Abstract
OBJECTIVES
This study aimed to review the safety of a reusable sealing instrument, BiClamp®, as an alternative to the mechanical stapler for interlobar fissure division in pulmonary lobectomy.
METHODS
A retrospective review was conducted of 95 patients who underwent pulmonary lobectomy performed by a single surgeon between November 2005 and March 2010. The patients were divided into two groups according to the period before and after introduction of the BiClamp®: 29 patients who underwent fissure division with staples only (staple group) and 66 patients who underwent the same procedure mainly with the instrument (BiClamp® group).
RESULTS
There were 60 (63.2%) male and 35 (36.8%) female patients, with a mean ± SD age of 67.5 ± 10.8 years. Comparison of the characteristics of the two groups revealed that the BiClamp® group included significantly more cases of lobectomy by video-assisted thoracic surgery and far fewer completely lobulated lungs; 6 of 66 patients (9.1%) compared with 9 of 29 (31.0%) of the staple group. Except for 18 patients who underwent division using staples owing to thick parenchyma of the interlobar fissure, we attempted to divide the fissure of 42 patients in the BiClamp® group. Solo use of the BiClamp® was possible for 25 of 60 patients (41.7%) with an incomplete fissure. Eight patients (13.3%) needed one staple cartridge in combination with BiClamp®, five (8.3%) needed two cartridges and four (6.7%) patients needed three (combined use). In most cases, except for right upper or middle lobectomy, the division of the interlobar fissure could be performed by sole use of the BiClamp®. Incidence rates of prolonged air leakage and pneumonia were not significantly different between the two groups (respectively, 6.9 and 3.4% in the staple group vs 10.6 and 9.1% in the BiClamp® group).
CONCLUSIONS
The study results demonstrate that the division of the interlobar fissure in pulmonary lobectomy with BiClamp® is safe and feasible in most cases. While the results point out the limitation that division of the right upper or middle lobe may still be a challenge, they show the potential benefit of staple reduction. Less use of staples results in reduced medical costs and carbon dioxide emission, contributing to ‘ecosurgery’, which ultimately conserves the global environment.
Keywords: BiClamp®, Lung, Lobectomy, Ecosurgery, SOFT COAG
INTRODUCTION
Automatic stapling devices, as a gold standard, have been routinely used for interlobar fissure division for pulmonary lobectomy [1, 2]. These useful and safe devices have not only contributed to the development of pulmonary surgery but have also made it possible to perform video-assisted thoracic surgery (VATS).
As an alternative method to the use of automatic stapling devices, we have reported the use of a reusable sealing instrument, BiClamp®, for pulmonary resection [3]. In brief, this sealing device, activated by the VIO300D electrosurgical system (ERBE Elektromedizin GmbH, Tübingen, Germany), is microprocessor-controlled, and the output voltage is maintained below 200 V in order to avoid generating sparks. In this way, the maximal tissue temperature remains below boiling point, resulting in no tissue carbonization. Using this reusable device, we reported bullectomy, lung wedge resection and fissure division [3]. We introduced the BiClamp® method for division of the fissure between the lobes in lobectomy.
The mechanism of sealing the lung parenchyma is tissue-thermofusion by alveolar collapse and the pressurized fusion of the clamped lesion [3], without the use of a physical back-up, such as staples. Although we had confidence in the tight closure of the lung parenchyma [3], we followed up the patients who underwent pulmonary surgery using the BiClamp®.
We carefully observed the occurrence of associated complications, such as prolonged air leakage, late air leakage and pneumonia, because these are the most frequent complications after pulmonary resection. Even in the era of the staple prime, there have been a lot of reports concerning the incidence of air leakage, which ranges widely between 1.0 and 16.9% [4–9]. While BiClamp® applies relatively low-temperature heating to the lung parenchyma, we were concerned about possible burns around the clamped lesion, which could cause lung damage, resulting in pneumonia or pneumonitis.
In the present study, we demonstrate the safety of this alternative method to mechanical staples for fissure division in pulmonary lobectomy. We also present the concept of ‘ecosurgery’ [3, 10–12], which ultimately conserves the global environment by reduced use of staples, in this instance in pulmonary resection.
MATERIALS AND METHODS
Patients
Our Institutional Review Board approved the study, and the enrolled patients provided full informed written consent. A retrospective review was conducted of 95 patients who underwent pulmonary lobectomy performed by a single surgeon (T.S.) between November 2005 and March 2010; data were collected from our unit's computer database and archives (Table 1). For the purpose of this analysis, patients were excluded if they underwent lobectomy associated with partial resection of the adjacent lobe owing to invasion of the tumour.
Table 1:
Patient characteristics
| Variables | n, 95 patients total |
|---|---|
| Age (years; mean ± SD, range) | 67.5 ± 10.8, 32–88 |
| Male | 60 (63.2%) |
| Disease | |
| Primary lung cancer | 90 (94.7%) |
| Metastatic lung cancer | 4 (4.2%) |
| Cryptococcosis | 1 (1.1%) |
| Resected lobe | |
| Right upper lobe | 34 (35.8%) |
| Right middle lobe | 7 (7.4%) |
| Right lower lobe | 19 (20.0%) |
| Right upper and middle lobes | 3 (3.2%) |
| Left upper lobe | 18 (18.9%) |
| Left lower lobe | 14 (14.7%) |
| Approach | |
| Open thoracotomy | 85 (89.5%) |
| Video-assisted thoracic surgery | 10 (10.5%) |
| Mediastinal lymph node dissection | 63 (66.3%) |
| Lobulation | |
| Complete | 15 (15.8%) |
| Incomplete | 80 (84.2%) |
| Adhesion | |
| No adhesion or mild | 72 (75.8%) |
| Hard to all around the lung | 23 (24.2%) |
| Partial resectiona | 9 (9.5%) |
| Bronchoplasty | 3 (3.2%) |
| Combined rib resection | 1 (1.1%) |
| Division of interlobar fissure | |
| Staple only (staple group) | 29 (30.5%) |
| BiClamp® innovated (BiClamp® group) | 66 (69.5%) |
| Operation time (min; median, range) | 281, 139–481 |
| Intraoperative bleeding (ml; median, range) | 125, 20–940 |
| Drainage duration (days; median, range) | 3, 1–28 |
aPartial resection for a frozen section to diagnose the tumour in the lobe to be resected or for other nodule(s) in the other lobe.
We have introduced the BiClamp® method to divide the fissure between the lobes in lobectomy since June 2007. We divided the 95 patients into two groups according to the period before and after introduction of the BiClamp® method; the staple group consisted of 29 patients who underwent division of the fissure with staples only, and the BiClamp® group consisted of 66 patients who underwent the same procedure with the BiClamp®. It should be noted, however, that the BiClamp® group included some patients with an incomplete fissure of the parenchyma that was divided using staples owing to the thickness of the parenchyma.
Patient demographics, disease, operative procedure, various parameters (volume of blood lost, duration of surgery and concomitant staple use) and complications (pneumonia and prolonged air leakage) were recorded (Table 1).
Operative technique
General anaesthesia with selective lung ventilation was performed by the use of a double-lumen endotracheal tube. The patients were placed in a decubitus position. In the thoracotomy approach, an incision approximately 15 cm long was made in the fourth or fifth intercostal space. A metal chest retractor was used to open the wound and intercostal space. We performed VATS lobectomy purely by viewing a video monitor with three ports, as follows: a 4-cm-long skin incision was made in the fourth intercostal space in the anterior axillary line as a utility thoracotomy, and two other 2-cm-long incisions for ports were made in the sixth intercostal space in the posterior axillary line and in the seventh intercostal space in the midaxillary line mainly for the thoracoscope.
In the BiClamp® group, we actively used an innovative method with a novel reusable bipolar sealing device, BiClamp® (ERBE, Tübingen, Germany), activated by an electrosurgical system, VIO300D (ERBE, Tübingen, Germany), for pulmonary lobectomy. Different types of BiClamp® for open and laparoscopic procedures were used in thoracotomy and VATS cases, respectively. Using these devices, the fissure was divided by coagulating the lung parenchyma, and then resected with scissors (Supplementary Video 1). All the patients with BiClamp® underwent the procedure with the VIO300D system, which is featured with not only the BiClamp® device but also SOFT COAG [11, 13].
Supplementary Video 1:
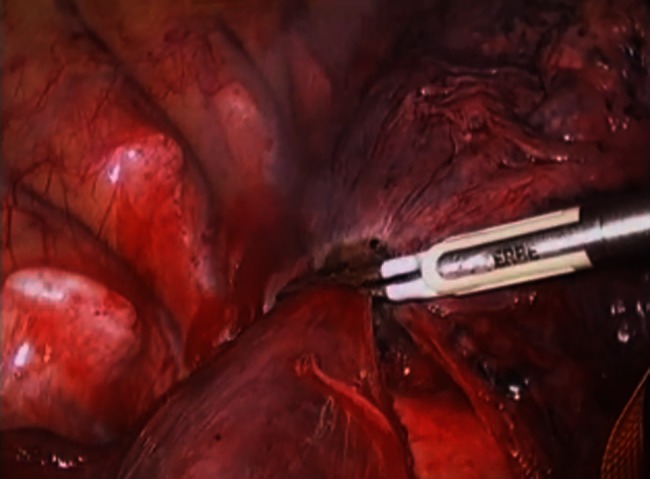
These are right lower lobectomies (the first half is in thoracotomy and the second half in video-assisted thoracic surgery). The lung parenchyma to be divided is treated by BiClamp® twice at the same point. Resection is then carried out along the coagulated part of the side to be resected. In these scenes, the left side is to be resected.
The forceps were connected to the multifunction output socket of the VIO300D electrosurgical system. Before the procedure, we routinely checked the resistance of the forceps to confirm that the device was working properly without a potential malfunction. The output settings of the BiClamp® were set at Effect 3 and Modulation 80. By measuring tissue resistance, the system determines the sealing duration/end-point and automatically stops the output with an acoustic signal.
Before introducing the BiClamp®, all the lung parenchyma with an unseparated interlobar fissure was treated with staples. After introducing the method, parenchyma to be separated with a thickness up to 3.5 mm became an indication for the BiClamp® method. The thickness of the parenchyma was confirmed by grasping palpation with atraumatic forceps. Determining factors for the use of staples were that those fissures with a parenchymal thickness of more than 3.5 mm required too much time for thermofusion and secured thermofusion, although this has not been firmly verified at this point. In order to ensure the safety of the procedure, we actively used the BiClamp® method for parenchyma with a thickness of up to 3.5 mm.
By taping and pulling up, away from other sensitive tissues, such as nerves and vessels, the unseparated interlobar fissure of the lung parenchyma to be divided was clamped and coagulated twice using the BiClamp® (until the acoustic signal indicated the end-point of sealing on each occasion) on the same point with the above-mentioned output settings. Sufficient volume of parenchyma in two or three adjacent areas was treated by the procedure to save the resected margin. After completing this phase, the lung was separated at the side of the resected lobe on the coagulated lesion (see Supplementary Video 1).
At the end of an operation, potential air leakage was examined by submerging the lung parenchyma in sterile saline and reinflating the lung to a sustained pressure of 25–30 cmH2O. Detected air leak, if significant, was managed by suturing for the thoracotomy cases and by BiClamp® for the VATS cases (see Supplementary Video 2) [12].
Supplementary Video 2:
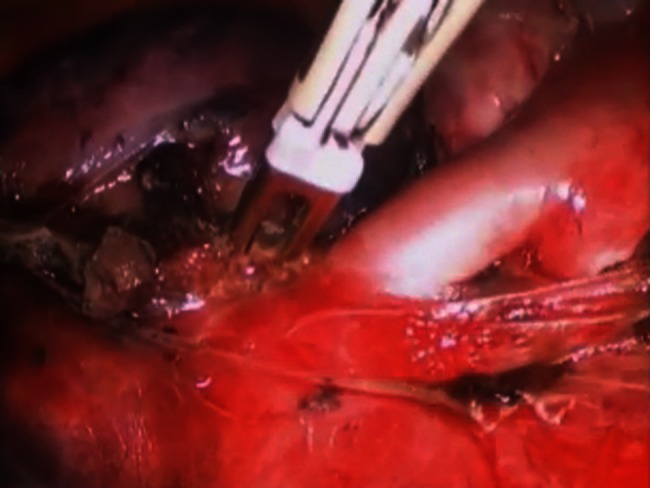
This is a case of air leakage after conducting a right upper lobectomy. Air leakage can be seen. The leakage point was grasped and treated by BiClamp®. After the treatment, there were no air leakages.
The divided area of the remnant lung after mechanical stapling or use of the BiClamp®, as well as any treated air leakage point, was covered with a biodegradable polyglycol acid (PGA) sheet and fibrin glue according to our routine procedure [3].
Statistical analysis
Statistical calculations were performed with SPSS (SPSS version 11 for Windows; SPSS Inc., Chicago, IL, USA). Clinical data are reported as the means ± SD and compared between the two groups using Student's unpaired t-test for normally distributed continuous data, or as the medians with the range and the Mann–Whitney U-test for non-normally distributed continuous data. Fisher's exact test was used to assess the categorical/binary data to detect the difference in proportion between the groups. A P-value of < 0.05 was accepted to be statistically significant.
RESULTS
Among the 66 patients in the BiClamp® group, six patients (9.1%) showed complete lobulation, which required no use of mechanical stapling or BiClamp®. We attempted to divide the fissure by using BiClamp® in 42 (70.0%) of 60 patients. Among these 60 patients, it was possible to separate the fissure for 25 patients (41.7%) with BiClamp® only (sole usage). Eight patients (13.3%) needed one staple cartridge in combination with BiClamp®, while five patients (8.3%) needed two cartridges and four (6.7%) needed three (combined usage). While those patients with an interlobar fissure thickness less than 3.5 mm were treated with the BiClamp® instead of the mechanical stapler, there were still 18 patients (30.0%) who were considered not to be BiClamp® subjects owing to thicker fissures.
Comparison of the staple and BiClamp® groups revealed that there were some differences in the characteristics of the patients (Table 2). First, we performed significantly more VATS lobectomies for the BiClamp® group. Second, patients in the BiClamp® group had far fewer completely lobulated lungs. Finally, there were statistically significant distributions of the resected lobes between the staple and BiClamp® groups.
Table 2:
Comparison of demographic and intraoperative data between the two groups
| Variables | Staple group (n = 29) | BiClamp® group (n = 66) | P-value |
|---|---|---|---|
| Age (years; mean ± SD, range) | 67.8 ± 13.2, 32–88 | 67.3 ± 9.7, 47–85 | 0.824a |
| Male | 17 (58.6%) | 43 (65.2%) | 0.351b |
| FEV1.0% | 71.3 ± 8.7 | 72.5 ± 10.5 | 0.569a |
| Resected lobe | |||
| Right upper lobe | 10 (34.5%) | 24 (36.4%) | 0.03c |
| Right middle lobe | 1 (3.4%) | 6 (9.1%) | |
| Right lower lobe | 7 (24.1%) | 12 (18.2%) | |
| Right upper and middle lobes | 0 (0.0%) | 3 (4.5%) | |
| Left upper lobe | 10 (34.5%) | 8 (12.1%) | |
| Left lower lobe | 1 (3.4%) | 13 (19.7%) | |
| Video-assisted thoracic surgery | 0 (0.0%) | 10 (15.2%) | 0.029b |
| Mediastinal lymph node dissection | 18 (62.1%) | 45 (68.2%) | 0.639b |
| Lobulation | |||
| Complete | 9 (31.0%) | 6 (9.1%) | 0.013b |
| Incomplete | 20 (69.0%) | 60 (90.9%) | |
| Adhesion | |||
| No adhesion or mild | 22 (75.9%) | 50 (75.8%) | 1.000b |
| Hard to all around the lung | 7 (24.1%) | 16 (24.2%) | |
| Partial resectiond | 4 (13.8%) | 5 (7.6%) | 0.448b |
| Bronchoplasty | 0 (0.0%) | 3 (4.5%) | 0.551b |
| Combined rib resection | 0 (0.0%) | 1 (1.5%) | 1.000b |
aStudent's unpaired t-test.
bFisher's exact test.
cChi-squared test.
dPartial resection for a frozen section to diagnose the tumour in the lobe to be resected or for other nodule(s) in the other lobe.
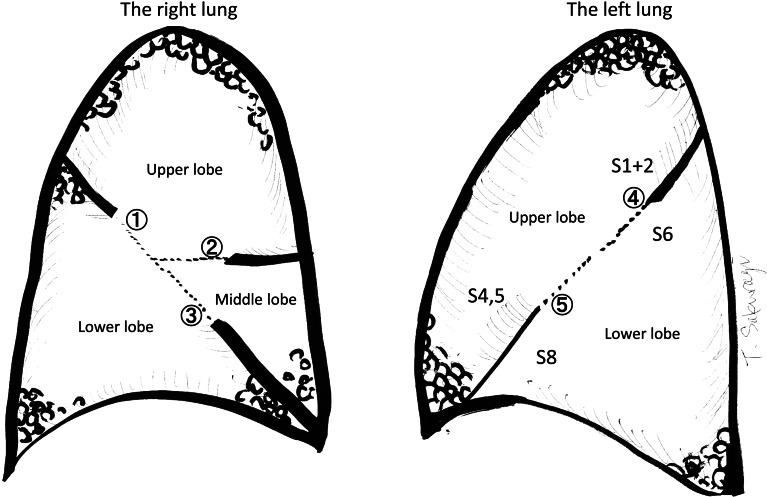
Figure 1 shows the unseparated fissures in an anatomical fashion. The specification of the relationship between the anatomical part and BiClamp® usage (solo usage or in combination with mechanical staples) is shown in Table 3. In most cases, except the right upper and middle lobe, the fissure division could be performed by sole use of the BiClamp®. This may result from the fact that the unseparated fissure between the right upper and middle lobe has a propensity to be thicker, which needs wider-opened mechanical staples than the 3.5-mm leg thickness cartridges.
Figure 1:
The fissures are described as follows. In the right lung:  upper–lower is the fissure separation for upper lobectomy, for lower lobectomy and for upper and middle bilobectomy;
upper–lower is the fissure separation for upper lobectomy, for lower lobectomy and for upper and middle bilobectomy;  upper–middle is for upper lobectomy and for middle lobectomy; and
upper–middle is for upper lobectomy and for middle lobectomy; and  lower–middle is for lower lobectomy and for middle lobectomy. In the left lung:
lower–middle is for lower lobectomy and for middle lobectomy. In the left lung:  upper is for the fissure separation between the upper segment of the upper lobe (S1 + 2) and the apical segment of the lower lobe (S6); and
upper is for the fissure separation between the upper segment of the upper lobe (S1 + 2) and the apical segment of the lower lobe (S6); and  lower is between the lingular (S4,5) segment and segment S8.
lower is between the lingular (S4,5) segment and segment S8.
Table 3:
Specification of the relationship between the anatomical part and the usage of BiClamp® for the 60 patients in the BiClamp® group
| Side | Divided fissurea | Sole usageb | Combined usagec |
|---|---|---|---|
| Right |
 Upper–lower (n = 20) Upper–lower (n = 20) |
17 (85.0%) | 3 (15.0%) |
 Upper–middle (n = 15) Upper–middle (n = 15) |
8 (53.3%) | 7 (46.7%) | |
 Lower–middle (n = 12) Lower–middle (n = 12) |
11 (91.7%) | 1 (8.3%) | |
| Left |
 Upper (n = 8) Upper (n = 8) |
8 (100%) | 0 (0%) |
 Lower (n = 7) Lower (n = 7) |
7 (100%) | 0 (0%) |
aThe circled numbers in the ‘divided fissure’ column are as illustrated in Fig. 1.
bSole usage, completion by using the BiClamp® without mechanical staples.
cCombined usage, completion by a combination of BiClamp® and mechanical staples.
Investigation of the incidence of intraoperative mechanical stapler use for interlobar fissure division shows that we achieved significantly less staple use after the introduction of the BiClamp® method (Fig. 2). For 65% of the patients treated using this method, the use of mechanical staples was reduced, with none or one cartridge per operation.
Figure 2:
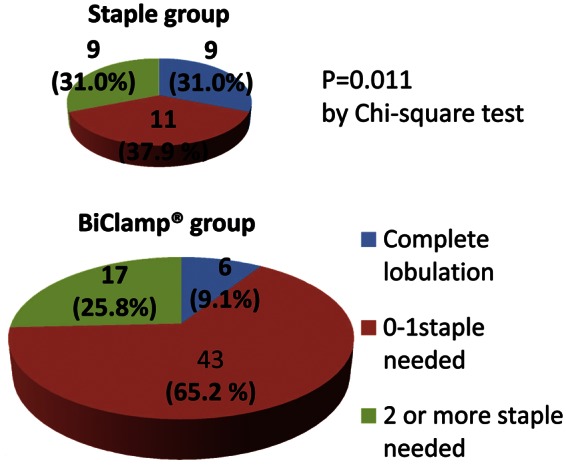
Number of intraoperative mechanical staples used for interlobar fissure division. In the BiClamp® group (lower pie chart), the use of mechanical staples was reduced to none or one cartridge per operation for 65.2% of the patients. Overall, after the introduction of the BiClamp® method, significantly less staple use was shown statistically (P = 0.011).
There were no significant differences in the duration of surgery or in the amount of intraoperative bleeding between the staple and BiClamp® groups (Table 4). A typical complication after separating the lung parenchyma, which is a pure, simple, air-rich organ, is prolonged air leakage, and there was no significant difference in incidence of this between the staple and BiClamp® groups. We were concerned that pneumonia could be associated with the low-temperature heating, which is the basic principle of this technique. However, there was also no significance in the frequency of pneumonia between the staple and BiClamp® groups (Table 4). These results show that the use of BiClamp® is feasible and safe for interlobar fissure division in lobectomy.
Table 4:
Comparison of postoperative parameters between the two groups
| Parameters | Staple group (n = 29) | BiClamp® group (n = 66) | P-value |
|---|---|---|---|
| Operation time (min; median, range) | 271, 152–397 | 281, 139–481 | 0.284a |
| Intraoperative bleeding (ml; median, range) | 103, 20–445 | 125, 20–940 | 0.277a |
| Prolonged air leak (>7 days) | 2 (6.9%) | 7 (10.6%) | 0.717b |
| Pneumonia | 1 (3.4%) | 6 (9.1%) | 0.672b |
aMann–Whitney U-test.
bFisher's exact test.
DISCUSSION
Although this study is a non-randomized, retrospective trial of an instrument, it is the first to investigate the safety of the BiClamp® as an alternative to the mechanical stapler for the division of unseparated interlobar fissures in pulmonary lobectomy. Our research shows that the BiClamp® method for fissure division, performed by a single surgeon, leads to no difference in the incidence of postoperative prolonged air leakage or pneumonia, the duration of surgery or intraoperative blood loss.
Comparison of the characteristics of patients in the two groups suggests that those in the BiClamp® group had more unseparated interlobar fissures. In addition, the BiClamp® group included more patients who underwent VATS, in which there seemed to be more prolonged air leakage than in conventional thoracotomy [6, 8, 9]. Despite these background differences in the distributions of the resected lobes and the VATS approach, there was no difference in the incidence of prolonged air leakage in both groups. This cohort of the BiClamp® group included a considerable number of cases from the initial period of introduction of the BiClamp® method. This implies that an even better outcome can be expected after some period of learning curve.
It is clear that there is no seam of a staple in the coagulated area following BiClamp® treatment. Theoretically, this means that there is no possibility of tearing of the seam, which could result in air leakage as the lung parenchyma is inflated. Therefore, less staple use may lead to less air leakage after pulmonary lobectomy. We adopted the air leakage control by BiClamp® [12] only in the last half of the BiClamp® group following introduction of the device (that is, air leakage control by BiClamp® was not our practice in the beginning). We believe that this method of controlling the air leakage is so effective that it could further reduce the incidence of prolonged air leakage in combination with BiClamp® fissure division.
During the initial phase of the BiClamp®, we had assumed that there might be undesirable effects of the heating on the lung parenchyma, which could lead to the risk of development of pneumonia or pneumonitis. It turned out, however, that there was no difference in the incidence of pneumonia before and after the introduction of the BiClamp® method. As the BiClamp® utilizes basic theory of SOFT COAG [11, 13] output of VIO300D, the rise in temperature is maintained below the boiling point, which is far lower than that caused by conventional electrosurgical systems. It should be considered that there should be less damage to the tissue with the BiClamp® method than with the conventional treatment methods for the lung parenchyma [14–16].
The results of the present study show that fissure division by the BiClamp® method, except for the fissure between the right upper and middle lobe, seems to be effective in pulmonary lobectomy, with use of few staples or none. As we have reported previously, the use of fewer staples results in lower medical costs and less carbon dioxide emission, contributing to ‘ecosurgery’ [3, 10–12], which ultimately conserves the global environment.
In conclusion, we report here the safety of the use of reusable BiClamp® forceps as an alternative to the use of staples for unseparated interlobar fissure division in pulmonary lobectomy. This method of fissure division seems promising, with a low incidence of prolonged air leakage after pulmonary lobectomy in combination with intraoperative air leakage control also by BiClamp®. The benefit of the use of fewer staples is termed ‘ecosurgery’, which is a concept to be shared globally.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Conflict of interest: none declared.
References
- 1.Hoyos AD, Santos RS, Patel A, Landreneau RJ. Instruments and techniques of video-assisted thoracic surgery. In: Shields TW, Joseph L, Ponn RB, Rusch VW, editors. General Thoracic Surgery. 6th edn. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 504–23. [Google Scholar]
- 2.McKenna RJ., Jr . Video-assisted thoracic surgery for wedge resection, lobectomy, and pneumonectomy. In: Shields TW, Joseph L, Ponn RB, Rusch VW, editors. General Thoracic Surgery, 6th edn. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 525–32. [Google Scholar]
- 3.Sakuragi T, Okazaki Y, Mitsuoka M, Yamasaki F, Masuda M, Mori D, et al. The utility of a reusable bipolar sealing instrument, BiClamp®, for pulmonary resection. Eur J Cardiothorac Surg. 2008;34:505–9. doi: 10.1016/j.ejcts.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 4.Asaph JW, Handy JR, Jr, Grunkemeier GL, Douville EC, Tsen AC, Rogers RC, et al. Median sternotomy versus thoracotomy to resect primary lung cancer: analysis of 815 cases. Ann Thorac Surg. 2000;70:373–9. doi: 10.1016/s0003-4975(00)01364-3. [DOI] [PubMed] [Google Scholar]
- 5.Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg. 2006;131:54–9. doi: 10.1016/j.jtcvs.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Lee PC, Korst RJ, Port JL, Kerem Y, Kansler AL, Altorki NK. Long-term survival and recurrence in patients with resected non-small cell lung cancer 1 cm or less in size. J Thorac Cardiovasc Surg. 2006;132:1382–9. doi: 10.1016/j.jtcvs.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 7.Gharagozloo F, Margolis M, Tempesta B. Robot-assisted thoracoscopic lobectomy for early-stage lung cancer. Ann Thorac Surg. 2008;85:1880–5. doi: 10.1016/j.athoracsur.2008.02.085. discussion 1885–6. [DOI] [PubMed] [Google Scholar]
- 8.Whitson BA, Groth SS, Duval SJ, Swanson SJ, Maddaus MA. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg. 2008;86:2008–16. doi: 10.1016/j.athoracsur.2008.07.009. discussion 2016–8. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto K, Ohsumi A, Kojima F, Imanishi N, Matsuoka K, Ueda M, et al. Long-term survival after video-assisted thoracic surgery lobectomy for primary lung cancer. Ann Thorac Surg. 2010;89:353–9. doi: 10.1016/j.athoracsur.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 10.Sitges-Serra A. Ecosurgery. Br J Surg. 2002;89:387–8. doi: 10.1046/j.0007-1323.2001.02032.x. [DOI] [PubMed] [Google Scholar]
- 11.Sakuragi T, Ohma H, Ohteki H. Efficacy of SOFT COAG for intraoperative bleeding in thoracic surgery. Interact Cardiovasc Thorac Surg. 2009;9:767–8. doi: 10.1510/icvts.2009.212696. [DOI] [PubMed] [Google Scholar]
- 12.Sakuragi T, Ohteki H. The utility of BiClamp® for intraoperative air leakage control in video-assisted thoracic surgery for pulmonary lobectomy. Gen Thorac Cardiovasc Surg. 2012;60:781–3. doi: 10.1007/s11748-012-0028-0. [DOI] [PubMed] [Google Scholar]
- 13.Sakuragi T, Okazaki Y, Mitsuoka M, Itoh T. Dramatic hemostasis of the transected pulmonary artery model using SOFT COAG electrosurgical output. Interact CardioVasc Thorac Surg. 2008;7:764–6. doi: 10.1510/icvts.2008.177923. [DOI] [PubMed] [Google Scholar]
- 14.Yoshikawa K, Tsubota N, Kodama K, Ayabe H, Taki T, Mori T. Prospective study of extended segmentectomy for small lung tumors: the final report. Ann Thorac Surg. 2002;73:1055–8. doi: 10.1016/s0003-4975(01)03466-x. discussion 1058–9. [DOI] [PubMed] [Google Scholar]
- 15.Okada M, Koike T, Higashiyama M, Yamato Y, Kodama K, Tsubota N. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg. 2006;132:769–75. doi: 10.1016/j.jtcvs.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 16.Okada M, Mimura T, Ikegaki J, Katoh H, Itoh H, Tsubota N. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg. 2007;133:753–8. doi: 10.1016/j.jtcvs.2006.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.