Abstract
Background:
The variable success of topical minoxidil in the treatment of androgenic alopecia has led to the hypothesis that other pathways could mediate this form of hair loss, including infection and/or microinflammation of the hair follicles. In this study, we prepared a multimodal microemulsion comprising minoxidil (a dihydrotestosterone antagonist), diclofenac (a nonsteroidal anti-inflammatory agent), and tea tree oil (an anti-infective agent). We investigated the stability and physicochemical properties of this formulation, and its therapeutic efficacy compared with a formulation containing minoxidil alone in the treatment of androgenic alopecia.
Methods:
We developed a multimodal oil/water (o/w) microemulsion, a formulation containing minoxidil alone, and another containing vehicle. A three-phase diagram was constructed to obtain the optimal concentrations of the selected oil, surfactant, and cosurfactant. Thirty-two men aged 18–30 years were randomized to apply 1 mL of microemulsion containing the multimodal formulation (formulation A, n = 11), minoxidil alone (formulation B, n = 11) or placebo (formulation C, n = 10) twice daily to the affected area for 32 weeks. Efficacy was evaluated by mean hair count, thickness, and weight on the targeted area of the scalp. Global photographs were taken, changes in the area of scalp coverage were assessed by patients and external investigators, and the benefits and safety of the study medications were evaluated. The physical stability of formula A was examined after a shelf storage period of 24 months.
Results:
Formulation A achieved a significantly superior response than formulations B and C in terms of mean hair count (P < 0.001), mean hair weight (P < 0.001), and mean hair thickness (P < 0.05). A patient self-assessment questionnaire demonstrated that the multimodal minoxidil formulation significantly (P < 0.001) slowed hair loss, increased hair growth, and improved appearance, and showed no appreciable side effects, such as itching and/or inflammation of the scalp compared with the minoxidil alone and placebo formulations. These improvements were in agreement with the photographic assessments made by the investigators. Formula A was shown to be an o/w formulation with consistent pH, viscosity, specific gravity, and homogeneity, and was physically stable after 24 months of normal storage.
Conclusion:
A multimodal microemulsion comprising minoxidil, diclofenac, and tea tree oil was significantly superior to minoxidil alone and placebo in terms of stability, safety, and efficacy, and achieved an earlier response in the treatment of androgenic alopecia compared with minoxidil alone in this 32-week pilot study.
Keywords: androgenic alopecia, diclofenac, microemulsion, minoxidil, nonsteroidal anti-inflammatory agents, tea tree oil
Video abstract
Introduction
The success rate of treatment for androgenic alopecia barely exceeds 30% using antihypertensive agents or modulators of androgen metabolism, implying that other pathophysiologic pathways may be involved in this condition.1–4 Androgenic alopecia is considered to be an alteration of hair growth and/or premature aging of the pilosebaceous unit, with a multifactorial and even polygenic etiology.1
Various hypotheses have been put forward to explain the causes of androgenic alopecia, of which none can be said to account fully for the condition. Infection and microinflammation of the hair follicles has been considered to contribute to the pathogenesis of androgenic alopecia.5–9 However, only 55% of patients with male-pattern androgenic alopecia and microinflammation were shown to have hair regrowth in response to treatment with minoxidil, which was less than the 77% recorded for patients with no signs of inflammation.3 This suggests that perifollicular inflammation may be a contributing factor in some cases of androgenic alopecia that do not respond to minoxidil. Inflammation may be effective in initiating androgenic alopecia by altering local androgenic hormone metabolism.2 Several studies of hair follicles taken from subjects with androgenic alopecia have reported moderate lymphohistiocytic inflammation in 30% of subjects with androgenic alopecia versus 15% of controls.9
Localization of the inflammatory infiltrate at the level of the upper follicle suggests that the primary causal event triggering inflammation might occur in the vicinity of the follicular infundibulum. One could speculate that colonization of the infundibulum by microbes and/or antigens could be involved in generation of an inflammatory response due to oxidative tissue injury.9,10 In line with this hypothesis, Piérard et al11 found that use of topical antimicrobials may be beneficial for the treatment of androgenic alopecia. Combinations containing minoxidil-pyrithione zinc,12 minoxidil-ketoconazole,13 and minoxidil-hydrocortisone14 have been shown to improve hair growth compared with use of minoxidil alone.
The aim of the present investigation was to prepare and evaluate a pharmaceutically stable and therapeutically effective multimodal topical treatment that can address the multifactorial etiology of androgenic alopecia, including excessive sensitivity of the hair follicle to androgen, microbial infection, and/or microinflammation. The formula tested was a microemulsion containing minoxidil (an antiandrogenic agent), tea tree oil (a widely used natural antifungal/antibacterial agent),15,16 and diclofenac (a topical nonsteroidal anti-inflammatory agent marked in the form of Voltaren® Emulgel™ 1% and Voltaren Ophthalmic 0.1% eyedrops by Novartis, Basel, Switzerland). Diclofenac was chosen to diminish hair follicle microinflammation because it has less pronounced side effects in comparison with cortisone derivatives. A microemulsion formulation was used because of its many advantages, including ease of preparation, improved drug solubility, ability to protect drugs with poor stability from environmental conditions, better transdermal drug delivery, and long shelf-life.19,20 The physical properties of a freshly prepared multimodal microemulsion, including pH, viscosity, refractive index, dilution test, dye staining test, optical transparency, specific gravity, and centrifugal stress, were compared with those after a shelf storage period of 24 months. A pilot study was also performed in men with androgenic alopecia to compare the efficacy and safety of microemulsions containing multimodal minoxidil, minoxidil alone, and placebo.
Materials and methods
Materials
Minoxidil USP was sourced from Alan Pharmaceuticals (London, UK), pharmaceutical grade diclofenac sodium from Ningbo Samreal Chemical Co, Ltd (Zhejiang, People’s Republic of China), premium grade tea tree oil (Malaleuca alternifolia) from Malaleuca Plantation of Bungawalbyn Pty Limited (Coraki, NSW, Australia), and high-performance liquid chromatography grade acetone and pharmaceutical grade propylene glycol and absolute alcohol from Sigma-Aldrich (St Louis, MO, USA). Labrasol® (containing caprylocaproyl polyoxyl-8 glycerides EP) was obtained from Gattefossé (Saint-Priest, Cedex, France).
Preparation of microemulsions
Construction of ternary phase diagram
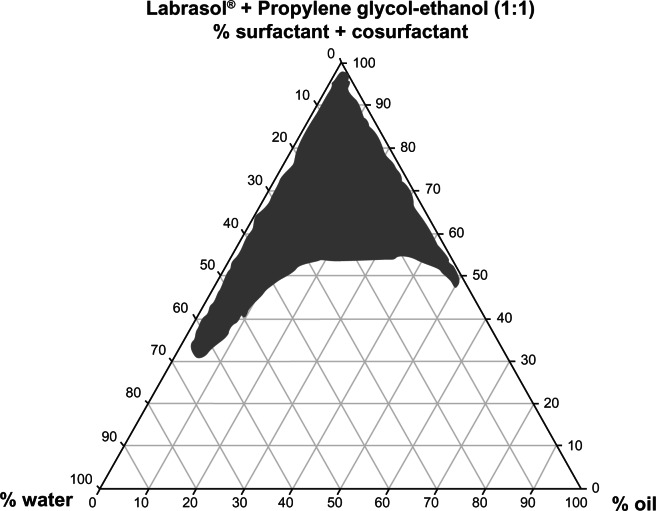
The ternary phase diagram shown in Figure 1 was constructed to investigate the range of concentrations needed for the components of the microemulsions using the water titration method and different oil/surfactant/cosurfactant ratios. The optimum composition was 0.5% diclofenac, 5% tea tree oil, 5% minoxidil, 5% lauryl alcohol, and 35% water in combination with 55% Labrasol® [(surfactant)/(propylene glycol-ethanol mixture at 1:1 as cosurfactant) at (1:1)].
Figure 1.
Ternary phase diagram of the oil, surfactant/cosurfactant mixture-water system at a 1:1 weight ratio of Labrasol® + propylene glycol-ethanol at ambient temperature.
Note: Shaded area shows microemulsion zone.
The compositions of the microemulsions containing multimodal minoxidil, minoxidil alone, and placebo are shown in Table 1. The microemulsion containing multimodal minoxidil was prepared by homogenously mixing 5% w/v of tea tree oil with 5% w/v of lauryl alcohol, chosen for its good drug solubilizing capacity. Specified amounts of minoxidil and diclofenac were dissolved in the Labrasol® surfactant/propylene glycol-ethanol mixture as a cosurfactant. Next, the two mixtures were combined by gentle stirring. A designated amount of double-distilled water was added to the mixtures dropwise, and the multimodal microemulsion was obtained at ambient temperature. The procedure was repeated to prepare the microemulsions containing minoxidil alone and placebo. The preparations obtained were individually packed in 50 mL amber brown glass bottles each equipped with a calibrated dropper. All bottles were labeled in a blinded fashion as A (multimodal minoxidil), B (minoxidil alone), or C (placebo).
Table 1.
Composition of formulations A, B, and C
| Ingredients | Formulation A | Formulation B | Formulation C |
|---|---|---|---|
| Minoxidil | 5% w/v (50 mg/mL) | 5% w/v (50 mg/mL) | – |
| Diclofenac sodium | 0.5% w/v (5 mg/mL) | – | – |
| Tea tree oil | 5% w/v (50 mg/mL) | – | – |
| Lauryl alcohol | 5% (50 mg/mL) | 5% (50 mg/mL) | 5% (50 mg/mL) |
| Propylene glycol | 13.75% w/v (0.1375 mg/mL) | 13.75% w/v (0.1375 mg/mL) | 13.75% w/v (0.1375 mg/mL) |
| Ethanol | 30% w/v (0.30 mg/mL) | 30% w/v (0.30 mg/mL) | 30% w/v (0.3 mg/mL) |
| Labrasol® | 27.5% w/v | 27.5% w/v | 27.5% w/v |
| Purified water | 35–100 mL | 35–100 mL | 35–100 mL |
Notes: w/v represents weight/volume. Formulation A, minoxidil, diclofenac and tea tree oil; formulation B, minoxidil only; formulation C, placebo.
Characterization and physical stability of multimodal microemulsion
Characterization of microemulsions is difficult because of the complex structures and components involved in these systems, as well as the limitations of the assessment techniques that can be used, but such knowledge is essential for their successful commercial exploitation. Therefore, studies using a combination of complementary techniques are usually required to obtain a comprehensive understanding of the physicochemical properties and structure of microemulsions. Because this was a pilot study, the multimodal microemulsion was characterized on the basis of its physical properties, which can not only explain the performance of the system but also help in predicting its physical stability over time, prior to and after 24 months of storage.
The multimodal microemulsions (formulation A) were examined for basic physical stability after 24 months of normal storage conditions and compared with the results obtained for the freshly prepared multimodal samples. The tests were carried out on five specimens from each preparation and observed for phase separation, flocculation, or precipitation. The microemulsions were centrifuged for 30 minutes at 25°C and inspected for any change in their homogeneity after storage for 24 months under ambient conditions and away from direct light. Chemical assays and testing of the active ingredients are planned in further broader investigations of the study formulation.
Staining/dye solubility tests
A few drops of the microemulsion were spread on a slide after mixing with a diluted drop of aqueous solution containing methylene blue as a water-soluble dye or a drop of Sudan III solution as an oil-soluble dye, and observed under an optical microscope. The blue color of the water-soluble dye appeared as a continuous phase within which microscopic oil droplets were scattered. The red color of the oil-soluble dye appeared as dispersible red microscopic droplets in a noncolored continuous phase, indicating that the multimodal formulation was an oil/water (o/w) microemulsion. These tests were repeated on the stored samples, and the results were similar, indicating no phase conversion.
Transparency/translucency
The droplets of the microemulsions being smaller than 1/4th the wavelength of visible light, permit white light to pass through the dispersed system. The microemulsion systems were inspected for optical transparency and homogeneity prior to and after storage by routine observation under strong light. The formulations were also checked for the presence of undissolved drug or other solid ingredients.
Dilution testing
O/w microemulsions are homogeneous when diluted with water, whereas a w/o microemulsion is not and undergoes phase inversion into an o/w microemulsion. Dilution tests were used to compare the freshly prepared A, B, and C microemulsions with corresponding formulations that had been stored for 24 months to determine if the microemulsion underwent phase inversion from o/w to w/o during storage. To do this, a few drops of the freshly prepared and stored microemulsions were placed on clean glass slides. A couple of drops of water were mixed in using a glass rod, and the transparency or turbidity of the microemulsions were examined.19
Centrifugation stress testing
Next, 2.5 mL samples of the freshly prepared and stored microemulsions were aliquoted into centrifuge tubes and centrifuged at 3000 rpm and 5000 rpm for 30 minutes at ambient temperature to assess their physical instability, including phase separation, phase inversion, aggregation, creaming, and cracking.
Measurements of pH
pH changes could affect product stability and drug bioavailability from a microemulsion at the site of permeation. Accordingly, the pH of the freshly prepared and stored microemulsions was determined using a microprocessor pH meter (pH 211, Hanna Instruments, Cluj-Napoca, Romania) calibrated using pH buffer solution. The pH of each microemulsion was determined in triplicate.20
Assessment of specific gravity
The specific gravity of the freshly prepared and stored multimodal samples was determined using a specific gravity bottle. The bottle was washed, dried completely, and weighed empty at room temperature (about 25°C). The bottle was then filled with the microemulsion and weighed. Specific gravity (g/mL) was calculated using the following formula:
| (1) |
Measurement of viscosity
Viscosity measurements were performed using a viscometer (Brookhaven Instruments Corporation, Holtsville, NY, USA) operating in single mode (Spindle C50).21 All measurements were done in triplicate for 60 seconds at a temperature of about 25°C.
Selection of subjects
Thirty-two men aged 25–30 years with early signs of stage II–IV frontal or vertex pattern androgenic alopecia based on the Norwood-Hamilton scale of baldness in men22 were enrolled into the study. Men with stage V–VII androgenic alopecia were excluded. All subjects were healthy Saudis or Egyptians with fair skin and black hair who also complained of a noticeably oily and slightly itchy scalp. Subjects who were using hair restorers or systemically active drugs, including steroids, nonsteroidal anti-inflammatories, cytotoxic agents, topical antiseptic and/or antifungals, vasodilators, antibiotics, antihypertensive agents, diuretics, or specifically contraindicated agents (such as spironolactone, cimetidine, diazoxide, cyclosporine, finasteride, or ketoconazole) during the previous six months were also excluded. All patients were informed of the likely pharmacological and therapeutic effects of the study formulations as well as their potential adverse effects, based on available drug information. Institutional review board approval and written informed consent were obtained before the participants were enrolled in the study.
Study design
This was a randomized, 32-week, placebo-controlled, double-blind investigation of the safety and efficacy of a multimodal emulsion containing minoxidil, diclofenac, and tea tree oil (n = 11) versus minoxidil formulation alone (n = 11) and a placebo control formulation (n = 10) for the treatment of early-stage frontal and vertex male-pattern androgenic alopecia in men.22 The patients applied 1 mL of each test solution at approximately 12-hour intervals to the frontoparietal and vertex areas of the scalp for 32 weeks. All cases were followed up and evaluated weekly by an external investigator and by the patients themselves.
Hair sampling
Sites for hair sampling were selected from the frontal and vertex scalp area most affected by alopecia.23 Hair in the designated area was hand-clipped during the initial baseline screening procedure and at eight-week intervals thereafter, for a total period of 32 weeks. Treatment was started immediately after the first clipping and continued for four successive clippings at eight-week intervals.
Hair clipping technique
A 2 cm2 rigid transparent plastic template was placed over the chosen scalp site. The hair within the square area was carefully pulled through, then grasped and hand-clipped close to the scalp with small straight surgical scissors. After removing the template, two diagonal corners of the outlined square were marked permanently with sterile Indian ink from a 21-gauge needle.23 Hair clipping was performed at eight-week intervals, and the cut hairs were carefully placed on collection paper. Before removing the template, the clipped area was inspected with a magnifying lens for any missed or loose hairs that should be included.
Application of treatment
Following hair clipping, the subjects were provided with the test solutions in a randomized manner. The assigned medications were provided in identically appearing prelabeled bottles. The subjects were instructed to use a calibrated dropper for application of 1 mL of each test microemulsion twice daily to the frontoparietal or vertex area of the scalp, starting at the clipped site. The test products were applied to a dry scalp and spread with a finger tip, and the hair was allowed to dry naturally without a hair dryer.
Assessment
Numerous methods are available for assessment of hair variables, ie, density, growth rate, and mean hair weight, count, and thickness. These techniques can be classified as invasive (eg, biopsy), semi-invasive (trichogram, unit area trichogram), or noninvasive (eg, global hair count, global photographic assessment, and phototrichogram).
The phototrichogram24 is a simple, reproducible, and sensitive noninvasive technique that is used to determine the rate of hair growth, size of hair fibers, and proportion of hair follicles in telogen phase, and to quantify the amount of hair shed. All hairs in a 2 cm2 area are trimmed 1 mm from the skin surface and a baseline photograph is taken. After a week, the same region is photographed and the hairs are trimmed again. This process is repeated until an adequate number of photographs are available for comparison. Comparison with baseline photographs identifies hair fibers that have grown (follicles in anagen) and those that have not (follicles in telogen), rate of hair growth over seven days, hair density (number of hairs counted in the photograph), and which hair fibers are missing five days later (hair shedding).
In 2001, Hoffmann developed a commercial fully computerized phototrichogram technique using automated software25 whereby a 1.8 cm2 area of hair loss showing a transition between normal hair growth and a balding area for men with androgenic alopecia and an area in the mid vertex for women with diffuse hair loss is chosen for clipping. The area on the scalp is marked with a central black tattoo. Clipped hairs within the target area are dyed and photographs are taken immediately afterwards and then 2–3 days after shaving using a digital closeup camera with epiluminescence microscopy. Two photographs are then compared using a software system that can recognize images of individual hair fibers. By comparing the two photographs, the computer can determine which hairs are growing and which are not. Because the results are reproducible, this software can be used to monitor the success of treatment. However, in this pilot study, we used previously reported noninvasive techniques,4,23 including determination of mean hair weight, hair count, and hair thickness, as well as a global photographic method.
Determination of mean hair weight, count, and thickness
The hair samples were degreased and then weighed in high-performance liquid chromatography grade acetone for at least one hour. When completely dry, the hair was transferred to a light aluminum weighing pan with a 5 digits readability balance. The relative humidity of the balance chamber was measured using a digital hygrometer calibrated at 20% and 90% relative humidity using saturated solutions of potassium acetate and zinc sulfate, respectively. The relative humidity of the balance chamber was recorded at 65% after conditioning for two hours, and the hair samples were then weighed. The entire hair samples were then individually laid out for counting in groups of ten on a marked grid using a wide-field zone projection microscope.23 After counting, subsamples containing 25 hairs each were measured for thickness. For convenience and reproducibility, only two subsamples were taken from each subject at baseline and after 32 weeks of treatment. The hair subsamples were mounted on large slides and covered with a piece of thin plastic wrap film to enable simple visual observation. Hair thickness was measured at the midpoint of each individual hair by tracking the projected image with a 10 × reticle, and dividing by a lens magnification of 71.2.23
Patient self-assessment
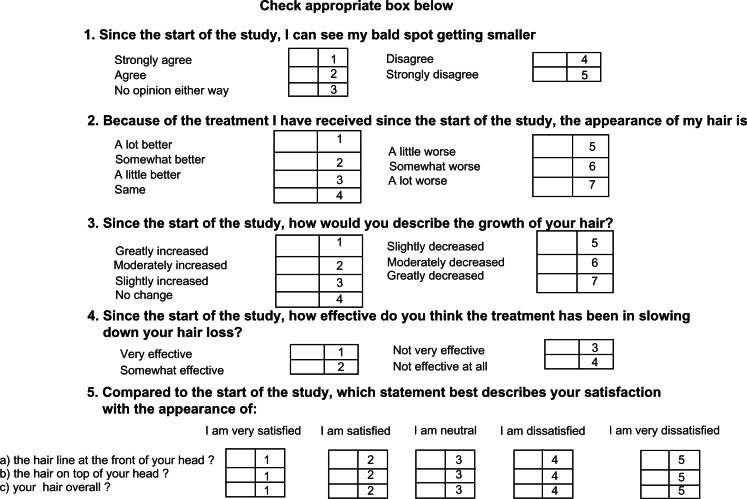
Patients assessed their scalp hair using a validated, self-administered hair growth questionnaire26 containing four questions on treatment efficacy and three questions on satisfaction with appearance (Figure 2). Mean ranking of responses by patients treated with the different microemulsions and their significance were assessed using a computerized Kruskal–Wallis test accounting for different weighting of answers and covariance between the questions.
Figure 2.
Patient self-assessment questionnaire concerning changes in their scalp hair after treatment.
Investigator assessment
Investigators assessed the participants at all time points using a standardized seven-point rating scale of hair growth compared with baseline (−3, greatly decreased; −2, moderately decreased; −1, slightly decreased; 0, no change; +1, slightly increased; +2, moderately increased; +3, greatly increased). Baseline global photographs for each patient were given to the investigator for reference.24
Global photographic assessment
Standardized global color photographs of the frontal and vertex scalp were taken. Paired baseline and post-treatment slides were examined independently by two investigators using the standardized seven-point rating scale.26 This technique has previously been demonstrated to have excellent test-retest reproducibility.27
Statistical analysis
Statistical analysis of the results for hair count, responses to the individual patient self-assessment questionnaire, and the investigator and global photographic assessments were done using analysis of variance.
Results
The ternary phase diagram shown in Figure 1 indicates that the composition and ratios of the surfactant, cosurfactant, and water used for preparation of the microemulsions existing in the designated region of the microemulsion zone. The compositions chosen for microemulsions A, B, and C (Table 1) were identical, except for the number of active ingredients in each formulation.
Physical properties and stability
The results in Table 2 shows the physical properties, including appearance, color, odor, viscosity, water dilution, optical transparency, dye staining tests, specific gravity, pH and centrifugal stress tests, for the freshly prepared multimodal microemulsion and the corresponding values after 24 months of storage. It is clear that the multimodal formulation is an o/w emulsion and that both the freshly prepared and stored formulations did not show any appreciable differences in physical properties, indicating good stability.
Table 2.
Results of stability testing for formulation A containing minoxidil, diclofenac, and tea tree oil at baseline and after 24 months of storage
| Test | Initial product | After 24 months of storage |
|---|---|---|
| Appearance | Clear and transparent | Clear and transparent |
| Color | Conforms | |
| Odor | Characteristically aromatic | Conforms |
| Specific gravity | 1.21 ± 0.015 g/mL | 1.25 ± 0.01 g/mL |
| pH | 5.7 ± 0.01 | 5.9 ± 0.025 |
| Water dilution test | Clear/transparent o/w | Clear/transparent o/w |
| Staining tests | Confirm o/w emulsion | Confirm o/w emulsion with no phase inversion |
| Optical transparency | Transparent/translucent/clear | Transparent/translucent/clear |
| Mean viscosity (PaS) | 0.79 ± 0.012 | 0.81 ± 0.071 |
| Centrifugation at 3000 rpm | No phase separation, turbidity, cracking, or creaming (stable) | No phase separation, turbidity, cracking, or creaming (stable) |
| Centrifugation at 5000 rpm | No phase separation, turbidity, cracking, or creaming (stable) | No phase separation, turbidity, cracking, or creaming (stable) |
Hair growth
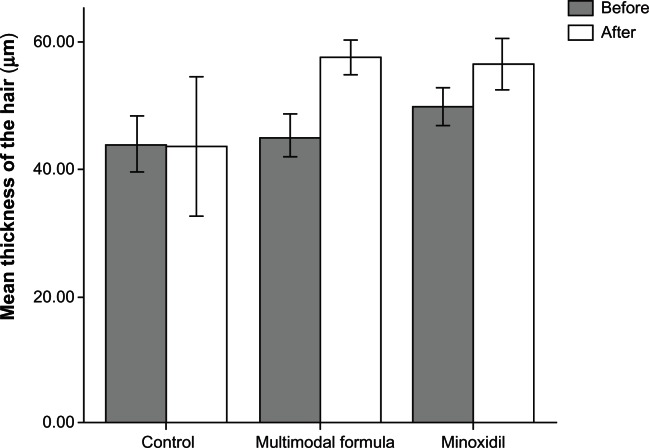
A summary of the results for hair growth measured on four occasions at eight-week intervals is provided in Figures 3–7 and Tables 3–6. Figure 3 compares the mean ± standard deviation thickness (μm) of hair at baseline and after 32 weeks of treatment with formulation A, B, or C. The differences in column height before and after treatment were in the order of multimodal microemulsion > minoxidil > placebo.
Figure 3.
Comparison of mean hair thickness (μm) at baseline and after 32 weeks of treatment.
Figure 7.
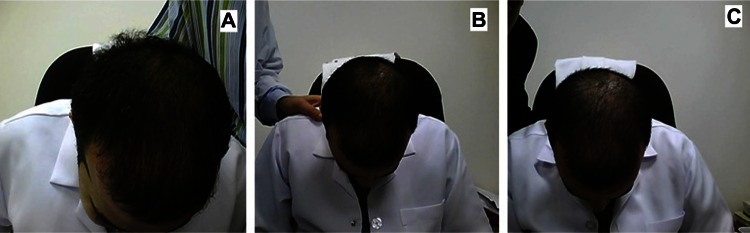
Representative photographs of a subject with frontal alopecia treated with placebo. (A) Baseline, (B) at week 8 (slight decrease in hair growth), and (C) week 16 (moderate decrease in hair growth).
Table 3.
Statistical evaluation of effects of formulations A, B, and C on mean hair count
|
Hair count (mean ± standard
deviation)
|
F | P-value | |||
|---|---|---|---|---|---|
| Formulation A | Formulation B | Formulation C | |||
| Baseline | 133.6 ± 26a | 122.3 ± 14.9a | 139.8 ± 23.9a | 1.597 | 0.221 |
| 8 weeks | 157.2 ± 29.9b | 136.1 ± 11.4a,b | 124.2 ± 23.1a | 5.599 | 0.009** |
| 16 weeks | 157.8 ± 29.9b | 136.1 ± 11.3c | 111.1 ± 21.5a | 23.333 | 0.000*** |
| 24 weeks | 201.4 ± 28.3b | 159.4 ± 12.4c | 100.0 ± 16.7a | 63.146 | 0.000*** |
| 32 weeks | 217.3 ± 27.9b | 170.5 ± 11.9c | 82.9 ± 13.5a | 126.165 | 0.000*** |
Notes: F and P-value calculated using one-way analysis of variance;
difference significant at P < 0.01;
difference significant at P < 0.001.
denote significance of difference at P < 0.05 calculated by Scheffe’s post hoc test.
Table 6.
Percentages of patients with improvement in scalp hair as per the investigator’s evaluation and global photographic assessment
|
Multimodal
|
Minoxidil
|
Placebo
|
Difference
|
Difference
|
|
|---|---|---|---|---|---|
| Formulation A | Formulation B | Formulation C | A versus C | B versus C | |
| Investigator assessment | |||||
| Increased hair growth | 75% | 37% | 16% | 59 (43, 76) | 21 (17, 30) |
| Photographic assessment | |||||
| Increased hair growth | 79% | 41% | 13% | 66 (63, 84) | 28 (21, 48) |
The effect of microemulsions A, B, and C on mean hair count is shown in Table 3, indicating that, as the treatment period increased from baseline to 32 weeks, the hair count increased progressively in the group treated with the multimodal emulsion and to a greater extent than in the group treated with the microemulsion containing minoxidil alone, while the placebo group showed a marked decrease in hair count. The last column in Table 3 shows that the effect of the multimodal emulsion on hair count is highly significant compared with that of the minoxidil and placebo formulations (P = 0.009 and P < 0.001, respectively).
Table 4 shows the effects of emulsions A, B, and C on mean hair weight, and are in agreement with those seen in Table 3, ie, during the 32-week treatment period, there was a progressive increase in hair weight, which was significantly better for the multimodal minoxidil microemulsion than for the formulations containing minoxidil alone or placebo (P < 0.001).
Table 4.
Statistical evaluation of effects of formulations A, B, and C on mean hair weight
|
Hair weight (mg, mean ± standard
deviation)
|
F | P-value | |||
|---|---|---|---|---|---|
| Formulation A | Formulation B | Formulation C | |||
| Baseline | 8.06 ± 1.09 | 7.80 ± 0.53 | 7.37 ± 0.76 | 1.765 | 0.190 |
| 8 weeks | 9.24 ± 1.46a | 8.40 ± 0.71a | 6.95 ± 0.75a | 12.554 | 0.000*** |
| 16 weeks | 10.62 ± 1.59b | 9.13 ± 0.95b | 6.48 ± 0.60a | 34.918 | 0.000*** |
| 24 weeks | 11.83 ± 1.74b | 9.77 ± 0.94c | 5.99 ± 0.52a | 62.755 | 0.000*** |
| 32 weeks | 12.30 ± 1.76b | 10.32 ± 1.02c | 5.34 ± 0.41a | 89.650 | 0.000*** |
Notes: F and P-value calculated using one-way analysis of variance;
denotes difference significant at P < 0.001.
denote significant difference at P < 0.05 calculated using the Scheffe’s post hoc test.
Table 5 shows the statistical analysis of results for the patient evaluation questionnaire. The multimodal microemulsion containing minoxidil showed the best rank values, followed by the minoxidil only and placebo microemulsions, with highly statistically significant treatment differences (P = 0.000). Table 6 shows the percentages of patients with improvement in hair growth on the scalp as assessed by the investigators and global photographic evaluation. The multimodal treatment microemulsion showed a 75%–79% increase in hair growth versus 37%–41% for minoxidil alone and 13%–16% for placebo.
Table 5.
Statistical evaluationa,b of the mean ranks between the three treatment groups based on patient evaluation questionnaire
| Questions | Placebo (mean rank) | Minoxidil (mean rank) | Multimodal (mean rank) | DF | Chi square | P-value |
|---|---|---|---|---|---|---|
| Patient self-assessment | ||||||
| Q1 | 27.05 | 14.00 | 9.41 | 2 | 22.237 | 0.000*** |
| Q2 | 27.10 | 16.23 | 7.14 | 2 | 25.065 | 0.000*** |
| Q3 | 26.90 | 17.09 | 6.45 | 2 | 26.027 | 0.000*** |
| Q4 | 27.30 | 16.27 | 6.91 | 2 | 26.899 | 0.000*** |
| Q5 | 27.50 | 16.09 | 6.91 | 2 | 26.513 | 0.000*** |
| Q6 | 28.54 | 17.45 | 7.06 | 2 | 27.55 | 0.000*** |
| Q7 | 28.65 | 17.99 | 7.46 | 2 | 27.85 | 0.000*** |
Notes:
Kruskal–Wallis test;
grouping variable;
denotes difference significant at P < 0.001; Q1, size of bald spots; Q2, appearance of hair; Q3, growth of hair; Q4, slowing hair loss; Q5, satisfaction with hair line at front of head; Q6, satisfaction with hair on top of head; Q7, hair overall.
Abbreviation: DF, degrees of freedom.
Figure 4 shows representative photographs for subjects with vertex alopecia treated using the multimodal minoxidil microemulsion at baseline (Figure 4A), indicating a moderate increase in hair growth at week 8 (Figure 4B) and greatly increased hair growth at week 16 (Figure 4C). Figure 5 shows similarly representative photographs for subjects with frontal alopecia treated using the multimodal minoxidil microemulsion at baseline (Figure 5A), indicating a moderate increase in hair growth at week 8 (Figure 5B) and markedly increased hair growth at week 16 (Figure 5C). Figure 6 shows representative photographs for subjects with frontal alopecia treated with the microemulsion containing minoxidil alone at baseline (Figure 6A), indicating a slight increase in hair growth at week 8 (Figure 6B) and a moderate increase in hair growth at week 16 (Figure 6C). Figure 7 shows representative photographs of placebo-treated subjects with frontal alopecia at baseline (Figure 7A), indicating a slight decrease in hair growth at week 8 (Figure 7B) and a moderate decrease in hair growth at week 32 (Figure 7C).
Figure 4.
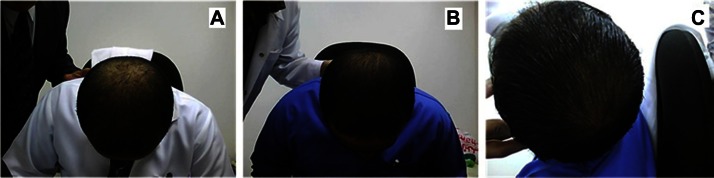
Representative photographs of a subject with vertex alopecia treated with the multimodal minoxidil microemulsion. (A) Baseline, (B) at week 8 (moderately increased hair growth), and (C) week 16 (markedly increased hair growth).
Figure 5.

Representative photographs of a subject with frontal alopecia treated with the multimodal minoxidil microemulsion. (A) Baseline, (B) at week 8 (moderately increased hair growth), and (C) week 16 (markedly increased hair growth).
Figure 6.

Representative photographs of a subject with frontal alopecia treated with minoxidil alone. (A) Baseline, (B) at week 8 (slightly increased hair growth), and (C) week 16 (moderately increased hair growth).
Discussion
Physical and stability tests for microemulsion A
The results of physical testing of the freshly prepared and stored multimodal minoxidil microemulsions were similar, with no marked changes seen. The microemulsions remained translucent and visually clear. Dilatability with aqueous phase and dye staining tests showed that the products were o/w microemulsions with no sign of phase inversion. Physical stability tests were done 10 and 30 days after preparation and again after 24 months of storage to detect any appreciable changes in consistency, color, odor, pH, viscosity, specific gravity, and centrifugal stress. The stored samples are found to be physically stable, with no signs of creaming, cracking, or phase separation.
Hair growth
Mean hair count
Table 3 shows a steady increase in mean hair count in the group treated with the multimodal minoxidil microemulsion which was more pronounced from baseline onwards throughout the study than in the group treated with the microemulsion containing minoxidil only, which in turn was more pronounced than in the placebo group. One-way analysis of variance showed no significant difference in mean hair count between the three treatment groups at baseline (P > 0.2). Improvement in hair count with the multimodal minoxidil microemulsion was significantly greater than with minoxidil alone or placebo throughout the treatment period (P < 0.01 and P < 0.001, respectively).
Mean hair weight
The mean hair weight results shown in Table 4 are consistent with those for mean hair count in Table 3. Mean hair weight for the multimodal minoxidil microemulsion was greater from baseline onwards compared with that for the minoxidil only and placebo formulations. One-way analysis of variance showed no statistically significant difference between the three treatment formulations at baseline (P > 0.19). However, improvement in mean hair weight for the multimodal minoxidil formulation was significantly greater (P < 0.001) than that from the minoxidil only and placebo formulations throughout the study.
Mean hair thickness
Figure 3 shows mean hair thickness in the three treatment groups at baseline and at the end of the 32-week treatment period. Variation in hair thickness measurements taken at eight-week intervals was found to be erratic, irreproducible, and too time-consuming a procedure for comparison between the different treatment groups. There was no appreciable change in column height in the placebo group before and after treatment. However, statistically significant differences in mean hair thickness were found between baseline and the end of treatment using the multimodal minoxidil microemulsion (P < 0.05, paired t-test). These findings are in agreement with the abovementioned evaluation results and are indicative of the superiority of the multimodal minoxidil formulation versus the minoxidil only and placebo formulations.
Patient self-assessment
Statistical evaluations of the mean ranks between the treatment groups for the patient self-evaluation questionnaire are presented in Table 5, and the results for responses to the patient self-assessment questionnaire are shown in Table 6. The results indicate that the multimodal minoxidil formulation was significantly superior (P < 0.001) according to the patients, and had an earlier onset of action in comparison with the minoxidil only and placebo formulations. Patients treated with the multimodal minoxidil formulation reported alleviation of scalp itching, reduced sebum production, and a pronounced reduction in amount of hair shedding during the first week of treatment compared with the subjects treated with minoxidil only, who reported a delayed effect, starting after 4–5 weeks of treatment. Treatment with the placebo formulation resulted in significant hair shedding.
Investigator and photographic assessments
Figures 4 and 5 show representative photographs of subjects with vertex and frontal alopecia, respectively, treated with the multimodal minoxidil microemulsion at baseline and after 8 and 16 weeks of treatment. These were rated as moderately improved at week 16 and greatly improved at week 32. Figure 5 shows representative photographs for subjects treated with the minoxidil only formulation, which were rated as slightly improved at week 16 and moderately improved at week 32. Figure 6 shows a representative example from the placebo-treated group, which was rated as showing a slight and moderate decrease in hair growth at weeks 16 and 32, respectively. The results for patient self-assessment shown in Table 5 are further confirmed by the results of investigator and photographic assessment shown in Table 6, indicating a 75%, 37%, and 16% improvement in scalp hair growth according to investigator assessment in the multimodal minoxidil, minoxidil only, and placebo groups, respectively, and a 79%, 41%, and 13% improvement according to photographic assessment, all favoring the multimodal minoxidil microemulsion.
Both the investigator assessment and patient reports indicated that signs and symptoms of contact dermatitis (ie, stinging, burning, itching, dryness, scaling) were more prevalent in the group treated with the 5% minoxidil solution (seven of 11 patients, about 64%) than in the placebo group (four of 10 patients, about 40%). In contrast, symptoms in the group treated with the multimodal minoxidil formulation were less prevalent (one of 11 patients, about 9%). These results are indicative of better tolerability and safety using the multimodal minoxidil formulation in comparison with the minoxidil only and placebo options. The superior tolerability of the multimodal minoxidil formulation could be attributed to the inclusion of ibuprofen, a nonsteroidal anti-inflammatory agent, that ameliorates inflammation of both the scalp and hair follicles. Reduced inflammation could minimize sebum production, which provides a good nutrition medium for bacterial and fungal infections. Inclusion of tea tree oil has the additional advantage of helping to eliminate bacterial and fungal organisms that could modify androgen metabolism and lead to hair fall. It appears that the multimodal formulation further potentiates the effect of minoxidil in increasing hair density, improving hair growth, and reducing inflammation and risk of bacterial infection.
Conclusion
All evaluations showed highly significant improvements using the multimodal minoxidil microemulsion in comparison with the minoxidil only formulation which, in turn, was significantly more effective than placebo. The pronounced effects of the multimodal minoxidil formulation compared with minoxidil alone could be attributed to the anti-inflammatory effect of diclofenac in reducing scalp and follicular microinflammation and the tea tree oil in resolving any existing microbial or fungal colonization of the hair follicles. These results indicate that androgenic alopecia is multifactorial and perhaps polygenic in nature. Hence, an effective multimodal microemulsion comprising minoxidil, an anti-inflammatory agent, and an anti-infective agent is recommended as being more promising than minoxidil alone. Diclofenac was used in the multimodal formulation instead of a cortisone derivative to reduce the risk of side effects, and tea tree oil was used on the basis that it is a highly effective natural anti-infective agent.15,16 Further study is planned in a larger patient population for an extended period to monitor the long-term effects in individuals with androgenic alopecia accompanied by hair follicle infection and/or microinflammation.
Acknowledgments
The authors thank Abdullah Al Shammery from the Riyadh Colleges of Dentistry and Pharmacy for providing the encouragement and support that made this work possible. They are also grateful to H Mosadomi from the Research Center, and to Sharat Pani and other colleagues for their constructive observations and suggestions throughout this investigation.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mahe YF, Michelet JF, Billoni N, et al. Androgenetic alopecia and microinflammation. Int J Dermatol. 2000;39:576–584. doi: 10.1046/j.1365-4362.2000.00612.x. [DOI] [PubMed] [Google Scholar]
- 2.Millikan LE. Androgenetic alopecia: The role of inflammation and demodex. Int J Dermatol. 2001;40:475–476. doi: 10.1046/j.1365-4362.2001.01173-4.x. [DOI] [PubMed] [Google Scholar]
- 3.Zari J, Abdolmajid F, Masood M, Vahid M, Yalda N. Evaluation of the relationship between androgenetic alopecia and demodex infestation. Indian J Dermatol. 2008;53:64–67. doi: 10.4103/0019-5154.41647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol. 1998;39:578–589. doi: 10.1016/s0190-9622(98)70007-6. [DOI] [PubMed] [Google Scholar]
- 5.Mahe YF. Inflammatory perifollicular fibrosis and alopecia. Int J Dermatol. 1998;37:416–417. doi: 10.1046/j.1365-4362.1998.00514.x. [DOI] [PubMed] [Google Scholar]
- 6.Vollmer RT. Demodex-associated folliculitis. Am J Dermatopathol. 1996;18:589–591. doi: 10.1097/00000372-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Whiting DA. Diagnostic and predictive value of horizontal sections of scalp biopsy specimens in male pattern androgenetic alopecia. J Am Acad Dermatol. 1993;28:755–763. doi: 10.1016/0190-9622(93)70106-4. [DOI] [PubMed] [Google Scholar]
- 8.Whiting DA. Chronic telogen effluvium: increased scalp hair shedding in middle aged women. J Am Acad Dermatol. 1996;35:899–906. doi: 10.1016/s0190-9622(96)90113-9. [DOI] [PubMed] [Google Scholar]
- 9.Sinclair RD. Alopecia: Common baldness and androgenetic alopecia. In: Burn T, Breathnach S, Cox N, Griffiths C, editors. Rook’s Textbook of Dermatology. 7th ed. London, UK: Blackwell Science; 2004. [Google Scholar]
- 10.Ralph MT. Is androgenetic alopecia a photoaggravated dermatosis? Dermatology. 2003;207:343–348. doi: 10.1159/000074111. [DOI] [PubMed] [Google Scholar]
- 11.Piérard G, Piérard-Franchimont C, Tassoudji N, et al. Improvement in the inflammatory aspect of androgenetic alopecia: a pilot study with an antimicrobial lotion. J Dermatol Treat. 1996;7:153–157. [Google Scholar]
- 12.Berger RS, Fu JL, Smiles KA, et al. The effects of minoxidil, 1% pyrithione zinc and a combination of both on hair density: a randomized controlled trial. Br J Dermatol. 2003;149:354–362. doi: 10.1046/j.1365-2133.2003.05435.x. [DOI] [PubMed] [Google Scholar]
- 13.Khandpour S, Suman M, Reddy BS. Comparative efficacy of various treatment regimens for androgenetic alopecia in men. J Dermatol. 2002;29:489–498. doi: 10.1111/j.1346-8138.2002.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 14.Kligman AM, inventor. Combination of minoxidil and an anti-inflammatory agent for treating patterned alopecia. 1991. Jun 25, United States patent US 5026691A.
- 15.Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50–62. doi: 10.1128/CMR.19.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Concha JM, Moore LS, Holloway WJ. Antifungal activity of Melaleuca alternifolia (tea-tree) oil against various pathogenic organisms. J Am Podiatr Med Assoc. 1998;88:489–492. doi: 10.7547/87507315-88-10-489. [DOI] [PubMed] [Google Scholar]
- 17.Keilgaard M. Influence of microemulsions on cutaneous drug delivery. Adv Drug Deliv Rev. 2002;54:77–98. doi: 10.1016/s0169-409x(02)00116-3. [DOI] [PubMed] [Google Scholar]
- 18.Moghimipour E, Salimi A, Leis F. Preparation and evaluation of tretinoin microemulsion based on pseudo-ternary phase diagram. Adv Pharm Bull. 2012;2:141–147. doi: 10.5681/apb.2012.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonia K, Anupama D. Microemulsion based transdermal drug delivery of tea tree oil. Int J Drug Dev Res. 2011;3:191–198. [Google Scholar]
- 20.Behera J, Keservant RK, Yadav A, Tripathi M, Chadoker A. Methoxsalen loaded chitosan coated microemulsion for effective treatment of psoriasis. Int J Drug Deliv. 2010;2:159–167. [Google Scholar]
- 21.Baroli B, Lopez-Quitela MA, Delgado-Charro MB, Fadda AM, Blanco-Mendez J. Microemulsions for topical delivery of 8-methoxsalen. J Control Release. 2000;69:209–218. doi: 10.1016/s0168-3659(00)00309-6. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton JB. Patterned loss of hair in men: types and incidence. Ann N Y Acad Sci. 1951;53:708–728. doi: 10.1111/j.1749-6632.1951.tb31971.x. [DOI] [PubMed] [Google Scholar]
- 23.Price VH, Menefee E. Quantitative estimation of hair growth I. Androgenetic alopecia in women: effect of minoxidil. J Invest Dermatol. 1990;95:683–687. doi: 10.1111/1523-1747.ep12514348. [DOI] [PubMed] [Google Scholar]
- 24.Rolf H. TrichoScan: a novel tool for the analysis of hair growth in vivo. J Invest Dermatol. 2003;8:109–115. doi: 10.1046/j.1523-1747.2003.12183.x. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann R. TrichoScan: combining epiluminescence microscopy with digital image analysis for the measurement of hair growth in vivo. Eur J Dermatol. 2001;11:362–368. [PubMed] [Google Scholar]
- 26.Barber BL, Kaufman KD, Kozolff RC, Girman CJ, Guess HA. A hair growth questionnaire for use in the evaluation of therapeutic effects in men. J Dermatol Treat. 1998;9:181–186. [Google Scholar]
- 27.Kaufmann K, Binkowitz B, Savin R, Canfield D. Reproducibility of global photographic assessments of patients with male pattern baldness in clinical trial with finasteride. J Invest Dermatol. 1995;104:659. [Google Scholar]