Abstract
In a previous study we examined whether frontal patients with impaired decision-making on the Iowa Gambling Task (IGT) would recover over time if retested on the IGT. However, a major limitation of repeated administration of the IGT is practice effects, where control participants show improvement with retesting (Bechara, Damasio, & Damasio, 2000). Therefore, the primary goal of this study was to design two alternative versions of the IGT to eliminate practice effects. We found that control participants did not show improvement in performance across the different versions of the task, thus reflecting success in our attempt to design alternative versions of the IGT. Compared to control participants, patients with damage to the ventromedial prefrontal cortex (vmPFC) performed worse on all three versions of the IGT, even after controlling for age, sex, and education. The development of alternative versions of the IGT provides a valuable tool for clinicians and researchers to utilize the IGT as a way to track how the decision-making abilities of patients change over time. Additionally, these results are consistent with findings from the original studies using the IGT with patients with damage to the vmPFC which showed that decision-making impairments do not recover over time.
MeSH words: Decision Making, Prefrontal Cortex, Iowa Gambling Task, Functional Recovery, Practice Effects
Introduction
Disturbances of decision-making are among the most devastating consequences of focal brain damage. More so than impairments of language or movement, impaired decision-making leads to a loss of independence and delays recovery (Damasio, 1994). Damage to the ventromedial prefrontal cortex (vmPFC), specifically, is associated with profound impairments in decision-making, yet there is a paucity of research investigating whether spontaneous recovery of decision-making function occurs. For example, we do not know whether other brain regions provide a plastic compensation, nor do we know the timing of recovery, if it does occur. Recently we began to examine this important issue using the Iowa Gambling Task (IGT). In order to test the stability of decision-making ability over time, patients with bilateral vmPFC damage were administered the IGT at three different time points. In this study, we found that impaired IGT performance after vmPFC damage does not appear to recover over time (Waters-Wood, Xiao, Denburg, Hernandez, & Bechara, 2012).
One of the limitations with repeated use of the IGT is that healthy participants show improvement in performance secondary to practice effects (Bechara, Damasio, & Damasio, 2000; Waters-Wood et al., 2012). This improvement is typical of frontal lobe measures in general (e.g., the Wisconsin Card Sorting Test), in that once the subject gradually discovers the rules of the task, the task becomes easier and performance improves over time (Bartels, 2010; Krenk, Rasmussen, Siersma, & Kehlet, 2012). While our previous study showed that vmPFC patients do not show improvement over time on the IGT despite this susceptibility to practice effects (Waters-Wood et al., 2012), the fact remains that this improved performance as a result of practice poses a concern, at least in other clinical patients with impairments similar to those of individuals with bilateral damage to the vmPFC. Such patients might show improvement after repeated IGT administration, however this improvement may not necessarily be due to recovery of decision-making function. Indeed, Buelow and Suhr (2009) pointed out that practice effects pose a particular concern for assessing interventions, since patients need to be evaluated before and after clinical interventions. If patient performance always improves with retesting, interventions may appear to be artificially effective (Buelow & Suhr, 2009). Hence, there is the need for developing alternative versions of the IGT to circumvent this problem.
Therefore, one primary objective of this study was to design alternative versions of the IGT that could offset the issue of improved IGT performance secondary to practice effects. We designed two alternative versions of the IGT: K-IGT, an alternative version of the IGT with decks K′L′M′N′, and Q-IGT, an alternative version of the IGT with decks Q′R′S′T′. The alternative versions are identical to the original IGT (A-IGT) in terms of immediate rewards versus probabilistic losses in the long term. However, we manipulated the fraction of the inconsistent rewards such that the A-IGT is the least difficult, the K-IGT is moderately difficult, and the Q-IGT is the most difficult. By increasing the level of difficulty of each subsequent version of the IGT, we aimed to offset the improvement in performance secondary to practice effects. We studied a group of patients with bilateral damage to the vmPFC and a group of healthy participants and tested them on all three versions of the IGT (i.e., A-IGT, K-IGT, and Q-IGT), administered in a serial fashion. With this manipulation, it was hypothesized that the performance of participants would not improve with repeated testing using the alternative versions of the IGT. Additionally, in order to confirm again that impaired IGT performance after vmPFC damage does not recover over time, another objective of this study was to use these alternative versions of the IGT to test the hypothesis that bilateral damage to the vmPFC is associated with poor recovery of complex decision-making function over time.
Methods
Participants
Sixty-four non-neurological participants were relatives or friends who accompanied the patients to the hospital. The selection criteria for non-neurological, hereafter referred to as “control” participants, was the absence of a history of mental retardation, learning disability, neurological disorder, psychiatric disorder, substance abuse, or any systemic disease capable of affecting the central nervous system. Individuals with self-reported history of cerebral lesions due to neurological disorders (e.g., tumors, stroke, traumatic brain injury, epilepsy, neurodegenerative disease) were excluded. The criteria were also to exclude individuals with a history of head trauma (open head injuries or closed head trauma with loss of consciousness), as well as those who are currently on psychotropic medications or other medications that affect the central nervous system (e.g., Prozac or antihistamines) that should not be discontinued.
Thirteen patients with focal bilateral damage to the vmPFC were tested. Participants with damage to the vmPFC were recruited from the Cognitive Neuroscience Patient Registry at the University of Iowa from a larger sample. Of the participants with vmPFC damage, brain lesions were due to: frontal meningioma (n = 3), anterior communicating artery (ACoA) aneurysm and clip (n = 8), a cyst (n = 1), and a surgically removed pituitary tumor that invaded the orbital frontal cortex region (n = 1). All vmPFC patients had undergone basic neuropsychological and neuroanatomical characterization and conformed to the inclusion criteria of the Patient Registry. The selection of vmPFC lesion patients conformed to the above criteria for controls (except the neurological disease) with the following additional criteria: (1) a stable and chronic lesion (onset was at least 3 months before the experiment) acquired in adulthood, and (2) bilateral involvement of the ventromedial prefrontal cortices. Participants had focal, stable lesions that could be clearly identified on brain scans, and they had no premorbid histories of abnormal social conduct, emotional maladjustment, or other psychological disturbance. All participants gave written informed consent approved by the Institutional Review Board at the University of Iowa.
Behavioral Tasks
A-IGT: The original IGT with decks A′B′C′D′
The general premise of the IGT is that participants must choose between decks of cards that yield high immediate rewards but larger probabilistic losses or decks that yield smaller immediate rewards but smaller probabilistic losses (Bechara, 2007). The two decks with higher rewards and higher losses have a net value that is negative. Participants who favor these “disadvantageous” decks will lose money over the course of the game. Thus, the higher immediate rewards make these decks tempting, but they are ultimately poor choices. Conversely, the two decks that have smaller rewards and losses have an overall positive net value. The decks are presented on a computer screen and labelled A′, B′, C′, and D′. Every time the participant picks a card from a deck, a message is displayed on the screen indicating the amount of money the participant has won or lost. Specifically, after selecting a deck with a reward, the following message is displayed: “Win $ X!”. When the gain is followed by a loss/punishment, the following message is displayed: “Win $ X! but lose $ Y”. Different audio feedback is also given for gains and losses. A green bar at the top of the screen displays the cumulative monetary reward. Once the money is added or subtracted from the cumulative reward, the face of the card disappears, and the participant can select another card. The sequence of gains and losses and the amount of each gain or loss encountered in each of the four decks are detailed in Table 1.
Table 1.
A-IGT reward schedule
| 1st Block (10 cards) Selections | Increase with each block of 10 Selections | 5th Block Selections | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average Immediate Reward | Number of Unpredictable Losses | Total Loss | Net Value | Average Immediate Reward | Number of Unpredictable Losses | Total Loss | Net Value | Average Immediate Reward | Number of Unpredictable Losses | Total Loss | Net Value | |
| Deck A | $100 | 5 | $1,250 | −$250 | $10 | 1 | $250 | −$150 | $150 | 10 | $2,500 | −$1,000 |
|
| ||||||||||||
| Deck B | $100 | 1 | $1,250 | −$250 | $10 | 0 | $250 | −$150 | $150 | 0 | $2,500 | −$1,000 |
|
| ||||||||||||
| Deck C | $50 | 5 | $250 | $250 | $5 | 1 | $25 | $25 | $75 | 10 | $375 | $375 |
|
| ||||||||||||
| Deck D | $50 | 1 | $250 | $250 | $5 | 0 | $25 | $25 | $75 | 0 | $375 | $375 |
In the A-IGT, Decks A′ and B′ are disadvantageous because they yield high immediate gains but greater losses in the long run (e.g., a net loss of $250 for the first 10 cards), and Decks C′ and D′ are advantageous in that they yield lower immediate gains but smaller losses in the long run (e.g., a net gain of $250 for the first 10 cards). After the A-IGT is completed, a net score is obtained by subtracting the total number of selections from the disadvantageous decks (A′ + B′) from the total number selections from the advantageous decks (C′ + D′).
It is important to note that the “original” or A-IGT discussed here matches the current computerized version of the task (Bechara, 2007). This version of the task has been used since the task became computerized (Bechara, Tranel, & Damasio, 2000). The details of these schedules are published in the professional manual of the IGT (Bechara, 2010).
IGT performance has proven resistant to changes, such as switching from a manually administered to a computer-administered version (Bechara et al., 2000). In fact, in a battery of revealing studies, the IGT has been robust enough to detect deficits when other parameters of the task have been altered. These changes include using real money as opposed to facsimile money (Bowman & Turnbull, 2003), using different time delays between trials on the task (Bowman, Evans, & Turnbull, 2005), and having subjects perform executive functioning tasks simultaneously (Turnbull, Evans, Bunce, Carzolio, & O’Connor, 2005).
K-IGT: An alternative version of the IGT with decks K′L′M′N′
The principles of the alternate IGT versions are identical to the original task (A-IGT) except for one key change. In the original version (A-IGT), the advantageous decks (C′ and D′) yield smaller immediate rewards than the disadvantageous decks (A′ and B′) 100% of the time. This percentage is reduced to 70% in the alternate version (K-IGT), so that 30% of the time, the advantageous decks yield rewards that resemble the average reward of the disadvantageous decks, and 30% of the time the disadvantageous decks yield rewards that resemble the average of the advantageous decks. This change circumvents the problem of practice effects in retest situations, when participants have experience with the original IGT and discover the rules of the task. Upon learning the original IGT rules, a simple heuristic to succeed would be to avoid decks with higher initial gains. Therefore, in this IGT manipulation, 3 out of 10 cards from each deck would yield a gain that would contradict this simple heuristic. The net score of K-IGT is obtained by subtracting the total number of selections from the disadvantageous decks (L′+N′) from the total number selections from the advantageous decks (K′+M′).
Q-IGT: An alternative version of the IGT with decks Q′R′S′T′
The alternate version Q-IGT is identical to the K-IGT except that the fraction of the inconsistent rewards is 40%. In the K-IGT version, 30% of the time the advantageous decks (K′ and M′) yield rewards that resemble the average reward of the disadvantageous decks, and the disadvantageous decks (L′ and N′) yield rewards that resemble the average of the advantageous decks. However, in the Q-IGT version, the advantageous decks (Q′ and T′) yield rewards that resemble the average reward of the disadvantageous decks, and the disadvantageous decks (R′ and S′) yield rewards that resemble the average of the advantageous decks 40% of the time. Therefore, the alternate version Q-IGT is more difficult than the alternate version K-IGT.
Procedures
For each IGT administration, participants were instructed to select one card at a time from any of the four decks visible on the screen. They were not told how much money could be won or lost, when the game would end, or the reward schedule of the decks. Furthermore, participants were asked to treat the play money in the game as if it were real money. The mean interval between Time 1 (A-IGT) and Time 2 (K-IGT) was 13.57± 3.7 months. The mean interval between Time 2 (K-IGT) and Time 3 (Q-IGT) was 49.83± 5.1months.
Order of administration
Because the alternative K-IGT and Q-IGT versions were specifically developed to mitigate the learning and practice effects of the original IGT version (A-IGT), the original version was always administrated first. Moreover, since the Q-IGT version was the most difficult among the three versions, it was always administrated last. Therefore, the order of administration was always A-IGT first, followed by K-IGT, and finishing with Q-IGT.
Data Analysis
Data were analyzed with the Statistical Package for the Social Sciences for Windows, Version 17.0 (SPSS Inc., IBM, Chicago, IL). Demographic variables were compared between control participants and vmPFC patients using independent samples t-tests and Chi-square tests. There was a significant difference in age and education between the control group and the patient group, therefore analyses were first performed separately for each group. That is, correlations among age, education, sex, and performance on the three IGT tasks were performed separately either among control participants or among vmPFC patients. In order to plot the learning curve of the IGT performance for each version, we subdivided the 100 card selections into five blocks of 20 cards each. Repeated measures ANOVAs were performed (3 sessions × 5 blocks) separately, either among the control group or among the vmPFC patient group. Next, the IGT total net scores were compared to the subjective score of 0 using one-sample t-tests. We used the conservative score of zero because it also represents the random choice of the task. Finally, to analyze the profile of the IGT performance between control participants and patients, we conducted repeated measure ANCOVA tests (3 sessions × 5 blocks × 2 groups) with age, sex, and education, as covariables.
Results
Demographic variables
Thirteen patients with vmPFC damage and 64 control subjects participated in this study. Table 2 shows the demographics for each group. There were more female participants in the control group than in the vmPFC patient group (χ2 (1)= 4.29, p < 0.05). The vmPFC patients were significantly older than control participants (t(75) = 5.90, p < 0.001). The control participants had more years of education than the vmPFC patients (t(75) = 4.20, p < 0.001).
Table 2.
Demographic data of subjects participating in the A-IGT, K-IGT and Q-IGT
| Patients | Controls | ||
|---|---|---|---|
| N | 13 | 64 | |
| Gender (male, female) | (7, 6) | (16, 48) | χ2 (1) =4.29, p <0.05 |
| Age (years; mean±SD) | 55.6±17.2 | 34.0±10.5 | t (75) = 5.90, p < 0.001 |
| Education (years; mean±SD) | 12.3±2.6 | 15.6±2.5 | t(75) = 4.20, p < 0.001 |
Correlations among demographic variables and IGT performance in three versions of the IGT in control participants
The upper panel in Table 3 reports correlations among demographic variables and IGT performance in three versions of the IGT in control participants. The results show that males had more years of education than females (p < 0.05). As expected, a significant correlation was found between A-IGT and K-IGT scores (r = 0.62, p <0.001), between K-IGT and Q-IGT scores (r = 0.74, p < 0.001), and between A-IGT and Q-IGT scores (r = 0.64, p < 0.001). Moreover, none of the demographic variables (age, sex, and education) were correlated with IGT performance for any of the three versions in control participants (all r < 0.1).
Table 3.
Correlations among demographic variables and IGT performance in three versions of the IGT in control participants (N=64) and VMPFC patients (N=13)
| Measures for control participants | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|
| 1. Age | −0.04 | −0.14 | −0.05 | −0.06 | −0.05 |
| 2. Gender a | 3 | −0.37* | −0.05 | 0.01 | −0.07 |
| 3. Education | 3 | −0.01 | 0.09 | 0.05 | |
| 4. A-IGT | 3 | 0.62*** | 0.74*** | ||
| 5. K-IGT | 3 | 0.64*** | |||
| 6. Q-IGT | |||||
| Measures for VMPFC patients | |||||
| 1. Age | −0.10 | −0.13 | −0.14 | −0.11 | 0.02 |
| 2. Gender a | 3 | −0.19 | −0.02 | 0.14 | −0.13 |
| 3. Education | 3 | 0.17 | 0.10 | 0.15 | |
| 4. A-IGT | 3 | 0.66*** | 0.63*** | ||
| 5. K-IGT | 3 | 0.88*** | |||
| 6. Q-IGT | |||||
Note:
Male was coded as 0, and female was coded as 1. Results of two-tailed significance tests are denoted by superscripts.
p<0.001,
p<0.05,
p<0.001, IGT=Iowa Gambling Task.
Behavioral performance in three versions of the IGT in control participants
Figure 1 shows performance on the three IGT versions in control participants. A net score above zero implied that the participants were selecting cards advantageously, and a net score below zero implied disadvantageous selection. In Figure 1A, we subdivided the 100 card selections into five blocks of 20 cards each for the original version of the IGT. For each block, we counted the number of selections from disadvantageous cards and the number of selections from advantageous cards, and then derived a net score for that block. Figure 1A presents the scores on the three versions of the IGT across five blocks in control participants. The performance of the IGT in the control participants was similar in each session. In every version of IGT, control participants gradually switched their preferences toward the advantageous decks and away from the disadvantageous decks in each testing session, as reflected by increasingly positive scores across blocks. A repeated measures ANOVA (3 sessions × 5 blocks) revealed a significant effect of block (Greenhouse-Geisser adjusted F(3.3, 226.8) = 26.14, p < 0.001) and a significant interaction between block and session (Greenhouse-Geisser adjusted F(6.3, 428.5) = 7.83, p < 0.001). However, there was no statistically significant effect of session (Greenhouse-Geisser adjusted F(1.6, 105) = 0.95, p = 0.39). The significant interaction between block and session was a result of the fluctuation among blocks in different sessions in controls. For example, controls scored high above 0 in Block 5 of A-IGT and K-IGT. However, they scored below zero in Block 1 of K-IGT and Q-IGT. Indeed, when compared to a score of zero (random choice), the scores from the first block of the three versions of the IGT were significantly lower than a score of zero, and the scores from all other blocks (except the fourth block of the Q-IGT) were significantly higher than a score of zero (p < 0.05).
Figure 1.
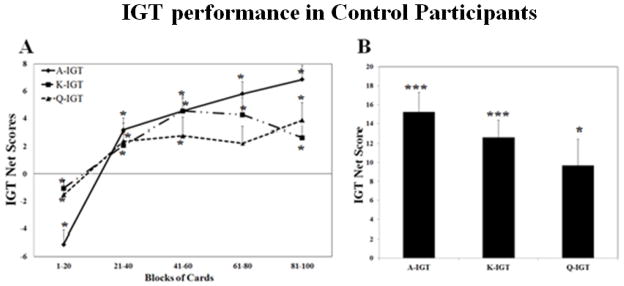
The performance on three versions of the IGT tasks in control participants. (A). The IGT net scores across five blocks of 20 cards expressed as mean+S.E. (B). The IGT total net scores expressed as mean+S.E.. Positive net scores reflect advantageous (non-impaired performance) while negative net scores reflect disadvantageous (impaired) performance. *p<0.05, ***p<0.001 comparing to a score of 0 (random choice). IGT=Iowa Gambling Task.
Figure 1B shows the total IGT net scores across the three versions of the IGT in control participants. Results from one-sample t-tests show that the total net scores on the three versions of the IGT in control participants were all significantly greater than a score of zero (p < 0.05).
These results suggest that the control participants showed a normal learning curve, performing better on the task within each session. Furthermore, there were no practice effects found across sessions when using the alternative versions of the IGT.
Correlations among demographic variables and IGT performance in three versions of the IGT in vmPFC patients
The lower panel in Table 3 reports correlations among demographic variables and IGT performance in the three versions of the IGT in vmPFC patients. The results showed that A-IGT correlated significantly with K-IGT (r = 0.66, p < 0.001) and Q-IGT scores (r = 0.63, p < 0.001). K-IGT also significantly correlated with Q-IGT scores (r= 0.88, p < 0.001). Moreover, none of the demographic variables (age, sex, and education) were correlated with IGT performance in any of the three versions in vmPFC patients (all r < 0.15).
Behavioral performance in three versions of the IGT in the vmPFC patients
Figure 2A presents the net scores as a function of block across the three versions in vmPFC patients. The performance of the IGT in the vmPFC patients was similar in each session. In every version of the IGT, vmPFC patients could not learn from the task, and they mainly chose from the disadvantageous decks for every block. A repeated measures ANOVA (3 sessions × 5 blocks) did not reveal a significant effect by block (Greenhouse-Geisser adjusted F(3.03, 20.79) = 1.24, p = 0.30) or an interaction between block and session (Greenhouse-Geisser adjusted F(1.73, 20.79) = 0.72, p = 0.68). Moreover, there was no statistically significant effect by session (Greenhouse-Geisser adjusted F(1.22, 20.79) = 1.34, p = 0.28). When compared to a score of zero (random choice), the scores from the first block of the three versions of the IGT were significantly lower than a score of zero, and the scores from several other blocks of three versions of the IGT were significantly higher than a score of zero (p < 0.05).
Figure 2.
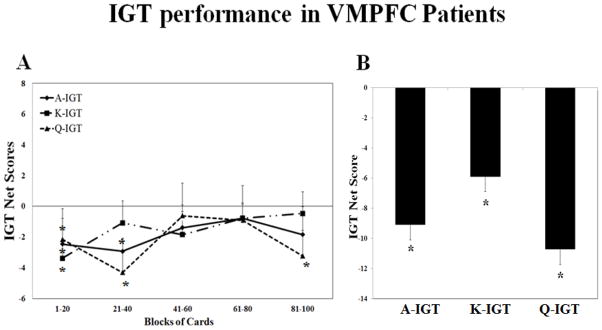
The performance on three versions of the IGT tasks in VMPFC patients. (A). The IGT net scores across five blocks of 20 cards expressed as mean+S.E. (B). The IGT total net scores expressed as mean+S.E.. Positive net scores reflect advantageous (non-impaired performance) while negative net scores reflect disadvantageous (impaired) performance. *p<0.05 comparing to the score of 0 (random choice). IGT=Iowa Gambling Task.
Figure 2B shows the total IGT net scores in three versions of the IGT in vmPFC patients. Results from one-sample t-tests show that the total net scores from the three versions of the IGT in vmPFC patients were significantly less than a score of zero (p < 0.05).
These results suggest that vmPFC patients did not show a normal learning curve in any of three versions of the IGT, and there was no significant improvement of performance across the three sessions. Moreover, vmPFC patients performed worse on all three versions of the IGT, as reflected by IGT net scores that were significantly lower than a score of zero (random choice).
Impaired IGT performance in patients relative to control participants
The correlation analysis in either control participants or vmPFC patients above shows that there was no statistically significant correlation between age, sex, or education and scores on the three versions of the IGT (all r < 0.15). This suggests that the slightly different demographic variables among the groups are not confounding factors to making a direct comparison of the IGT performance between control participants and vmPFC patients. To confirm this notion, repeated measure ANCOVA tests (3 sessions × 5 blocks × 2 groups) were performed with age, education, and sex as covariables. The results demonstrate that there were no significant effects of within-subjects test sessions, sessions × age, sessions × education, sessions × sex or sessions × group (all p > 0.05). There were no between-subjects effects of age, sex, or education (all p > 0.05). As predicted, the only significant effect was the between-subjects group effect (F(1, 72) = 5.7, p < 0.05).
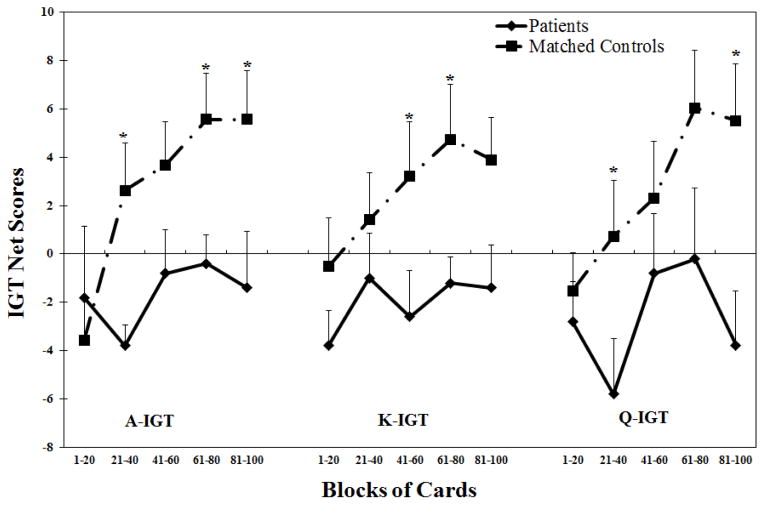
Moreover, we conducted additional analyses comparing 10 vmPFC patients and 19 controls that were matched by age, sex, and education. We excluded three patients due to their low levels of education (between 7 and 8 years), as we could not find any matched controls for these patients. We found that the matched controls still performed significantly better than patients on all three versions of the IGT (See Appendix Table 1 & Figure 1).
In conclusion, these findings suggest that relative to controls, vmPFC patients performed worse on all three versions of the IGT as reflected by significantly lower IGT net scores, even after adjusting for age, sex, and education level.
Discussion
The results of this study confirm our hypothesis that patients with bilateral lesions to the vmPFC do not show recovery of decision-making capabilities over time and are persistent in making disadvantageous decisions as measured by the original and alternative versions of the IGT. Patients with bilateral vmPFC lesions persisted in demonstrating significant impairment in performance relative to controls across all versions of the task. These results are consistent with an earlier study using repeated tests with only one version of the IGT (the original version) (Waters-Wood et al., 2012), and they also confirm clinical observations that the decision-making impairment of patients with vmPFC damage does not recover over time, even when the damage is acquired very early on in life (e.g., Anderson, Bechara, Damasio, Tranel, & Damasio, 1999). Although recovery of function is often observed in several other domains, these findings support the notion that brain plasticity cannot compensate for the decision-making impairment associated with vmPFC damage.
The development of alternative versions of the IGT with increasing difficulty was successful in accomplishing the goal of eliminating the typical improvement seen in scores for healthy subjects when retested on the same original version. Our results demonstrate that control participants showed the expected learning curve for all versions of the IGT. That is, they incrementally selected more choices from the advantageous decks within each IGT version. Furthermore, there was no improvement in scores across the three sessions among control participants. By contrast, the vmPFC patients did not learn to choose advantageously for any of the three versions of the IGT, and they did not show any improvement in performance across the three sessions.
Given the strong correlations among the different versions of the IGT, it is reasonable to assume that these alternative versions of the IGT carry the same fidelity in detecting decision-making impairments as the original version. This is also corroborated by the fact that control participants chose significantly more often from the advantageous decks, as opposed to making random choices (i.e., compared to score of 0) in each of the three sessions, whereas vmPFC patients selected significantly more from disadvantageous decks as compared to random choice (a score of zero) in each session. The results also demonstrated that vmPFC patients performed significantly worse than control participants after controlling for the demographic variables of age, sex, and education.
Besides the implication of the current results for understanding recovery of decision-making function after vmPFC damage, the development of alternative, parallel versions of the IGT should offer potential tools for researchers and clinicians who need to retest participants following behavioral or pharmacological interventions (see Buelow & Suhr, 2009). Developing such tools has significant implications, given the fact that disturbances of decision-making are among the most devastating consequences of focal brain damage. Although recent years have witnessed a significant growth in test instruments designed to measure decision-making capacity, the IGT can be set apart from other decision-making tasks based on the clinical characteristics of the IGT. It is important to highlight these clinical characteristics that differentiate it from other tests of decision-making.
Some criticisms of the IGT were made on the basis that the task might not be specific to the vmPFC (Manes, Sahakian, Clark, Rogers, Antoun et al., 2002). This issue was addressed extensively in subsequent studies which revealed that poor IGT scores should not automatically imply a specific vmPFC dysfunction (Bechara, 2004b; Bechara & Damasio, 2005; Bechara et al., 2000). Individuals who have memory problems and perform poorly on other executive function tests, such as the Wisconsin Card Sorting Test (Berg, 1948), are very likely to perform poorly on the IGT (Torralva, Kipps, Hodges, Clark, Bekinschtein et al., 2009). However, inferring a vmPFC diagnosis is reasonable when the patient performs normally on most or all memory/executive function tests but performs poorly on the IGT. Poor IGT scores combined with relatively normal memory and executive function test scores imply poor decision-making linked to vmPFC damage or dysfunction. In contrast, poor IGT scores combined with poor memory, especially working memory, and other executive function scores imply poor decision-making linked to damage or dysfunction of the dorsolateral prefrontal cortex, the insular cortex, the parietal cortex, or the anterior and medial temporal lobe, including the amygdala and the hippocampus (Bechara, 2004b; Bechara & Damasio, 2005; Bechara et al., 2000). The specificity of the IGT to vmPFC dysfunction increases when impairments on other cognitive tests are ruled out.
Previous studies have demonstrated that the relationship between decision-making on one hand and working memory (including the executive process of working memory such as response inhibition or reversal learning) on the other hand are asymmetrical in nature (Bechara, 2004a; Bechara, Damasio, Tranel, & Damasio, 2005). That is, working memory and/or reversal learning can be normal, even when deficits in decision-making are present. Lesions or impaired functioning of the dorsolateral prefrontal cortex, an area critical to working memory function, leads to impaired performance on the IGT (Manes et al., 2002; Suhr & Hammers, 2010). However, when working memory and/or reversal learning deficits are present, decision-making capacity becomes compromised. In support of this notion, many patients with vmPFC lesions who are severely impaired in decision-making, as measured by the IGT, were shown to have normal working memory, as measured by delayed non-matching to sample tasks (Bechara, Damasio, Tranel, & Anderson, 1998). Furthermore, these patients also show normal performance on simple (as opposed to probabilistic) reversal learning tasks (Clark, Cools, & Robbins, 2004). By contrast, decision-making is abnormal in brain lesion patients with any other brain damage associated with impairments in working memory (Bechara et al., 1998; Clark, Manes, Antoun, Sahakian, Robbins et al., 2003; Manes et al., 2002), or impairments in reversal learning (Fellows & Farah, 2003).
Implications
The implications of the current study go well beyond the recovery of decision-making function in neurological patients given that the IGT is currently used to detect impairment in decision-making in numerous other neuropsychiatric conditions. For example, impaired performance on the IGT has been associated with a number of clinical diagnoses, including addiction (Bechara & Martin, 2004), pathological gambling (Cavedini, Riboldi, Keller, D’Annucci, & Bellodi, 2002), obsessive-compulsive disorder (Whitney, Fastenau, Evans, & Lysaker, 2004), schizophrenia (Sevy, Burdick, Visweswaraiah, Abdelmessih, Lukin et al., 2007), attention-deficit/hyperactivity disorder (ADHD) (Malloy-Diniz, Fuentes, Borges-Leite, Correa, & Bechara, 2007), frontotemporal dementia (Torralva, Kipps, Hodges, Clark, Bekinschtein et al., 2007), impulsive aggressive disorders (Best, Williams, & Coccaro, 2002), and neurodegenerative diseases (Kobayakawa, Koyama, Mimura, & Kawamura, 2008; Mimura, Oeda, & Kawamura, 2006; Sinz, Zamarian, Benke, Wenning, & Delazer, 2008; Stout, Rodawalt & Siemers, 2001). Interestingly, recent research has used the IGT to detect possible decision-making abnormalities among individuals who otherwise have not been diagnosed with any neurological or neuropsychiatric condition, such as obese individuals (Davis, Levitan, Muglia, Bewell, & Kennedy, 2004), older adults (Denburg, Tranel, & Bechara, 2005), and “fun-seeking” personalities (Suhr & Tsanadis, 2007), among others. Moreover, the IGT has also been used in studies of normal adolescents (Hooper, Luciana, Conklin & Yarger, 2004; Overman, 2004).
Some limitations exist in this study. First, the controls were older and had higher education levels than the patients. However, several statistical tests and an additional analysis of a subgroup of control participants and patients who were matched on age and education confirmed that these demographic differences did not exert a meaningful change in the interpretation of our results. Therefore, our results are consistent with previous studies showing that age and education are not significantly correlated with IGT performance (Evans, Kemish, & Turnbull, 2004, Wood, Busemeyer, Koling, Cox, & Davis, 2005). Second, compared to previous studies that examined practice effects using the intervals of tests by weeks or one year (Bartels, 2010; Krenk et al., 2012), the intervals between tests in our study were much longer. We chose a more extended period of time due to findings from our previous work that indicates that even after a five-year interval between administrations, the control participants still demonstrated strong practice effects on the original version of the IGT (A-IGT) (Waters-Wood et al., 2012). Future studies should be done to test the practice effects with much shorter time intervals. Third, the order of the three tasks was fixed in this study, therefore, a crossover design is needed in future studies in order to address the possible order effects of the tasks. Finally, the number of patients was relatively small in our study. We calculated the effect size and the needed sample size for sufficient power across two of the variants (A versus K-IGT, A versus Q-IGT), and we found that the effect size is so small to the point that we would need a sample size of several hundreds of individuals to detect a meaningful difference (see Appendix Table 2). Therefore, while these analyses demonstrate that potentially there are some differences that could be detected significantly with a very large sample, and this would constitute another limitation of the study, the fact remains that in practice, with such a small effect size, any comparison among the different tasks in a clinical population would not yield an interpretable effect. Regardless (i.e., be it there are some minute differences or not), the primary interpretation of the current results that frontal lesion patients do not recover upon repeated testing with variants of the IGT would remain the same.
In conclusion, this study improves our understanding of the recovery, or lack thereof, from impairments in decision-making caused by bilateral damage of the vmPFC. The development of alternative IGT tasks that reliably measure decision-making impairments in vmPFC lesion patients, and which circumvent the problem of practice effects with repeated testing in healthy individuals, should provide a valuable tool for clinicians and researchers who need to use the IGT to track how patients’ performance changes over time.
Acknowledgments
The research described in this article was supported by grants from the National Institute on Drug Abuse (NIDA) R01 DA023051, National Cancer Institute (NCI) R01CA152062 and a Center Grant from the National Institute of Neurological Disorders and Strokes (NINDS) P50 NS019632. While the original version of the IGT is now the property of PAR, Inc. and can only be obtained from PAR, Inc., copies of the alternate versions of the IGT used in this study can be obtained for use in research projects only by contacting the senior author of this study Antoine Bechara.
Appendix
Table 1.
Demographic data of patients and matched controls participating in the A-IGT, K-IGT and Q-IGT
| Patients | Controls | Difference between groups | |
|---|---|---|---|
| N | 10 | 19 | |
| Gender (male, female) | (6, 4) | (7, 12) | χ2 (1) =2.23, p=0.23 |
| Age (years; mean±SD) | 58.8±12.6 | 53.7±12.7 | t (27) = 1.03, p =0.31 |
| Education (years; mean±SD) | 14.2±1.6 | 14.4±2.2 | t(27) = 0.33, p =0.74 |
Table 2.
IGT net score at three times across groups
| Time 1 A-IGT | Time 2 K-IGT | Time 3 Q-IGT | Effect Size (T1, T2) (Cohen’s d) | N* for (T1, T2) | Effect Size (T1, T3) (Cohen’s d) | N* for (T1, T3) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||||
| Controls | 14.88 | 30.98 | 12.82 | 30.30 | 9.91 | 39.15 | 0.08 | 1229 | 0.19 | 220 |
| Patients | −9.08 | 15.84 | −5.88 | 14.03 | −10.73 | 21.09 | −0.26 | 119 | 0.10 | 787 |
Note:
The sample size needed to reach 80% of the power based on the effect size.
Figure 1.
The performance on three versions of the IGT tasks in patients and matched controls. (A). The IGT net scores across five blocks of 20 cards expressed as mean+S.E. (B). The IGT total net scores expressed as mean+S.E.. Positive net scores reflect advantageous (non-impaired performance) while negative net scores reflect disadvantageous (impaired) performance. *p<0.05, comparing to patient group. IGT=Iowa Gambling Task.
Footnotes
All authors declare no conflict of interest.
References
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Bartels C, Wegrzyn M, Wiedl A, Ackermann V, Ehrenreich H. Practice effects in healthy adults: longitudinal study on frequent repetitive cognitive testing. BMC Neuroscience. 2010;11:1–12. doi: 10.1186/1471-2202-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Disturbances of emotion regulation after focal brain lesions. International Review of Neurobiology. 2004a;62:159–193. doi: 10.1016/S0074-7742(04)62006-X. [DOI] [PubMed] [Google Scholar]
- Bechara A. The role of emotion in decision-making: Evidence from neurological patients with orbitofrontal damage. Brain and Cognition. 2004b;55:30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Bechara A. Iowa gambling task professional manual. Lutz: Psychological Assessment Resources; 2010. [Google Scholar]
- Bechara A, Damasio AR. The somatic marker hypothesis: A neural theory of economic decision. Games and Economic Behavior. 2005;52:336–372. [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation of working memory from decision making within the human prefrontal cortex. Journal of Neuroscience. 1998;18:428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends in Cognitive Sciences. 2005;9:159–162. doi: 10.1016/j.tics.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18:152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Berg EA. A simple objective technique for measuring flexibility in thinking. The Journal General Psychology. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Best M, Williams JM, Coccaro EF. Evidence for a dysfunctional prefrontal circuit in patients with an impulsive aggressive disorder. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8448–8453. doi: 10.1073/pnas.112604099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Fujiwara E, Borsutzky S, Kalbe E, Kessler J, Markowitsch HJ. Decision-making deficits of Korsakoff patients in a new gambling task with explicit rules: Associations with executive functions. Neuropsychology. 2005;19:267–277. doi: 10.1037/0894-4105.19.3.267. [DOI] [PubMed] [Google Scholar]
- Bowman CH, Evans CE, Turnbull OH. Artificial time constraints on the Iowa Gambling Task: the effects on behavioural performance and subjective experience. Brain and Cognition. 2005;57:21–25. doi: 10.1016/j.bandc.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Bowman CH, Turnbull OH. Real versus facsimile reinforcers on the Iowa Gambling Task. Brain and Cognition. 2003;53:207–210. doi: 10.1016/s0278-2626(03)00111-8. [DOI] [PubMed] [Google Scholar]
- Buelow MT, Suhr JA. Construct Validity of the Iowa Gambling Task. Neuropsychology Review. 2009;19:102–114. doi: 10.1007/s11065-009-9083-4. [DOI] [PubMed] [Google Scholar]
- Cavedini P, Riboldi G, Keller R, D’Annucci A, Bellodi L. Frontal lobe dysfunction in pathological gambling patients. Biological Psychiatry. 2002;51:334–341. doi: 10.1016/s0006-3223(01)01227-6. [DOI] [PubMed] [Google Scholar]
- Clark L, Manes F, Antoun N, Sahakian BJ, Robbins TW. The contributions of lesion laterality and lesion volume to decision-making impairment following frontal lobe damage. Neuropsychologia. 2003;41:1474–1483. doi: 10.1016/s0028-3932(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Davis C, Levitan RD, Muglia P, Bewell C, Kennedy JL. Decision-making deficits and overeating: a risk model for obesity. Obesity Research. 2004;12:929–935. doi: 10.1038/oby.2004.113. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Tranel D, Bechara A. The ability to decide advantageously declines prematurely in some normal older persons. Neuropsychologia. 2005;43:1099–1106. doi: 10.1016/j.neuropsychologia.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Evans EY, Kemish K, Turnbull OH. Paradoxical effects of education on the Iowa Gambling Task. Brain and Cognition. 2004;53:240–244. doi: 10.1016/j.bandc.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Fellows L, Farah M. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Hooper CJ, Luciana M, Conklin HM, Yarger RS. Adolescents’ performance on the Iowa Gambling Task: implications for the development of decision making and ventromedial prefrontal cortex. Developmental Psychology. 2004;40:1148–1158. doi: 10.1037/0012-1649.40.6.1148. [DOI] [PubMed] [Google Scholar]
- Huettel S, Stowe C, Gordon E, Warner B, Platt M. Neural signatures of economic preferences for risk and ambiguity. Neuron. 2006;49:765–75. doi: 10.1016/j.neuron.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Knight FH. Risk, uncertainty and profit. Boston and New York: Boston, MA: Hart, Schaffner & Marx; Houghton Mifflin Co; 1921. [Google Scholar]
- Kobayakawa M, Koyama S, Mimura M, Kawamura M. Decision making in Parkinson’s disease: analysis of behavioral and physiological patterns in the Iowa gambling task. Movement Disorder. 2008;23:547–52. doi: 10.1002/mds.21865. [DOI] [PubMed] [Google Scholar]
- Krenk L, Rasmussen LS, Siersma VD, Kehlet H. Short-term practice effects and variability in cognitive testing in a healthy elderly population. Experimental Gerontology. 2012 doi: 10.1016/j.exger.2012.03.011. (in press) [DOI] [PubMed] [Google Scholar]
- Malloy-Diniz L, Fuentes D, Leite WB, Correa H, Bechara A. Impulsive behavior in adults with attention deficit/hyperactivity disorder: Characterization of attentional, motor and cognitive impulsiveness. Journal of the International Neuropsychological Society. 2007;13:693–698. doi: 10.1017/S1355617707070889. [DOI] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, et al. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Mimura M, Oeda R, Kawamura M. Impaired decision-making in Parkinson’s disease. Parkinsonism & related disorders. 2006;12:169–175. doi: 10.1016/j.parkreldis.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Overman WH, Frassrand K, Ansel S, Trawalter S, Bies B, Redmond A. Performance on the IOWA card task by adolescents and adults. Neuropsychologia. 2004;42:1838–1851. doi: 10.1016/j.neuropsychologia.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, et al. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. Journal of Neuroscience. 1999;19:9029–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevy S, Burdick KE, Visweswaraiah H, Abdelmessih S, Lukin M, Yechiam E, et al. Iowa Gambling Task in schizophrenia: A review and new data in patients with schizophrenia and co-occurring cannabis use disorders. Schizophrenia Research. 2007;92:74–84. doi: 10.1016/j.schres.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinz H, Zamarian L, Benke T, Wenning GK, Delazer M. Impact of ambiguity and risk on decision making in mild Alzheimer’s disease. Neuropsychologia. 2008;46:2043–2055. doi: 10.1016/j.neuropsychologia.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Stout JC, Rodawalt WC, Siemers ER. Risky decision making in Huntington’s disease. Journal of International Neuropsychological Society. 2001;7:92–101. doi: 10.1017/s1355617701711095. [DOI] [PubMed] [Google Scholar]
- Suhr J, Hammers D. Who fails the Iowa Gambling Test (IGT)? Personality, neuropsychological, and near-infrared spectroscopy findings in healthy young controls. Archives of Clinical Neuropsychology. 2010;25:293–302. doi: 10.1093/arclin/acq017. [DOI] [PubMed] [Google Scholar]
- Torralva T, Kipps CM, Hodges JR, Clark L, Bekinschtein T, Roca M, et al. The relationship between affective decision-making and theory of mind in the frontal variant of fronto-temporal dementia. Neuropsychologia. 2007;45:342–349. doi: 10.1016/j.neuropsychologia.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Torralva T, Roca M, Gleichgerrcht E, Bekinschtein T, Manes F. A neuropsychological battery to detect specific executive and social cognitive impairments in early frontotemporal dementia. Brain. 2009;132:1299–1309. doi: 10.1093/brain/awp041. [DOI] [PubMed] [Google Scholar]
- Turnbull OH, Evans CEY, Bunce A, Carzolio B, O’Connor J. Emotion-based learning and central executive resources: An investigation of intuition and the Iowa Gambling Task. Brain and Cognition. 2005;57:244–247. doi: 10.1016/j.bandc.2004.08.053. [DOI] [PubMed] [Google Scholar]
- Waters-Wood SM, Xiao L, Denburg NL, Hernandez M, Bechara A. Failure to learn from repeated mistakes: Persistent decision-making impairment as measured by the Iowa Gambling Task in patients with ventromedial prefrontal cortex lesions. Journal of the International Neuropsychological Society. 2012;18:927–930. doi: 10.1017/S135561771200063X. [DOI] [PubMed] [Google Scholar]
- Weller JA, Levin IP, Shiv B, Bechara A. Neural correlates of adaptive decision making for risky gains and losses. Psychological Science. 2007;18:958–964. doi: 10.1111/j.1467-9280.2007.02009.x. [DOI] [PubMed] [Google Scholar]
- Whitney KA, Fastenau PS, Evans JD, Lysaker PH. Comparative neuropsychological function in obsessive-compulsive disorder and schizophrenia with and without obsessive-compulsive symptoms. Schizophrenia Research. 2004;69:75–83. doi: 10.1016/j.schres.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Wood S, Busemeyer J, Koling A, Cox CR, Davis H. Older adults as adaptive decision makers: evidence from the Iowa gambling task. Psychology and Aging. 2005;20:220–225. doi: 10.1037/0882-7974.20.2.220. [DOI] [PubMed] [Google Scholar]