Abstract
Several studies indicate abnormalities in the hypothalamic-pituitary-adrenal axis (HPA) during acute opiate withdrawal, but protracted withdrawal has gotten less study. The current study further characterized the 24-hour time course of plasma cortisol levels in heroin-dependent individuals who were abstinent for 10–15 days, which is beyond the 5 days of acute withdrawal, compared to demographically matched healthy controls using samples collected every 3 hours over 24 hours and assessed with radioimmunoassay (RIA). The abstinent heroin-dependent participants had significantly higher plasma cortisol levels nocturnally suggesting a loss of diurnal variation in these heroin subjects.
Keywords: Circadian rhythm, cortisol, detoxification, heroin abstainers
INTRODUCTION
Abnormalities of the hypothalamic-pituitary-adrenal axis (HPA) responses to physical and psychological stress have long been associated with altered cortisol levels. Activation of the HPA axis involves a cascade of releasing corticotropin releasing hormone (CRH) from the hypothalamus, adrenocorticotropic hormone (ACTH) from the anterior pituitary, and, ultimately, the synthesis/release of cortisol from the adrenal cortex (1). Receptors for CRH, ACTH and corticosteroid are located in key brain regions beyond the hypothalamus, including the hippocampus, limbic system, and prefrontal cortex (2, 3). Indeed, the widespread distributions of these receptors confirm the broad brain effects of the HPA response to psychological stress (4).
Since stress is a component of opiate withdrawal, changes in cortisol levels have been used as stress assessments during acute withdrawal and the protracted withdrawal period that follows (5). In humans, HPA function is suppressed when heroin is acutely or chronically administered with significantly lower cortisol levels than controls (1, 6–8). In non-heroin-using individuals, hypocortisolism is observed after administration of codeine or the met-enkephalin analog FK 33-824 (9, 10). Conversely, patients undergoing acute heroin withdrawal show a marked and persistent increase in salivary cortisol (11–13). Kreek and Koob (1) have proposed that abnormal activity of the HPA during dependence and withdrawal could be among the mechanisms contributing to a “spiraling distress cycle” that leads to repeated drug use and relapse.
Diurnal variation in plasma cortisol levels have not been fully characterized following acute opiate withdrawal when protracted withdrawal may contribute to relapse. The current study assessed these changes in plasma cortisol throughout a 24-hour period in opioid-dependent patients compared to demographically similar healthy controls.
SUBJECTS AND METHODS
Subjects
Sixteen male heroin-dependent individuals between 22 to 44 years of age (mean ± SD, 33.5 ± 6.48 years) were recruited from the Center for Addiction Treatment of Guiyang, Guiyang, China. The subjects were undergoing lofexidine-assisted heroin detoxification and had remained drug-free for 10–15 days upon recruitment. The exclusion criteria were: (1) current use of cocaine or other illicit drugs (conformed by urine drug screens); (2) clinically evident cognitive impairment; (3) diastolic blood pressure less than 60 mmHg and heart rate less than 60 pulse per minute; (4) HIV positive; (5) current or past serious physical illness (e.g., active tuberculosis, acute hepatitis or cirrhosis, renal, cardiovascular illness, and unstable diabetes). Prior to study participation, each patient provided signed consent form, a urine sample, detailed drug, and medical history. Complete physical and psychiatric evaluations were conducted. The participants met Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychiatric Association) for opioid dependence. Subjects reported 65.4 (±14.5) months of drug dependence and daily heroin use of .1 to 2 g (mean ± SD; .8 ± .2 g). Ten participants reported “chasing the dragon” in administering heroin, four used intravenous injections, and two used both. No participants reported using alcohol and heroin together, and all abstainers reported smoking tobacco, which was allowed throughout the study.
Control participants were eight physically and mentally healthy volunteers, recruited from the local community, and matched for age (mean ± SD; 30.5 ± 4.66). The healthy volunteers were also monitored with urine drug screens. The following criteria were exclusionary: (1) any of the exclusion criteria for the experimental group, (2) past or present DSM-IV Axis I or Axis II disorders (including alcohol/substance abuse or dependence), (3) sleep disturbance, (4) smoking, (5) age less than 21 years, (6) use of any medication within the past 7 days, (7) any current or past physical illness that would be aggravated or reappear if the individual participated in the study (in the judgment of our team’s physician), and (8) positive breath alcohol or urine drug screen. The characteristics of all participants are summarized in Table 1.
Table 1.
Demographic characteristics of participants
| Healthy controls | Abstinent heroin patients | |
|---|---|---|
| Number of participants | 8 | 16 |
| Age (years; mean ± SD) | 30.5 ± 4.66 | 33.5 ± 6.48 |
| Gender, % male | 100 | 100 |
| Marital status, % married | 50.00 | 37.5 |
| Education (years; mean ± SD) | 9.13 ± 1.55 | 8.19 ± 2.56 |
| Number of cigarettes/day (mean ± SD) | 0 | 9.94 ± 4.95 |
| Number of drinks/day (mean ± SD) | 0 | 0 |
Potential participants who appeared to meet inclusion criteria completed the Structured Clinical Interview for DSM-IV disorders (SCID), a physical examination, electrocardiogram, and urine drug screening. All the research procedures had been approved by the ethics committee of Sichuan University, Sichuan, China, and all participants provided written informed consent before participating. Each participant was paid 300.00 RMB ($38.07) for participation upon completion, and the opioid-dependent participants remained in the rehabilitation center after the study.
Experimental Procedure
The study was conducted over two consecutive days. Participants were required to arrive at the clinic before 9:00 on the first day and remained in the clinic until the afternoon of the following day (approximately 15:00). At 9:00, an intravenous catheter was inserted into a forearm vein. From 9:00 to 17:00, participants relaxed in a recliner to adapt to the experimental environment but were not allowed to sleep. At 17:00, the first blood sample was drawn; a total of eight blood samples were collected from each participant (collection every three hours: 17:00, 20:00, 23:00, 2:00, 5:00, 8:00, 11:00, and 14:00). Lights were turned off at 23:00, and all participants were woken up at 7:00–7:30. A total of 19 ml blood was collected from each participant throughout the study. The tubing system was kept patent by continuous infusion of heparinized isotonic saline between blood samplings. Safety was monitored by the nursing stuff throughout blood collection. Participants received standardized meals and were allowed to watch television, read books, and talk to each other. Smoking was discouraged in the heroin-dependent individuals, but every 8 heroin-dependent participants were permitted to share up to 40 cigarettes during the session.
Laboratory Procedures
Blood samples were kept in nonheparinized tubes immediately after being drawn, allowed to clot at 37°C for 5 min, and then centrifuged at 2500 rpm, at 15°C. Serum was separated and stored at −70°C until assay. Serum concentration of cortisol was detected by a radioimmunoassay (RIA) kit (Furui biotechnology company, Beijing, China). The intra-and inter-assay coefficients of variation were less than 5% and 10%, respectively.
Data Analyses
Repeated-measures analysis of variance (ANOVA) compared the changes of plasma cortisol levels across 24 hours and specifically during the nocturnal (20:00, 23:00, 2:00, and 5:00) and the daytime phases (8:00, 11:00, 14:00, and 17:00) between the experimental and control group (SPSS 13.0).
RESULTS
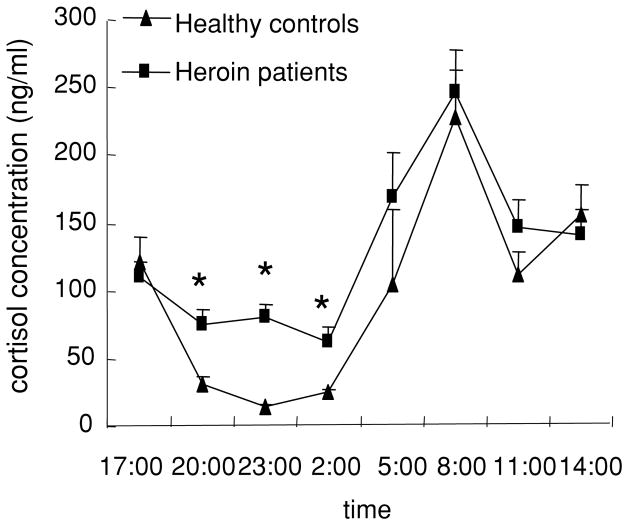
All participants completed the two-day experiment. The 24-hour time course of cortisol concentration is shown in Figure 1. All participants showed significant changes in plasma cortisol across 24 hours (time: F (7,126) = 10.32, p < .01). There was a trend of a higher level of cortisol in abstinent heroin-dependent patients than in controls (group: F(1,18) = 3.32, p = .085). Independent t-tests found higher levels of cortisol at 20:00, 23:00, and 2:00 hours in the abstinent heroin-dependent patients than in healthy controls (t-test, p < .05).
Figure 1.
A 24-hour time course of cortisol concentrations in health controls and heroin-dependent patients. Blood samples were collected every three hours for a period of 24 hours. Data are presented as mean ± SEM cortisol concentrations for each time point. *p < .05, compared with the health control group in the same time point.
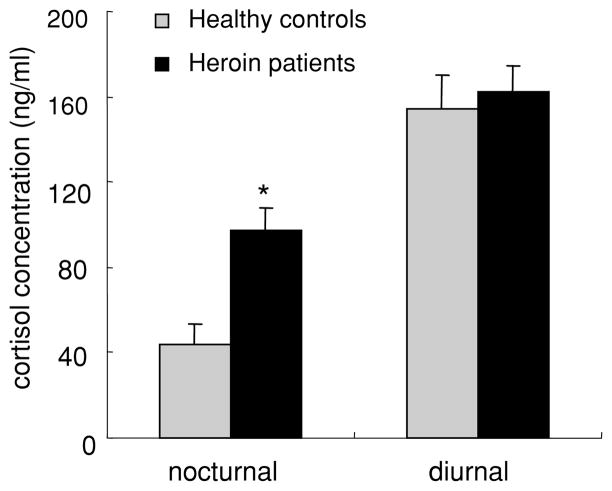
When data were collapsed into nocturnal and daytime phases (Figure 2), nocturnal cortisol levels were significantly higher in heroin-dependent patients than in controls (F(1,78) = 5.99, p = .02); in contrast, the daytime cortisol levels were not significantly different between groups (F(1,78) = .08, p = .79).
Figure 2.
Cortisol concentrations during nocturnal and diurnal phases in health controls and heroin-dependent patients. Data are presented as the means ± SEM cortisol levels during the nocturnal and diurnal phases respectively. *p < .05, compared with health control group during the same phase.
DISCUSSION
The present study was conducted to assess the 24-hour time course of cortisol secretion in heroin-dependent individuals who had undergone detoxification 10–15 days previously. As expected, both abstinent heroin-dependent patients and healthy controls showed significant changes in plasma cortisol across 24 hours, but the heroin-dependent patients showed a loss of diurnal variation compared to normals.
The loss of diurnal variation in cortisol levels is the earliest sign of hypercortisolism and is consistent with previous findings in heroin-dependent patients (12–15). Prior studies that collected blood samples at only one time point would not be sensitive to this loss of diurnal variation (12, 13).
The relationships among withdrawal syndromes, negative affect, and HPA function remain potential avenues for relapse (16–18). Opioid-dependent patients with higher salivary cortisol levels also have reported more severe protracted withdrawal symptoms during 25-days of abstinence after acute withdrawal (13). Higher cortisol levels and more depressive episodes have also been found in cocaine and heroin users, compared to nonusers (12). Thus, the elevated cortisol levels probably are reactive aspects of the neuron-hormonal system in attempting to correct broader brain abnormalities associated with protracted withdrawal (5).
The current study’s limitations included first that protracted withdrawal symptoms were not systematically assessed in order to relate them to cortisol levels. However, the acute withdrawal was completed at least a week before these cortisol levels were obtained, and symptom levels were minimal with disturbed sleep being the most prominent. Second, the healthy volunteers were non-smokers, whereas all heroin-dependent patients were smokers and continued doing so throughout the study. Elevated cortisol levels occur in regular smokers (19–23), but loss of diurnal variation is not described with smoking, and our heroin patients did not smoke during the night, when levels were abnormally high. Thus, the elevated cortisol levels were not related to acute nicotine effects. Third, because withdrawal treatment used only Lofexidine and not methadone or buprenorphine, our findings may not apply to patients who are treated with these opiates for detoxification.
In summary, the present study supports a loss of diurnal variation in plasma cortisol among heroin addicts during the early period of protracted withdrawal, and interventions to reverse this abnormality might be examined to reduce stress-related relapse (24, 25).
Acknowledgments
This work was supported in part by the grants from the program for New Century Excellent Talents in University, the National High Technology Research and Development Program of China (863 program, 2006AA02Z4D1), the Post-doc foundation from Sichuan University and U.S. National Institute on Drug Abuse K05-DA0454 (TRK).
References
- 1.Kreek MJ, Koob GF. Drug dependence: Stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51(1–2):23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS, Weiss JM, Schwartz LS. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220(5170):911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: Relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci. 2000;20(12):4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovallo WR. Cortisol secretion patterns in addiction and addiction risk. Int J Psychophysiol. 2006;59(3):195–202. doi: 10.1016/j.ijpsycho.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satel SL, Kosten TR, Schuckit MA, Fischman MW. Should protracted withdrawal from drugs be included in DSM-IV? Am J Psychiatry. 1993;150(5):695–704. doi: 10.1176/ajp.150.5.695. [DOI] [PubMed] [Google Scholar]
- 6.Allolio B, Schulte HM, Deuss U, Kallabis D, Hamel E, Winkelman W. Effect of oral morphine and naloxone on pituitary-adrenal response in man induced by human corticotropin-releasing hormone. Acta Endocrinologica. 1987;114(4):509–514. doi: 10.1530/acta.0.1140509. [DOI] [PubMed] [Google Scholar]
- 7.Facchinetti F, Grasso A, Petraglia F, Parrini D, Volpe A, Genazzani AR. Impaired circadian rhythmicity of beta-lipotrophin, beta-endorphin and ACTH in heroin addicts. Acta Endocrinologica. 1984;105(2):149–155. doi: 10.1530/acta.0.1050149. [DOI] [PubMed] [Google Scholar]
- 8.Rittmaster RS, Cutler GB, Jr, Sobel DO, Goldstein DS, Koppelman MC, Loriaux DL, Chrousos GP. Morphine inhibits the pituitary-adrenal response to ovine corticotropin-releasing hormone in normal subjects. J Clin Endocrinol Metab. 1985;60(5):891–895. doi: 10.1210/jcem-60-5-891. [DOI] [PubMed] [Google Scholar]
- 9.Allolio B, Deuss U, Kaulen D, Leonhardt U, Kallabis D, Hamel E, Winkelmann W. FK 33-824, a met-enkephalin analog, blocks corticotropin-releasing hormone-induced adrenocorticotropin secretion in normal subjects but not in patients with Cushing’s disease. J Clin Endocrinol Metab. 1986;63(6):1427–1431. doi: 10.1210/jcem-63-6-1427. [DOI] [PubMed] [Google Scholar]
- 10.Garland EJ, Zis AP. Effect of codeine and oxazepam on afternoon cortisol secretion in men. Psychoneuroendocrinology. 1989;14(5):397–402. doi: 10.1016/0306-4530(89)90009-7. [DOI] [PubMed] [Google Scholar]
- 11.Cami J, Gilabert M, San L, de la Torre R. Hypercortisolism after opioid discontinuation in rapid detoxification of heroin addicts. Br J Addict. 1992;87(8):1145–1151. doi: 10.1111/j.1360-0443.1992.tb02001.x. [DOI] [PubMed] [Google Scholar]
- 12.Wisniewski AB, Brown TT, John M, Cofranceso J, Jr, Golub ET, Ricketts EP, Wand G, Dobs AS. Cortisol levels and depression in men and women using heroin and cocaine. Psychoneuroendocrinology. 2006;31(2):250–255. doi: 10.1016/j.psyneuen.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Bearn J, Buntwal N, Papadopoulos A, Checkley S. Salivary cortisol during opiate dependence and withdrawal. Addict Biol. 2001;6(2):157–162. doi: 10.1080/13556210020040235. [DOI] [PubMed] [Google Scholar]
- 14.Kosten TR, Kreek MJ, Ragunath J, Kleber HD. Cortisol levels during chronic naltrexone maintenance treatment in ex-opiate addicts. Biol Psychiatry. 1986;21(2):217–220. doi: 10.1016/0006-3223(86)90150-2. [DOI] [PubMed] [Google Scholar]
- 15.Kosten TR, Morgan C, Kreek MJ. Beta endorphin levels during heroin, methadone, buprenorphine, and naloxone challenges: Preliminary findings. Biol Psychiatry. 1992;32(6):523–528. doi: 10.1016/0006-3223(92)90220-t. [DOI] [PubMed] [Google Scholar]
- 16.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164(8):1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33(1):13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- 18.Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology. 2003;170(1):62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- 19.al’Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug Alcohol Depend. 2004;73(3):267–278. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Baron JA, Comi RJ, Cryns V, Brinck-Johnsen T, Mercer NG. The effect of cigarette smoking on adrenal cortical hormones. J Pharmacol Exp Ther. 1995;272(1):151–155. [PubMed] [Google Scholar]
- 21.Kirschbaum C, Wust S, Strasburger CJ. ‘Normal’ cigarette smoking increases free cortisol in habitual smokers. Life Sci. 1992;50(6):435–442. doi: 10.1016/0024-3205(92)90378-3. [DOI] [PubMed] [Google Scholar]
- 22.Seyler LE, Jr, Fertig J, Pomerleau O, Hunt D, Parker K. The effects of smoking on ACTH and cortisol secretion. Life Sci. 1984;34(1):57–65. doi: 10.1016/0024-3205(84)90330-8. [DOI] [PubMed] [Google Scholar]
- 23.Wilkins JN, Carlson HE, Van Vunakis H, Hill MA, Gritz E, Jarvik ME. Nicotine from cigarette smoking increases circulating levels of cortisol, growth hormone, and prolactin in male chronic smokers. Psychopharmacology. 1982;78(4):305–308. doi: 10.1007/BF00433730. [DOI] [PubMed] [Google Scholar]
- 24.Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE. Neural activity associated with stress-induced cocaine craving: A functional magnetic resonance imaging study. Psychopharmacology. 2005;183(2):171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- 25.Sinha R, Kimmerling A, Doebrick C, Kosten TR. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: Preliminary findings. Psychopharmacology. 2007;190(4):569–574. doi: 10.1007/s00213-006-0640-8. [DOI] [PubMed] [Google Scholar]