Abstract
Expression of adhesin to collagen of Enterococcis faecalis (ace), a known virulence factor, is increased by environmental signals such as the presence of serum, high temperature, and bile salts. Currently, the enterococcal regulator of survival (Ers) of E. faecalis strain JH2-2 is the only reported repressor of ace. Here, we show that for strain OG1RF, Ers is not involved in the regulation of ace. Our data showed similar levels of ace expression by OG1RF and its Δers derivative in the presence of bile salts, serum, and high temperature. Using ace promoter-lacZ fusions and site-directed mutagenesis, we confirmed these results and further showed that, while the previously designated Ers box is important for increased expression from the ace promoter of OG1RF, the region responsible for the increase is bigger than the Ers box. In summary, these results indicate that, in strain OG1RF, Ers is not a repressor of ace expression. Although JH2-2 and OG1RF differ by 6 nucleotides in the region upstream of ace as well as in production of Fsr and gelatinase, the reason(s) for the difference in ace expression between JH2-2 and OG1RF and for increased ace expression in bile, serum and at 46°C remain(s) to be determined.
Keywords: Enterococcus faecalis, ace, regulation, bile salts, virulence
INTRODUCTION
Enterococcus faecalis is a gram-positive commensal bacterium recognized as an important cause of nosocomial infections (Arias & Murray, 2012). At the first step of infection, bacteria adhere to host tissue and the family of bacterial surface proteins known as MSCRAMMs (Microbial Surface Components Recognizing Adhesive Matrix Molecules) appear to play an important role in this process (Patti et al., 1994). The most studied MSCRAMM of E. faecalis is Ace (Adhesin to collagen of E. faecalis), which plays a major role in experimental E. faecalis infections and is presumed to mediate its attachment to host tissues via its interaction with collagen I and IV (Rich et al., 1999). In different models such as the murine macrophage, urinary tract infection and experimental endocarditis, ace null mutants were clearly attenuated suggesting that Ace is an important virulence factor (Lebreton et al., 2009; Singh et al., 2010; Nallapareddy et al., 2011). In addition, anti-recombinant Ace antibodies showed a protective effect against E. faecalis experimental endocarditis (Singh et al., 2010).
It was recently shown that the presence of Ace on the cell surface of E. faecalis varies during the growth cycle in a strain-dependent way (Pinkston et al., 2011). In E. faecalis strain OG1RF, Ace increases on the cell surface in early exponential phase and diminishes in stationary phase (Hall et al., 2007; Pinkston et al., 2011); the stationary phase decrease was shown to be dependent on a post-translational process mediated by gelatinase (GelE), which cleaves Ace from the cell surface (Nallapareddy et al., 2000; Pinkston et al., 2011). Strains that do not produce GelE, such as JH2-2, maintain Ace on their surface even in stationary phase (Pinkston et al., 2011).
Expression of ace by E. faecalis is also well known to be increased by environmental stimuli such as the presence of serum, bile salts, urine, and at 46°C (Shepard & Gilmore, 2002; Nallapareddy & Murray, 2006; Lebreton et al., 2009), but how ace is regulated is largely unknown. Lebreton and coworkers (Lebreton et al., 2009) showed that, with E. faecalis strain JH2-2, the enterococcal regulator of survival (Ers) negatively regulates ace expression while it positively regulates other genes (Riboulet-Bisson et al., 2008, 2009; Lebreton et al., 2009). When bile salts were added, expression of ers by JH2-2 decreased and ace expression increased. However, ers expression was reported as not being changed by high temperature (46°C), nor the addition of collagen or horse serum, suggesting that other factors regulate ace expression under these conditions (Lebreton et al., 2009).
To better understand the regulation of this important virulence factor, we studied ace expression in the well studied E. faecalis strain OG1RF (Bourgogne et al., 2008) and in its ers deletion mutant (Δers) by northern and western blot analysis. In addition, we studied the importance of the putative Ers binding motif using lacZ fusion plasmids with deletions/substitutions in and adjacent to this motif.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions
The strains and plasmids used in this study are listed in Table 1. Bacteria were grown at 37°C in Luria-Bertani (LB) broth, Brain Heart Infusion (BHI) broth, M17 medium supplemented with 0.5% glucose (M17-G) or MM9YEG with 10 mM p-chloro-phenylalanine (p-Cl-Phe, Sigma-Aldrich Co.) (Kristich et al., 2007). For some experiments, BHI was supplemented with 40% (v/v) horse serum (BHIS). To investigate ace expression with bile salts, strain OG1RF and its Δers mutant were grown in M17-G media. At the early exponential phase of growth (2.5 hr), bile salts (1:1 mixture of sodium cholate and sodium deoxycholate, Sigma-Aldrich Co.) were added to the final concentrations of 0.02, 0.04, 0.06, and 0.08% as indicated in Fig. 1A. The antibiotic concentrations used for bacterial selection were as follows: 200 µg/ml erythromycin, 50 µg/ml ampicillin, and 25 µg/ml gentamicin (all from Sigma-Aldrich Co.) for Escherichia coli and 10 µg/ml erythromycin and 125 µg/ml of gentamicin for E. faecalis.
Table 1.
Bacteria strains and plasmids used in this study.
| Strain/plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| E. faecalis | ||
| OG1RF | Fusr, Rifr | (Bourgogne et al., 2008) |
| TX5696 | OG1RF Δers (EF0074); non-polar, in-frame deletion of ers; Fusr, Rifr | This study |
| E. coli | ||
| DH5α | E. coli host strain used for routine cloning | Stratagene |
| EC1000 | E. coli host strain, provides RepA | (Leenhouts et al., 1996) |
| Plasmids | ||
| pBluescript | E. coli cloning vector; Apr | Agilent Technologies |
| pHOU1 | Plasmid for mutagenesis; Gmr | (Panesso et al., 2011) |
| pKAF7 | Reporter plasmid with lacZ from pCJK47; Emr | (Fox et al., 2009) |
| pTEX6075b | P435; pKAF7 with 435 bp upstream of the ace start codon and 27 bp of the ace gene; Emr | This study |
| pTEX6075c | P176; pKAF7 with 176 bp upstream promoter region of the ace start codon and 27 bp of the ace gene; Emr | This study |
| pTX6080f | PΔCAAA; P435 with 4 bp CAAA deleted near the Ers box; Emr | This study |
| pTX6080g | PΔTTGTA; P435 with 5 bp TTGTA deleted from the Ers box; Emr | This study |
| pTX6080h | PGTC; P435 substitution of ACA in the Ers box with GTC; Emr | This study |
| pTX6080i | PGGGC; substitution of TAAT near the Ers box with GGGC, Emr | This study |
Ap, ampicillin; Em, erythromycin; Fus, fusidic acid; Gm, gentamicin; Rif, rifampicin.
Figure 1.
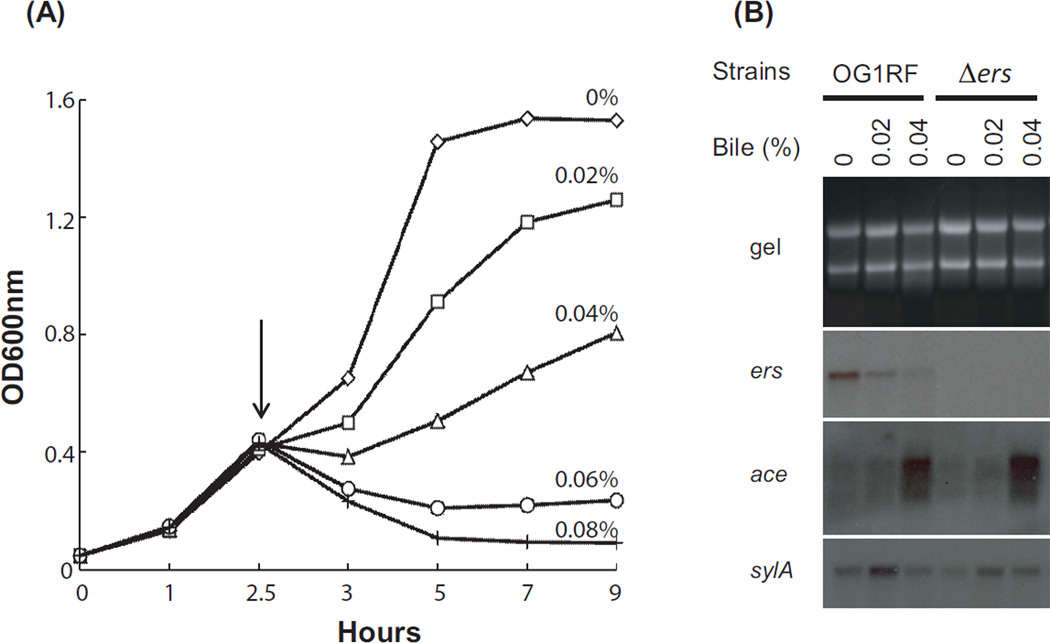
Effect of bile salts on growth and gene expression of E. faecalis. (A) Growth curve of E. faecalis OG1RF grown in M17-G medium with and without bile salts and (B) northern blot analysis. Bile salts were added at 2.5 hr (indicated by arrow) and sampled at 3 hr (after 30 minutes incubation). Final concentrations of bile salts are indicated with different symbols: diamonds (0% bile salts), squares (0.02%), triangles (0.04%), circles (0.06%), and crosses (0.08%). Total RNA was separated in 1% agarose gel (gel), transferred, and hybridized with internal probes of ers, ace, and sylA genes.
Construction of E. faecalis mutants
A non-polar, unmarked, in-frame deletion of ers was created using pHOU1 as described previously (Panesso et al., 2011). Briefly, pHOU1 containing the ers deletion construct (Supplementary Table for construction primers) was introduced into strain OG1RF by electroporation, and selected on BHI agar with 125 µg/ml gentamicin and 200 µg/ml X-Gal (Gold Biotechnology Inc.). Colonies containing the presumed first crossover recombination event were then streaked onto MM9YEG supplemented with 10 mM p-Cl-Phe and incubated at 37°C overnight to allow excision of pHOU1 from the E. faecalis chromosome. Resulting colonies were screened for loss of the plasmid (loss of both gentamicin resistance and β-galactosidase activity) and deletion of the target gene was confirmed by PCR using gene flanking primers (Supplementary Table). Mutants were confirmed by DNA sequencing of the deleted region and by pulsed field gel electrophoresis (PFGE) after SmaI digestion as reported previously (Murray et al., 1993).
RNA analysis and identification of the transcription initiation site
Total RNA was isolated as follows: an overnight culture was re-inoculated into 20 ml BHI (or M17-G) to a starting OD600 of 0.05. At mid-exponential phase (OD600 = 0.5), 5 ml of culture was mixed with 10 ml RNAprotect reagent (Qiagen) and collected (3,900 rpm for 10 min) for RNA isolation. The pellet was resuspended in 1ml RNAwiz (Ambion) and disrupted by bead beating for 1 min using a Mini BeadBeater (BioSpec Products). RNA was extracted according to the protocol of RNAwiz and cleaned with the RNeasy Mini kit (Qiagen). Total RNA (10 µg/lane) was separated in a 1% agarose gel. 16S and 23S rRNA bands in the gel were stained by ethidium bromide and used as loading controls. Northern blot analysis was performed with internal probes for ace, ers and sylA genes. The transcriptional regulator gene, sylA, was used as a control for RNA quality and bile salts effect because its expression was previously reported to be increased in the presence of bile salts (Michaux et al., 2011). Primers to amplify an internal probe of each gene are shown in Supplementary Table. RadPrime DNA labeling kit (Invitrogen) was used to labeling the probes with [α-32P]-dCTP (PerkinElmer Inc.).
The 5’/3’ RACE kit 2nd Generation (Roche) was used to map the transcriptional start site of the ace gene. RNA was extracted from strain OG1RF grown in BHIS at 37 °C to mid-exponential phase (OD600 = 0.5). cDNA of the ace gene was generated using primer AceSP1, followed by addition of a poly(A) tail by a terminal transferase. Nested primer AceSP2 and either ACEF1099R or AceSP3 primer were used for further PCR. cDNA generated was subcloned in pBluescript and sequenced by Genewiz Inc. (South Plainfield, NJ).
Construction of the ace::lacZ fusion plasmids and β-galactosidase assay
Six lacZ fusion plasmids were created containing the following variations of the ace promoter: P435, P176, PΔCAAA, PΔTTGTA, PGTC, and PGGGC; the fusions were constructed in pKAF7, a promoter-less lacZ plasmid (Fox et al., 2009). The upstream promoter region of ace was amplified with primer pairs, AcePromF2b/AcePromR and AcePromF3b/AcePromR, and ligated into pKAF7 to make P435 and P176, respectively. P435 includes 101 bp of the upstream gene EF1098, the 334 bp intergenic region, and 27 bp of the ace gene. P176 consists of 176 bp from the ace start codon up to the predicted Ers box previously described (Lebreton et al., 2009) and 27 bp of the ace gene. Plasmids designated PΔCAAA, PΔTTGTA, PGTC, and PGGGC were created by site-directed mutagenesis using P435 as a template. Plasmids PΔCAAA and PΔTTGTA are deletion derivatives of the Ers box region whereas plasmids PGTC and PGGGC are replacement constructs (GTC replaces ACA and GGGC replaces AATG). Deletion/substitution constructs were created using the primer pairs DelErsF1/DelErsR, DelupErsF/DelupErsR, ErsGGCF/ErsGGCR and CovGGGCF/CovGGGCR (Supplementary Table). Fusion constructs were electroporated into the parent strain OG1RF and its Δers mutant. Independent duplicate/triplicate cultures were assessed for β-galactosidase activity as described previously (Hammerstrom et al., 2011).
Mutanolysin extraction and western blotting
Surface proteins of E. faecalis were prepared by mutanolysin treatment described previously (Nallapareddy et al., 2000) and separated in 10% SDS-PAGE (10 µg/lane). Proteins transferred to a nitrocellulose membrane were incubated with anti-Ace monoclonal antibody 70 (Pinkston et al., 2011) and developed with SuperSignal West Pico chemiluminescent substrate (Pierce).
RESULTS AND DISCUSSION
Currently, Ers of E. faecalis JH2-2 is the only transcriptional regulator reported for regulation of ace expression. Lebreton, et al., showed an interaction between Ers and the ace promoter using electrophoretic mobility shift assay and that purified recombinant Ers protein bound a 400 bp DNA fragment containing the ace promoter, but also required the presence of crude extract of the Δers strain (Lebreton, et al., 2009). In addition, RT-qPCR data showed that out of the 8 genes of JH2-2 containing a putative Ers box in its promoter region, only ace expression was significantly (5–7 fold) increased in the Δers strain while the other genes were expressed similarly in both JH2-2 and its Δers mutant (Lebreton et al., 2009). Therefore, Ers was inferred to be a repressor of ace expression (Lebreton et al., 2009).
To further investigate how Ers is involved in ace expression, we generated an ers null (Δers) strain in E. faecalis OG1RF. Comparison of the ers ORF sequence of OG1RF and JH2-2 showed 100% identity between strains. The mutant strain, Δers, contains a non-polar in-frame deletion and lacks 534 bp (out of 654 bp) of the ers ORF (Supplementary Table for primer sequence). Comparison of growth of OG1RF and Δers in BHI, in BHI with serum, bile salts, and high temperature using BHI broth showed no significant differences between two strains (data not shown).
Effect of bile salts on ace and ers expression
The effect of bile salts on the expression of ace and ers was studied by northern blot analysis of OG1RF and its Δers mutant grown in M17-G supplemented with increased amounts of bile salts (0 - 0.08%). Addition of bile salts inhibited the growth of OG1RF (Fig. 1A) and Δers (data not shown) in the same concentration dependent manner. High concentrations of bile salts (0.06% and 0.08%) severely inhibited growth of both strains that had not recovered by 24 hrs (data not shown). Lower concentrations of bile salts (0.02 and 0.04%) initially inhibited growth but then growth resumed (Fig. 1A). The growth decrease of OG1RF in 0.02 and 0.04% is in agreement with the growth curve of JH2-2 in 0.08% bile salt condition previously reported (Michaux et al., 2011).
Figure 1B shows the northern blot analysis of OG1RF and its Δers derivative grown in 0%, 0.02%, and 0.04% bile salts concentrations. Expression of ers in strain OG1RF was decreased by increasing the bile salts concentration; Δers did not show an ers signal, as expected. Expression of ace in both OG1RF and its Δers mutant was very low and similar to each other without bile salts (0%) or in 0.02% suggesting Ers is not a repressor in these conditions. In 0.04% bile salts concentration, ace expression increased approximately equally in both OG1RF and its Δers mutant, further indicating that Ers is not a repressor of ace in strain OG1RF. The increase in ace expression of OG1RF in 0.04% bile salts appears similar to the increase in ace expression by JH2-2 at the single concentration previously reported (0.08%) (Lebreton et al., 2009); our results for OG1RF differ from those previously published for JH2-2 showing 5–7 fold increase of ace expression of Δers strain compared to its parental strain (Lebreton et al., 2009).
Effect of serum and high temperature on ace and ers expression
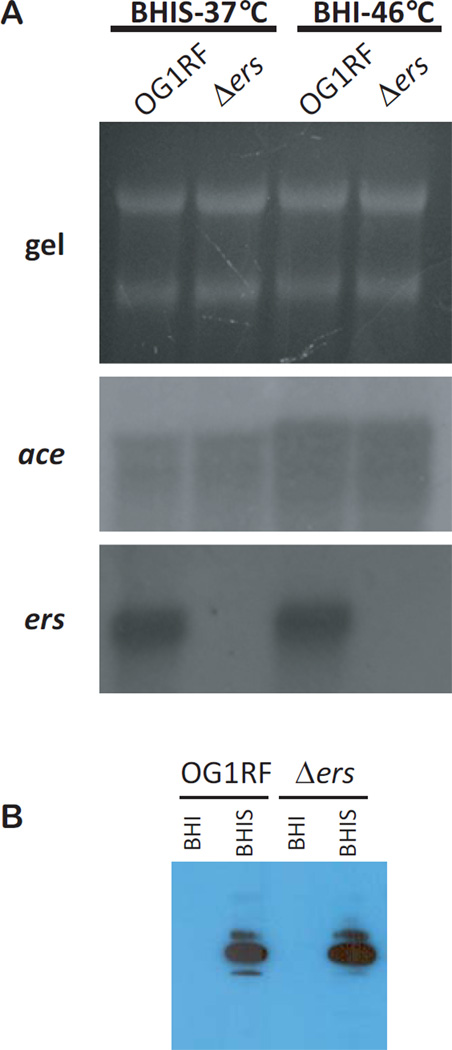
In addition to bile salts, the effect of Ers on ace expression was investigated under two other stress conditions previously reported to induce ace expression: growth in the presence of serum and growth at 46°C. Figure 2A shows northern blots of ace and ers transcripts from OG1RF and Δers grown in BHIS at 37 °C and in BHI broth at 46 °C. Strain Δers grown in BHIS showed similar ace levels to the parent strain OG1RF (Fig. 2A). Also, after growth at 46°C, ace expression of the Δers mutant was similar to that of OG1RF (Fig. 2A). Expression of ace was about 3-fold higher in both strains grown in BHI at 46°C compared to BHIS. However, expression of ers was similar for OG1RF grown in both stress conditions indicating again that Ers is not a repressor of ace expression. As previously reported (Nallapareddy & Murray, 2006), ace expression was increased in OG1RF grown in BHIS compared to BHI alone (Fig. 1B and Fig. 3). We also performed western blotting using OG1RF and its Δers mutant grown in BHIS with anti-Ace monoclonal antibody 70 (Pinkston et al., 2011) (Fig. 2B). Addition of serum increased the presence of Ace on the cell surface but Ace amounts in OG1RF and Δers were similar when grown in BHIS condition. These observations were comparable to the changes in transcriptional activity shown in Figures 1 and 3.
Figure 2.
Expression of ace/Ace by E. faecalis OG1RF and its Δers derivative. (A) Transcriptional changes by strains grown in BHI with serum at 37°C and in BHI at 46°C. Northern blot analysis was performed with total RNA (gel, 10 µg/lane) and hybridized with internal probes of ace and ers. (B) Surface expression of Ace after growth with serum. Western blot analysis was performed as described in the Materials and Methods.
Figure 3.
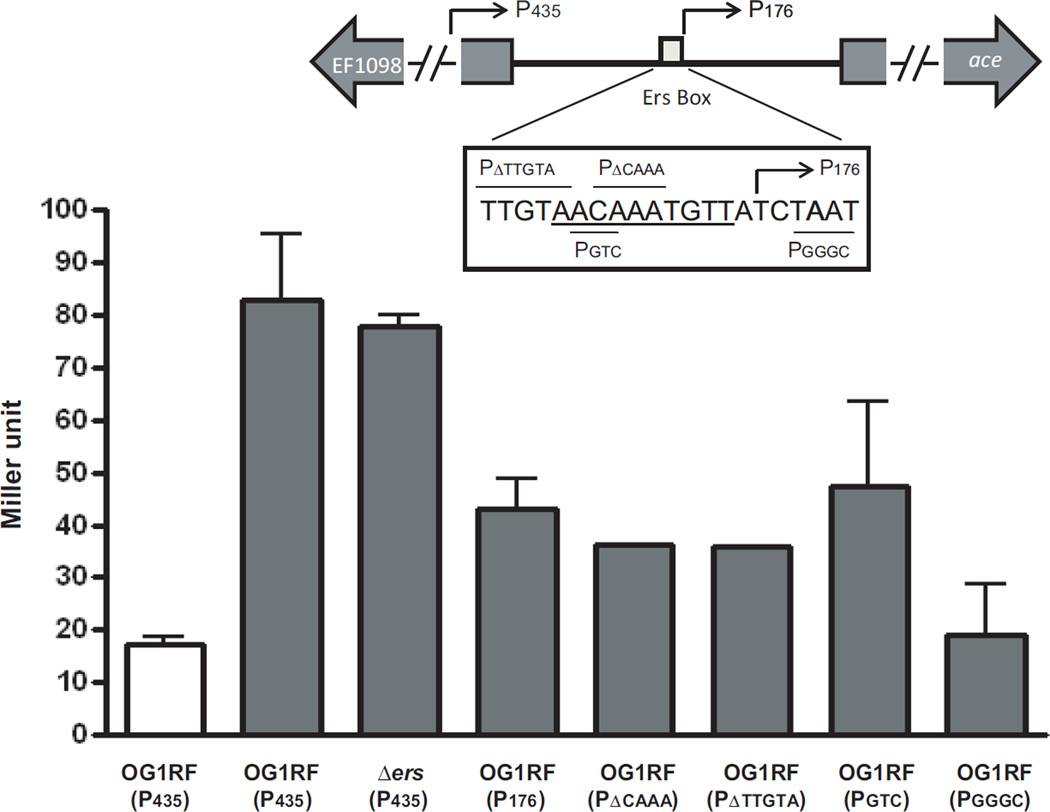
Effect of changes around the putative Ers box on expression from the ace promoter. When grown in the presence of serum, β-galactosidase activities of P435 and its derivatives were determined. Sequence information of fusion plasmids is indicated in the box. The putative Ers binding site (AACAAATGTTA) is underlined. Deletion constructs are indicated above the sequence whereas substitution constructs are indicated below the sequence. P176, indicated with the arrow, extends from the Ers box to 27 bp inside the ace gene. Bars with standard error indicate the means of the duplicate/triplicate assay using strains collected at early exponential phase. Strains were grown in BHI (white bar) or BHIS (gray bar).
Promoter region and requirement for an intact Ers box for increased expression of ace
To characterize the promoter structure of ace, we first determined the transcriptional start site of ace using E. faecalis OG1RF grown in BHIS. Using 5’/3’ RACE kit, the transcriptional start site (+1) of ace was identified at 57 bp upstream of the start codon (data not shown). This site was previously identified as the transcriptional start site of ace in strain JH2-2 grown in the presence of bile salts (Lebreton et al., 2009). Therefore, the transcriptional start site of ace appears to be the same regardless of different conditions (serum or bile salts) or clonal lineages (ST1 for OG1RF and ST8 for JH2-2, Ruiz-Garbajosa et al., 2006).
Expression of ace after growth in BHIS was also investigated by β-galactosidase reporter gene assay (Fig. 3). We generated 6 different ace promoter fusions using the promoter-less lacZ plasmid pKAF7: P435, P176, PΔCAAA, PΔTTGTA, PGTC, and PGGGC (Fig. 3 and Supplementary Figure) and transformed them into E. faecalis OG1RF and into Δers. β-galactosidase activitity was first determined for OG1RF(P435) grown in BHI and in BHIS; results showed that β-galactosidase activities increased about 4–5 fold when OG1RF(P435) was grown in BHIS compared to BHI alone (Fig. 3). Production of β-galactosidase activity by Δers(P435) in BHIS was similar to that in OG1RF(P435) and consistent with the northern results in Fig. 2.
OG1RF(P176) (contains 176 bp upstream of ace start codon but lacks the predicted Ers box) showed about half the β-galactosidase activity compared to OG1RF(P435) suggesting that the upstream region containing the previously designated Ers box is important for ace induction. These results were corroborated using deletions derived from P435 (PΔCAAA, and PΔTTGTA) and substitutions (PGTC and PGGGC) within the predicted Ers box and its surrounding region (Fig. 3). Like OG1RF(P176), the two deletion constructs, PΔCAAA and PΔTTGTA, showed reduced β-galactosidase activities as did the substitution constructs, PGTC and PGGGC (Fig. 3). Among the constructs, OG1RF(PGGGC) showed the lowest β-galactosidase activity in BHIS indicating that the region immediately downstream of the Ers box is also very important for the ace induction. Therefore, unknown transcriptional regulator(s) other than Ers could bind to the putative Ers binding site and regulate ace expression by responding to environmental signals.
Although we showed that Ers in OG1RF is not involved in the regulation of ace, we also recognize that there is a difference in the ace upstream regions of JH2-2 and OG1RF, as shown in the Supplementary Figure, with 6 mismatches out of 334 nucleotides, including one mismatch located in the putative -35 box (Supplementary Figure). The 6 different nucleotides might play a role in the regulation of this important virulence factor. Another notable difference between JH2-2 and OG1RF is that the former is phenotypically a gelatinase (GelE) non-producer. Maintenance of Ace on the cell surface depends on the expression of GelE, which in turns depends on the expression of a complete Fsr system, a system that is present in OG1RF but largely absent in JH2-2. The influence of the loss of this global regulator (Bourgogne et al., 2006) on the interaction of Ers and ace expression is unknown.
In summary, we showed that regulation of ace of E. faecalis OG1RF under stress conditions requires the putative Ers box, but not the transcriptional regulator Ers. Contrary to JH2-2, in OG1RF, the increase in ace expression in the presence of bile salts occurs independently of Ers. Future studies are needed to address these differences as well as the mechanism for the effect of stress conditions on ace expression.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Danielle A. Garsin for providing us with the plasmid pKAF7 and Dr. Caná Ross for the helpful discussions.
This work was supported by National Institutes of Health grant AI047923 to BEM from the Division of Microbiology and Infectious Diseases, NIAID to BEM.
REFERENCES
- Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10:266–278. doi: 10.1038/nrmicro2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgogne A, Hilsenbeck SG, Dunny GM, Murray BE. Comparison of OG1RF and an isogenic fsrB deletion mutant by transcriptional analysis: the Fsr system of Enterococcus faecalis is more than the activator of gelatinase and serine protease. J Bacteriol. 2006;188:2875–2884. doi: 10.1128/JB.188.8.2875-2884.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgogne A, Garsin DA, Qin X, et al. Large scale variation in Enterococcus faecalis illustrated by the genome analysis of strain OG1RF. Genome Biol. 2008;9:R110. doi: 10.1186/gb-2008-9-7-r110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KA, Ramesh A, Stearns JE, Bourgogne A, Reyes-Jara A, Winkler WC, Garsin DA. Multiple posttranscriptional regulatory mechanisms partner to control ethanolamine utilization in Enterococcus faecalis. Proc Natl Acad Sci U S A. 2009;106:4435–4440. doi: 10.1073/pnas.0812194106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AE, Gorovits EL, Syribeys PJ, Domanski PJ, Ames BR, Chang CY, Vernachio JH, Patti JM, Hutchins JT. Monoclonal antibodies recognizing the Enterococcus faecalis collagen-binding MSCRAMM Ace: conditional expression and binding analysis. Microb Pathog. 2007;43:55–66. doi: 10.1016/j.micpath.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Hammerstrom TG, Roh JH, Nikonowicz EP, Koehler TM. Bacillus anthracis virulence regulator AtxA: oligomeric state, function and CO(2)-signalling. Mol Microbiol. 2011;82:634–647. doi: 10.1111/j.1365-2958.2011.07843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristich CJ, Chandler JR, Dunny GM. Development of a host-genotype-independent counterselectable marker and a high-frequency conjugative delivery system and their use in genetic analysis of Enterococcus faecalis. Plasmid. 2007;57:131–144. doi: 10.1016/j.plasmid.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton F, Riboulet-Bisson E, Serror P, Sanguinetti M, Posteraro B, Torelli R, Hartke A, Auffray Y, Giard JC. ace, which encodes an adhesin in Enterococcus faecalis, is regulated by Ers and is involved in virulence. Infect Immun. 2009;77:2832–2839. doi: 10.1128/IAI.01218-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenhouts K, Buist G, Bolhuis A, ten Berge A, Kiel J, Mierau I, Dabrowska M, Venema G, Kok J. A general system for generating unlabelled gene replacements in bacterial chromosomes. Mol Gen Genet. 1996;253:217–224. doi: 10.1007/s004380050315. [DOI] [PubMed] [Google Scholar]
- Michaux C, Martini C, Hanin A, Auffray Y, Hartke A, Giard JC. SlyA regulator is involved in bile salts stress response of Enterococcus faecalis. FEMS Microbiol Lett. 2011;324:142–146. doi: 10.1111/j.1574-6968.2011.02390.x. [DOI] [PubMed] [Google Scholar]
- Murray BE, Singh KV, Ross RP, Heath JD, Dunny GM, Weinstock GM. Generation of restriction map of Enterococcus faecalis OG1 and investigation of growth requirements and regions encoding biosynthetic function. J Bacteriol. 1993;175:5216–5223. doi: 10.1128/jb.175.16.5216-5223.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallapareddy SR, Murray BE. Ligand-signaled upregulation of Enterococcus faecalis ace transcription, a mechanism for modulating host-E. faecalis interaction. Infect Immun. 2006;74:4982–4989. doi: 10.1128/IAI.00476-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallapareddy SR, Singh KV, Duh RW, Weinstock GM, Murray BE. Diversity of ace, a gene encoding a microbial surface component recognizing adhesive matrix molecules, from different strains of Enterococcus faecalis and evidence for production of Ace during human infections. Infect Immun. 2000;68:5210–5217. doi: 10.1128/iai.68.9.5210-5217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallapareddy SR, Qin X, Weinstock GM, Hook M, Murray BE. Enterococcus faecalis adhesin, Ace, mediates attachment to extracellular matrix proteins collagen type IV and laminin as well as collagen type I. Infect Immun. 2000;68:5218–5224. doi: 10.1128/iai.68.9.5218-5224.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallapareddy SR, Singh KV, Sillanpaa J, Zhao M, Murray BE. Relative contributions of Ebp pili and the collagen adhesin ace to host extracellular matrix protein adherence and experimental urinary tract infection by Enterococcus faecalis OG1RF. Infect Immun. 2011;79:2901–2910. doi: 10.1128/IAI.00038-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panesso D, Montealegre MC, Rincon S, Mojica MF, Rice LB, Singh KV, Murray BE, Arias CA. The hylEfm gene in pHylEfm of Enterococcus faecium is not required in pathogenesis of murine peritonitis. BMC Microbiol. 2011;11:20. doi: 10.1186/1471-2180-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti JM, Allen BL, McGavin MJ, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- Pinkston KL, Gao P, Diaz-Garcia D, Sillanpaa J, Nallapareddy SR, Murray BE, Harvey BR. The Fsr quorum-sensing system of Enterococcus faecalis modulates surface display of the collagen-binding MSCRAMM Ace through regulation of gelE. J Bacteriol. 2011;193:4317–4325. doi: 10.1128/JB.05026-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riboulet-Bisson E, Hartke A, Auffray Y, Giard JC. Ers controls glycerol metabolism in Enterococcus faecalis. Curr Microbiol. 2009;58:201–204. doi: 10.1007/s00284-008-9308-4. [DOI] [PubMed] [Google Scholar]
- Riboulet-Bisson E, Sanguinetti M, Budin-Verneuil A, Auffray Y, Hartke A, Giard JC. Characterization of the Ers regulon of Enterococcus faecalis. Infect Immun. 2008;76:3064–3074. doi: 10.1128/IAI.00161-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich RL, Kreikemeyer B, Owens RT, LaBrenz S, Narayana SV, Weinstock GM, Murray BE, Hook M. Ace is a collagen-binding MSCRAMM from Enterococcus faecalis. J Biol Chem. 1999;274:26939–26945. doi: 10.1074/jbc.274.38.26939. [DOI] [PubMed] [Google Scholar]
- Ruiz-Garbajosa P, Bonten MJ, Robinson DA, et al. Multilocus sequence typing scheme for Enterococcus faecalis reveals hospital-adapted genetic complexes in a background of high rates of recombination. J Clin Microbiol. 2006;44:2220–2228. doi: 10.1128/JCM.02596-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard BD, Gilmore MS. Differential expression of virulence-related genes in Enterococcus faecalis in response to biological cues in serum and urine. Infect Immun. 2002;70:4344–4352. doi: 10.1128/IAI.70.8.4344-4352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KV, Nallapareddy SR, Sillanpaa J, Murray BE. Importance of the collagen adhesin Ace in pathogenesis and protection against Enterococcus faecalis experimental endocarditis. PLoS Pathog. 2010;6:e1000716. doi: 10.1371/journal.ppat.1000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.