Abstract
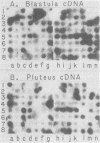
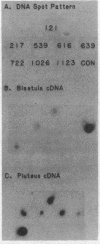
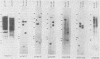
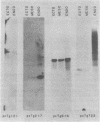
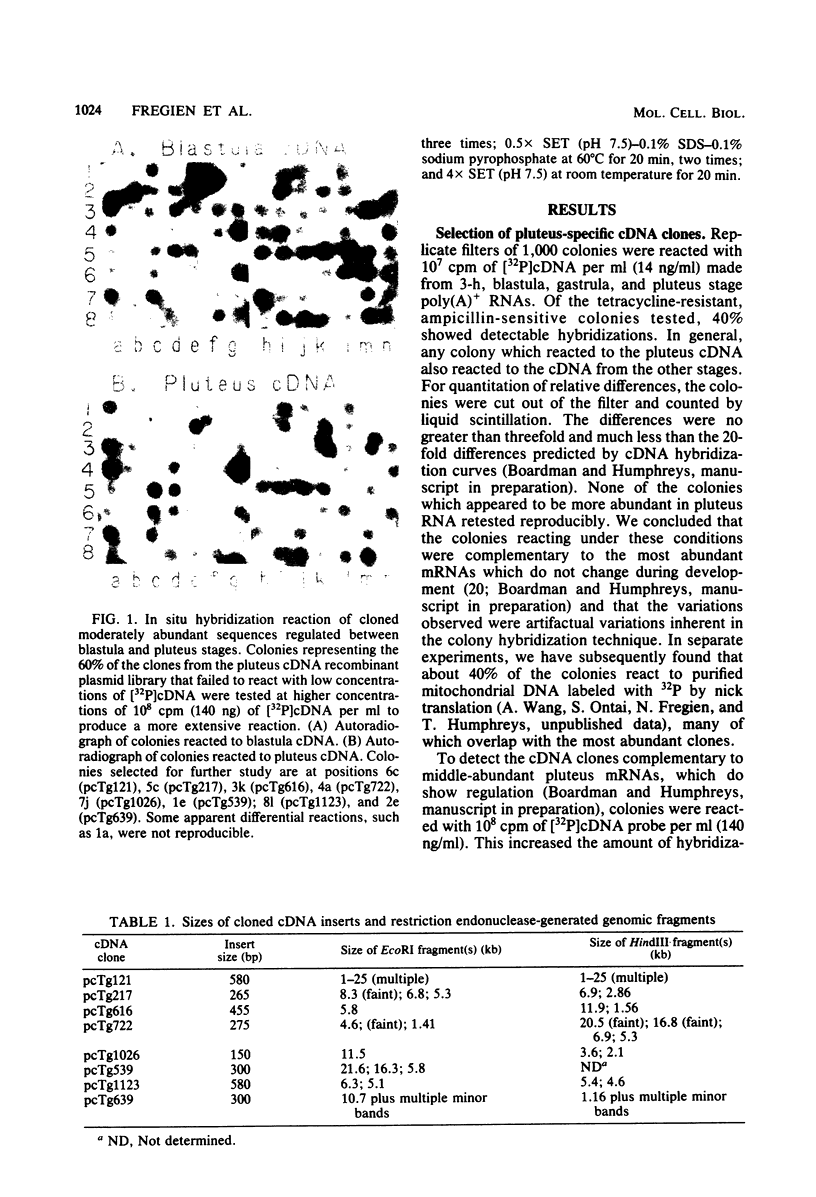
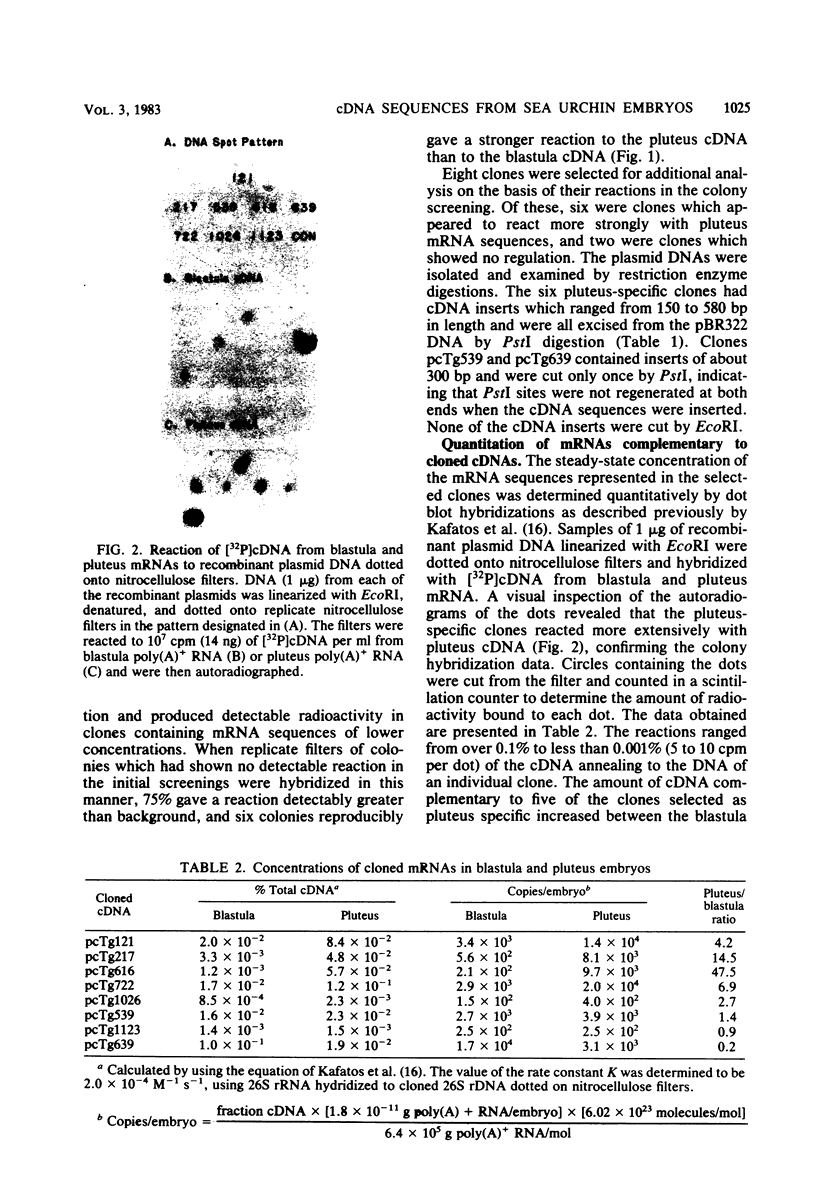
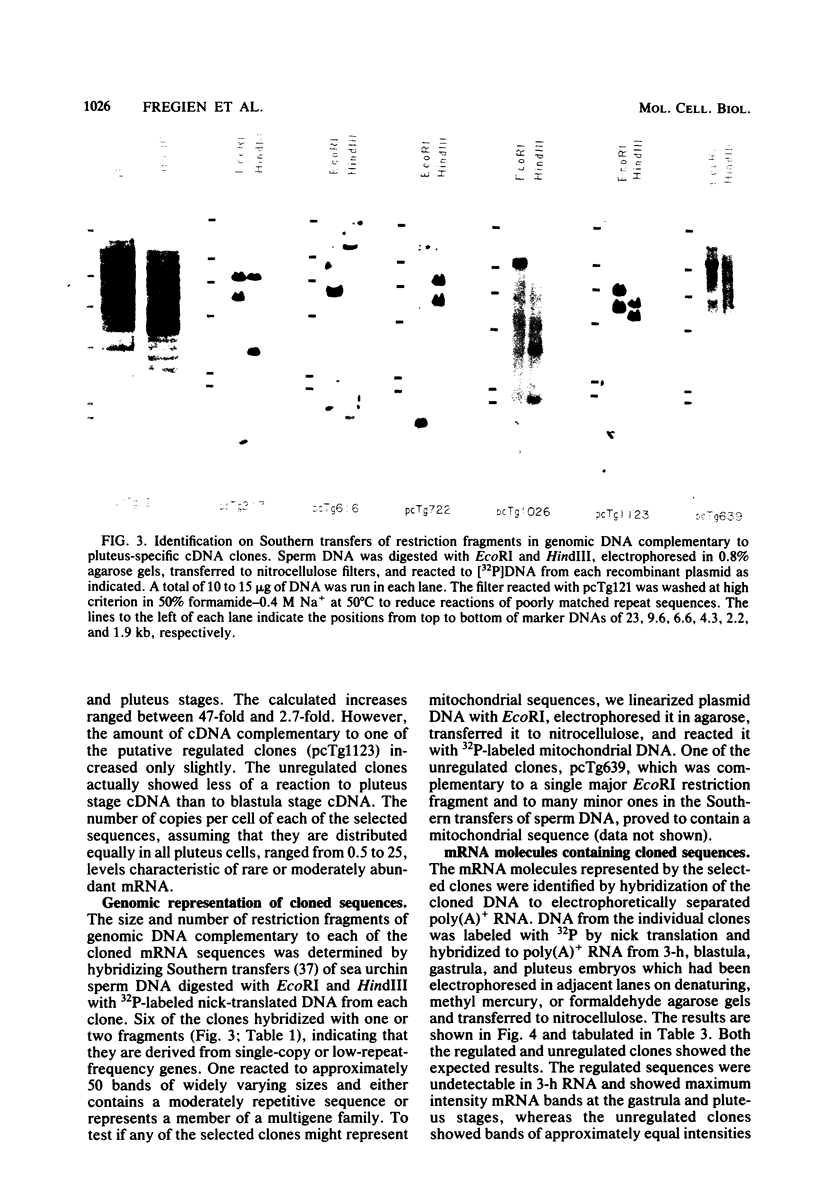
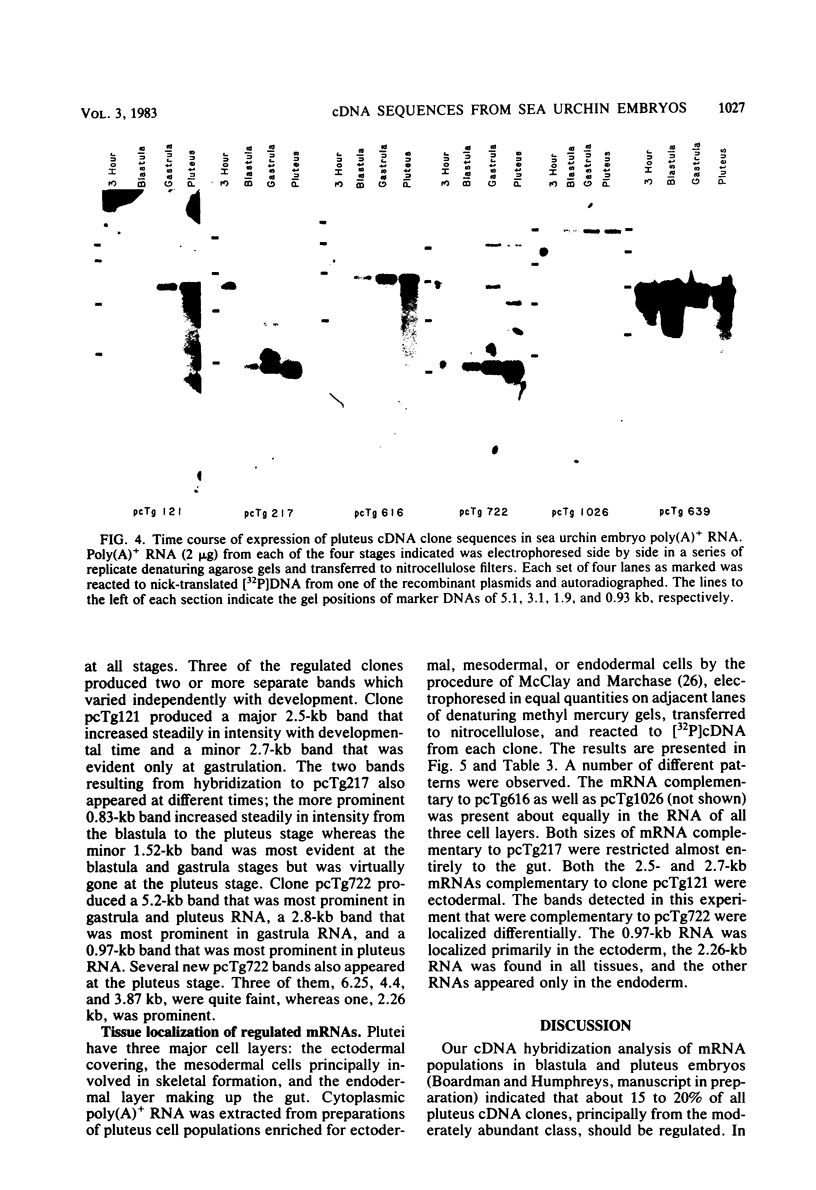
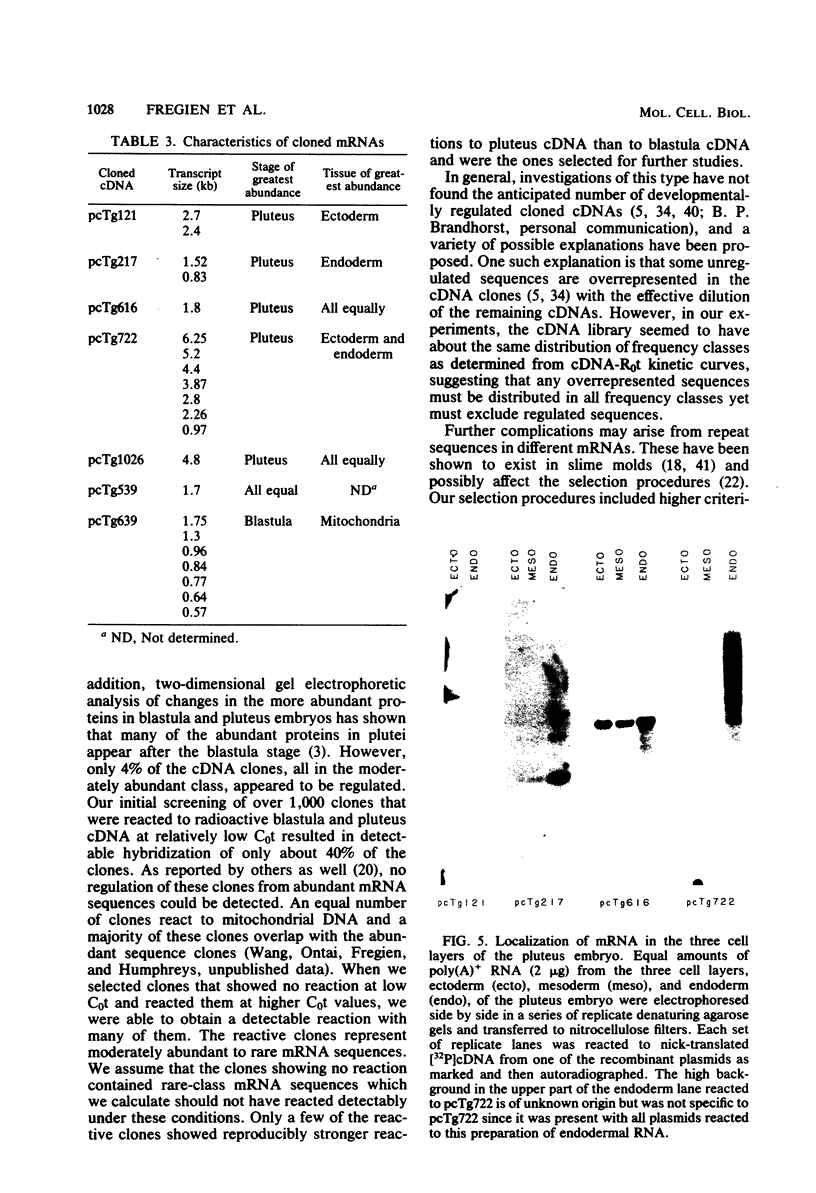
Five developmentally regulated sea urchin mRNA sequences which increase in abundance between the blastula and pluteus stages of development were isolated by molecular cloning of cDNA. The regulated sequences all appeared in moderately abundant mRNA molecules of pluteus cells and represented 4% of the clones tested. There were no regulated sequences detected in the 40% of the clones which hybridized to the most abundant mRNA, and the screening procedures were inadequate to detect possible regulation in the 20 to 30% of the clones presumably derived from rare-class mRNA. The reaction of 32P[cDNA] from blastula and pluteus mRNA to dots of the cloned DNAs on nitrocellulose filters indicated that the mRNAs complementary to the different cloned pluteus-specific sequences were between 3- and 47-fold more prevalent at the pluteus stage than at the blastula stage. Polyadenylated RNA from different developmental stages was transferred from electrophoretic gels to nitrocellulose filters and reacted to the different cloned sequences. The regulated mRNAs were undetectable in the RNA of 3-h embryos, became evident at the hatching blastula stage, and reached a maximum in abundance by the gastrula or pluteus stage. Certain of the clones reacted to two sizes of mRNA which did not vary coordinately with development. Transfers of RNA isolated from each of the three cell layers of pluteus embryos that were reacted to the cloned sequences revealed that two of the sequences were found in the mRNA of all three layers, two were ectoderm specific, and one was endoderm specific. Four of the regulated sequences were complementary to one or two major bands and one to at least 50 bands on Southern transfers of restriction endonuclease-digested total sea urchin DNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandraki D., Ruderman J. V. Sequence heterogeneity, multiplicity, and genomic organization of alpha- and beta-tubulin genes in sea urchins. Mol Cell Biol. 1981 Dec;1(12):1125–1137. doi: 10.1128/mcb.1.12.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., Brenner D. J., Neufeld B. R., Britten R. J. Reduction in the rate of DNA reassociation by sequence divergence. J Mol Biol. 1973 Dec 5;81(2):123–135. doi: 10.1016/0022-2836(73)90184-8. [DOI] [PubMed] [Google Scholar]
- Brandhorst B. P., Humphreys T. Stabilities of nuclear and messenger RNA molecules in sea urchin embryos. J Cell Biol. 1972 May;53(2):474–482. doi: 10.1083/jcb.53.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst B. P. Two-dimensional gel patterns of protein synthesis before and after fertilization of sea urchin eggs. Dev Biol. 1976 Sep;52(2):310–317. doi: 10.1016/0012-1606(76)90248-7. [DOI] [PubMed] [Google Scholar]
- Bruskin A. M., Tyner A. L., Wells D. E., Showman R. M., Klein W. H. Accumulation in embryogenesis of five mRNAs enriched in the ectoderm of the sea urchin pluteus. Dev Biol. 1981 Oct 30;87(2):308–318. doi: 10.1016/0012-1606(81)90154-8. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P., Metz C. B. Mitochondrial RNA synthesis in sea urchin embryos. J Mol Biol. 1972 Mar 14;64(3):593–607. doi: 10.1016/0022-2836(72)90085-x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Hughes S. H., Stubblefield E., Kirschner M. W., Varmus H. E. Multiple alpha and beta tubulin genes represent unlinked and dispersed gene families. J Biol Chem. 1981 Mar 25;256(6):3130–3134. [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain W. R., Jr, Durica D. S., Van Doren K. Actin gene expression in developing sea urchin embryos. Mol Cell Biol. 1981 Aug;1(8):711–720. doi: 10.1128/mcb.1.8.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Duncan R., Humphreys T. Most sea urchin maternal mRNA sequences in every abundance class appear in both polyadenylated and nonpolyadenylated molecules. Dev Biol. 1981 Dec;88(2):201–210. doi: 10.1016/0012-1606(81)90164-0. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Kindle K. L., Davidson N., Kindle K. L. The actin genes of Drosophila: a dispersed multigene family. Cell. 1980 Feb;19(2):365–378. doi: 10.1016/0092-8674(80)90511-5. [DOI] [PubMed] [Google Scholar]
- GROSS P. R., MALKIN L. I., MOYER W. A. TEMPLATES FOR THE FIRST PROTEINS OF EMBRYONIC DEVELOPMENT. Proc Natl Acad Sci U S A. 1964 Mar;51:407–414. doi: 10.1073/pnas.51.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedes L. H., Cohn R. H., Lowry J. C., Chang A. C., Cohen S. N. The organization of sea urchin histone genes. Cell. 1975 Nov;6(3):359–369. doi: 10.1016/0092-8674(75)90185-3. [DOI] [PubMed] [Google Scholar]
- Kindle K. L., Firtel R. A. Evidence that populations of Dictyostelium single-copy mRNA transcripts carry common repeat sequences. Nucleic Acids Res. 1979 Jun 11;6(7):2403–2422. doi: 10.1093/nar/6.7.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene K. C., Humphreys T. Similarity of hnRNA sequences in blastula and pluteus stage sea urchin embryos. Cell. 1977 Sep;12(1):143–155. doi: 10.1016/0092-8674(77)90192-1. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Lev Z., Xin J. H., Britten R. J., Davidson E. H. Messenger RNA prevalence in sea urchin embryos measured with cloned cDNAs. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5317–5321. doi: 10.1073/pnas.77.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Chung S., Zuker C., Lodish H. F. Selection and analysis of cloned developmentally-regulated Dictyostelium discoideum genes by hybridization-competition. Nucleic Acids Res. 1981 Feb 25;9(4):947–963. doi: 10.1093/nar/9.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Kee S. G., Efstratiadis A., Kafatos F. C. Amplification and characterization of a beta-globin gene synthesized in vitro. Cell. 1976 Jun;8(2):163–182. doi: 10.1016/0092-8674(76)90001-5. [DOI] [PubMed] [Google Scholar]
- McClay D. R., Marchase R. B. Separation of ectoderm and endoderm from sea urchin pluteus larvae and demonstration of germ layer-specific antigens. Dev Biol. 1979 Aug;71(2):289–296. doi: 10.1016/0012-1606(79)90170-2. [DOI] [PubMed] [Google Scholar]
- Norgard M. V., Emigholz K., Monahan J. J. Increased amplification of pBR322 plasmid deoxyribonucleic acid in Escherichia coli K-12 strains RR1 and chi1776 grown in the presence of high concentrations of nucleoside. J Bacteriol. 1979 Apr;138(1):270–272. doi: 10.1128/jb.138.1.270-272.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikó L., Blair D. G., Tyler A., Vinograd J. Cytoplasmic DNA in the unfertilized sea urchin egg: physical properties of circular mitochondrial DNA and the occurrence of catenated forms. Proc Natl Acad Sci U S A. 1968 Mar;59(3):838–845. doi: 10.1073/pnas.59.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rowekamp W., Firtel R. A. Isolation of developmentally regulated genes from Dictyostelium. Dev Biol. 1980 Oct;79(2):409–418. doi: 10.1016/0012-1606(80)90126-8. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Jan;3(1):101–116. doi: 10.1093/nar/3.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G., Telford J., Baldari C., Pirrotta V. Isolation of cloned genes differentially expressed at early and late stages of Drosophila embryonic development. Dev Biol. 1981 Sep;86(2):438–447. doi: 10.1016/0012-1606(81)90202-5. [DOI] [PubMed] [Google Scholar]
- Shepherd G. W., Nemer M. Developmental shifts in frequency distribution of polysomal mRNA and their posttranscriptional regulation in the sea urchin embryo. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4653–4656. doi: 10.1073/pnas.77.8.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M., Hirsh J., Davidson N. The cuticle genes of drosophila: a developmentally regulated gene cluster. Cell. 1981 Jul;25(1):165–177. doi: 10.1016/0092-8674(81)90241-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker C., Lodish H. F. Repetitive DNA sequences cotranscribed with developmentally regulated Dictyostelium discoideum mRNAs. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5386–5390. doi: 10.1073/pnas.78.9.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]