Abstract
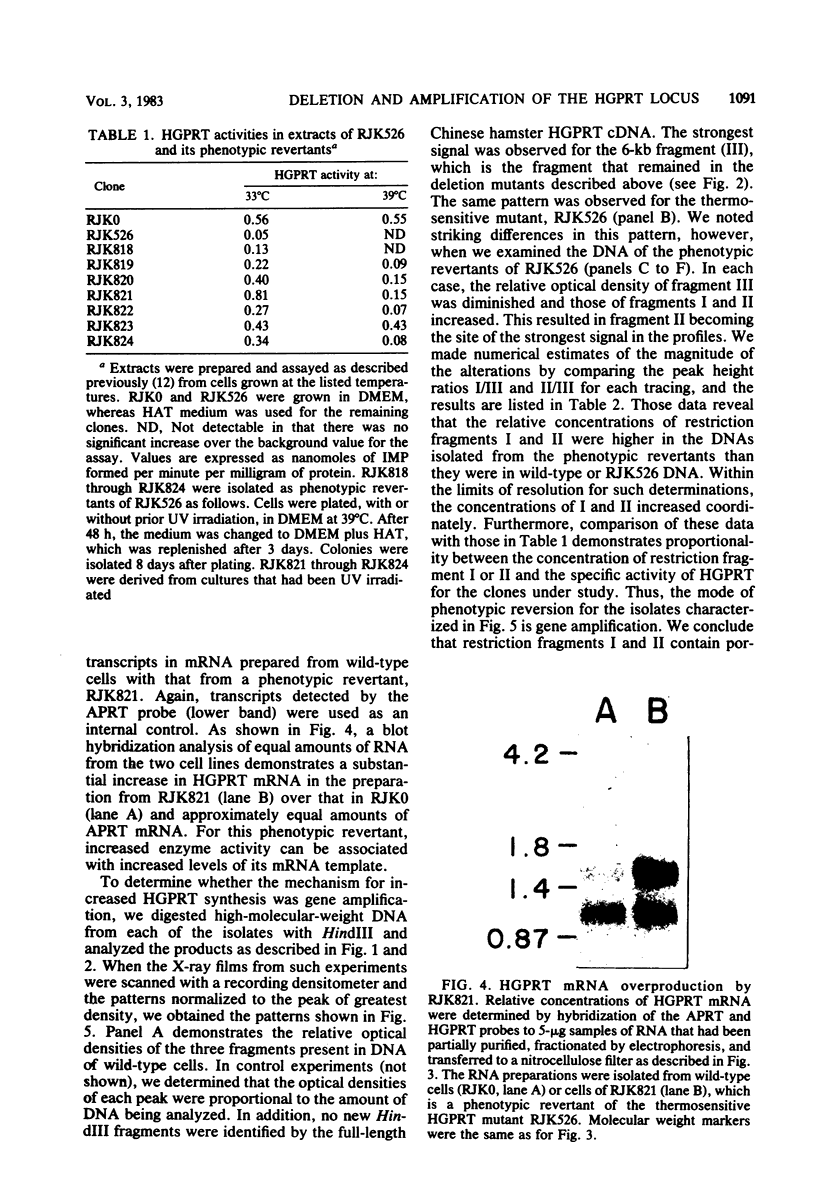
Somatic cell selective techniques and hybridization analyses with a cloned cDNA probe were used to isolate and identify Chinese hamster cell lines in which the X-linked gene for hypoxanthine-guanine phosphoribosyltransferase (HGPRT) has been altered. Two of 19 HGPRT-deficient mutants selected were found to have major DNA deletions affecting the HGPRT locus. Cytogenetic studies revealed that the X chromosome of each deletion mutant had undergone a translocation event, whereas those from the remaining 17 mutants were normal. Phenotypic revertants of the thermosensitive HGPRT mutant RJK526 were isolated, and amplification of the mutant allele was shown to be the predominant mechanism of reversion. Comparisons of restriction enzyme fragments of DNA from deletion versus amplification strains identified two regions of the Chinese hamster genome that contained homology to the cDNA probe. One was shown to be much larger than the 1,600-nucleotide mRNA for HGPRT and to be comprised of linked fragments that contained the functional HGPRT gene. The second was neither transcribed nor tightly linked to the functional gene. These initial studies of HGPRT alterations at the level of DNA thus identified molecular mechanisms of phenotypic variation.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach L. R., Palmiter R. D. Amplification of the metallothionein-I gene in cadmium-resistant mouse cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2110–2114. doi: 10.1073/pnas.78.4.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudet A. L., Roufa D. J., Caskey C. T. Mutations affecting the structure of hypoxanthine: guanine phosphoribosyltransferase in cultured Chinese hamster cells. Proc Natl Acad Sci U S A. 1973 Feb;70(2):320–324. doi: 10.1073/pnas.70.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand J., Chinault A. C., Konecki D. S., Melton D. W., Caskey C. T. Cloned cDNA sequences of the hypoxanthine/guanine phosphoribosyltransferase gene from a mouse neuroblastoma cell line found to have amplified genomic sequences. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1950–1954. doi: 10.1073/pnas.79.6.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caskey C. T., Kruh G. D. The HPRT locus. Cell. 1979 Jan;16(1):1–9. doi: 10.1016/0092-8674(79)90182-x. [DOI] [PubMed] [Google Scholar]
- Cox R., Masson W. K. Do radiation-induced thioguanine-resistant mutants of cultured mammalian cells arise by HGPRT gene mutation or X-chromosome rearrangement? Nature. 1978 Dec 7;276(5688):629–630. doi: 10.1038/276629a0. [DOI] [PubMed] [Google Scholar]
- Farrell S. A., Worton R. G. Chromosome loss is responsible for segregation at the HPRT locus in Chinese hamster cell hybrids. Somatic Cell Genet. 1977 Sep;3(5):539–551. doi: 10.1007/BF01539124. [DOI] [PubMed] [Google Scholar]
- Fenwick R. G., Jr, Caskey C. T. Mutant chinese hamster cells with a thermosensitive hypoxanthine-guanine phosphoribosyltransferase. Cell. 1975 Jun;5(2):115–122. doi: 10.1016/0092-8674(75)90019-7. [DOI] [PubMed] [Google Scholar]
- Fenwick R. G., Jr Reversion of a mutation affecting the molecular weight of HGPRT: intragenic suppression and localization of X-linked genes. Somatic Cell Genet. 1980 Jul;6(4):477–494. doi: 10.1007/BF01539151. [DOI] [PubMed] [Google Scholar]
- Fenwick R. G., Jr, Wasmuth J. J., Caskey C. T. Mutations affecting the antigenic properties of hypoxanthine-guanine phosphoribosyl transferase in cultured Chinese hamster cells. Somatic Cell Genet. 1977 Mar;3(2):207–216. doi: 10.1007/BF01551815. [DOI] [PubMed] [Google Scholar]
- Fuscoe J. C., O'Neill J. P., Machanoff R., Hsie A. W. Quantification and analysis of reverse mutations at the hgprt locus in Chinese hamster ovary cells. Mutat Res. 1982 Sep;96(1):15–30. doi: 10.1016/0027-5107(82)90013-6. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Roufa D. J., Beaudet A. L., Caskey C. T. 8-Azaguanine resistance in mammalian cells. I. Hypoxanthine-guanine phosphoribosyltransferase. Genetics. 1972 Oct;72(2):239–252. doi: 10.1093/genetics/72.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf L. H., Jr, Chasin L. A. Direct demonstration of genetic alterations at the dihydrofolate reductase locus after gamma irradiation. Mol Cell Biol. 1982 Jan;2(1):93–96. doi: 10.1128/mcb.2.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecki D. S., Brennand J., Fuscoe J. C., Caskey C. T., Chinault A. C. Hypoxanthine-guanine phosphoribosyltransferase genes of mouse and Chinese hamster: construction and sequence analysis of cDNA recombinants. Nucleic Acids Res. 1982 Nov 11;10(21):6763–6775. doi: 10.1093/nar/10.21.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruh G. D., Fenwick R. G., Jr, Caskey C. T. Structural analysis of mutant and revertant forms of Chinese hamster hypoxanthine-guanine phosphoribosyltransferase. J Biol Chem. 1981 Mar 25;256(6):2878–2886. [PubMed] [Google Scholar]
- Lowy I., Pellicer A., Jackson J. F., Sim G. K., Silverstein S., Axel R. Isolation of transforming DNA: cloning the hamster aprt gene. Cell. 1980 Dec;22(3):817–823. doi: 10.1016/0092-8674(80)90558-9. [DOI] [PubMed] [Google Scholar]
- Melton D. W. Cell fusion-induced mouse neuroblastomas HPRT revertants with variant enzyme and elevated HPRT protein levels. Somatic Cell Genet. 1981 May;7(3):331–344. doi: 10.1007/BF01538858. [DOI] [PubMed] [Google Scholar]
- Melton D. W., Konecki D. S., Ledbetter D. H., Hejtmancik J. F., Caskey C. T. In vitro translation of hypoxanthine/guanine phosphoribosyltransferase mRNA: characterization of a mouse neuroblastoma cell line that has elevated levels of hypoxanthine/guanine phosphoribosyltransferase protein. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6977–6980. doi: 10.1073/pnas.78.11.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuth M., Arrand J. E. Alterations of gene structure in ethyl methane sulfonate-induced mutants of mammalian cells. Mol Cell Biol. 1982 Nov;2(11):1459–1462. doi: 10.1128/mcb.2.11.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicer A., Wigler M., Axel R., Silverstein S. The transfer and stable integration of the HSV thymidine kinase gene into mouse cells. Cell. 1978 May;14(1):133–141. doi: 10.1016/0092-8674(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Przybyla A. E., MacDonald R. J., Harding J. D., Pictet R. L., Rutter W. J. Accumulation of the predominant pancreatic mRNAs during embryonic development. J Biol Chem. 1979 Mar 25;254(6):2154–2159. [PubMed] [Google Scholar]
- Ray M., Mohandas T. Proposed banding nomenclature for the Chinese hamster chromosomes (Cricetulus griseus). Cytogenet Cell Genet. 1976;16(1-5):83–91. doi: 10.1159/000130559. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roberts J. M., Axel R. Gene amplification and gene correction in somatic cells. Cell. 1982 May;29(1):109–119. doi: 10.1016/0092-8674(82)90095-2. [DOI] [PubMed] [Google Scholar]
- Shepard H. M., Polisky B. Measurement of Plasmid copy number. Methods Enzymol. 1979;68:503–513. doi: 10.1016/0076-6879(79)68039-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thacker J. The chromosomes of a V79 Chinese hamster line and a mutant subline lacking HPRT activity. Cytogenet Cell Genet. 1981;29(1):16–25. doi: 10.1159/000131547. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]








