Abstract
Naturally occurring impulsive choice has been found to positively predict alcohol consumption in rats. However, the extent to which experimental manipulation of impulsive choice may modify alcohol consumption remains unclear. In the present study, we sought to: (a) train low levels of impulsive choice in rats using early, prolonged exposure to reward delay, and (b) determine the effects of this manipulation on subsequent alcohol consumption. During a prolonged training regimen, three groups of male, adolescent Long-Evans rats (21-22 days old at intake) responded on a single lever for food rewards delivered after either a progressively increasing delay, a fixed delay, or no delay. Post-tests of impulsive choice were conducted, as was an evaluation of alcohol consumption using a limited-access, two-bottle test. Following delay-exposure training, both groups of delay-exposed rats made significantly fewer impulsive choices than did rats in the no-delay group. In addition, fixed-delay rats consumed significantly more alcohol during daily, 30-min sessions than no-delay rats. Possible mechanisms of these effects are discussed, as is the significance of these findings to nonhuman models of addiction.
Keywords: impulsive choice, delay discounting, alcohol self-administration, lever press, rat
Impulsivity comprises an array of potentially discrete behavioral forms, including motor disinhibition, inattention, excessive risk-taking, and deficits in intertemporal decision-making (Bickel, Jarmolowicz, Mueller, Gatchalian, & McClure, 2012). The latter form describes a preference for smaller, sooner over larger, later rewards. This form of impulsivity involves an explicit choice between reward alternatives and is often referred to as impulsive choice to distinguish it from other forms of impulsivity.
In humans, a growing research literature reveals that greater impulsive choice in laboratory tasks is strongly associated with drug abuse and dependence (for meta-analysis, see MacKillop et al., 2011). This relation remains robust across many drugs of abuse, including alcohol (e.g., Vuchinich & Simpson, 1998), opioid drugs (e.g., Madden, Petry, Badger, & Bickel, 1997), cocaine (e.g., Coffey, Gudleski, Saladin, & Brady, 2003), methamphetamine (e.g., Hoffman et al., 2006), and nicotine (e.g., Reynolds, Richards, Horn, & Karraker, 2004).
One possible account of this relation is that impulsivity plays an etiological role in drug abuse and dependence (for reviews, see Perry & Carroll, 2008; Stein & Madden, in press). That is, individuals who disproportionately value reward immediacy over reward magnitude may be more motivated by immediate drug effects than the temporally distant (but objectively more valuable) benefits of abstinence (e.g., social, occupational, or financial rewards). Provisional support for this hypothesis comes from longitudinal studies in which impulsive choice in varying screening tasks in childhood precedes and predicts the subsequent adoption of cigarette smoking (Audrain-McGovern et al., 2009) or cocaine use (Ayduk et al., 2000).
More evidence that impulsive choice precedes drug abuse and dependence comes from nonhuman laboratory models in which a sample of adult rats is screened on an impulsive-choice task, divided into sample-dependent quantiles, and subsequently assessed under various drug self-administration (SA) tasks (for review, see Stein & Madden, in press). Most relevant to the present study, Poulos, Le, and Parker (1995) first reported that degree of impulsive choice in rats positively predicted consumption of a 12% (wt/vol) alcohol solution in a two-bottle test (Richter & Campbell, 1940). Greater impulsive choice in rats has also been shown to predict greater drug intake during many discrete SA phases, including acquisition and escalation of cocaine SA (Anker, Perry, Gliddon, & Carroll, 2009; Perry, Larson, German, Madden, & Carroll, 2005; Perry, Nelson, & Carroll, 2008) and cue- and drug-induced reinstatement of cocaine and nicotine SA (Broos, Diergaarde, Schoffelmeer, Pattij, & de Vries, 2012; Diergaarde et al., 2008; Perry, Nelson, & Carroll, 2008).
Despite the apparent predictive validity of impulsive choice in these nonhuman models, a direct etiological role of impulsive choice in drug SA has not been established because these two variables could co-vary with a third—and ultimately causal—variable. Stronger statements might be made if impulsive choice could be experimentally manipulated before nonhumans were given drug SA opportunities. If experimental reduction of impulsive choice reduces drug SA relative to subjects not exposed to this manipulation, then a direct etiological role of impulsive choice in drug SA would be further supported. By extension, this finding would suggest that therapies designed to reduce impulsivity might also reduce drug abuse and dependence in humans. However, if experimental reduction of impulsive choice does not result in reduced drug SA, then the predictive relation between these variables more likely owes to an as yet unknown third variable.
In the present study, we examined: (a) a behavioral method of training low levels of impulsive choice in rats, and (b) concomitant effects of this manipulation on alcohol SA. Two experimental groups of adolescent rats were first trained to respond (on a single lever) for delayed food pellets, and subsequently completed 120 training sessions (spanning into mid-adulthood) in which lever pressing initiated either a progressively increasing delay or a fixed delay to food pellets. Following this training, we compared impulsive choice and alcohol consumption in these two delay-exposed groups to a group that responded for immediate pellets throughout training.
Methods
Subjects
Subjects were 44 experimentally naïve, male Long-Evans rats (Harlan Sprague-Dawley, Indianapolis, IN). Rats were of post-natal days (PNDs) 21-22 at intake and were housed individually in polycarbonate cages in a temperature- and humidity-controlled room on a 12-hr light/dark cycle (lights on at 7:00 am) throughout the experiment. Water was available continuously in the home cage. Following three days of ad-libitum food access, rats were weighed daily and food-restricted to the strain's average, age-adjusted 85% free-feeding weight (calculated from the vendor-supplied growth curve). Rats were randomly assigned to either no-delay (ND; n = 14), fixed-delay (FD; n = 14), or progressive-delay (PD; n = 16) groups. More rats were intentionally assigned to the PD group to accommodate potential increased between-subject variability in this group's dependent measures as a result of variability in terminal training delays. Food restriction continued throughout all experimental phases, with the exception of the alcohol SA test (see below) in which all rats received ad-libitum food access in their home cages. After alcohol SA, 85% free-feeding weights were recalculated for individual rats using mean body weight over the last three days of a post-alcohol period. In all phases described below, rats completed sessions 7 days per week between the hours of 7:00 am and 1:00 pm, with individual rats completing sessions at the same time each day (time of day counterbalanced across groups).
Apparatus
Twelve identical operant conditioning chambers were used (24.1 × 30.5 × 21 cm; Med Associates, St. Albans, VT). Each was equipped with a white-noise speaker and housed within a sound-attenuating cubicle. Centered on the rear wall and 6.5 cm above the grid floor was a retractable response lever. Identical left and right levers were positioned at the same height on the front wall; above each was a 28-V DC cue light. A pellet feeder (Coulbourn Instruments, Allentown, PA), equipped with an infrared pellet detector (Pinkston, Ratzlaff, Madden, & Fowler, 2008), delivered grain-based pellets (45 mg; Bio-Serv, Frenchtown, NJ) into a receptacle between the levers.
Twelve identical polycarbonate home cages were used in the alcohol SA test. Cages were equipped with two glass drinking tubes (Dyets, Inc., Bethlehem, PA), each affixed to the left and right walls on one end of the cage. Drinking tubes were positioned above small, glassware bowls (Pyrex; World Kitchen, LLC, Rosemont, IL) to contain potential leakage. The room was equipped with a white-noise speaker and illuminated by a 40W red light.
Procedures
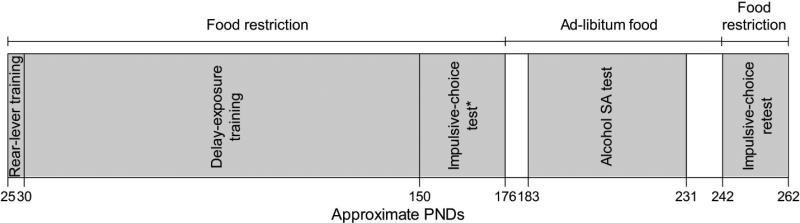
Figure 1 depicts the order and duration of all experimental conditions described below.
Figure 1.
Depiction of experimental conditions across postnatal days (PNDs). See text for details. *Duration of initial impulsive-choice test includes side-lever and choice training.
Rear-lever training (Approximate PNDs 25-29)
An autoshaping procedure was used to establish rear-lever pressing. For ND rats, the intertrial interval (ITI) was 55 s (during which all levers were retracted and cue lights extinguished), followed by 5 s of concurrent rear-lever insertion and rear cue-light illumination. Following this 5-s period, the cue light was extinguished, the lever was retracted, and two food pellets were delivered immediately to the receptacle; however, the rat could earn the reward at any time during this period with a single lever press.
These parameters provided a ratio of ITI to trial duration (I:T) of 11:1. In contrast, autoshaping with PD and FD rats involved delayed rewards. Following a 247.5-s ITI, the rear-lever and cue light were activated. After 5 s or a single lever press, the rear lever retracted but its cue light remained illuminated for 17.5 s prior to reward delivery (an I:T ratio of 11:1, equated across groups to increase the probability that lever training would be completed in a comparable number of sessions; Gibbon, Baldock, Locurto, Gold, & Terrace, 1977). Training continued until individual rats pressed the lever to earn ≥ 90% of the scheduled rewards for two consecutive sessions.
Delay-exposure training (Approximate PNDs 30-149)
The next 120 sessions were composed of 80 trials, each 60 s in duration. Trials began when the rear lever was inserted into the chamber and its cue light was illuminated. A single lever press retracted the lever and initiated a delay to the delivery of two food pellets. The cue light remained on throughout the delay. If the lever was not pressed within 20 s of trial onset, the trial was terminated (lever retracted and cue light extinguished for the remainder of the trial) and was scored as an omission. Following pellet delivery (or omissions), no stimuli were presented until the beginning of the next trial.
For the rats assigned to the FD and ND groups, the delays to food were, respectively, 17.5 s and 0.01 s (henceforth referred to as 0 s). The delay for PD rats was initially 17.5 s and was gradually increased based on performance. Specifically, at every 4th trial, the computer queried a moving window of the last 120 trials. If the mean response latency across these trials was less than 4 s and fewer than 12 omissions had occurred, the delay was increased by 0.057%. This schedule of delay adjustments allowed for a maximum terminal delay of 68.36 s over the course of 120 sessions. Beginning with session 100, trial duration was increased to 80 s for all rats to accommodate the adjusted delays of the PD group.
Impulsive-choice test (Approximate PNDs 150-175)
In the next several sessions, in order to train responding on the side levers, the left or right levers on the front wall of the chamber were presented individually in random order with the constraint that each was presented 40 times per session. Pressing the lever once led to two pellets delivered after the terminal delay from the delay-exposure training phase. Once consistent side-lever pressing was trained (≥ 90% of trials completed for two consecutive sessions), several choice-training sessions were conducted in which both levers were inserted at the beginning of each trial. The purpose of these sessions was to ensure sensitivity to differences in reward amount. Thus, pressing one lever led to one pellet and pressing the other led to three pellets (assignment counterbalanced within each group). The delays to both rewards were identical and were unchanged from the terminal delay-exposure training phase. These sessions continued until each rat chose the larger reward on ≥ 90% of the trials, and made no more than five omissions, for two consecutive sessions.
Next, impulsive choice was assessed in 20 sessions using a within-session, increasing-delay procedure (Evenden & Ryan, 1996). Sessions were composed of two 20-trial blocks with a 7-min inter-block blackout period. The first six trials in each block were forced-exposure trials in which only one lever and its associated cue light were presented (order determined randomly every two trials). The remaining 14 trials in a block were choice trials, in which both levers were presented. Trials began with the insertion of the rear lever and the illumination of its associated cue light. Following a single rear-lever response, the rear lever was retracted and its cue light was extinguished. One or both side levers (depending on trial type) on the opposite wall were then inserted into the chamber and their associated cue lights were illuminated. Retaining the lever assignments from choice-training sessions, pressing one lever led to one food pellet and the other led to three pellets. In the first trial block, the delay to both rewards was 0 s. In the second trial block, the delay to the larger reward was increased to 15 s (cue light on during the delay). An ITI ensured that trials began every 80 s regardless of the reward chosen. As in previous phases, a 20-s omission criterion was used.
Two sessions in which the delay to both rewards remained at 0 s across both trial blocks (Evenden & Ryan, 1996) were pseudorandomly interspersed among those of the impulsive-choice test. These no-delay sessions were otherwise identical to those described above, but were not programmed over the final six sessions analyzed.
Initiation of ad-libitum food access (Approximate PNDs 176-182)
Upon completion of the impulsive-choice test, rats were provided with ad-libitum food and water access in the home cage for 7 days prior to, and throughout, the alcohol SA test.
Alcohol SA test (Approximate PNDs 183-230)
Alcohol SA procedures closely followed those used by Poulos et al. (1995). Rats were weighed prior to each session and placed in prepared cages for 30 min. The cages were equipped with glass drinking tubes, one containing deionized water and the other an alcohol solution. The left or right position of the solution within the polycarbonate cage alternated strictly across sessions. Following each session, the weights of the remaining alcohol solution and water (plus leakage, if present) were recorded.
Four alcohol concentrations (3%, 6%, 12%, and 24% wt/vol) were assessed in ascending order: 8 days at the 3% concentration and 10 days each at the 6%, 12%, and 24% concentrations.
Continuation/termination of ad-libitum food access (Approximate PNDs 231-241)
After completion of the alcohol SA test, rats continued to receive ad-libitum food and water access in their home cages for 11 days prior to the reinstatement of food restriction.
Impulsive-choice retest (Approximate PNDs 242-261)
When rats returned to 85% of post-alcohol free-feeding weights, the impulsive-choice retest was conducted using the same parameters as in the initial test of impulsive choice.
Statistical analysis
All statistical tests were conducted using SPSS (ver. 19.0, SPSS Inc., Chicago, IL). In all analyses, an alpha level of .05 was considered statistically significant. All pairwise comparisons were examined using Bonferroni correction. Unless otherwise noted, data obtained in the last six sessions of each condition were analyzed.
Separate one-way ANOVAs were used to evaluate between-group differences in the following behavioral outcomes: (a) number of sessions required to acquire rear- and side-lever pressing, (b) the number of sessions required to demonstrate ≥ 90% choice of the larger number of pellets (in choice-training sessions), and (c) mean response latencies and omissions at the conclusion of delay-exposure training.
In the impulsive-choice test and retest, dependent measures were percent large-reward choice in the first and second trial blocks (0-s and 15-s delays, respectively). In the alcohol SA test, dependent measures were mean consumption of alcohol (g/kg) and water (mL/kg), as well as mean body weight (g), at each alcohol concentration. Dependent measures in the impulsive-choice and alcohol SA tests were non-normally distributed (positive skew) and were not amenable to transformation. Group differences in each of the measures above were therefore examined using separate generalized estimating equation (GEE) models, a generalized regression technique that allows analysis of correlated repeated measures, but makes fewer parametric assumptions than do traditional methods (for overview, see Ballinger, 2004).
In each GEE model, main effects of group and the relevant within-subjects variable (e.g., test type in the impulsive-choice model, alcohol concentration in the alcohol SA model) were included, as well as group x within-subject variable interactions. GEE models were implemented using first-order auto-regressive working correlation matrices. In the impulsive-choice GEE model, mean alcohol consumption (collapsed across concentration) was included as a covariate to examine the possibility that alcohol exposure influenced impulsive choice between test and retest. In the alcohol SA GEE model, mean body weight at each concentration was included as a covariate to examine the possibility that between-group differences in alcohol consumption were mediated by differing metabolic or motivational processes (e.g., calorie seeking) between groups unrelated to the value of drug reward.
Results
As shown in Table 1, acquisition of rear-lever pressing was undifferentiated between groups, F(2, 41) = 1.54, p > .05. At the conclusion of 120 days of delay-exposure training, the mean adjusted delay for the PD group was 44.82 s (±1.66; range: 34.13 - 57.60 s). A significant main effect of group, F(2, 41) = 3.86, p < .05, was detected on rear-lever response latencies (see Table 2); however, pairwise comparisons revealed no significant differences between groups (p > .05, in all cases). Table 2 also shows a significant main effect of group on response omissions, F(2, 41) = 52.15, p < .0001, with PD rats omitting more trials than FD or ND rats (p < .001, in both cases).
Table 1.
Mean number of sessions required to meet the acquisition criteria during rear-lever, side-lever, and choice training for all groups (±SEM).
| Group |
|||
|---|---|---|---|
| Training Phase | PD | FD | ND |
| Rear lever | 4.19 (0.44) | 5.14 (0.50) | 5.29 (0.54) |
| Side levers | 4.00 (0.40)Ω≡ | 2.57 (0.17) | 2.29 (0.16) |
| Choice | 5.69 (0.51)Ω≡ | 4.10 (0.46) | 3.50 (0.40) |
indicate PD/FD and PD/ND differences, respectively, in pairwise comparisons (p < .01).
indicate PD/FD and PD/ND differences, respectively, in pairwise comparisons (p < .01).
Table 2.
Mean rear-lever response latencies and omissions per session over the last six delay-exposure training sessions for all groups (±SEM).
| Group |
|||
|---|---|---|---|
| Dependent measure | PD | FD | ND |
| Response latencies (s) | 1.96 (0.24) | 1.26 (0.19) | 1.25 (0.19) |
| Omissions | 3.28 (0.30)Ω≡ | 0.69 (0.14) | 0.58 (0.12) |
indicate PD/FD and PD/ND differences, respectively, in pairwise comparisons (p < .01).
indicate PD/FD and PD/ND differences, respectively, in pairwise comparisons (p < .01).
Prior to the impulsive-choice test, a main effect of group, F(2, 41) > 6.08, p < .01, was detected in the number of sessions required to acquire side-lever pressing (see Table 1), with PD rats requiring about 1.5 more sessions than FD or ND rats (p < .01). Likewise, PD rats required more sessions than the FD or ND groups to demonstrate ≥ 90% preference for the larger reward just prior to the impulsive-choice test (p < .01; see Table 1).
Impulsive-choice Test
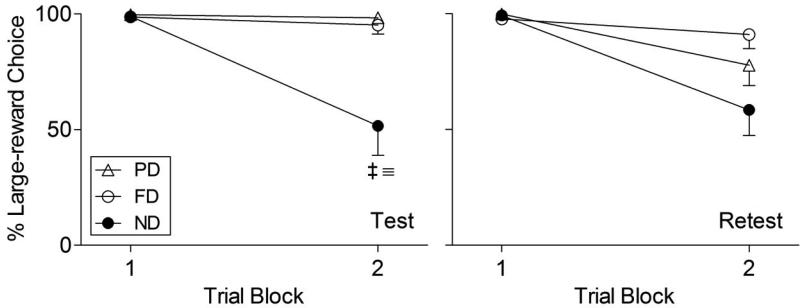
In the first trial block, when neither reward was delayed, there was no significant main effect of group on percent large-reward choice in either the test, Wald χ2 = 3.05; p = .22 (left panel of Figure 2), or retest, Wald χ2 = 0.20; p = .63 (right panel of Figure 2); thus, all groups were equally able to discriminate reward amounts (one vs. three pellets) in the absence of delay. For this reason, choice in the first trial block was excluded from all subsequent analyses.
Figure 2.
Percent large-reward choice across trial blocks in the impulsive-choice test (left panel) and retest (right panel). ≡ and ‡ indicate, respectively, PD/ND and FD/ND differences (p < .01, in both cases). Error bars represent SEM.
The effects of delay-exposure training were evident in the second trial block (15-s delay) in the initial impulsive-choice test (left panel of Figure 2), with pairwise comparisons in the GEE model indicating that both PD and FD rats made fewer impulsive choices than ND rats (p = .003, in both cases).
Alcohol SA Test
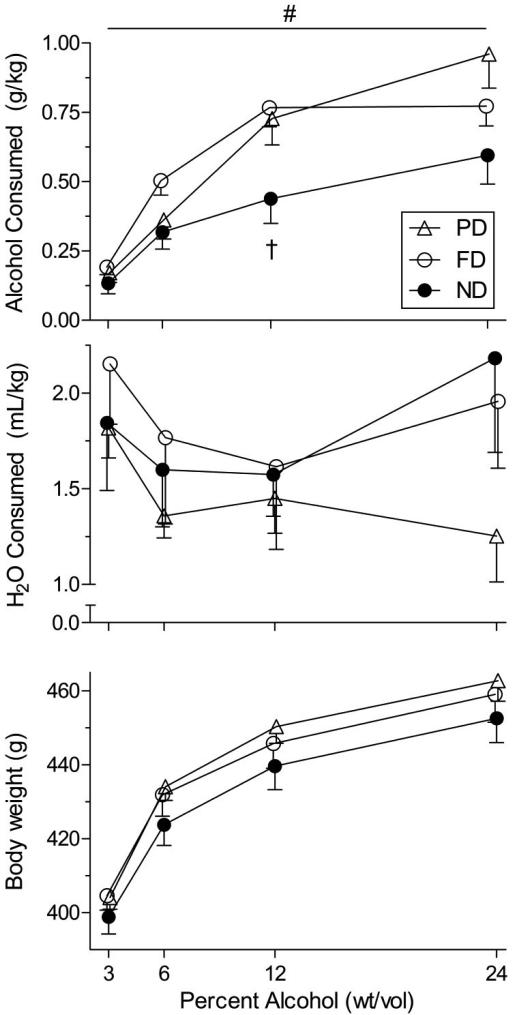
The top, middle, and bottom panels of Figure 3 depict, respectively, mean alcohol consumption (g/kg), water consumption (mL/kg), and body weight (g) collapsed across all sessions at each concentration in the alcohol SA test. Significant main effects of group, Wald χ2 = 8.43; p = .02, and concentration, Wald χ2 = 48.75; p < .001, on alcohol consumption were detected, as was a group × concentration interaction, Wald χ2 = 13.84; p = .03. No effect of body weight on alcohol consumption was observed, Wald χ2 = .01; p = .94.
Figure 3.
Mean consumption of alcohol (top panel) and water (middle panel) at each alcohol concentration. Also depicted is mean body weight at each alcohol concentration (bottom panel). # indicates FD/ND difference (p < .05), when data were collapsed across concentration. † indicates FD/ND difference at individual concentrations (p < .05). Data points have been displaced slightly on the x-axes, for clarity. Error bars represent SEM.
Collapsed across concentration, pairwise comparisons indicated that FD rats consumed more alcohol than ND rats (p = .02), but no other overall between-group differences were significant (PD/ND difference: p = .09). Pairwise comparisons at individual concentrations revealed greater alcohol consumption in FD rats at 12% wt/vol alcohol compared to ND rats (p = .02), but FD/ND differences at other concentrations were not significant (p > .15, in all cases). No other pairwise comparisons were statistically significant at any concentration (PD/ND difference at 12% and 24% wt/vol alcohol: p = .14 and .07, respectively).
No significant main effects of group were detected in either water consumption or body weight, Wald χ2 < 2.51; p > .29 in both cases, and the group × concentration interactions were not significant, Wald χ2 < 8.05; p > .23 in both cases. However, a main effect of concentration was detected on water consumption, Wald χ2 = 9.15; p = .03, and body weight, Wald χ2 = 665.60; p = < .001. No significant between-group differences were observed in pairwise comparisons of either dependent measure either when collapsed across concentration (p > .51, in all cases) or at individual concentrations (p > .95, in all cases).
Impulsive-choice Retest
The right panel of Figure 2 depicts percent large-reward choice in the impulsive-choice retest. Pairwise comparisons in a GEE model revealed no between-group differences in large-reward choice in the retest at the second trial block (p > .15, in all cases).
Across the test and retest of impulsive choice (second trial block only), a GEE model revealed significant main effects of group, Wald χ2 = 8.48; p = .02, and test type, Wald χ2 = 4.97; p = .03, on percent large-reward choice, and a group x test type interaction, Wald χ2 = 7.83; p = .02. The latter reflects a significant decline in large-reward choice in the PD group (p = .04) relative to the other groups (p > .25, in both cases). Finally, although the analysis was not specific to PD rats (the only group in which a significant difference was observed in impulsive choice between test and retest), alcohol consumption in the intervening alcohol SA test was a trend-level predictor of change in percent large-reward choice, Wald χ2 = 3.63; p = .06.
Discussion
The present study demonstrates that early and prolonged exposure to reward delay decreases impulsive choice in rats. In the initial test of impulsive choice, rats in both experimental groups (FD & PD) made significantly fewer impulsive choices than did ND rats. Thus, the present findings extend a relatively small literature on training variables known to impact impulsive choice in nonhuman animals (Logue, Rodriguez, Peña-Correal, & Mauro, 1984; Mazur & Logue, 1978). The present methodology is most similar to that reported by Eisenberger, Masterson, and Lowman (1982), who exposed adult rats to progressively increasing intervals between response-independent food pellets (0-78 s) across 24 training sessions. In a subsequent test, rats exposed to these increasing inter-pellet intervals made significantly fewer impulsive choices than did rats exposed to shorter intervals (5 s). Although the effect observed by Eisenberger et al. in their impulsive-choice test (i.e., approximately 38% vs. 18% large-reward choice, across groups) was relatively smaller than that in the present study, any number of methodological differences (e.g., duration of the training regimen, age of rats during training, or the use of response-independent vs. dependent food delivery) prohibit direct, quantitative comparison between studies.
While the effects of delay-exposure training on impulsive-choice were largely expected, its effects on alcohol consumption were not. In humans, accumulating evidence demonstrates that greater impulsive choice is strongly associated with alcohol abuse and dependence (e.g., Petry et al., 2001; Vuchinich & Simpson, 1998). In rats, Poulos et al. (1995) reported that impulsive choice positively predicted subsequent alcohol consumption in a two-bottle test almost identical to the one used here. Likewise, selectively bred, alcohol-preferring rat and mouse lines make more impulsive choices do non-alcohol-preferring comparison lines (Oberlin & Grahame, 2009; Wilhelm & Mitchell, 2008). Thus, in the absence of experimental manipulation, greater impulsive choice in rats appears strongly related to greater alcohol consumption. However, in the present study, when impulsive choice was experimentally manipulated, rats in the FD group consumed more alcohol than did ND rats; the difference between PD and ND rats only approached significance (p = .09 overall, and .07 at the 24% concentration).
The finding that PD rats (exposed to substantially longer delays than FD rats) were undifferentiated from ND rats in alcohol consumption, whereas FD rats were, is itself a matter of interest. Inclusion of PD training was intended as a parametric manipulation of delay exposure, and was thus hypothesized to produce behavioral effects greater than those observed in FD rats. However, that PD rats made significantly more response omissions during delay-exposure training and required significantly more sessions to acquire delayed side-lever pressing than FD rats suggests that the continuous challenge of progressively increasing delays may have obstructed the effects of delay-exposure training on alcohol consumption.
Although the finding that FD rats consumed significantly more alcohol than ND rats was unexpected, a recent study conducted by Broos et al. (2012; Experiment 2) reports a qualitatively consistent outcome. Broos et al. used an acute dose of methylphenidate (1.0 mg/kg ip) to significantly decrease impulsive choice in rats and reported a concomitant increase in cue-induced reinstatement of cocaine seeking. Conversely, increases in impulsive choice following acute doses of SCH-23390 (0.01 mg/kg sc) were accompanied by decreases in cue-induced cocaine reinstatement relative to saline. Broos et al.'s findings, however, should be interpreted with caution if one considers potential neuropharmacological interaction between experimenter- and self-administered drugs. For instance, in humans, both methylphenidate and cocaine produce similar physiological and subjective drug effects, and cannot be differentiated in a drug-discrimination task (Rush & Baker, 2001). In rats, Schenk and Partridge (1999) reported that a priming dose of methylphenidate produced reinstatement of cocaine SA. Thus, the increases in cocaine reinstatement observed by Broos et al. may have simply been primed by methylphenidate—a possibility that would have nothing to do with impulsive choice. Likewise, the reduction of cocaine SA reinstatement following SCH-23390 may have been a product of this drug's motor-suppressing effects at doses similar or equal to the one used by Broos et al. (e.g., 0.01 mg/kg, Hoffman & Beninger, 1985; 0.17 mg/kg, Morelli & Di Chiara, 1985).
A similar interpretational problem may be found in a study by Oberlin, Bristow, Heighton, and Grahame (2010), who reported that reduction of impulsive choice in high-alcohol-preferring (HAP) mice following an acute dose of amphetamine (1.2 mg/kg) was not accompanied by concomitant changes in alcohol consumption in a two-bottle test. However, in light of the present study's counterintuitive effects of experimentally-reduced impulsive choice on alcohol consumption, an alternative explanation for Oberlin et al.'s null finding exists. Namely, acute amphetamine has been shown to produce a dose-related decrease in two-bottle alcohol consumption in rats (Linseman, 1990). Thus, the impact of competing mechanisms that simultaneously increase and decrease alcohol consumption may have washed out any significant effects of amphetamine on alcohol consumption. In light of the considerations above, manipulation of impulsive choice via training variables (as in the present study) may be preferred over pharmacological methods.
Future research may be designed to address the precise determinants of the effects of delay-exposure training on alcohol consumption. Such an analysis would bear directly on the validity of training-related SA behavior as a measure of drug seeking in nonhuman models, as opposed to an otherwise unrelated or more general behavioral process. No between-group differences were apparent in water consumption in the present study, suggesting that our observed effect was not mediated, in general, by differential consummatory behavior between groups. Further, potential differences in calorie seeking were controlled statistically in the present study by assuming body weight as a relevant proxy measure. We also varied the feeding regimen between the impulsive-choice and alcohol SA tests (restriction vs. ad-libitum access) to minimize the potential that consumption across groups would be differentially motivated by alcohol's caloric properties—a practice common among studies that have shown a relation between impulsive choice and alcohol SA (Oberlin & Grahame, 2009; Poulos et al., 1995; Wilhelm & Mitchell, 2008), and even when the drug examined has no caloric properties (Diergaarde et al., 2008; Marusich & Bardo, 2009; Yates et al., 2011; cf. Anker et al., 2009; Koffarnus & Woods, 2011; Perry et al., 2005, 2008). Nonetheless, a more stringent experimental control may be employed in future studies. For example, investigating consumption of an isocaloric sucrose solution in separate cohorts of delay-exposed and delay-naïve rats would allow examination of caloric or taste variables as alternative explanations for our findings.
As a second alternative explanation for our findings, training-related increases in alcohol consumption may have been mediated by stress exposure. Prior to the alcohol SA test, experimental rats had been exposed to reward delay from early adolescence through middle adulthood (PNDs 25-150). If reward delay is a stressor, then the results of the present study may be placed in the context of a larger experimental literature on the effects of acute and chronic stress on drug SA (for reviews, see Koob, 2008; Piazza & LeMoal, 1998; Sinha, Shaham, & Heilig, 2008). For example, stressors such as restraint, foot shock, and social isolation have increased SA of multiple drugs of abuse in rats, including alcohol (Bozarth, Murray, & Wise, 1989; Goeders & Guerin, 1994; Erb, Shaham, & Stewart, 1996; Shaham, 1993; Shaham & Stewart, 1995). This effect has been linked to several neurochemical and neuroendocrine systems, including limbic dopamine and hypothalamic-pituitary-adrenal axis function (e.g., Schulkin, McEwen, & Gold, 1994; Shepard, Barron, & Myers, 2000). Future studies in this line may be designed to examine behavioral and neurobiological indicators of stress in delay-exposed rats, such as exploratory behavior in an open-field maze or the stress-related steroid corticosterone. However, we note that no work to our knowledge has identified delay as an explicit source of stress in rats. Further, why delay-exposed rats wouldn't have actively avoided this putative stressor throughout impulsive-choice testing, by choosing the immediate reward, remains a paradox.
As a final alternative explanation for our results, training-related increases in alcohol consumption may have been mediated by the relatively slow pharmacokinetic profile of oral alcohol. Onset of drug action varies directly as a function of route of administration (Fowler et al., 2008; Parasrampuria et al., 2007; Volkow et al., 2000). Absorption of orally ingested alcohol in rats sufficient to produce pharmacologically active blood alcohol concentrations requires significant delays (e.g., Livy, Parnell, & West, 2003; Spirduso, Mayfield, Grant, & Schallert, 1989). Thus, prior experience in detecting and exploiting contingent relations between responding and delayed rewards may have better prepared rats to detect and exploit the contingent relation between alcohol consumption and its delayed pharmacological effects.
A few potential limitations of the present study deserve comment. First, impulsive choice was assessed at only one non-zero delay. The purpose of this was to minimize testing-related exposure to delay—our putative independent variable—in ND rats. However, the use of only one non-zero delay may have limited our ability to detect parametric differences in impulsive choice between PD and FD rats—differences that may have emerged had longer delays to the larger reward been explored.
Second, we used only male rats, thus preventing identification of potential sex differences in our dependent variables. Examinations of the relation between naturally occurring impulsive choice and nonhuman drug SA have predominantly been conducted with male rats (e.g., Broos et al., 2012; Diergaarde et al., 2008; Koffarnus & Woods, 2011; Marusich & Bardo, 2009; Poulos et al., 1995), presumably to avoid any uncontrolled effect of estrous cycle. While there are known sex differences in absolute levels of drug SA (for review, see Lynch, 2006), we note that the relation between impulsive choice and drug SA appears the same as that observed in males when female rats have been examined (Anker et al., 2009; Oberlin & Grahame, 2009; Perry et al., 2005, 2008). Nonetheless, the literature would benefit from systematic investigation of potential sex differences as they may uniquely pertain to the effects of delay-exposure training.
Third, in confounding the passage of time with alcohol exposure, the generality of the effects of delay-exposure on impulsive choice across time are difficult to interpret. In the impulsive-choice retest (approximately 65 days following the initial test), we observed no significant differences between delay-exposed and ND rats. In addition, PD rats made significantly more impulsive choices in the retest compared to the initial test. However, whether these findings were due to the passage of time, or to differential alcohol exposure between groups, is unanswerable from the experimental design used. Thus, we defer firm conclusions regarding the effects of alcohol on impulsive choice (e.g., Evenden & Ryan, 1999; Olmstead, Hellemans, & Paine, 2006; Poulos, Parker, & Le, 1998), or the effects of delay exposure on impulsive choice across time, to studies designed explicitly to test such relations.
Fourth, prior literature documents anxiogenic effects and neurobiological deficits in adolescent and adult rats exposed to chronic and severe food restriction (e.g., 50-60% ad-libitum food intake; Gur, Newman, Avraham, Dremencov, & Berry, 2003; Huether, Zhou, Schmidt, Wiltfang, & Rüther, 1997; Jahng et al., 2007). Rats in the present experiment were subjected to food restriction from early adolescence to middle adulthood (PNDs 25-175) in order to encourage operant responding. However, we believe it is unlikely that our use of food restriction substantially impacted our findings, as the level of restriction in the present study was much milder (approximately 85% of ad-libitum food intake) than has been widely found to produce behavioral and neurochemical abnormalities in the studies cited above. Relatively little research has been designed to examine such neurobehavioral effects as a result of the mild food restriction employed in the study of operant food responding (cf. Carr, Tsimberg, Berman, & Yamamoto, 2003). Further, the use of adolescent food restriction in the present study was a variable held constant across all groups, and thus did not likely pose a threat to internal validity.
As a final limitation, delay exposure in the present study was a composite variable consisting of both delayed-reward autoshaping and a prolonged, 120-day training regimen. In addition, training began during adolescence (a period of highly plastic responsiveness to experimental variables; Chapillon, Patin, Roy, Vincent, & Caston, 2002) to increase the likelihood that delay exposure would produce stable, trait-like patterns of behavior in adulthood. The primary goal of this multi-faceted approach was to create distinct groups of varying levels of impulsivity to explore related group differences in alcohol SA. The extent to which any variable in the delay-exposure regimen weighed independently on our observed effects cannot be resolved from the experimental design used. However, future studies may be designed to isolate these variables, or parametrically manipulate the duration of the training regimen, to determine their effects on impulsive choice and alcohol consumption.
In conclusion, the present data suggest that the relation between impulsive choice and alcohol SA is not a straightforward one—experimentally reducing impulsive choice did not decrease alcohol consumption in rats; to the contrary, it appears to have increased it. Thus, the present data do not accord with previous findings suggesting that impulsive choice precedes and predicts drug SA in rats (e.g., Diergaarde et al., 2008; Perry et al., 2005, 2008; Koffarnus & Woods, 2011; Poulos et al., 1995). Nonetheless, further investigation will be required to determine the generality of the present findings across other nonhuman drug SA models (e.g., iv cocaine SA), in which many of the variables reviewed above (e.g., oral alcohol's slow pharmacokinetic profile or caloric properties) would not play a role. Whether these future investigations yield findings similar, or opposite, to those of the present study might yield further evidence for, or against, respectively, a direct causal relation between impulsive choice and drug SA.
Acknowledgements
This research was supported financially by a grant from the National Institutes of Health: 1R01DA029605, awarded to the last author (G. J. Madden).
The second author (P. S. Johnson) is now at the Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine.
All authors would like to thank Shayne M. Barker for assistance in data collection.
Footnotes
All authors contributed in a significant way and have read and approved the final manuscript.
Disclosures
None of the authors have any real or potential conflict(s) of interest, including financial, personal, or other relationships with organizations or pharmaceutical/biomedical companies that may inappropriately influence the research and interpretation of the findings.
References
- Anker JJ, Perry JL, Gliddon LA, Carroll ME. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacology, Biochemistry, and Behavior. 2009;93:343–348. doi: 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, Wileyto EP. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug and Alcohol Dependence. 2009;103:99–106. doi: 10.1016/j.drugalcdep.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayduk O, Mendoza-Denton R, Mischel W, Downey G, Peake PK, Rodriguez M. Regulating the interpersonal self: Strategic self-regulation for coping with rejection sensitivity. Journal of Personality and Social Psychology. 2000;79:776–792. doi: 10.1037//0022-3514.79.5.776. [DOI] [PubMed] [Google Scholar]
- Ballinger GA. Using generalized estimating equations for longitudinal data analysis. Organizational Research Methods. 2004;7:127–150. [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Gatchalian KM, McClure SM. Are executive function and impulsivity antipodes? A conceptual reconstruction with special reference to addiction. Psychopharmacology. 2012;221:367–387. doi: 10.1007/s00213-012-2689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth MA, Murray A, Wise RA. Influence of housing conditions on the acquisition of intravenous heroin and cocaine self-administration in rats. Pharmacology, Biochemistry, and Behavior. 1989;33:903–937. doi: 10.1016/0091-3057(89)90490-5. [DOI] [PubMed] [Google Scholar]
- Broos N, Diergaarde L, Schoffelmeer AN, Pattij T, de Vries TJ. Trait impulsive choice predicts resistance to extinction and propensity to relapse to cocaine seeking: A bidirectional investigation. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2011.323. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience. 2003;119:1157–1168. doi: 10.1016/s0306-4522(03)00227-6. [DOI] [PubMed] [Google Scholar]
- Chapillon P, Patin V, Roy V, Vincent A, Caston J. Effects of pre-and postnatal stimulation on developmental, emotional, and cognitive aspects in rodents: A review. Developmental psychobiology. 2002;41:373–387. doi: 10.1002/dev.10066. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Experimental and Clinical Psychopharmacology. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer ANM, de Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biological Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Eisenberger R, Masterson FA, Lowman K. Effects of previous delay of reward, generalized effort, and deprivation on impulsiveness. Learning and Motivation. 1982;13:378–389. [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology. 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behavior in rats: The effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behavior in rats VI: The effects of ethanol and selective serotonergic drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1999;146:413–421. doi: 10.1007/pl00005486. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Logan J, Alexoff D, Telang F, Wang GJ, Apelskog K. Fast uptake and long-lasting binding of methamphetamine in the human brain: Comparison with cocaine. Neuroimage. 2008;43:756–63. doi: 10.1016/j.neuroimage.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon J, Baldock MD, Locurto C, Gold L, Terrace HS. Trial and intertrial durations in autoshaping. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:264–284. [Google Scholar]
- Goeders NE, Guerin GF. Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology. 1994;114:63–70. doi: 10.1007/BF02245445. [DOI] [PubMed] [Google Scholar]
- Gur E, Newman ME, Avraham Y, Dremencov E, Berry EM. The differential effects of food restriction on 5-HT1A and 5-HT1B receptor mediated control of serotonergic transmission in the hippocampus and hypothalamus of rats. Nutritional Neuroscience. 2003;6:169–175. doi: 10.1080/1028415031000115936. [DOI] [PubMed] [Google Scholar]
- Hoffman DC, Beninger RJ. The D1 dopamine receptor antagonist, SCH-23390 reduces locomotor activity and rearing in rats. Pharmacology, Biochemistry, and Behavior. 1985;22:341–342. doi: 10.1016/0091-3057(85)90401-0. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology. 2006;188:162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- Huether G, Zhou D, Zchmidt S, Wiltfang J, Ruther E. Long-term food restriction down-regulates the density of serotonin transporters in the rat frontal cortex. Biological Psychiatry. 1997;41:1174–1180. doi: 10.1016/s0006-3223(96)00265-x. [DOI] [PubMed] [Google Scholar]
- Jahng JW, Kim JG, Kim HJ, Kim BT, Kang DW, Lee JH. Chronic food restriction in young rats results in depression- and anxiety-like behaviors with decreased expression of serotonin reuptake transporter. Brain Research. 2007;1150:100–107. doi: 10.1016/j.brainres.2007.02.080. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Woods JH. Individual differences in discount rate are associated with demand for self-administered cocaine, but not sucrose. Addiction Biology. 2011 doi: 10.1111/j.1369-1600.2011.00361.x. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linseman MA. Effects of dopaminergic agents on alcohol consumption by rats in a limited access paradigm. Psychopharmacology. 1990;100:195–200. doi: 10.1007/BF02244405. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Parnell SE, West JR. Blood ethanol concentration profiles: A comparison between rats and mice. Alcohol. 2003;29:165–171. doi: 10.1016/s0741-8329(03)00025-9. [DOI] [PubMed] [Google Scholar]
- Logue AW, Rodriguez ML, Peña-Correal TE, Mauro BC. Choice in a self-control paradigm: Quantification of experience-based differences. Journal of the Experimental Analysis of Behavior. 1984;41:53–67. doi: 10.1901/jeab.1984.41-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafó MR. Delayed reward discounting and addictive behavior: A meta-analysis. Psychopharmacology. 2011;216:305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: Drug and monetary rewards. Experimental and Clinical Psychopharmacology. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Bardo MT. Differences in impulsivity on a delay discounting task predict self-administration of a low unit dose of methylphenidate in rats. Behavioural pharmacology. 2009;20:447–454. doi: 10.1097/FBP.0b013e328330ad6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE, Logue AW. Choice in a “self-control” paradigm: Effects of a fading procedure. Journal of the Experimental Analysis of Behavior. 1978;30:11–17. doi: 10.1901/jeab.1978.30-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli M, Di Chiara G. Catalepsy induced by SCH 23390 in rats. European Journal Pharmacology. 1985;117:179–185. doi: 10.1016/0014-2999(85)90602-8. [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Bristow RE, Heighton ME, Grahame NJ. Pharmacologic dissociation between impulsivity and alcohol drinking in high alcohol preferring mice. Alcoholism: Clinical and Experimental Research. 2010;64:1363–1375. doi: 10.1111/j.1530-0277.2010.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Grahame NJ. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcoholism: Clinical and Experimental Research. 2009;33:1294–1303. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Hellemans KG, Paine TA. Alcohol-induced impulsivity in rats: An effect of cue salience. Psychopharmacology. 2006;184:221–228. doi: 10.1007/s00213-005-0215-0. [DOI] [PubMed] [Google Scholar]
- Parasrampuria DA, Schoedel KA, Schuller R, Gu J, Ciccone P, Silber SA, Sellers EM. Assessment of pharmacokinetics and pharmacodynamic effects related to abuse potential of a unique oral osmotic-controlled extended-release methylphenidate formulation in humans. Journal of Clinical Pharmacology. 2007;47:1476–1488. doi: 10.1177/0091270007308615. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of i.v. cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Carroll ME. Impulsive choice as a predictor of acquisition of IV cocaine self-administration and reinstatement of cocaine-seeking behavior in male and female rats. Experimental and Clinical Psychopharmacology. 2008;16:165–177. doi: 10.1037/1064-1297.16.2.165. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends in Pharmacological Science. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Pinkston JW, Ratzlaff KL, Madden GJ, Fowler SC. An inexpensive infrared detector to verify the delivery of food pellets. Journal of the Experimental Analysis of Behavior. 2008;90:249–255. doi: 10.1901/jeab.2008.90-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behavioural Pharmacology. 1995;6:810–814. [PubMed] [Google Scholar]
- Poulos CX, Parker JL, Le AD. Increased impulsivity after injected alcohol predicts later alcohol consumption in rats: Evidence for “loss-of-control drinking” and marked individual differences. Behavioural Neuroscience. 1998;112:1247–1257. doi: 10.1037//0735-7044.112.5.1247. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behavioural Processes. 2004;65:35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Richter CP, Campbell KH. Alcohol taste thresholds and concentrations of solution preferred by rats. Science. 1940;91:507–508. doi: 10.1126/science.91.2369.507. [DOI] [PubMed] [Google Scholar]
- Rush CR, Baker RW. Behavioral pharmacological similarities between methylphenidate and cocaine in cocaine abusers. Experimental and Clinical Psychopharmacology. 2001;9:59–73. doi: 10.1037/1064-1297.9.1.59. [DOI] [PubMed] [Google Scholar]
- Schenk SS, Partridge B. Cocaine-seeking produced by experimenter-administered drug injections: Dose-effect relationships in rats. Psychopharmacology. 1999;147:285–290. doi: 10.1007/s002130051169. [DOI] [PubMed] [Google Scholar]
- Schulkin J, McEwen BS, Gold PW. Allostasis, amygdala, and anticipatory angst. Neuroscience and Biobehavioral Reviews. 1994;18:385–396. doi: 10.1016/0149-7634(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Shaham Y. Immobilization stress-induced oral opioid self-administration and withdrawal in rats: Role of condition factors and the effect of stress on “relapse” to opioid drugs. Psychopharmacology. 1993;111:477–485. doi: 10.1007/BF02253539. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: An effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Research. 2000;861:288–295. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology. 2011;218:69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirduso WW, Mayfield D, Grant M, Schallert T. Effects of route of administration of ethanol on high-speed reaction time in young and old rats. Psychopharmacology. 1989;97:413–417. doi: 10.1007/BF00439461. [DOI] [PubMed] [Google Scholar]
- Stein JS, Madden GJ. Delay discounting and drug abuse: Empirical, conceptual, and methodological considerations. In: Mackillop J, de Wit H, editors. The Wiley-Blackwell Handbook of Addiction Psychopharmacology. Wiley-Blackwell; Oxford, UK: in press. [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin R, Fowler JS, Franceschi D, Pappas N. Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sciences. 2000;67:1507–1515. doi: 10.1016/s0024-3205(00)00731-1. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Experimental and Clinical Psychopharmacology. 1998;6:292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH. Rats bred for high alcohol drinking are more sensitive to delayed and probabilistic outcomes. Genes, Brain and Behavior. 2008;7:705–713. doi: 10.1111/j.1601-183X.2008.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Marusich JA, Gipson CD, Beckmann JS, Bardo MT. High impulsivity in rats predicts amphetamine conditioned place preference. Pharmacology Biochemistry and Behavior. 2012;100:370–376. doi: 10.1016/j.pbb.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]