faraone SV, Seidman LJ. Towards further understanding of the co-morbidity between attention deficit hyperactivity disorder and bipolar disorder: a MRI study of brain volumes. Psychol Med. 2008;38:1045–56. doi: 10.1017/S0033291707001791. [DOI] [PubMed] [Google Scholar]
- Brown A, Biederman J, Valera E, Makris N, Doyle A, Whitfield-Gabrieli S, Mick E, Spencer T, Faraone S, Seidman L. Relationship of DAT1 and adult ADHD to task-positive and task-negative working memory networks. Psychiatry Research: Neuroimaging. 2011;193:7–16. doi: 10.1016/j.pscychresns.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AB, Biederman J, Valera EM, Doyle AE, Bush G, Spencer T, Monuteaux MC, Mick E, Whitfield-Gabrieli S, Makris N, LaViolette PS, Oscar-Berman M, Faraone SV, Seidman LJ. Effect of dopamine transporter gene (SLC6A3) variation on dorsal anterior cingulate function in attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:365–75. doi: 10.1002/ajmg.b.31022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJ, Rotrosen J, Adler LA, Milham MP. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–7. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Forman SD, Braver TS, Casey BJ, Serven-Schrelber D, Noll DC. Activation of the prefrontal cortex in a nonspatial working memory task with functional MRI. Human Brain Mapping. 1994;1:293–304. doi: 10.1002/hbm.460010407. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Rauch SL, Buhlmann U, Ostacher MJ, Beucke JC, Nierenberg AA, Sachs G, Dougherty DD. An fMRI investigation of working memory and sadness in females with bipolar disorder: a brief report. Bipolar Disord. 2008;10:928–42. doi: 10.1111/j.1399-5618.2008.00633.x. [DOI] [PubMed] [Google Scholar]
- Drapier D, Surguladze S, Marshall N, Schulze K, Fern A, Hall MH, Walshe M, Murray RM, McDonald C. Genetic liability for bipolar disorder is characterized by excess frontal activation in response to a working memory task. Biol Psychiatry. 2008;64:513–20. doi: 10.1016/j.biopsych.2008.04.038. [DOI] [PubMed] [Google Scholar]
- Eichele T, Debener S, Calhoun VD, Specht K, Engel AK, Hugdahl K, von Cramon DY, Ullsperger M. Prediction of human errors by maladaptive changes in event-related brain networks. Proc Natl Acad Sci U S A. 2008;105:6173–8. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabagh S, Premkumar P, Anilkumar AP, Kumari V. A longer duration of schizophrenic illness has sex-specific associations within the working memory neural network in schizophrenia. Behav Brain Res. 2009;201:41–7. doi: 10.1016/j.bbr.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Feighner JA, Monuteaux MC. Assessing symptoms of attention deficit hyperactivity disorder in children and adults: which is more valid? J Consult Clin Psychol. 2000;68:830–42. [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Mennin D, Wozniak J, Spencer T. Attention-deficit hyperactivity disorder with bipolar disorder: a familial subtype? J Am Acad Child Adolesc Psychiatry. 1997;36:1378–87. doi: 10.1097/00004583-199710000-00020. discussion 1387–90. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Monuteaux MC. Attention deficit hyperactivity disorder with bipolar disorder in girls: further evidence for a familial subtype? J Affect Disord. 2001;64:19–26. doi: 10.1016/s0165-0327(00)00213-5. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–28. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, D.C: American Psychiatric Press; 1997. [Google Scholar]
- Frangou S, Kington J, Raymont V, Shergill SS. Examining ventral and dorsal prefrontal function in bipolar disorder: a functional magnetic resonance imaging study. Eur Psychiatry. 2008;23:300–8. doi: 10.1016/j.eurpsy.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Friston K, Ashburner J, Kiebel S, Nichols T, Penny W. Statistical Parametric Mapping: The Analysis of Functional Brain Images. London: Academic Press; 2007. [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Anagnoson R, Breiter HC, Makris N, Goodman JM, Tsuang MT, Seidman LJ. Sex differences in prefrontal cortical brain activity during fMRI of auditory verbal working memory. Neuropsychology. 2005;19:509–19. doi: 10.1037/0894-4105.19.4.509. [DOI] [PubMed] [Google Scholar]
- Gruber O, Tost H, Henseler I, Schmael C, Scherk H, Ende G, Ruf M, Falkai P, Rietschel M. Pathological amygdala activation during working memory performance: Evidence for a pathophysiological trait marker in bipolar affective disorder. Hum Brain Mapp. 2010;31:115–25. doi: 10.1002/hbm.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale TS, Bookheimer S, McGough JJ, Phillips JM, McCracken JT. Atypical brain activation during simple & complex levels of processing in adult ADHD: an fMRI study. J Atten Disord. 2007;11:125–40. doi: 10.1177/1087054706294101. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–43. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF. Neuropsychology of adults with attention-deficit/hyperactivity disorder: a meta-analytic review. Neuropsychology. 2004;18:485–503. doi: 10.1037/0894-4105.18.3.485. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–37. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, Zaslavsky AM. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716–23. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagopoulos J, Ivanovski B, Malhi GS. An event-related functional MRI study of working memory in euthymic bipolar disorder. J Psychiatry Neurosci. 2007;32:174–84. [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Rich BA, Vinton DT, Nelson EE, Fromm SJ, Berghorst LH, Joshi P, Robb A, Schachar RJ, Dickstein DP, McClure EB, Pine DS. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry. 2007;164:52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson M, Michael ES, Terry JE, Breeze JL, Hodge SM, Tang L, Kennedy DN, Moore CM, Makris N, Caviness VS, Frazier JA. Subcortical differences among youths with attention-deficit/hyperactivity disorder compared to those with bipolar disorder with and without attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19:31–9. doi: 10.1089/cap.2008.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppelman LF. EdITS Manual for the Profile of Mood States (POMS) San Diego, CA: EdITS/Education and Industrial Testing Service; 1992. [Google Scholar]
- Monks PJ, Thompson JM, Bullmore ET, Suckling J, Brammer MJ, Williams SC, Simmons A, Giles N, Lloyd AJ, Harrison CL, Seal M, Murray RM, Ferrier IN, Young AH, Curtis VA. A functional MRI study of working memory task in euthymic bipolar disorder: evidence for task-specific dysfunction. Bipolar Disord. 2004;6:550–64. doi: 10.1111/j.1399-5618.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Miyahara S, Spencer T, Wisniewski SR, Otto MW, Simon N, Pollack MH, Ostacher MJ, Yan L, Siegel R, Sachs GS. Clinical and diagnostic implications of lifetime attention-deficit/hyperactivity disorder comorbidity in adults with bipolar disorder: data from the first 1000 STEP-BD participants. Biol Psychiatry. 2005;57:1467–73. doi: 10.1016/j.biopsych.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Orvaschel H, Puig-Antich J. Schedule for Affective Disorders and Schizophrenia for School-Age Children: Epidemiologic Version. Fort Lauderdale, FL: Nova University; 1987. [Google Scholar]
- Paloyelis Y, Mehta MA, Kuntsi J, Asherson P. Functional MRI in ADHD: a systematic literature review. Expert Rev Neurother. 2007;7:1337–56. doi: 10.1586/14737175.7.10.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Potenza MN, Wang Z, Zhu H, Martin A, Marsh R, Plessen KJ, Yu S. An FMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. Am J Psychiatry. 2009;166:1286–94. doi: 10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Bearden CE, Monkul ES, Tordesillas-Gutierrez D, Velligan DI, Frangou S, Glahn DC. Fronto-temporal dysregulation in remitted bipolar patients: an fMRI delayed-non-match-to-sample (DNMS) study. Bipolar Disord. 2009;11:351–60. doi: 10.1111/j.1399-5618.2009.00703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LJ, Thompson JM, Gallagher P, Goswami U, Young AH, Ferrier IN, Moore PB. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J Affect Disord. 2006;93:105–15. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ. Impact of ADHD on the neurocognitive functioning of adolescents with bipolar disorder. Biol Psychiatry. 2006;60:921–8. doi: 10.1016/j.biopsych.2006.03.067. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Biederman J, Liang L, Valera EM, Monuteaux MC, Brown A, Kaiser J, Spencer T, Faraone SV, Makris N. Gray matter alterations in adults with Attention-Deficit/Hyperactivity Disorder identified by voxel based morphometry. Biol Psychiatry. 2011;69:857–866. doi: 10.1016/j.biopsych.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Hinshaw S, D’Esposito M. Efficiency of the prefrontal cortex during working memory in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:1357–66. doi: 10.1097/chi.0b013e31812eecf7. [DOI] [PubMed] [Google Scholar]
- Silverstone PH, Bell EC, Willson MC, Dave S, Wilman AH. Lithium alters brain activation in bipolar disorder in a task- and state-dependent manner: an fMRI study. Ann Gen Psychiatry. 2005;4:14. doi: 10.1186/1744-859X-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamam L, Karakus G, Ozpoyraz N. Comorbidity of adult attention-deficit hyperactivity disorder and bipolar disorder: prevalence and clinical correlates. Eur Arch Psychiatry Clin Neurosci. 2008;258:385–93. doi: 10.1007/s00406-008-0807-x. [DOI] [PubMed] [Google Scholar]
- Thermenos HW, Goldstein JM, Milanovic SM, Whitfield-Gabrieli S, Makris N, Laviolette P, Koch JK, Faraone SV, Tsuang MT, Buka SL, Seidman LJ. An fMRI study of working memory in persons with bipolar disorder or at genetic risk for bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:120–31. doi: 10.1002/ajmg.b.30964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thermenos HW, Makris N, Whitfield-Gabrieli S, Brown AB, Giuliano AJ, Lee EH, Faraone SV, Tsuang MT, Seidman LJ. A functional MRI study of working memory in adolescents and young adults at genetic risk for bipolar disorder: preliminary findings. Bipolar Disord. 2011;13:272–286. doi: 10.1111/j.1399-5618.2011.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirion B, Pinel P, Meriaux S, Roche A, Dehaene S, Poline JB. Analysis of a large fMRI cohort: Statistical and methodological issues for group analyses. Neuroimage. 2007;35:105–20. doi: 10.1016/j.neuroimage.2006.11.054. [DOI] [PubMed] [Google Scholar]
- Thompson JM, Gallagher P, Hughes JH, Watson S, Gray JM, Ferrier IN, Young AH. Neurocognitive impairment in euthymic patients with bipolar affective disorder. Br J Psychiatry. 2005;186:32–40. doi: 10.1192/bjp.186.1.32. [DOI] [PubMed] [Google Scholar]
- Townsend J, Bookheimer SY, Foland-Ross LC, Sugar CA, Altshuler LL. fMRI abnormalities in dorsolateral prefrontal cortex during a working memory task in manic, euthymic and depressed bipolar subjects. Psychiatry Res. 2010;182:22–9. doi: 10.1016/j.pscychresns.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Margulies DS, Shehzad Z, Shaw D, Ghaffari M, Rotrosen J, Adler LA, Castellanos FX, Milham MP. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods. 2008;169:249–54. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Valera EM, Brown A, Biederman J, Faraone SV, Makris N, Monuteaux MC, Whitfield-Gabrieli S, Vitulano M, Schiller M, Seidman LJ. Sex differences in the functional neuroanatomy of working memory in adults with ADHD. Am J Psychiatry. 2010;167:86–94. doi: 10.1176/appi.ajp.2009.09020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Biederman J, Poldrack RA, Seidman LJ. Functional neuroanatomy of working memory in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:439–47. doi: 10.1016/j.biopsych.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Vance A, Silk TJ, Casey M, Rinehart NJ, Bradshaw JL, Bellgrove MA, Cunnington R. Right parietal dysfunction in children with attention deficit hyperactivity disorder, combined type: a functional MRI study. Mol Psychiatry. 2007;12:826–32. 793. doi: 10.1038/sj.mp.4001999. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–8. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–84. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo AP, Ghaemi SN. A systematic review of rates and diagnostic validity of comorbid adult attention-deficit/hyperactivity disorder and bipolar disorder. J Clin Psychiatry. 2007;68:1776–84. doi: 10.4088/jcp.v68n1118. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Plichta MM, Sambataro F, Fallgatter AJ, Jacob C, Lesch KP, Herrmann MJ, Schonfeldt-Lecuona C, Connemann BJ, Gron G, Vasic N. Regional brain activation changes and abnormal functional connectivity of the ventrolateral prefrontal cortex during working memory processing in adults with attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2009;30:2252–66. doi: 10.1002/hbm.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.