Abstract
OBJECTIVES
The total cavopulmonary connection (TCPC), the current palliation of choice for single-ventricle heart defects, is typically created with a single cylindrical tunnel or conduit routing inferior vena caval (IVC) flow to the pulmonary arteries. Previous studies have shown the haemodynamic efficiency of the TCPC to be sub-optimal due to the collision of vena caval flow, thus placing an extra energy burden on the single ventricle. The use of a bifurcated graft as the Fontan baffle (i.e. the ‘Optiflo’) has previously been proposed on the basis of theoretically improved flow efficiency; however, anatomical constraints may limit its effectiveness in some patients.
METHODS
In this study, an alternative approach to flow bifurcation is proposed, where a triangular insert is placed at the distal end of the IVC graft. The proof of concept for this design is demonstrated in two steps: first, determining the optimal insert size at a fixed Fontan graft size through a parametric study; then, characterizing the efficiency as a function of graft size when compared with a TCPC control. TCPC power loss and IVC flow distribution were the primary metrics of interest and were evaluated under both resting and simulated exercise conditions using an in-house computational fluid dynamics solver.
RESULTS
Results demonstrated that there was an optimal insert size that improved efficiency compared with the TCPC. For an 18-mm Fontan baffle, TCPC power loss was 4.1 vs 3.7 mW with the optimal flow-divider. The optimal insert was then scaled up for a 20-mm graft, with a similar reduction in power loss observed. Flow distribution results were inconsistent, based on sensitivity to the placement of the insert within the baffle.
CONCLUSION
This study demonstrated proof of concept that the flow-divider has the potential to reduce power loss and streamline IVC flow through the TCPC. An appropriate size for the insert in proportion to the Fontan baffle size was identified that reduced losses compared with a TCPC control under both resting and simulated exercise flow conditions.
Keywords: Congenital heart, Single ventricle, Fontan, Haemodynamics
INTRODUCTION
The total cavopulmonary connection (TCPC) is currently the most common approach to the Fontan operation for single-ventricle heart defects [1, 2]. This connection uses a cylindrical ‘tunnel’ or conduit to route inferior vena caval (IVC) flow to the pulmonary arteries (PAs). The TCPC has been shown to promote sub-optimal haemodynamic efficiency due to the collision of caval flows, which can increase cardiovascular workload and decrease exercise tolerance [3–6].
Previous studies have sought to reduce TCPC power loss by manipulating the vena cavae positions and thereby minimize the energy dissipation due to the caval flow collision [7]. Caval offsetting was one suggested solution to prevent head-on collision [4]; however, despite favourable haemodynamics, it is possible that the unidirectional flow that could arise with such an offset can lead to an irregular distribution of hepatic proteins carried through the Fontan pathway, potentially resulting in the development of pulmonary arterio-venous malformations and abnormal vascular development in the lungs [8].
To circumvent these problems, a novel surgical approach to Fontan connection, called the ‘Optiflo’ (US Patent #7811244), was proposed by Soerensen et al. [9]. This connection design calls for the bifurcation of both vena cavae prior to connections to both PAs, such that the connection takes on a diamond shape rather than the ‘+’ configuration of the TCPC. Such a design avoids caval flow collision, streamlines flow from the vena cava to the PAs and promotes an even IVC flow distribution. Subsequent studies stemming from this idea have focused primarily on using a bifurcated Y-graft for only the IVC to reduce the complexity of the design while promoting the beneficial effects of the original Optiflo [10]. Although the original studies using idealized models produced favourable results, translating these designs to patient-specific geometries is non-trivial as there are significant limitations that could affect surgical outcome. These include inadequate space due to the surrounding anatomy and an increase in the amount of suturing required.
Kanter et al. [11] recently reported on a series of 6 patients who received a Y-graft, showing its feasibility. While acute postoperative outcomes were all favourable, some haemodynamic limitations related to branch placement were noted, as well as increased surgical time required for the additional stitching. Haemodynamic analyses by Haggerty et al. [12] showed that the geometric shortcomings of these in vivo connections limited the efficiency gains when compared with virtual extracardiac TCPC controls. Surgical modifications were suggested to improve the energetic performance, which need to be evaluated through future study. Concurrently, this study seeks to evaluate an alternative modification of the Optiflo design: rather than altering the design of the Fontan baffle, we propose to incorporate a flow-dividing element directly into the distal end of the surgical baffle prior to surgery. We hypothesize that this design will similarly improve the haemodynamic efficiency of the Fontan connection compared with standard TCPCs with minimal increases in surgical complexity.
As a first step, computational fluid dynamics (CFD) simulations are used to identify an ideal insert size for such an element based on resting and simulated exercise efficiency results. Secondly, we determine a relationship between the IVC baffle size and its optimal insert size for use with alternate conduit sizes.
MATERIALS AND METHODS
Magnetic resonance imaging (MRI) data from an 8-year old patient were selected from an institutional review board-approved retrospective review of the Georgia Tech Fontan MRI database as a relatively simple and idealized, yet representative TCPC model. The anatomy was reconstructed from an axial stack of static, steady-state MRI images by semi-automatically segmenting the vessels of interest [13, 14]. The patient-specific flow conditions were measured from phase-contrast MRI for use as boundary conditions in the computational models.
A state-of-the-art virtual surgical tool was used to create two idealized Fontan baffles of varying diameters: 18 and 20 mm [15]. The baffles were connected to the native geometry with no lateral offset with respect to the superior vena cava (SVC) using Geomagic Studio (Geomagic, Inc., Research Triangle Park, NC, USA) to best align the opposing SVC flow with the downstream face of the flow-divider upon insertion.
Flow-dividers
Solidworks (Waltham, MA, USA) was used to create various sizes for the baffle insert to determine the optimal size characteristics. A triangular prism was selected as the shape of choice given its similarity to the Optiflo design and its ability to divide IVC flow with minimal disruption. The flow-divider was inserted just below the intersection of the vena cavae and PA, such that, in reality, it would be contained within the artificial graft and not require additional stitching to the native vessels.
Initially, parametric studies were conducted using the 18-mm diameter IVC baffle in order to determine the optimal base and height of a triangle insert for this baffle geometry. The various flow-divider geometries (base–height, mm) included: 12-18, 12-16, 12-14, 10-16, 10-14, 10-12. Each was visually positioned to align the centre of the base with the centre of the IVC baffle. Once the optimal size characteristics were determined, the new design was translated to the 20-mm baffle geometry both with and without insert scaling to confirm the haemodynamic findings.
Computational fluid dynamics analysis
An in-house computational solver based on the method of immersed boundaries with an unstructured, fractional step time advancing implementation was used to simulate the haemodynamics associated with each geometry, including power loss and hepatic (i.e. IVC) flow distribution (HFD) [16]. Triangular surface meshes were created using Gambit (ANSYS, Inc.) before registering within the Cartesian immersed boundary domain; grid spacing was set to 2% of IVC diameter, which has been shown to be sufficiently dense to achieve grid-independent results [16]. Unsteady simulations of Navier-Stokes equations were conducted using time-averaged inflow volume rates with flat velocity profiles, MRI-derived outflow ratios as boundary conditions, and rigid vessel walls. The HFD was determined by uniformly seeding streamlines across the IVC and quantifying the flux distribution to the left and right PAs. Power loss (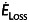 ) was calculated using a control volume approximation:
) was calculated using a control volume approximation:
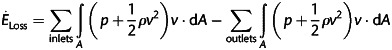 |
where P is the static pressure, ρ the blood density, A area of the inlet/outlet and v the velocity. The specific boundary conditions for this patient are included in Table 1.
Table 1:
Specific boundary conditions
| Resting | Exercise | |
|---|---|---|
| IVC (l/min) | 2.38 (69%) | 6.18 (81%) |
| SVC (l/min) | 1.06 (31%) | 1.42 (19%) |
| LPA (l/min) | 1.8 (70%) | 1.8 (70%) |
| RPA (l/min) | 0.78 (30%) | 0.78 (30%) |
Exercise tolerance has been shown to be unfavourable for Fontan patients [17, 18], and studies have shown that increased cardiac output results in non-linear increases in power loss [6]. Therefore, simulations that model exercise conditions allow for a more complete assessment of the haemodynamics and provide insight on the stress tolerance level of a given surgical geometry. Exercise conditions were tested by doubling the resting aortic flow rate and imposing the difference between resting and exercise flow rate as additional IVC flow. The pulmonary flow splits were maintained constant through the example of previous studies [19].
RESULTS
Insert size optimization
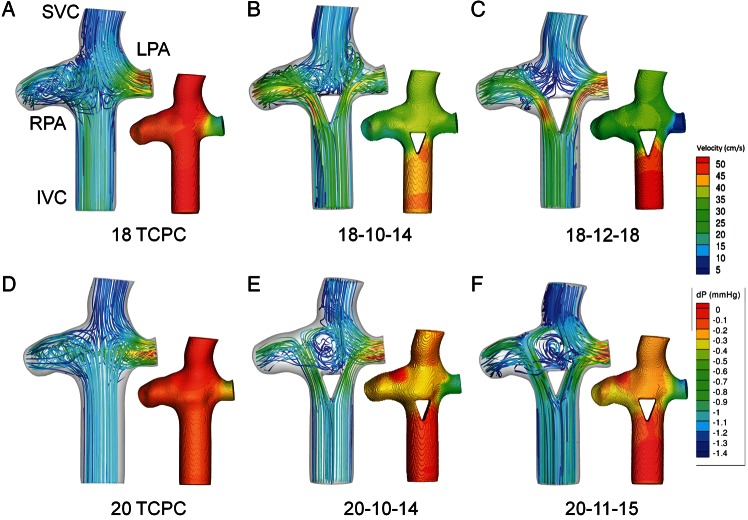
Power loss results are provided in Fig. 1, comparing the 18-mm diameter TCPC standard with the flow-divider options. Losses were found to be sensitive to the size of the flow-divider, with the TCPC being more efficient than 12 mm base designs, but less efficient than the 10-mm base designs. In particular, the insert with a base of 10 mm and a height of 14 mm (18-10-14) resulted in the lowest power loss. The velocity streamlines for the TCPC geometry (Fig. 2A) display the typical collision and mixing of caval inflows at the centre of the connection. On the other hand, the addition of the 10-14-mm flow-divider (Fig. 2B) and 12-18-mm flow-divider (Fig. 2C), redirected IVC flow away from the connection centre to reduce such collisions. However, local acceleration of flow on the lateral sides of the divider was observed.
Figure 1:
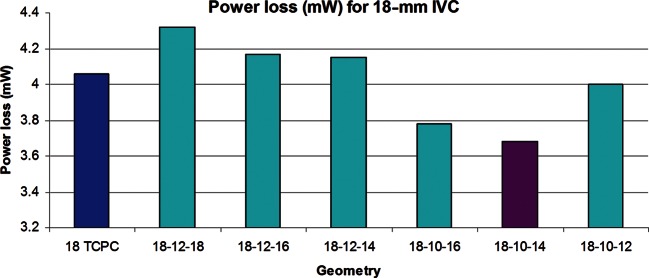
Bar graph showing power loss obtained from the parametric study conducted on various flow-dividers for the 18-mm IVC baffle compared with the 18-mm TCPC.
Figure 2:
Velocity field with inset pressure profile for (A) 18 TCPC, (B) 18-10-14 flow-divider geometry, (C) 18-12-18 flow-divider geometry, (D) 20 TCPC, (E) 20-10-14 flow-divider geometry and (F) 20-11-15 flow-divider geometry. Pressure drops and local velocity changes represent the regions of flow inefficiency.
With regard to flow distribution (Table 2), the TCPC geometry had an HFD of 59% to the left pulmonary artery (LPA). The 10-16 option had the most even flow distribution, with a 49-51 split into the PAs. On the other hand, the 10-14 insert had an HFD of 35%.
Table 2:
Hepatic flow distribution (HFD) for the 18-mm IVC baffle
| 18-mm IVC | HFD (%LPA) |
|---|---|
| 12-18 | 48 |
| 12-16 | 53 |
| 12-14 | 41 |
| 10-16 | 50 |
| 10-14 | 35 |
| 10-12 | 58 |
20-mm IVC
Based on the finding of the 10-14 insert being energetically optimal for the 18-mm IVC, additional simulations were performed with a 20-mm Fontan baffle using: (a) the same 10-14 divider, and (b) a scaled-up design to maintain the previous ratio of cross-sectional areas (insert base area/graft area = 70.9%), yielding an 11-15 base/height design. The height of the insert was determined by keeping the same base length-to-height proportions as the 10-14 insert, resulting in a 15-mm height. In comparison with the TCPC control (3.6 mW), the scaled design resulted in lower power loss (3.2 mW), while the 10-14 insert had the same energetic performance (3.6 mW). As seen in the 18-mm IVC case, Figs. 2E and F show local acceleration by the inserts as a result of a progressive decrease in cross-sectional area for the 20-mm IVC baffle. Furthermore, as with the 18-mm TCPC geometry, there is a flow collision present at the cross-section of the vena cavae and PAs for the 20-mm TCPC control (Fig. 2D). While the insert design in this case led to a significant low-flow recirculation region inside the connection (Fig. 2E and F), it was still found to be energetically favourable compared with the TCPC in at least one case.
Flow distributions for all three geometries are summarized in Table 3. The TCPC had an HFD of 62% to the LPA. On the other hand, the 10-14 flow-divider had an HFD of 82%, while the 11-15 insert had an HFD of 65%, thereby showing a mixed range of results for the flow-divider geometries.
Table 3:
Hepatic flow distribution (HFD) for the 20-mm IVC baffle
| 20-mm IVC | HFD (%LPA) |
|---|---|
| TCPC | 62 |
| 10-14 | 82 |
| 11-15 | 65 |
Exercise conditions
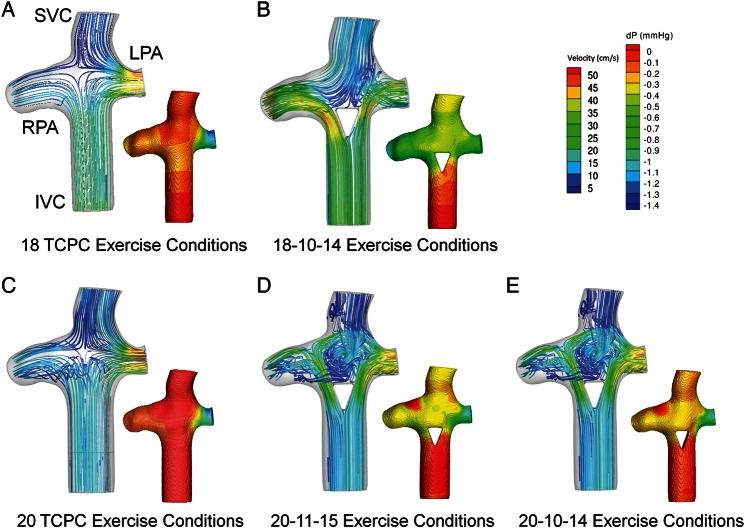
The most efficient flow-divider geometries (18-10-14 and 20-11-15) displayed improved performance under exercise conditions (Fig. 3) compared with the corresponding TCPC geometries. The efficiency gains with respect to controls were larger with exercise than under resting flow conditions, consistent with previous studies. It is particularly interesting to note that the 18-mm graft with the flow-divider was more efficient than the 20-mm TCPC control. Figure 4 shows pressure and velocity fields under exercise conditions for 18 and 20 TCPC and flow-divider geometries. The flow-divider geometries that were exposed to exercise conditions showed increased fluid acceleration and pressure changes as the area of the IVC decreased.
Figure 3:
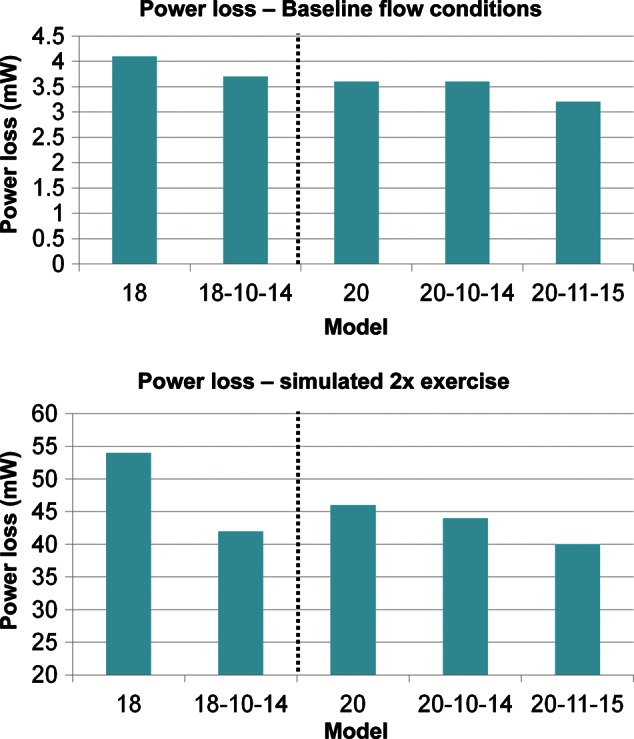
Bar graphs indicating power loss differences between TCPC and flow-divider geometries under resting (top) and simulated exercise (bottom) conditions.
Figure 4:
Velocity field with inset pressure profile for (A) 18 TCPC, (B) 18-10-14 flow-divider geometry, (C) 20 TCPC, (D) 20-11-15 flow-divider geometry, and (E) 20-10-14 flow-divider geometry under exercise conditions. Pressure drops and local velocity changes represent the regions of flow inefficiency.
DISCUSSION
This study proposed a novel Optiflo design for the TCPC that integrates a flow-divider at the centre of the Fontan baffle. The motivation for this design was the hypothesis that it would be able to approach the energetically beneficial effects of the original Optiflo design [9] by improving flow streaming through the connection, while also improving the pulmonary arterial distribution of hepatic flow, with less-surgical complexity. To demonstrate the proof of concept, CFD was used to determine the optimal insert size for the considered set of options based on the diameter of the Fontan baffle and to assess the haemodynamics of the flow-divider design relative to those of the TCPC.
Power loss
Results showed that the performance of the flow-divider was dependent on the insert size relative to the Fontan graft diameter. For the base 12-mm design in the 18-mm conduit, the losses due to convective acceleration (from decreased cross-sectional area) outweighed the improvements achieved by avoiding flow collision. However, once the optimal insert size was determined through the parametric study, improvements in flow efficiency compared with the TCPC were seen. The reduction in cross-sectional area still led to convective flow acceleration and pressure drop on the lateral sides of the divider, as seen in the inset pressure contour images. Yet, Table 4 shows that the advantages of avoiding flow collision outweighed these convective losses in some cases as the overall power loss for the 18-10-14 geometry was less than the TCPC. In addition, a power loss trend was observed solely based on the insert height sizes. A height of 14 showed the lowest amount of power loss independent of the insert base size.
Table 4:
Quantitative power loss
| Geometry | Power loss (mW) |
|---|---|
| 18-12-18 | 4.3 |
| 18-12-16 | 4.2 |
| 18-12-14 | 4.2 |
| 18-10-16 | 3.8 |
| 18-10-14 | 3.7 |
| 18-10-12 | 4.0 |
| 18 | 4.1 |
| 20 | 3.6 |
| 20-10-14 | 3.6 |
| 20-11-15 | 3.2 |
These gains in flow efficiency were accentuated during exercise conditions. Since small inefficiencies under resting conditions may be amplified and exacerbated with higher flows, these results confirm the robustness of this connection design for improving the streaming profile of blood flow in the TCPC. Theoretical improvements in power loss with exercise, when they are typically significantly increased, suggest that the flow-divider could help improve exercise tolerance in these patients [6].
An important finding was that the optimal insert size for the flow-divider geometry was relative to the size of the Fontan baffle for this particular patient. The reason that the 12 base inserts were sub-optimal for the 18-mm baffles was because the losses due to acceleration outweighed the improvements achieved by avoiding flow collision (Fig. 2C). After the parametric study determined the 10-14 flow-divider to be most advantageous for the 18-mm baffle, an 11-15 flow-divider was inserted into the 20-mm baffle proportionally. The resulting connection was more efficient than its respective control TCPC, but more baffle sizes would need to be tested to confirm this proportionality relationship. Yet, this study was able to determine a potential and promising relationship between the insert sizes and baffle diameter that could work for other patient geometries as well.
The real strength of the flow-divider, when compared with Y-graft connections, is the improved surgical efficiency. In the study by Marsden et al. [10], drastic decreases in cross-sectional area of the Y-graft resulted in greater power loss. Therefore, to reduce energy dissipation, larger baffles were supported to conserve the cross-sectional area. However, the increase in the size of the baffles would lead to an increase in suture lines and could be constrained by the surrounding anatomy, thereby making the surgical feasibility questionable, at least in some patients [12]. Conversely, less change in the surgical procedure would be needed for the Optiflo flow-divider compared with the current Fontan operation, and it does not require extra space for connection.
Flow distribution
Flow distribution into the PAs was calculated in order to observe another aspect the flow-divider would affect. Theoretically, the use of a flow-divider enforces the split of IVC flow into the PAs. Results were mixed, thus making this aspect of the study inconclusive. Intuitively, it is easy to see how the position of the insert could affect the split. In the 18-10-14 geometry, the insert was positioned slightly towards the LPA, resulting in a flow split that was biased towards the RPA. This example shows the sensitivity of insert placement. On the other hand, the 10-16 insert was placed directly in the middle of the IVC, leading to a nearly perfect IVC flow split. Therefore, a simple remedy would be to precisely place the insert at the centre of the IVC baffle before surgery. Another consideration could include a baffle that contained an oval-shaped, rather than circular, cross-sectional area in order to reduce the sensitivity. This would maximize the distance between the lateral walls of the divider and the graft, therefore reducing the effect the graft would have on flow acceleration. Also, in reality, the insert would be placed within the IVC baffle before the surgical procedure to minimize error. Furthermore, this study was conducted under the assumption that no caval offset would be most favourable. Therefore, another surgical concern is the placement of the Fontan baffle with regard to the SVC position.
As previously mentioned, hepatic proteins are crucial for proper lung development. However, the minimum amount required for successful lung progression is unknown, and reports of lung malformations are uncommon among most Fontan patients. Therefore, while distribution of hepatic proteins is a concern, it is a secondary element when considering the value of the flow-divider.
Future potential
With only a limited analysis in a semi-idealized geometric configuration, it is difficult to project the ultimate potential of this concept. The ability to robustly recreate the haemodynamic improvements presently demonstrated in a wide array of patient-specific connections will be critical. However, the sensitivity of the result to the placement/position of the divider within the baffle is more critical than the compatibility of the concept with the various different geometries: suitable patients can be selected based on determined criteria, but the consistency of performance must be the cornerstone.
The heterogeneity among patient-specific TCPCs suggests that there is no ‘one-size-fits-all’ solution to Fontan surgery, and the present proposal is no exception. Thus, the question is not ‘will this idea work for all single-ventricle patients’, but is instead ‘for which patients will this connection provide a haemodynamic improvement?’ The Fontan Y-graft has already demonstrated potential for successfully achieving Fontan flow bifurcation and improving haemodynamic efficiency in single-ventricle patients [12]. Realizable improvements with the flow-divider will therefore be achieved if either the flow-divider is found to be haemodynamically superior to the Y-graft, which appears unlikely, or it shows benefit in patients for whom a Y-graft is contraindicated (perhaps in cases with large aortic reconstructions).
Limitations
This study had several limitations. The primary limitation included the fact that it was conducted on a single geometry. This study was intended to be a proof of concept for the flow-divider modification, and thus future work will continue to explore these connections to better characterize the mediating factors. In addition, a small number of insert options were considered. An insert height of 14 mm showed lower power loss for the 18-mm IVC, yet an 11-14 insert was not examined for the 20-mm Fontan baffle. Another limitation was encountered with our computational methods as steady, time averaged flow boundary conditions were used that disregarded pulsatile and respiratory effects that normally occur in the SV physiology.
Finally, while recent studies have suggested that TCPC power loss is inversely associated with cardiac output and systemic venous haemodynamics [5, 20], on an individual patient level there is no guarantee that the improvement in power loss presently demonstrated through the use of a flow-divider would yield appreciable improvement in cardiac output. The multitude of other factors that mediate single-ventricle function (e.g. pulmonary vascular resistance, ventricular compliance) may instead have a dominant influence for a given patient.
SUMMARY
The proposal and proof of concept for a flow-divider as an alternative means of achieving flow bifurcation in the TCPC pathway has been shown for the first time. A parametric study determined the optimal proportion of a triangular insert for a given base size of the Fontan baffle, which was subsequently found to mediate energetic improvements in TCPC flow compared with a control model under both resting and simulated exercise conditions. As such, these results suggest that the flow-divider concept may successfully emulate the theoretical benefits of the Y-graft while improving the ease of surgical use and being feasible in patients lacking sufficient space for effective Y-graft implementation. The biggest concern remains where the insert is positioned within the baffle in order to promote proper flow distribution. Further studies will explore this modification to better determine the limitations and optimal patient candidates for this design.
Funding
This work was supported by the Georgia Tech President's Undergraduate Research Award, National Institutes of Health (HL67622 and HL098252), and American Heart Association (10PRE3720002).
Conflict of interest: none declared.
REFERENCES
- 1.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–48. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Leval MR, Kilner P, Gewillig M, Bull C. Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. J Thorac Cardiovasc Surg. 1988;96:682–95. [PubMed] [Google Scholar]
- 3.Ensley A, Lynch P, Chatzimavroudis GP, Lucas CL, Sharma S, Yoganathan AP. Toward designing the optimal total cavopulmonary connection: an in vitro study. Ann Thorac Surg. 1999;68:1384–90. doi: 10.1016/s0003-4975(99)00560-3. [DOI] [PubMed] [Google Scholar]
- 4.Sharma S, Goudy S, Walker P, Panchal S, Ensley A, Kanter K, et al. In vitro flow experiments for determination of optimal geometry of total cavopulmonary connection for surgical repair of children with functional single ventricle. J Am Coll Cardiol. 1996;27:1264–69. doi: 10.1016/0735-1097(95)00598-6. [DOI] [PubMed] [Google Scholar]
- 5.Sundareswaran KS, Pekkan K, Dasi LP, Whitehead K, Sharma S, Kanter K, et al. The total cavopulmonary connection resistance: a significant impact on single ventricle hemodynamics at rest and exercise. Am J Physiol Heart Circ Physiol. 2008;295:H2427–H35. doi: 10.1152/ajpheart.00628.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitehead KK, Pekkan K, Kitajima HD, Paridon SM, Yoganathan AP, Fogel MA. Nonlinear power loss during exercise in single-ventricle patients after the Fontan: insights from computational fluid dynamics. Circulation. 2007;116:I-165–I-71. doi: 10.1161/CIRCULATIONAHA.106.680827. [DOI] [PubMed] [Google Scholar]
- 7.Murakami H, Yoshimura N, Kitahara J, Otaka S, Ichida F, Misaki T. Collision of the caval flows caused early failure of the Fontan circulation. J Thorac Cardiovasc Surg. 2006;132:1235. doi: 10.1016/j.jtcvs.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Duncan B, Desai S. Pulmonary arteriovenous malformations after cavopulmonary anastomosis. Ann Thorac Surg. 2003;76:1759–66. doi: 10.1016/s0003-4975(03)00450-8. [DOI] [PubMed] [Google Scholar]
- 9.Soerensen DD, Pekkan K, de Zelicourt D, Sharma S, Kanter K, Fogel M, et al. Introduction of a new optimized total cavopulmonary connection. Ann Thorac Surg. 2007;83:2182–90. doi: 10.1016/j.athoracsur.2006.12.079. [DOI] [PubMed] [Google Scholar]
- 10.Marsden AL, Bernstein AJ, Reddy VM, Shadden SC, Spilker RL, Chan FP, et al. Evaluation of a novel Y-shaped extracardiac Fontan baffle using computational fluid dynamics. J Thorac Cardiovasc Surg. 2009;137:394–403. doi: 10.1016/j.jtcvs.2008.06.043. [DOI] [PubMed] [Google Scholar]
- 11.Kanter KR, Haggerty CM, Restrepo M, De Zelicourt D, Rossignac J, Parks WJ, et al. Preliminary clinical experience with a bifurcated Y-graft Fontan procedure–a feasibility study. J Thorac Cardiovasc Surg. 2012;144:383–89. doi: 10.1016/j.jtcvs.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haggerty CM, Kanter KR, Restrepo M, de Zelicourt D, Parks WJ, Rossignac J, et al. Simulating hemodynamics of the Fontan Y-graft based on patient-specific in vivo connections. J Thorac Cardiovasc Surg. 2013;145:663–670. doi: 10.1016/j.jtcvs.2012.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frakes D, Conrad C, Healy T, Monaco J, Fogel MA, Sharma S, et al. Application of an adaptive control grid interpolation technique to morphological vascular reconstruction. IEEE Trans Biomed Eng. 2003;50:197–206. doi: 10.1109/TBME.2002.807651. [DOI] [PubMed] [Google Scholar]
- 14.Frakes D, Smith M, Parks WJ, Sharma S, Fogel M, Yoganathan AP. New techniques for the reconstruction of complex vascular anatomies from MRI images. J Cardiovasc Magn Reson. 2005;7:425–32. doi: 10.1081/jcmr-200053637. [DOI] [PubMed] [Google Scholar]
- 15.Pekkan K, Whited B, Kanter K, Sharma S, de Zelicourt D, Sundareswaran KS, et al. Patient-specific surgical planning and hemodynamic computational fluid dynamics optimization through free-form haptic anatomy editing tool (SURGEM) Med Biol Eng Comput. 2008;46:1139–52. doi: 10.1007/s11517-008-0377-0. [DOI] [PubMed] [Google Scholar]
- 16.de Zelicourt D, Ge L, Wang C, Sotiropoulos F, Gilmanov A, Yoganathan AP. Flow simulations in arbitrarily complex cardiovascular anatomies—an unstructured Cartesian grid approach. Comput Fluids. 2009;38:1749–62. [Google Scholar]
- 17.Goldberg D, French B, McBridge MG, Marino BS, Mirarchi N, Hanna BD, et al. Impact of oral sildenafil on exercise performance in children and young adults after the Fontan operation. Circulation. 2011;123:1185–93. doi: 10.1161/CIRCULATIONAHA.110.981746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paridon SM, Mitchell PD, Colan SD, Williams RV, Blaufox A, Li JS, et al. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol. 2008;52:99–107. doi: 10.1016/j.jacc.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen EM, Stenbøg EV, Fründ T, Houlind K, Kromann O, Sørensen KE, et al. Flow during exercise in the total cavopulmonary connection measured by magnetic resonance velocity mapping. Heart. 2002;87:554–58. doi: 10.1136/heart.87.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haggerty CM, Restrepo M, Tang E, Mirabella L, de Zelicourt D, Bethel J, et al. Fontan fluid mechanics from 100 patient-specific magnetic resonance imaging scans: a computational analysis. Circulation. 2012;126:A13270. [Google Scholar]