Abstract
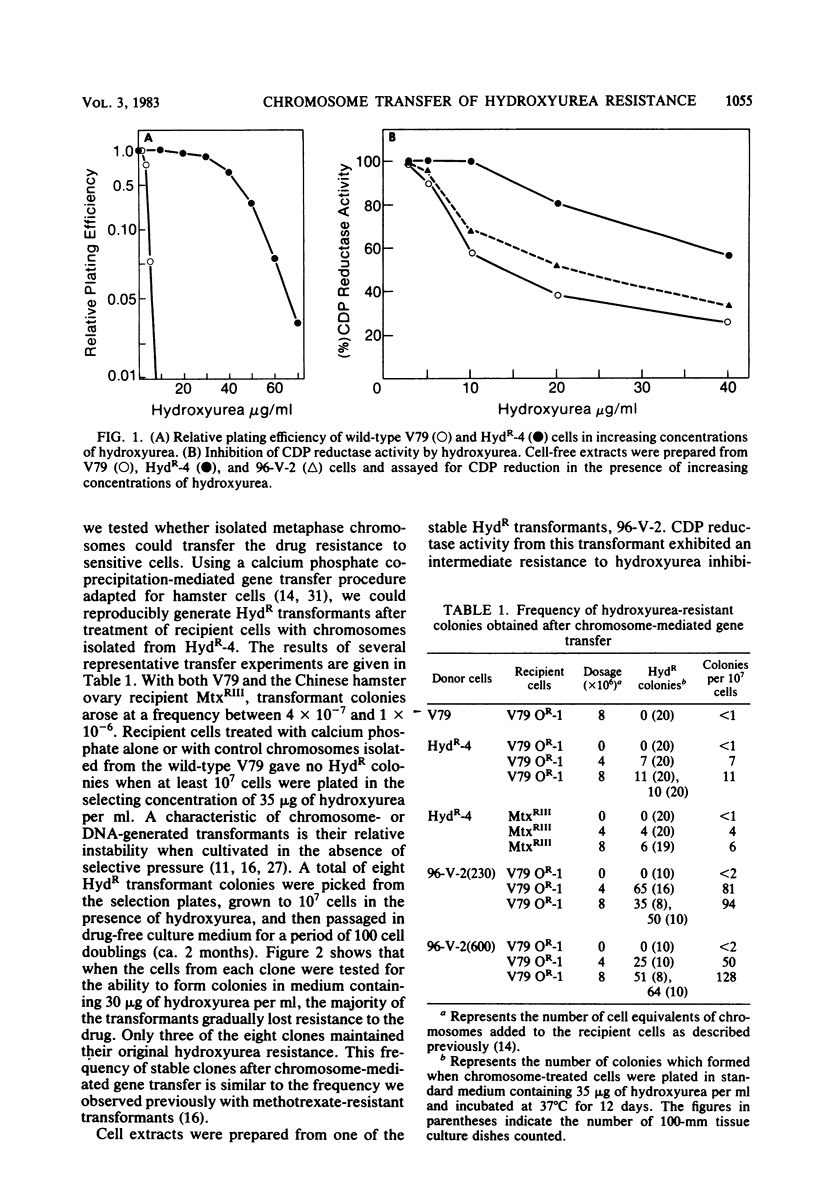
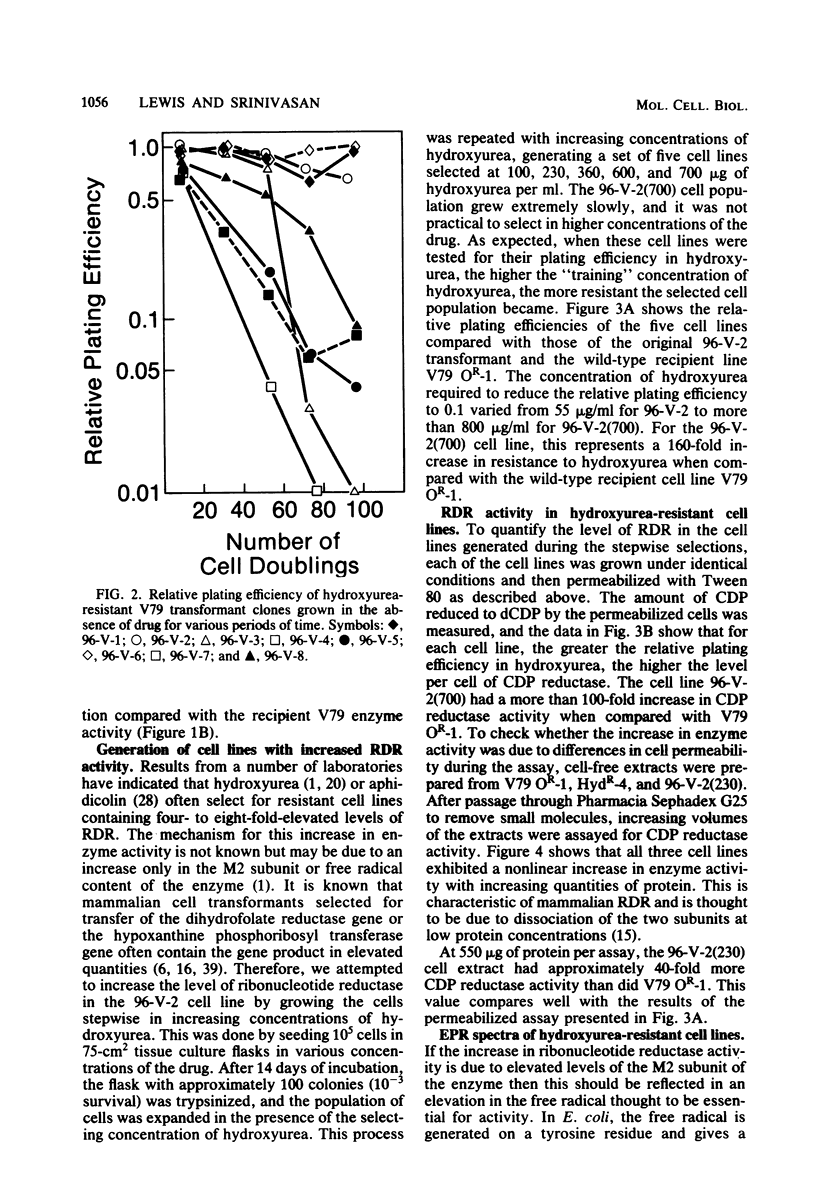
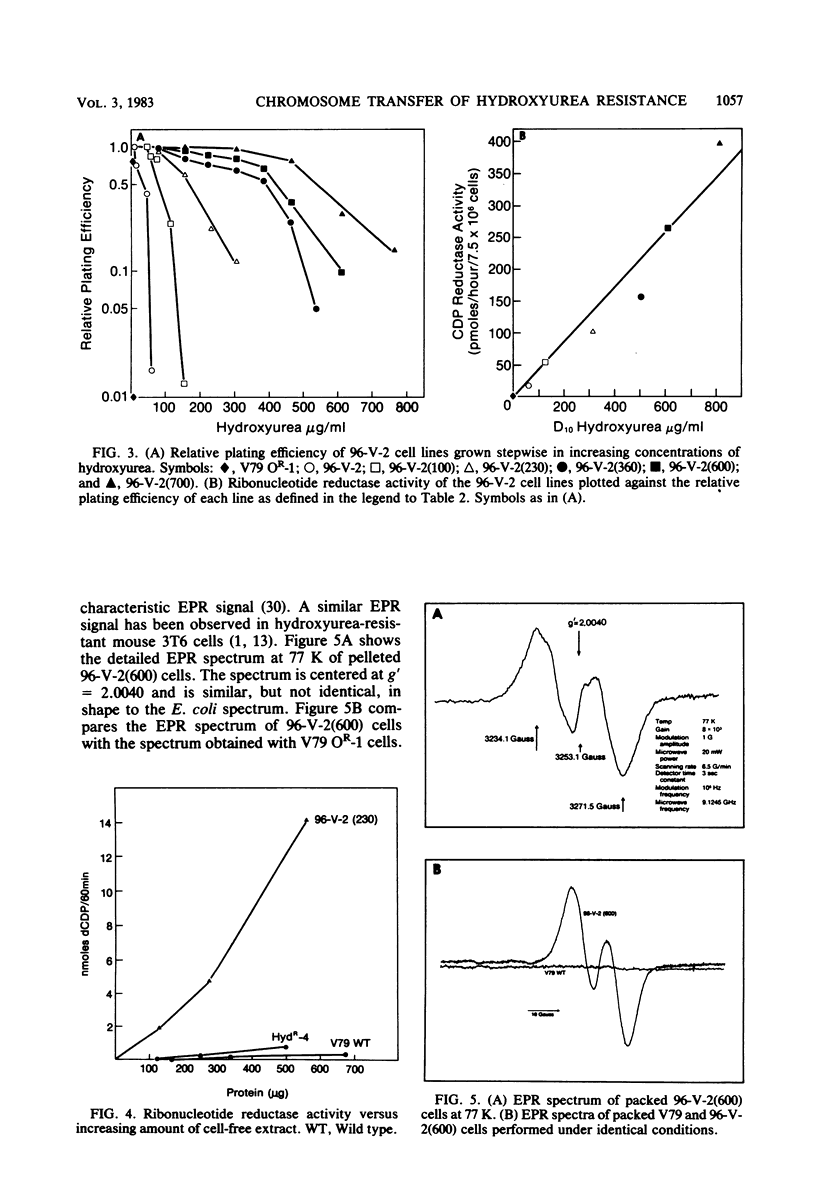
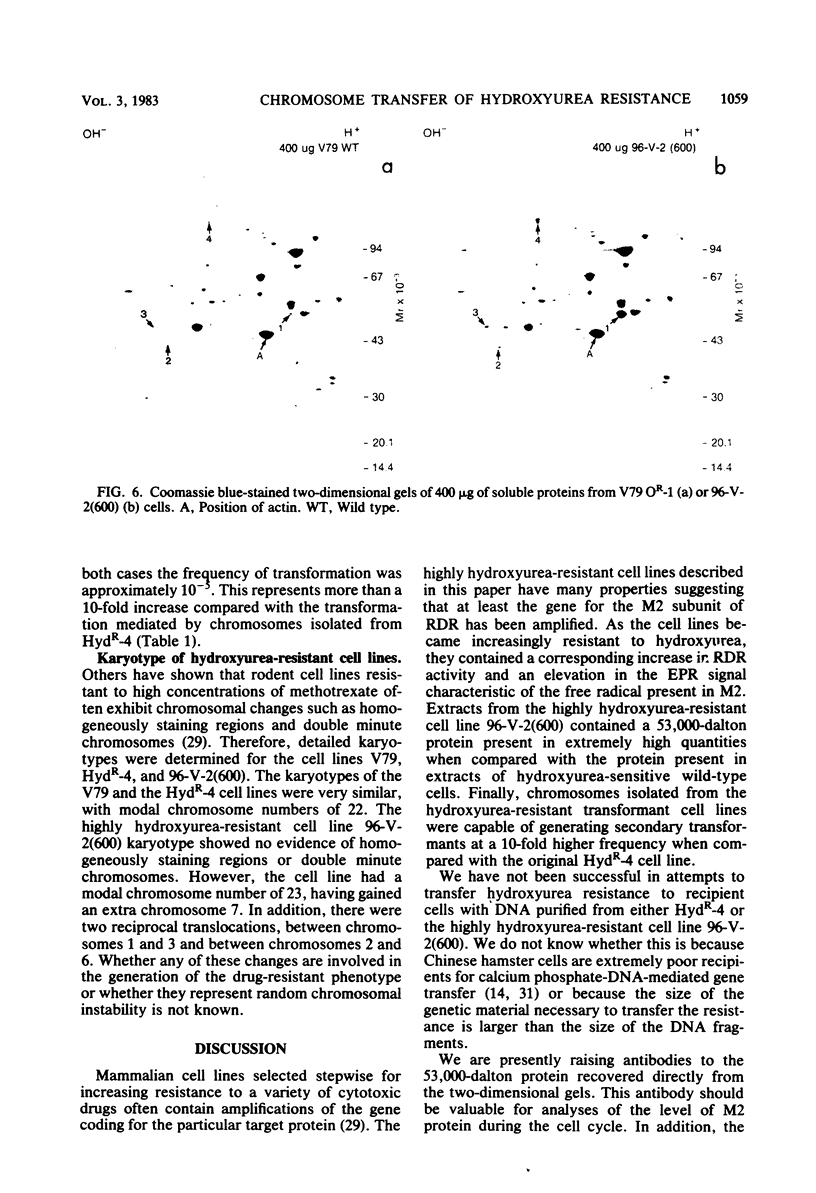
Metaphase chromosomes purified from a hydroxyurea-resistant Chinese hamster cell line were able to transform recipient wild-type cells to hydroxyurea resistance at a frequency of 10(-6). Approximately 60% of the resulting transformant clones gradually lost hydroxyurea resistance when cultivated for prolonged periods in the absence of drug. One transformant was subjected to serial selection in higher concentrations of hydroxyurea. The five cell lines generated exhibited increasing relative plating efficiency in the presence of the drug and a corresponding elevation in their cellular content of ribonucleotide reductase. The most resistant cell line had a 163-fold increase in relative plating efficiency and a 120-fold increase in enzyme activity when compared with the wild-type cell line. The highly hydroxyurea-resistant cell lines had strong electron paramagnetic resonance signals characteristic of an elevated level of the free radical present in the M2 subunit of ribonucleotide reductase. Two-dimensional electrophoresis of cell-free extracts from one of the resistant cell lines indicated that a 53,000-dalton protein was present in greatly elevated quantities when compared with the wild-type cell line. These data suggest that the hydroxyurea-resistant cell lines may contain an amplification of the gene for the M2 subunit of ribonucleotide reductase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerblom L., Ehrenberg A., Gräslund A., Lankinen H., Reichard P., Thelander L. Overproduction of the free radical of ribonucleotide reductase in hydroxyurea-resistant mouse fibroblast 3T6 cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2159–2163. doi: 10.1073/pnas.78.4.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Bellatin J., Bravo R., Celis J. E. Changes in the relative proportion of transformation-sensitive polypeptides in giant HeLa cells produced by irradiation with lethal doses of x-rays. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4367–4370. doi: 10.1073/pnas.79.14.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. C., Eliasson R., Reichard P., Thelander L. Spectrum and iron content of protein B2 from ribonucleoside diphosphate reductase. Eur J Biochem. 1969 Jul;9(4):512–518. doi: 10.1111/j.1432-1033.1969.tb00639.x. [DOI] [PubMed] [Google Scholar]
- Brown N. C., Reichard P. Ribonucleoside diphosphate reductase. Formation of active and inactive complexes of proteins B1 and B2. J Mol Biol. 1969 Nov 28;46(1):25–38. doi: 10.1016/0022-2836(69)90055-2. [DOI] [PubMed] [Google Scholar]
- Degnen G. E., Miller I. L., Adelberg E. A., Eisenstadt J. M. Overexpression of an unstably inherited gene in cultured mouse cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3956–3959. doi: 10.1073/pnas.74.9.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström Y., Eriksson S., Thelander L., Akerman M. Ribonucleotide reductase from calf thymus. Purification and properties. Biochemistry. 1979 Jul 10;18(14):2941–2948. doi: 10.1021/bi00581a004. [DOI] [PubMed] [Google Scholar]
- Eriksson S., Martin D. W., Jr Ribonucleotide reductase in cultured mouse lymphoma cells. Cell cycle-dependent variation in the activity of subunit protein M2. J Biol Chem. 1981 Sep 25;256(18):9436–9440. [PubMed] [Google Scholar]
- Eriksson S., Thelander L., Akerman M. Allosteric regulation of calf thymus ribonucleoside diphosphate reductase. Biochemistry. 1979 Jul 10;18(14):2948–2952. doi: 10.1021/bi00581a005. [DOI] [PubMed] [Google Scholar]
- Flintoff W. F., Davidson S. V., Siminovitch L. Isolation and partial characterization of three methotrexate-resistant phenotypes from Chinese hamster ovary cells. Somatic Cell Genet. 1976 May;2(3):245–261. doi: 10.1007/BF01538963. [DOI] [PubMed] [Google Scholar]
- Graf L. H., Jr, Urlaub G., Chasin L. A. Transformation of the gene for hypoxanthine phosphoribosyltransferase. Somatic Cell Genet. 1979 Nov;5(6):1031–1044. doi: 10.1007/BF01542658. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gräslund A., Ehrenberg A., Thelander L. Characterization of the free radical of mammalian ribonucleotide reductase. J Biol Chem. 1982 May 25;257(10):5711–5715. [PubMed] [Google Scholar]
- Lewis W. H. Chromosome-mediated gene transfer with the Chinese hamster ovary cell line. Exp Cell Res. 1983 Feb;143(2):309–318. doi: 10.1016/0014-4827(83)90056-3. [DOI] [PubMed] [Google Scholar]
- Lewis W. H., Kuzik B. A., Wright J. A. Assay of ribonucleotide reduction in nucleotide-permeable hamster cells. J Cell Physiol. 1978 Mar;94(3):287–298. doi: 10.1002/jcp.1040940306. [DOI] [PubMed] [Google Scholar]
- Lewis W. H., Srinivasan P. R., Stokoe N., Siminovitch L. Parameters governing the transfer of the genes for thymidine kinase and dihydrofolate reductase into mouse cells using metaphase chromosomes or DNA. Somatic Cell Genet. 1980 May;6(3):333–347. doi: 10.1007/BF01542787. [DOI] [PubMed] [Google Scholar]
- Lewis W. H., Wright J. A. Altered ribonucleotide reductase activity in mammalian tissue culture cells resistant to hydroxyurea. Biochem Biophys Res Commun. 1974 Oct 8;60(3):926–933. doi: 10.1016/0006-291x(74)90403-3. [DOI] [PubMed] [Google Scholar]
- Lewis W. H., Wright J. A. Genetic characterization of hydroxyurea-resistance in Chinese hamster ovary cells. J Cell Physiol. 1978 Oct;97(1):73–85. doi: 10.1002/jcp.1040970108. [DOI] [PubMed] [Google Scholar]
- Lewis W. H., Wright J. A. Isolation of hydroxyurea-resistant CHO cells with altered levels of ribonucleotide reductase. Somatic Cell Genet. 1979 Jan;5(1):83–96. doi: 10.1007/BF01538788. [DOI] [PubMed] [Google Scholar]
- Lewis W. H., Wright J. A. Ribonucleotide reductase from wild type and hydroxyurea-resistant chinese hamster ovary cells. J Cell Physiol. 1978 Oct;97(1):87–97. doi: 10.1002/jcp.1040970109. [DOI] [PubMed] [Google Scholar]
- Lowdon M., Vitols E. Ribonucleotide reductase activity during the cell cycle of Saccharomyces cerevisiae. Arch Biochem Biophys. 1973 Sep;158(1):177–184. doi: 10.1016/0003-9861(73)90611-5. [DOI] [PubMed] [Google Scholar]
- Lowy I., Pellicer A., Jackson J. F., Sim G. K., Silverstein S., Axel R. Isolation of transforming DNA: cloning the hamster aprt gene. Cell. 1980 Dec;22(3):817–823. doi: 10.1016/0092-8674(80)90558-9. [DOI] [PubMed] [Google Scholar]
- MOORE E. C., HURLBERT R. B. Reduction of 5'-cytidylic acid to deoxycytidylic acid by mammalian enzymes. Biochim Biophys Acta. 1960 May 20;40:371–372. doi: 10.1016/0006-3002(60)91371-8. [DOI] [PubMed] [Google Scholar]
- Murphree S., Stubblefield E., Moore E. C. Synchronized mammalian cell cultures. 3. Variation of ribonucleotide reductase activity during the replication cycle of Chinese hamster fibroblasts. Exp Cell Res. 1969 Nov;58(1):118–124. doi: 10.1016/0014-4827(69)90121-9. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Lipsich L., Wigler M. Isolation of the chicken thymidine kinase gene by plasmid rescue. Nature. 1980 May 22;285(5762):207–210. doi: 10.1038/285207a0. [DOI] [PubMed] [Google Scholar]
- Peterson J. L., McBride O. W. Cotransfer of linked eukaryotic genes and efficient transfer of hypoxanthine phosphoribosyltransferase by DNA-mediated gene transfer. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1583–1587. doi: 10.1073/pnas.77.3.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabourin C. L., Bates P. F., Glatzer L., Chang C. C., Trosko J. E., Boezi J. A. Selection of aphidicolin-resistant CHO cells with altered levels of ribonucleotide reductase. Somatic Cell Genet. 1981 Mar;7(2):255–268. doi: 10.1007/BF01567661. [DOI] [PubMed] [Google Scholar]
- Sjöberg B. M., Reichard P. Nature of the free radical in ribonucleotide reductase from Escherichia coli. J Biol Chem. 1977 Jan 25;252(2):536–541. [PubMed] [Google Scholar]
- Steeper J. R., Steuart C. D. A rapid assay for CDP reductase activity in mammalian cell extracts. Anal Biochem. 1970 Mar;34:123–130. doi: 10.1016/0003-2697(70)90092-8. [DOI] [PubMed] [Google Scholar]
- Thelander L., Eriksson S., Akerman M. Ribonucleotide reductase from calf thymus. Separation of the enzyme into two nonidentical subunits, proteins M1 and M2. J Biol Chem. 1980 Aug 10;255(15):7426–7432. [PubMed] [Google Scholar]
- Thelander L. Physicochemical characterization of ribonucleoside diphosphate reductase from Escherichia coli. J Biol Chem. 1973 Jul 10;248(13):4591–4601. [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Thirion J. P., Banville D., Noel H. Galactokinase mutants of Chinese hamster somatic cells resistant to 2-deoxygalactose. Genetics. 1976 May;83(1):137–147. doi: 10.1093/genetics/83.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Perucho M., Kurtz D., Dana S., Pellicer A., Axel R., Silverstein S. Transformation of mammalian cells with an amplifiable dominant-acting gene. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3567–3570. doi: 10.1073/pnas.77.6.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]




