Abstract
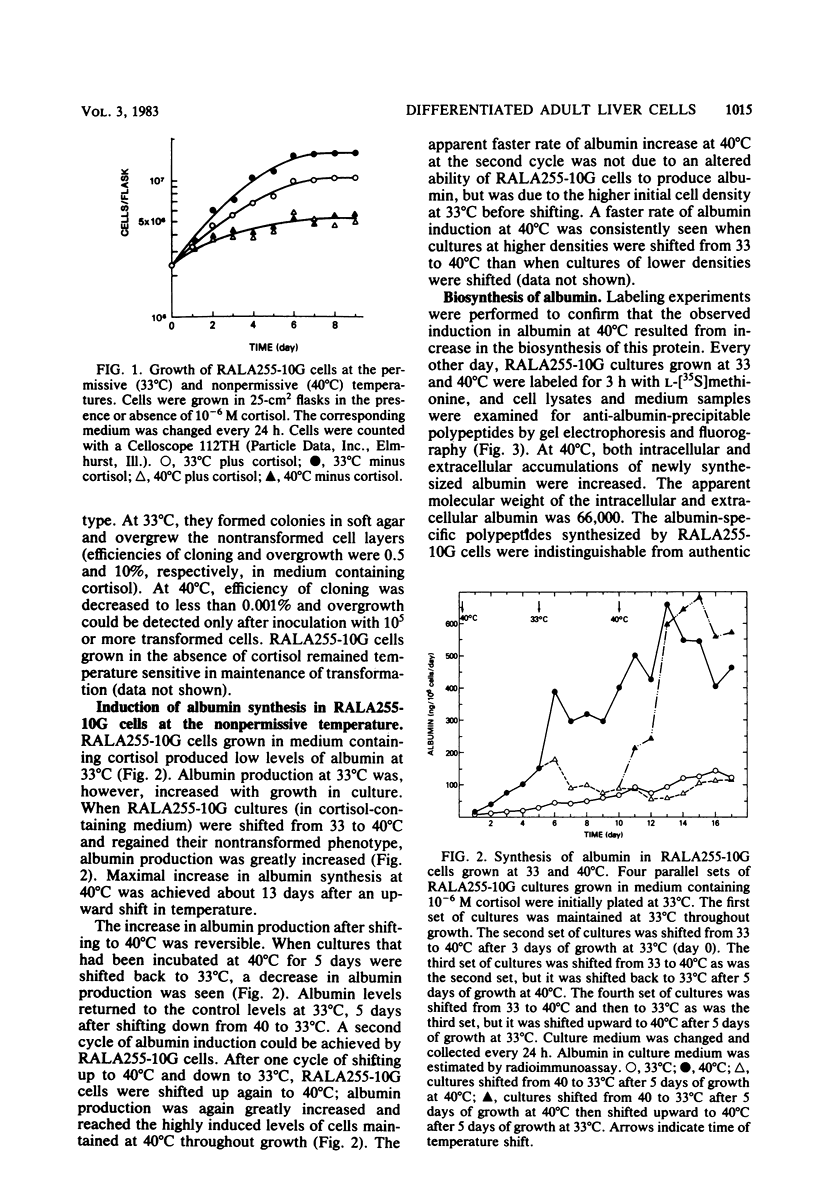
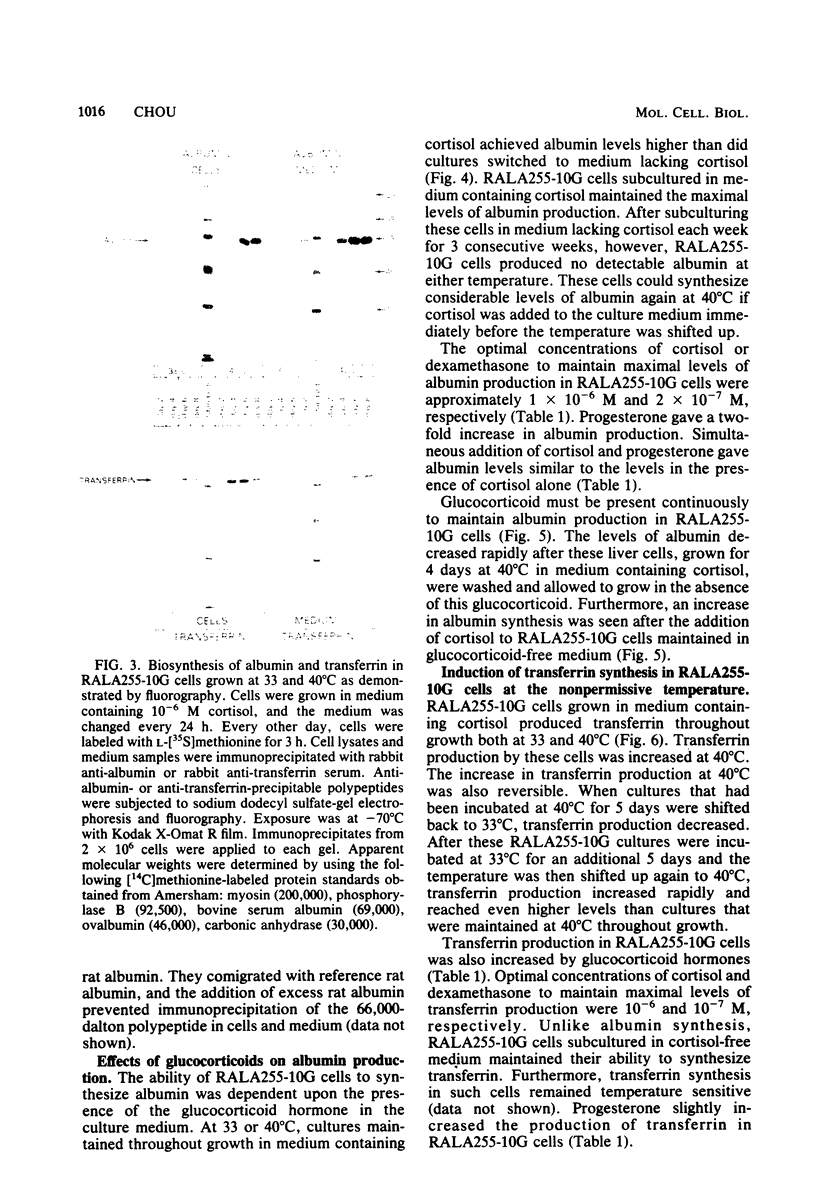
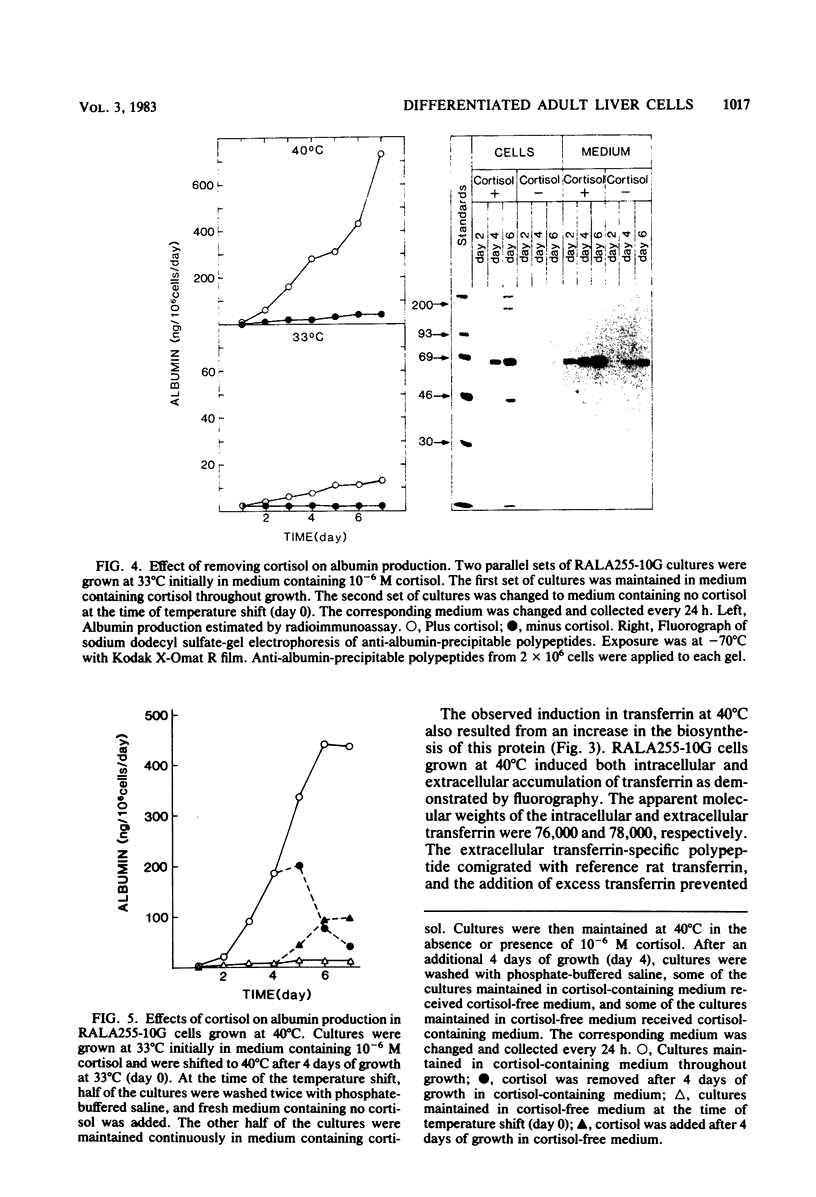
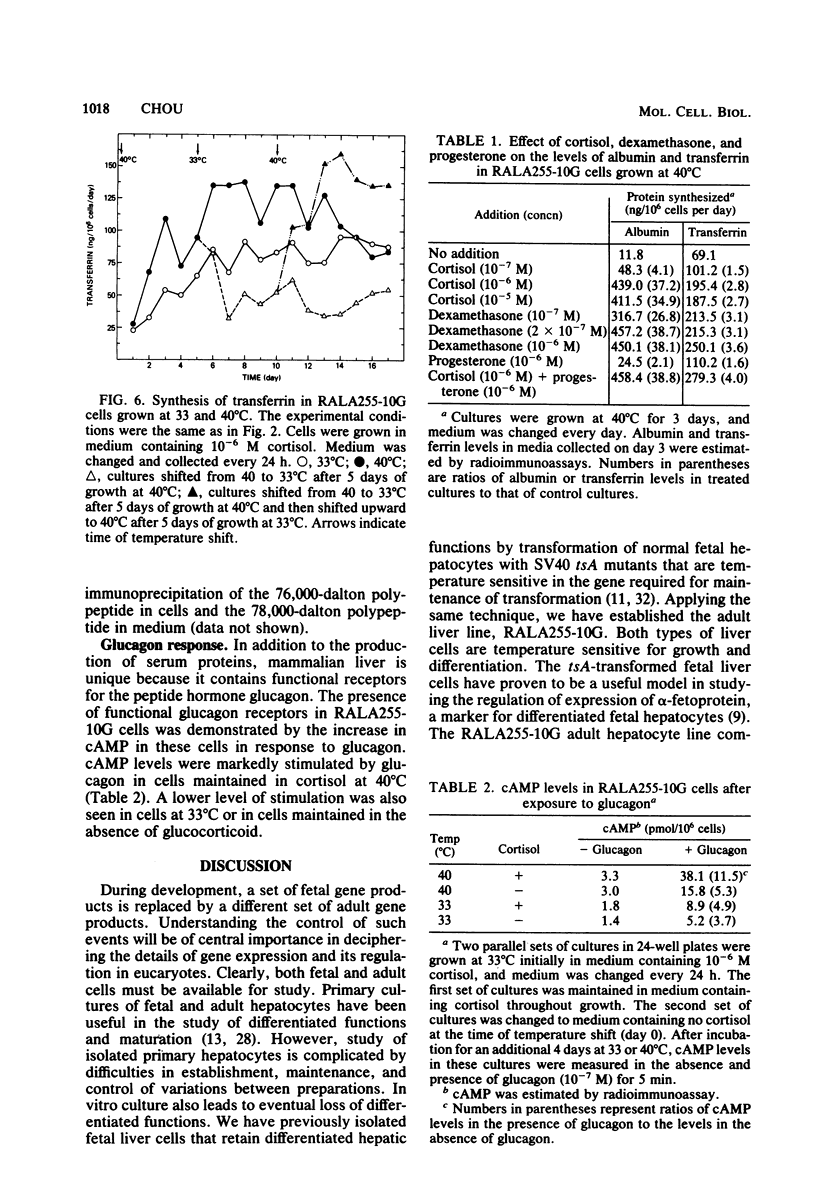
A clonal rat adult hepatocyte cell line (RALA255-10G) was shown to be temperature sensitive (ts) for growth and differentiation. Glucocorticoid was necessary to maintain the maximal levels of differentiated functions in these cells. The RALA255-10G cell line was established by transforming primary adult hepatocytes with simian virus 40 tsA255 virus that is temperature sensitive for maintenance of transformation. At the permissive temperature (33 degrees C), RALA255-10G cells showed characteristics of malignant transformation, synthesized low levels of albumin and transferrin, and contained low levels of functional receptors for glucagon. At the nonpermissive temperature (40 degrees C), these cells regain the normal differentiated phenotype, and the levels of these three hepatic functions were increased. Induction of albumin and transferrin production by RALA255-10G cells at 40 degrees C was shown to be the result of the increase in the biosynthesis of these proteins. Furthermore, the albumin and transferrin produced by these cells were immunologically and electrophoretically indistinguishable from authentic rat albumin and transferrin. Glucocorticoid, which reduced the growth rate and saturation density of RALA255-10G cells at 33 degrees C, was absolutely required by these cells to synthesize albumin at both temperatures. This hormone also enhanced transferrin production and glucagon response. Our data indicate that glucocorticoid hormone is one of the factors that maintain adult hepatocytes in a differentiated state.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelev G. I. Alpha-fetoprotein in ontogenesis and its association with malignant tumors. Adv Cancer Res. 1971;14:295–358. doi: 10.1016/s0065-230x(08)60523-0. [DOI] [PubMed] [Google Scholar]
- Baumann H., Held W. A. Biosynthesis and hormone-regulated expression of secretory glycoproteins in rat liver and hepatoma cells. Effect of glucocorticoids and inflammation. J Biol Chem. 1981 Oct 10;256(19):10145–10155. [PubMed] [Google Scholar]
- Belanger L., Hamel D., Lachance L., Dufour D., Tremblay M., Gagnon P. M. Hormonal regulation of alpha1 foetoprotein. Nature. 1975 Aug 21;256(5519):657–659. doi: 10.1038/256657a0. [DOI] [PubMed] [Google Scholar]
- Boettiger D., Roby K., Brumbaugh J., Biehl J., Holtzer H. Transformation of chicken embryo retinal melanoblasts by a temperature-sensitive mutant of Rous sarcoma virus. Cell. 1977 Aug;11(4):881–890. doi: 10.1016/0092-8674(77)90299-9. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brown P. C., Papaconstantinou J. Coordinated modulation of albumin synthesis and mRNA levels in cultured hepatoma cells by hydrocortisone and cyclic AMP analogs. J Biol Chem. 1979 Oct 10;254(19):9379–9384. [PubMed] [Google Scholar]
- Cain G. D., Mayer G., Jones E. A. Augmentation of albumin but not fibrinogen synthesis by corticosteroids in patients with hepatocellular disease. J Clin Invest. 1970 Dec;49(12):2198–2204. doi: 10.1172/JCI106438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y. Human placental cells transformed by tsA mutants of simian virus 40: a model system for the study of placental functions. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1409–1413. doi: 10.1073/pnas.75.3.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Ito F., Evans G., Chiu J. F., Feldman M. alpha-Fetoprotein biosynthesis and hepatocellular differentiation. Biochem Biophys Res Commun. 1982 Oct 29;108(4):1524–1530. doi: 10.1016/s0006-291x(82)80080-6. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Mano T., Feldman M. Inhibition of synthesis of alpha-fetoprotein by glucocorticoids in cultured hepatoma cells. J Cell Biol. 1982 May;93(2):314–317. doi: 10.1083/jcb.93.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Schlegel-Haueter S. E. Study of liver differentiation in vitro. J Cell Biol. 1981 May;89(2):216–222. doi: 10.1083/jcb.89.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commer P., Schwartz C., Tracy S., Tamaoki T., Chiu J. F. Dexamethasone inhibits alpha-fetoprotein gene expression in developing mouse liver. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1294–1299. doi: 10.1016/0006-291x(79)92149-1. [DOI] [PubMed] [Google Scholar]
- Freeman A. E., Engvall E., Hirata K., Yoshida Y., Kottel R. H., Hilborn V., Ruoslahti E. Differentiation of fetal liver cells in vitro. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3659–3663. doi: 10.1073/pnas.78.6.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham J. W. Cell types in long-term propagable cultures of rat liver. Ann N Y Acad Sci. 1980;349:128–137. doi: 10.1111/j.1749-6632.1980.tb29521.x. [DOI] [PubMed] [Google Scholar]
- Hussa R. O., Story M. T., Pattillo R. A. Regulation of human chorionic gonadotropin (hCG) secretion by serum and dibutyryl cyclic AMP in malignant trophoblast cells in vitro. J Clin Endocrinol Metab. 1975 Mar;40(3):401–405. doi: 10.1210/jcem-40-3-401. [DOI] [PubMed] [Google Scholar]
- Koga K., Tamaoki T. Developmental changes in the synthesis of alpha-fetoprotein and albumin in the mouse liver. Cell-free synthesis by membrane-bound polyribosomes. Biochemistry. 1974 Jul 16;13(15):3024–3028. doi: 10.1021/bi00712a004. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leffert H. L., Moran T., Boorstein R., Koch K. S. Procarcinogen activation and hormonal control of cell proliferation in differentiated primary adult rat liver cell cultures. Nature. 1977 May 5;267(5606):58–61. doi: 10.1038/267058a0. [DOI] [PubMed] [Google Scholar]
- Leffert H. L., Paul D. Studies on primary cultures of differentiated fetal liver cells. J Cell Biol. 1972 Mar;52(3):559–568. doi: 10.1083/jcb.52.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffert H. L., Sell S. Alpha1-fetoprotein biosynthesis during the growth cycle of differentiated fetal rat hepatocytes in primary monolayer culture. J Cell Biol. 1974 Jun;61(3):823–829. doi: 10.1083/jcb.61.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggins G. C. Adrenocortical-related maturational events in the fetus. Am J Obstet Gynecol. 1976 Dec 1;126(7):931–941. doi: 10.1016/0002-9378(76)90680-3. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Lee D. C., Palmiter R. D. Transferrin gene expression. Regulation of mRNA transcription in chick liver by steroid hormones and iron deficiency. J Biol Chem. 1980 Jan 10;255(1):148–153. [PubMed] [Google Scholar]
- Pacifici M., Boettiger D., Roby K., Holtzer H. Transformation of chondroblasts by Rous sarcoma virus and synthesis of the sulfated proteoglycan matrix. Cell. 1977 Aug;11(4):891–899. doi: 10.1016/0092-8674(77)90300-2. [DOI] [PubMed] [Google Scholar]
- Plas C., Nunez J. Glycogenolytic response to glucagon of cultured fetal hepatocytes. Refractoriness following prior exposure to glucagon. J Biol Chem. 1975 Jul 25;250(14):5304–5311. [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Trepat J. M., Dever J., Sargent T. D., Thomas K., Sell S., Bonner J. Changes in expression of albumin and alpha-fetoprotein genes during rat liver development and neoplasia. Biochemistry. 1979 May 29;18(11):2167–2178. doi: 10.1021/bi00578a006. [DOI] [PubMed] [Google Scholar]
- Schlegel-Haueter S. E., Schlegel W., Chou J. Y. Establishment of a fetal rat liver cell line that retains differentiated liver functions. Proc Natl Acad Sci U S A. 1980 May;77(5):2731–2734. doi: 10.1073/pnas.77.5.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]