Abstract
Intracardiac leiomyomatosis is rare but has been increasingly reported in recent years. Owing to its rarity, intracardiac leiomyomatosis has been reported only as isolated case reports and case series. This disorder is thought to be underestimated and easily overlooked in the clinic, while it is dangerous owing to the risk of sudden death caused by total outflow tract obstruction. We performed an electronic literature search for intracardiac leiomyomatosis and identified 194 cases that were reported in English from 1974 (the first reported case) to September 2012. Our aim is to provide a detailed and comprehensive review of the clinical presentation, diagnosis, histopathological characterization, treatment and prognosis of this disorder. According to our analysis, intracardiac leiomyomatosis is most common in the fifth decade, and the mean age of detection is ∼50 years. Most patients had undergone previous hysterectomy/myomectomy or had a coexisting uterine leiomyoma when admitted. The most common clinical presentations were dyspnoea, syncope, oedema of the lower extremities and palpitation. Transoesophageal echocardiography, computed tomography and magnetic resonance imaging are helpful in the preoperative diagnosis and to guide the surgical management. Complete removal guarantees an excellent outcome, with no recurrence or postoperative death, while incomplete removal leads to recurrence in one-third of patients. Anti-oestrogen therapy is not imperative after incomplete removal owing to its inability to prevent recurrence.
Keywords: Intracardiac leiomyomatosis, Secondary cardiac tumours, Metastasectomy
INTRODUCTION
Metastatic cardiac tumours are relatively common; however, intravenous tumour extension into the heart is very infrequent. Intracardiac leiomyomatosis (ICLM), a condition rarely seen, is characterized by histologically benign smooth muscle tumours of extracardiac origin reaching the right heart by direct extension into the venous channel. Owing to its rarity, the accumulated knowledge of ICLM has derived mainly from isolated case reports and case series. This tumour has been suggested to originate either from a uterine leiomyoma with vascular invasion or from the venous smooth muscle wall itself. When extending beyond the inferior vena cava (IVC), this intravenous tumour can reach as far as the right atrium (RA), the right ventricle (RV) or even the pulmonary arteries (PAs). Most patients are middle-aged women. Similar to other intracardiac tumours, the clinical presentation of ICLM is non-specific. The advent of echocardiography, computed tomography (CT) and magnetic resonance imaging (MRI) has allowed early and accurate evaluation of this lesion, while the final diagnosis must be made by postoperative pathological examination. Although histologically benign, ICLM is thought to be clinically aggressive owing to the risk of sudden death caused by total outflow tract obstruction.
MATERIALS AND METHODS
We performed an electronic literature search for ICLM in MEDLINE/PubMed, Web of Science and Cochrane Library. The search terms ‘intravenous leiomyoma’, ‘intravenous leiomyomatosis’, ‘intracardiac leiomyoma’, ‘intracardiac leiomyomatosis’, ‘intravascular leiomyoma’, ‘intravascular leiomyomatosis’ ‘cardiac extension’, ‘heart extension’ and ‘pulmonary extension’ were used to identify case reports and case series published in English. Cases with intravascular mass extension into the right heart and confirmed by histopathological examination as leiomyoma were included. All the references cited in each publication were evaluated for additional eligible cases.
We found 163 articles with cases that met the characterization of ICLM (see Supplementary material). Of these, any patient reported more than once in different articles was treated as one case, and the medical information was integrated into the analysis. Finally, we identified 194 cases of ICLM that were reported in English up to September 2012.
Adhesion was defined as the attachment of tumour to the vascular wall, and it may lead to blood vessel rupture or even fatal haemorrhage when the tumour is removed. Complete removal was confirmed by the surgeon during the operation or by postoperative imaging examination. Unless otherwise specified, incomplete removal mentioned here includes partial excision of the tumour and non-surgical treatment (in patients who refused surgery), because both of these left residual tumour in the patient. Recurrence was defined as the postoperative regrowth of the tumour detected by imaging examination. The recurrent and survival data were analysed with the standard Kaplan–Meier actuarial technique. A two-tailed probability value of <0.01 was considered to indicate statistical significance. All the data in our analysis, including demographics, clinical presentation, histopathological characterization, treatment and prognosis, were extracted from these 194 cases using Excel 2007 (Microsoft Corp., Redmond, WA, USA) and Prism 5.0 (Graphpad Software, La Jolla, CA, USA).
RESULTS
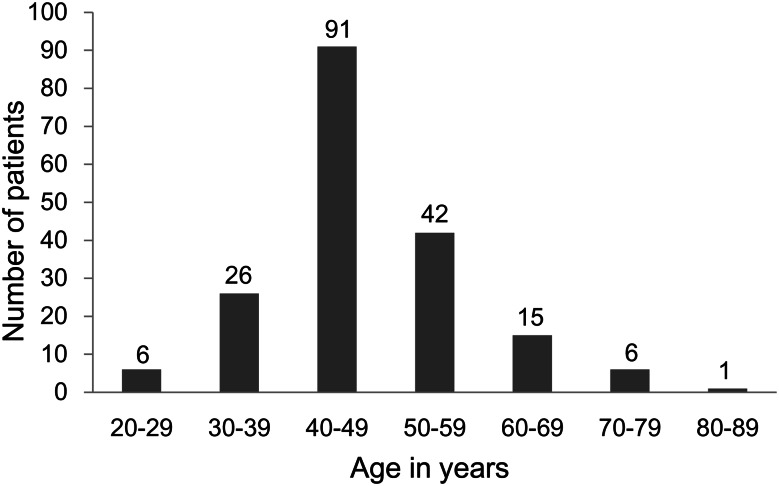
According to our collection of 194 patients, ICLM is seen exclusively in women. Recently, this disorder has been reported with increasing frequency, but it is certain that cases still go unreported. Half of the cases were reported within the last 10 years. Age prevalence was accurately available in 187 patients (Fig. 1), giving an average 47.6 years (range: 20–81 years). Reproductive history was not mentioned in 154 patients (79.4%), and of the remainder, 10 of 40 patients (25.0%) were nulliparous and 30 of 40 (75.0%) were multiparous. One hundred and four patients (53.6%) had undergone a previous hysterectomy/myomectomy, and 58 (29.9%) had a coexisting uterine leiomyoma on admission. Only four patients (2.1%) were reported with a normal uterus. The time between the hysterectomy/myomectomy and detection of ICLM was available for 84 patients, giving an average of 87.8 months (range: 3 months to 47 years).
Figure 1:
Age at the diagnosis of intracardiac leiomyomatosis (n = 187 patients).
The clinical presentation of ICLM varied (n = 177 patients) and is shown in Table 1. On auscultation, a heart murmur was heard in 65 patients (33.5%; 54 systolic, six diastolic and five unidentified). Tumour plop was noted in 11 patients (5.7%) and pericardial rub in only two (1.0%).
Table 1:
Summary of clinical details of intracardiac leiomyomatosis
| Clinical data | No. of patients (%) |
|---|---|
| Presenting symptoms (n = 177 patients) | |
| Dyspnoea | 65 (36.7) |
| Syncope | 47 (26.6) |
| Oedema of the lower extremities | 46 (26.0) |
| Palpitation | 36 (20.3) |
| Fatigue | 19 (10.7) |
| Ascites | 16 (9.0) |
| Jugular vein distension | 13 (7.3) |
| Chest pain | 13 (7.3) |
| Abdominal pain | 12 (6.8) |
| Asymptomatic | 23 (13.0) |
| Route of extension (n = 148 patients) | |
| Iliac vein | 98 (66.2) |
| Ovarian vein | 26 (17.6) |
| Renal vein | 21 (14.2) |
| Both iliac and ovarian veins | 10 (6.8) |
| Gonadal vein | 6 (4.1) |
| Hypogastric vein | 5 (3.4) |
| Uterine vein | 5 (3.4) |
| Hypohepatic or hepatic vein | 5 (3.4) |
| Pelvic vein | 2 (1.4) |
| Extent of involvement (n = 172 patients) | |
| Right atrium | 69 (40.1) |
| Right ventricle | 81 (47.1) |
| Pulmonary artery | 22 (12.8) |
Chest roentgenogram was mentioned for 51 patients, and the results were normal in 30 (58.8%) and showed heart enlargement in 21 (41.2%). Electrocardiographic results, which were available in 54 patients, were normal in 22 (40.7%), and showed a low voltage in 13 (24.1%), non-specific ST–T change in 11 (20.4%), sinus tachycardia in seven (13.0%) and arrhythmia in four (7.4%).
In 148 cases, the route of tumour extension was described and is shown in Table 1. Distant locations were recorded in 172 patients, with extension into the RA, RV and PAs (Table 1). The adhesion situation was available in 76 patients; 21 (27.6%) did not have any adhesions, while adhesion at the lower diaphragmatic level of the IVC was found in 29 (38.2%), upper diaphragmatic in 15 (19.7%), unidentified in 10 (13.2%), and one patient (1.3%) had a small area of attachment at the level of the diaphragm.
Of 105 patients for whom a preoperative diagnosis was made, ICLM was correctly diagnosed in 57 (54.3%), while 31 (29.5%) were misdiagnosed as RA myxomas, followed by thrombus-in-transit in four (3.8%), pulmonary embolism in three (2.9%) and IVC embolism in two (1.9%).
Treatment of 11 (5.7%) patients was not provided in detail. Apart for four patients (2.1%) who died before surgery and four (2.1%) who refused surgery, surgical excision was performed in 175 patients (90.2%). The surgical procedures are summarized in Table 2. In addition to surgical excision of the tumour, tricuspid valve replacement was performed in seven patients (4.0%) and repair in six (3.4%). The duration of postoperative hospital stay was available for 55 patients, with an average of 10.6 days (range: 4–33 days).
Table 2:
Summary of treatments of intracardiac leiomyomatosis
| Treatments (n = 194 patients) | No. of patients (%) |
|---|---|
| Complete removal | 104 (53.6) |
| One-stage operation | 44 (22.7) |
| Two-stage operation | 49 (25.3) |
| Thoracotomy alone | 6 (3.1) |
| Laparotomy alone | 5 (2.6) |
| Incomplete removala | 45 (23.2) |
| Degree of removal unspecified | 23 (11.9) |
| Not provided in detail | 11 (5.7) |
| Intraoperative death | 3 (1.5) |
| Preoperative death | 4 (2.1) |
| Refused operation | 4 (2.1) |
aDoes not include the four patients who refused surgery.
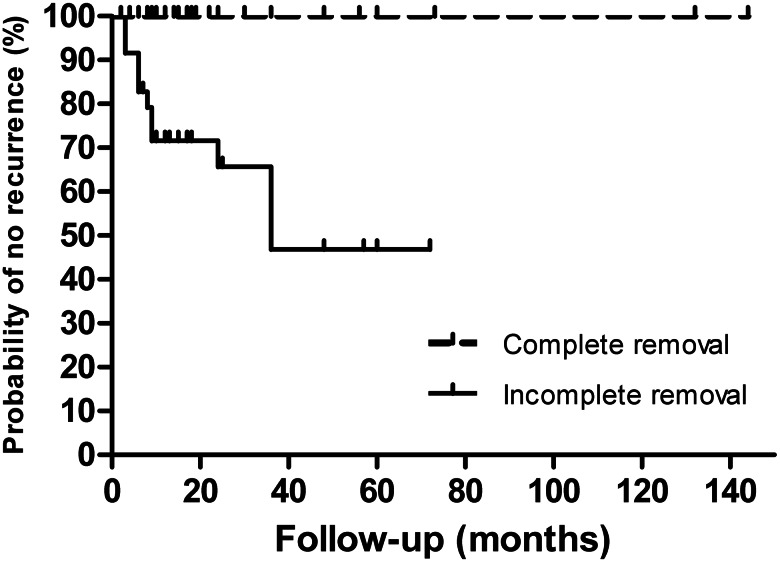
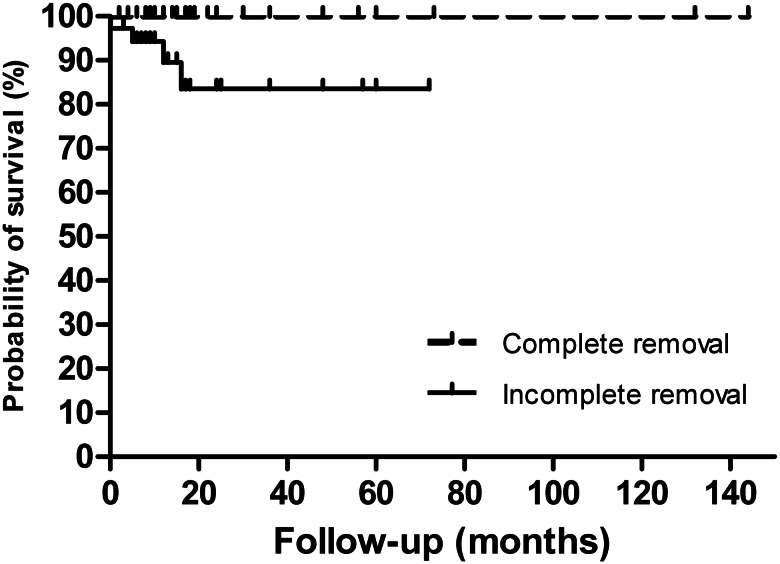
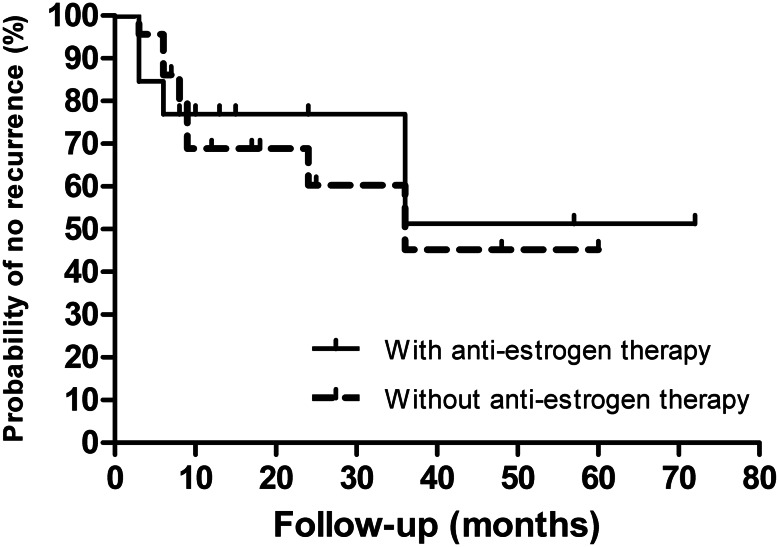
The follow-up time was provided for 123 patients, with an average of 27.3 months (range: 2 months to 12 years). Follow-up of complete removal was recorded for 79 patients (75 of them with follow-up time provided), and no recurrence was reported. The longest postoperative follow-up was 12 years. Follow-up of incomplete removal was recorded for 36 patients and, of these, recurrence occurred in 12 (33.3%; Fig. 2; log-rank P value < 0.0001) and death in four (11.1%; including one patient who died 3 days after surgery; Fig. 3; log-rank P value = 0.0022). The intracardiac location of recurrence was recorded for five patients (two re-extension into the RA, two into the RV and one into the PAs). Two of them underwent reoperation. Among the 36 patients with incomplete removal, out of those who had anti-oestrogen therapy (13), four had a reported recurrence, whereas out of those without anti-oestrogen therapy (23), eight had a recurrence (Fig. 4; log rank P value = 0.8259).
Figure 2:
Surgical removal and recurrence (n = 111 patients). Kaplan–Meier actuarial curves show that incomplete removal significantly impacts recurrence [hazard ratio (HR): 0.029; 95% confidence interval (CI): 0.008–0.103; log-rank P value < 0.0001].
Figure 3:
Surgical removal and survival (n = 112 patients). Kaplan–Meier survival curves show that postoperative death increases significantly after incomplete removal (HR: 0.035; 95% CI: 0.004–0.301; log-rank P value = 0.0022).
Figure 4:
Incomplete removal and recurrence (n = 36 patients). Kaplan–Meier actuarial curves of recurrence show no significant difference between patients with or without anti-oestrogen therapy after incomplete removal (HR: 0.872; 95% CI: 0.257–2.959; log-rank P value = 0.8259).
DISCUSSION
Historical perspective
In 1907, the initial cases of ICLM were described in German by Dürck and Hörmann, respectively [1, 2]. The first report of ICLM in English was by Mandelbaum et al. in 1974 and originated from the IVC [3]. In 1980, Timmis et al. noted the first case of ICLM originating from the uterus [4] and, in the same year, anti-oestrogen therapy was first used by Tierney et al. to prevent possible recurrence due to incomplete excision [5]. In 1982, Maurer and Nanda used the term ‘intracardiac leiomyomatosis’ and diagnosed this disease on the basis of transthoracic echocardiography (TTE) for the first time [6]. The first complete removal of an ICLM using a one-stage procedure was reported by Iverson et al. in 1983 [7] and using a two-stage procedure by Basso et al. in 1984 [8]. In 1989, Torres et al. were the first to use transoesophageal echocardiography (TOE) for preoperative evaluation of the intracardiac portion of an ICLM [9]. In 1998, Marom et al. reported this lesion in a pregnant woman [10]. Kullo et al., in 1999, provided the first description of recurrent ICLM due to incomplete excision [11]. In 2002, Quade et al. performed cytogenetic and molecular studies of ICLM [12]. The longest ICLM (75 cm) was reported by Lam et al. in 2004 [13]. In 2006, Jeanmart et al. reported the first successful complete excision via thoracoscopy alone [14]. More recently, Okada et al. reported an older patient (81 years old) who tolerated the one-stage complete removal procedure well in 2012 [15].
Epidemiology and characterization
Although ICLM occurs at ages ranging from 20 to 81 years, it is most common in the fifth decade (Fig. 1). The mean age of detection is ∼50 years. In our collection of cases, this disorder is seen exclusively in women. Ten patients were nulliparous, while 30 were multiparous. Given that the reproductive history was not mentioned in most cases (79.4%), its relation to this disorder cannot be specified. It is postulated that ICLM arises from an invasive uterine myoma or from the vascular wall [8, 16, 17]. Approximately 83.5% of the patients had undergone a previous hysterectomy/myomectomy or had a coexisting uterine leiomyoma, suggesting uterine leiomyoma with vascular invasion as the origin of this lesion. A few patients were found with a normal uterus [3, 18–20], which may support the theory of ICLM originating from the venous smooth muscle wall itself. The interval between hysterectomy/myomectomy and the detection of ICLM varied from merely 3 months [21] to as long as more than 40 years [22, 23]. Furthermore, two cases of pregnant women with ICLM have been described [10, 24].
Clinical manifestations
Even with intravenous tumour extension as far as the IVC, symptoms may be absent until cardiac insufficiency develops due to intracardiac extension [4, 25–27]. The clinical presentation of ICLM usually depends on the extension and size of the tumour [3, 28–30]. Right ventricular extension may cause complete outflow tract obstruction [31, 32], and patients with extensions as far as the PAs may develop severe pulmonary embolism [28, 33, 34] or even sudden death [35]. Asymptomatic manifestations were reported in 13.0% of ICLM patients because of little or no compromise of venous return. Symptomatic patients presented with a various symptoms, the most common being dyspnoea, syncope, oedema of the lower extremities and palpitations, followed by fatigue, ascites, jugular vein distension, chest pain, abdominal pain and hepatomegaly. In a few cases, other manifestations, such as dizziness [19, 33, 36–40], cyanosis [31, 41–43], cough [42, 44], vomiting [24, 43, 45], chest tightness [27, 33, 46], menstrual disorder [32, 46–48], orthopnoea [39, 49, 50], lethargy [51–53], headache [11, 54, 55], sweating [24, 43, 55], postmenopausal bleeding [56–58], weight loss [56, 59], shock [42, 60], nausea [43, 61], hypotension [24, 42], skin petechiae [31, 62], haemoptysis [63, 64], scleral icterus [62], facial oedema [65] and urinary incontinence [53], have been reported.
Although seldom seen, concomitant embolic phenomena in the distal vasculature occurred in a few patients. As an unusual clinical presentation, acute Budd–Chiari syndrome due to thrombotic occlusion of large hepatic veins has been reported [22, 56]. Another patient with embolism in an artery of the left parietal lobe was admitted with sudden onset of right hemiparesis and slurring of speech [66]. In addition, a case of ICLM with severe hypoxia culminating in generalized seizure activity in a pregnant woman has been described [24].
Depending on the size and mobility of the tumour, ICLM with RV extension (including intermittent prolapse into the RV) through the tricuspid orifice may damage the valve apparatus, leading to tricuspid insufficiency or stenosis. A systolic or diastolic murmur was noted in 33.5% of patients. In 5.7% of the reported patients, tumour plop was heard when the tumour was highly mobile in the flow of blood. Electrocardiographic findings showed normal results in 40.7% of patients, while low-voltage, non-specific ST–T change, sinus tachycardia and arrhythmia have also been reported.
Imaging examination
Imaging examination is helpful to the preoperative diagnosis and detection of recurrence of ICLM [37, 66, 67]. The chest roentgenogram, which was mentioned for 51 patients, presented non-specific cardiomegaly in 58.8% of these and was not helpful for the detection of the tumour unless there was extensive calcification [6, 68].
Echocardiography is a more convenient method and can provide real-time information on an intracardiac tumour [69]. Currently, TTE is widely used, but the visualization of an IVC tumour may be unsuccessful owing to a pattern of echo-free space caused by blood in a tubular tumour [8, 53]. Transoesophageal echocardiography is superior to TTE in evaluation of a right atrial mass by providing more accurate images [9, 11, 21, 26, 55]. Transoesophageal echocardiography has been used to show the fine details of the IVC, interatrial septum, tricuspid valve apparatus and attachments of the tumour [9, 26, 36]. To avoid vein injury during tumour removal, intraoperative TOE monitoring was suggested to guide the surgeons on localization and adherence [36, 39, 58].
Enhanced CT and MRI are usually used to evaluate the size, location, origin and extent of an ICLM, because they can provide excellent anatomical information [28, 47, 49, 66]. Magnetic resonance imaging is more useful because of its superior ability to demonstrate soft tissue and blood flow without contrast injection [27, 66]. Magnetic resonance imaging usually shows a mass of isosignal intensity on T1 weighting, and a heterogeneous and high-intensity signal in some regions on T2 weighting, due to cystic or hyaline degeneration [27, 47]. However, a patient with an ICLM that showed a mildly enhanced signal in both T1 and T2 weighting has been described [65]. Motion artifacts may be the main disadvantage of MRI. Cardiac catheterization is not usually required, because most of the time adequate information is provided by non-invasive imaging. Whole-body scans with radioactive elements can be used to exclude residual tumours in other locations.
Generally speaking, it is a challenge to make a correct diagnosis of ICLM before surgery, and its occurrence is considered to be underestimated [59], but with advances in imaging technologies, this situation has substantially improved.
Differential diagnosis
Differential diagnosis primarily encompasses right atrial myxoma, thrombus-in-transit, renal cell carcinoma, Wilms’ tumour, intracardiac leiomyosarcomatosis, benign metastasizing leiomyoma and other metastatic cardiac tumours. Almost 30% of the patients were misdiagnosed preoperatively as having RA myxomas. In contrast to the right-sided characterization of ICLM, myxoma has a left-sided predominance, with an incidence between 87.3 and 88.5% [70–72]. Although most left atrial masses are myxomas, they constitute only 43% of right atrial masses [18]. Most myxomas originate from the interatrial septum with a stalk and may attach to the walls of the cardiac chambers [36, 65, 69]. Renal cell carcinoma, which should be distinguished from ICLM by postoperative pathological examination, is the most common tumour that can extend into the IVC and right heart [49, 69]. A history of previous hysterectomy/myomectomy or a coexisting uterine leiomyoma may be helpful. Although, in rare instances, Wilms’ tumour with intracardiac extension in adult has been reported, it is commonly seen in children [21]. Renal cell carcinoma and Wilms’ tumour can also be distinguished by detection of renal tumour on imaging examination. Thrombus-in-transit, which typically occurs postoperatively and frequently leads to sudden death, usually gives a ‘popcorn’ appearance within the cardiac chambers [11, 42, 67]. Intracardiac leiomyosarcomatosis, a malignant type in the smooth muscle tumour spectrum, often shares clinical presentations with ICLM, depending on the size and site [49], and should be differentiated by histopathological examination and immunohistochemical staining [52, 62, 65]. Benign metastasizing leiomyoma generally originates from the uterus and metastasizes to other organs, such as the lung, lymph nodes, bone, skeletal muscle, subcutaneous tissue or retroperitoneal space. Although ICLM is rarely coincident with benign metastasizing leiomyoma in the lung [11, 16, 57], the latter alone usually shows no occupation of vascular channels. Other metastatic cardiac tumours commonly involve the pericardium/myocardium, and the primary nidus can be detected by whole-body positron emission computed tomography.
Treatment
Considering the risk of sudden death caused by total outflow tract obstruction, early treatment is recommended when ICLM is highly suspected [75]. Surgical removal of the ICLM is curative, and many surgical techniques have been applied in the treatment of this disease. A quaternary classification system has been described to choose the best surgical strategy for different patients [63].
Complete removal is strongly recommended, because no recurrence has been reported. Blind removal of the tumour via sternotomy or laparotomy alone is dangerous, because this may lead to incomplete retrieval or fatal retroperitoneal haemorrhage [31, 56].
A double approach, using a one- or two-stage procedure, is performed more commonly and thought to be safer. The two-stage procedure involves separate removal of the intracardiac and intra-abdominal components at intervals varying from 7 days to 2 years. Although the patient reported by Altinok et al. [47] was followed up every 3–6 months, an interval as long as 2 years should be avoided. The advantages of this procedure are as follows [39, 46]: (i) suitability for patients in poor condition who cannot tolerate a one-stage procedure; and (ii) it is recommended by some authors when the tumour is extensive or adheres tightly to the vascular wall. The interval can be decided according to the recovery status of the patient [46]; however, rapid growth leading to re-extension into the right cardiac chamber during the waiting interval has been reported [37, 47]. When the patient presents as an emergency [27, 39], a separate surgery is mandatory, with sternotomy first.
More recently, a one-stage operation, in which the sternotomy and laparotomy are performed simultaneously, is being increasingly reported. This approach needs the co-operation of a combined surgical team. The one-stage procedure has several advantages, as follows [40, 73]: (i) avoidance of haemodynamic complications during the interval between two stages; (ii) avoidance of the risk of a second general anesthetic; (iii) reduction of the risk of tumour embolism caused by incomplete resection in the first stage; and (iv) better economic benefits. Whether the risk of bleeding increases is still disputed, because the operative time is not excessively prolonged. Most one-stage operations are performed with cardiopulmonary bypass and total circulatory arrest; in only three cases was the on-pump beating-heart technique used when removing the intracardiac mass [74–76].
In 2011, a woman with an ICLM originating from the uterus was admitted to our department. The tumour was completely removed in a one-stage procedure by co-operation of cardiac surgeons, vascular surgeons and gynaecologists. The patient was asymptomatic, with no evidence of recurrence on 13 month follow-up. Our experience also confirmed that, if there is no adhesion to the IVC or heart at any point, a one-stage complete resection without cardiac arrest is feasible and safe, and leads to a satisfactory outcome [77].
Intraoperative TOE monitoring is used to guide the surgeons about the localization and adherence, to avoid vein injury [36, 39, 58]. Surgery with endovascular techniques has been successful in preventing distal embolization [37]. Furthermore, with the advent of minimally invasive techniques, laparoscopic excision of an abdominal tumour [40] and successful complete excision via thoracoscope alone [14] have been reported.
When the tricuspid valve apparatus is injured by the tumour, valve replacement or repair may be required. A total hysterectomy is recommended if ICLM is confirmed as originating from a coexisting uterine leiomyoma. Bilateral salpingo-oophorectomy should also be performed because of the oestrogen-dependent characteristic of the tumour. To prevent recurrence, ligation of the originating vein is imperative [52].
Whatever the surgical approach chosen, the best choice of treatment is complete removal. Neither medication (including anti-oestrogen therapy using tamoxifen or letrozole) nor radiotherapy alone has been reported to provide a satisfactory outcome. Long-term follow-up should be arranged in case of recurrence.
Incomplete removal is not recommended, because recurrence occurred in 12 patients (33.3%). When the resection is incomplete because of technical difficulty or the patient refuses further surgery, anti-oestrogen therapy (using tamoxifen or letrozole) has been suggested by some authors [21, 27, 33, 50]. Although the oestrogen-dependent characteristic of ICLM is widely recognized, our analysis showed that postoperative anti-oestrogen therapy does not help to prevent recurrence. The possible explanation seems to be that some ICLMs are not oestrogen dependent, but this supposition cannot be confirmed because the oestrogen level in the tumour was not available for most patients with recurrence (10 of 12). The side-effects of this medical treatment include associated menopause, which leads to osteoporosis and heart disease [24]. We conclude that anti-oestrogen therapy is not imperative after incomplete removal. We acknowledge that when dealing with case reports as the source of data, publication bias evidently favours cases with unique features. Moreover, follow-up of incomplete removal was provided in only 36 patients in our analysis, and no randomized controlled data are available, so this recommendation for postoperative anti-oestrogen therapy is still controversial and needs to be assessed further.
Pathology and pathogenesis
Macroscopically, ICLM originating from the uterus usually has a serpentine appearance, with iliac or ovarian vein extension into the IVC and RA. In contrast, a few patients with ICLM originating from the venous smooth muscle wall itself have been reported [3, 17–20, 28]. The tumour is generally smooth and rubbery, with a greyish-white or rusty colour, with or without an intumescent intracardiac head [21]. In patients with a long course of this disease, the tumour may be calcified [6, 19, 28, 32, 68] and extend more than 50 cm [13].
Intracardiac leiomyomatosis is histologically benign. Microscopic examination of atrial tumours often shows a bundled arrangement of spindle-shaped cells and fibromuscular tissues with thick-walled blood vessels [21]. Haemorrhage and necrosis are seldom seen. Mitoses are not evident, and hardly any cellular irregularity or nuclear pleomorphism is found. Occasionally, atypical histopathological features, such as angioleiomyoma [17, 19], lipoleiomyoma [48], lymphangioleiomyoma [78] and borderline leiomyoma [25, 49], have been described.
Immunohistochemical staining is positive for smooth muscle markers (desmin and smooth muscle actin) [33]. Intracardiac leiomyomatosis was reported positive for oestrogen or progestogen receptor markers in 17 patients. In ICLMs originating from the uterus, the uterine tumour shares pathological and immunohistochemical features with the intracardiac portion. A cytogenetic and molecular biological study revealed an abnormal karyotype of 45,XX,der(14)t(12;14)(q15;q24),-22 in all of their specimens [12]. However, owing to its rarity, the pathogenesis of ICLM remains unclear.
Prognosis
Four patients died before surgery due to heart failure [32, 56], septic shock [22] and pulmonary embolism [35]. Intraoperative death occurred in three patients [31, 47, 79], two of whom died from an IVC tear [31, 79]. Whether a one- or two-stage procedure is chosen, the short- and long-term outcome of complete removal is excellent, with no recurrence or postoperative death reported. Recurrence after incomplete removal was found in 33.3%, and four patients died during follow-up [20, 63, 80].
CONCLUSIONS
Although ICLM is rare and always occurs sporadically, it is more common than previously thought. It is most common in the fifth decade, and most patients had undergone a previous hysterectomy/myomectomy or had a coexisting uterine leiomyoma. When a right atrial mass is identified in middle-aged women with previous hysterectomy/myomectomy or uterine leiomyoma, a high degree of suspicion should be maintained, and an abdominal CT should be arranged to confirm the origin. Complete removal guarantees an excellent outcome, with no recurrence or postoperative death, while incomplete removal leads to recurrence in one-third of patients. Anti-oestrogen therapy is not imperative after incomplete removal, owing to its inability to prevent recurrence.
Supplementary material
Supplementary material is available at ICVTS online.
Acknowledgements
The authors thank Iain C. Bruce (Zhejiang University School of Medicine) for reading the manuscript.
Conflict of interest: none declared.
References
- 1.Dürck H. Ueber ein kontinvierlich durch die learned Hohlvene in das Herz vorwachsendes Fibromyom des Uterus. München Med Wochenschr. 1907;54:1154. [Google Scholar]
- 2.Hörmann K. Über einen Fall von myomatosem Uterus Tumor. Zentralbl Gynakol. 1907;51:1604–5. [Google Scholar]
- 3.Mandelbaum I, Pauletto FJ, Nasser WK. Resection of a leiomyoma of the inferior vena cava that produced tricuspid valvular obstruction. J Thorac Cardiovasc Surg. 1974;67:561–7. [PubMed] [Google Scholar]
- 4.Timmis AD, Smallpeice C, Davies AC, Macarthur AM, Gishen P, Jackson G. Intracardiac spread of intravenous leiomyomatosis with successful surgical excision. N Engl J Med. 1980;303:1043–4. doi: 10.1056/NEJM198010303031806. doi:10.1056/NEJM198010303031806. [DOI] [PubMed] [Google Scholar]
- 5.Tierney WM, Ehrlich CE, Bailey JC, King RD, Roth LM, Wann LS. Intravenous leiomyomatosis of the uterus with extension into the heart. Am J Med. 1980;69:471–5. doi: 10.1016/0002-9343(80)90022-4. doi:10.1016/0002-9343(80)90022-4. [DOI] [PubMed] [Google Scholar]
- 6.Maurer G, Nanda NC. Two-dimensional echocardiographic identification of intracardiac leiomyomatosis. Am Heart J. 1982;103:915–7. doi: 10.1016/0002-8703(82)90409-4. doi:10.1016/0002-8703(82)90409-4. [DOI] [PubMed] [Google Scholar]
- 7.Iverson LI, Lee J, Drew D, Sharp J, Ecker RR, Young JN, et al. Intravenous leiomyomatosis with cardiac extension. Tex Heart Inst J. 1983;10:275–8. doi: [PMC free article] [PubMed] [Google Scholar]
- 8.Basso LV, Gradman M, Finkelstein S, Gonzalez-Lavin L. Tricuspid valve obstruction due to intravenous leiomyomatosis. Clin Nucl Med. 1984;9:152–5. doi: 10.1097/00003072-198403000-00010. doi:10.1097/00003072-198403000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Torres F, Tye T, Urbanowicz J. Transesophageal echocardiographic identification of intracardiac leiomyomatosis. J Diagnostic Medical Sonography. 1989;5:258–60. [Google Scholar]
- 10.Marom D, Pitlik S, Sagie A, Ovadia Y, Bishara J. Intravenous leiomyomatosis with cardiac involvement in a pregnant woman. Am J Obstet Gynecol. 1998;178:620–1. doi: 10.1016/s0002-9378(98)70453-3. doi:10.1016/S0002-9378(98)70453-3. [DOI] [PubMed] [Google Scholar]
- 11.Kullo IJ, Oh JK, Keeney GL, Khandheria BK, Seward JB. Intracardiac leiomyomatosis: echocardiographic features. Chest. 1999;115:587–91. doi: 10.1378/chest.115.2.587. doi:10.1378/chest.115.2.587. [DOI] [PubMed] [Google Scholar]
- 12.Quade BJ, Dal Cin P, Neskey DM, Weremowicz S, Morton CC. Intravenous leiomyomatosis: molecular and cytogenetic analysis of a case. Mod Pathol. 2002;15:351–6. doi: 10.1038/modpathol.3880529. doi:10.1038/modpathol.3880529. [DOI] [PubMed] [Google Scholar]
- 13.Lam PM, Lo KW, Yu MY, Wong WS, Lau JY, Arifi AA, et al. Intravenous leiomyomatosis: two cases with different routes of tumor extension. J Vasc Surg. 2004;39:465–9. doi: 10.1016/j.jvs.2003.08.012. doi:10.1016/j.jvs.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Jeanmart H, Lecompte P, Casselman F, Coddens J, Van Vaerenberg G, Vanermen H. Endoscopic tumor resection of the inferior vena cava. J Thorac Cardiovasc Surg. 2006;132:687–8. doi: 10.1016/j.jtcvs.2006.04.029. doi:10.1016/j.jtcvs.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Okada M, Miyoshi Y, Kato G, Ochi Y, Shimizu S, Nakai M. Successful one-stage surgical removal of intravenous leiomyomatosis with cardiac extension in an elderly patient. Gen Thorac Cardiovasc Surg. 2012;60:153–6. doi: 10.1007/s11748-011-0791-3. doi:10.1007/s11748-011-0791-3. [DOI] [PubMed] [Google Scholar]
- 16.Stegmann T, Garcia-Gallont R, Döring W. Intravascular leiomyomatosis: report of a case and review of the literature. Thorac Cardiovasc Surg. 1987;35:157–60. doi: 10.1055/s-2007-1020220. doi:10.1055/s-2007-1020220. [DOI] [PubMed] [Google Scholar]
- 17.Ohmori T, Uraga N, Tabei R, Abe M, Sumimoto T, Hamada M, et al. Intravenous leiomyomatosis: a case report emphasizing the vascular component. Histopathology. 1988;13:470–2. doi: 10.1111/j.1365-2559.1988.tb02066.x. doi:10.1111/j.1365-2559.1988.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 18.Cleveland DC, Westaby S, Karp RB. Treatment of intra-atrial cardiac tumors. JAMA. 1983;249:2799–802. doi:10.1001/jama.1983.03330440037028. [PubMed] [Google Scholar]
- 19.Tamburino C, Russo G, Incognito C, Battaglia G, Monaca V, Lomeo A, et al. Intracardiac extension of a calcified ovarian hemangioma — a case report. Angiology. 1992;43:249–52. doi: 10.1177/000331979204300310. doi:10.1177/000331979204300310. [DOI] [PubMed] [Google Scholar]
- 20.Wang XW, Zhang L, Chen YJ, Zhang SJ, Qin JW, Wu YH, et al. Diagnosis and surgical treatment of intravenous leiomyomatosis extending into the heart: two cases report and review of the literature. J Nanjing Medical University. 2009;23:305–10. [Google Scholar]
- 21.Grella L, Arnold TE, Kvilekval KH, Giron F. Intravenous leiomyomatosis. J Vasc Surg. 1994;20:987–94. doi: 10.1016/0741-5214(94)90237-2. doi:10.1016/0741-5214(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 22.Kuenen BC, Slee PH, Seldenrijk CA, Wagenaar SS. Intravenous leiomyomatosis complicated by Budd–Chiari syndrome. Postgrad Med J. 1996;72:686–8. doi: 10.1136/pgmj.72.853.686. doi:10.1136/pgmj.72.853.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornélis F, Belleannée G, Lederlin M. Cardiac extension of an intravascular leiomyomatosis 43 years after hysterectomy. J Thorac Cardiovasc Surg. 2012;144:e3–5. doi: 10.1016/j.jtcvs.2012.03.022. doi:10.1016/j.jtcvs.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Ling FT, David TE, Merchant N, Yu E, Butany JW. Intracardiac extension of intravenous leiomyomatosis in a pregnant woman: a case report and review of the literature. Can J Cardiol. 2000;16:73–9. [PubMed] [Google Scholar]
- 25.Nagumo M, Kiso I, Misumi T, Yasudo M, Nakada K, Mukai M. Cardiac extension of intravenous leiomyomatosis with successful resection. Tokai J Exp Clin Med. 1997;22:125–31. [PubMed] [Google Scholar]
- 26.Gehr NR, Lund O, Alstrup P, Nielsen JS, Villadsen AB, Bartholdy NJ. Recurrence of uterine intravenous leiomyomatosis with intracardiac extension. Diagnostic considerations and surgical removal. Scand Cardiovasc J. 1999;33:312–4. doi: 10.1080/14017439950141597. doi:10.1080/14017439950141597. [DOI] [PubMed] [Google Scholar]
- 27.Wu CK, Luo JL, Yang CY, Huang YT, Wu XM, Cheng CL, et al. Intravenous leiomyomatosis with intracardiac extension. Intern Med. 2009;48:997–1001. doi: 10.2169/internalmedicine.48.1780. doi:10.2169/internalmedicine.48.1780. [DOI] [PubMed] [Google Scholar]
- 28.Marcus SG, Krauss T, Freedberg RS, Culliford AT, Weinreich DJ, Kronzon I. Pulmonary embolectomy for intravenous uterine leiomyomatosis. Am Heart J. 1994;127:1642–5. doi: 10.1016/0002-8703(94)90404-9. doi:10.1016/0002-8703(94)90404-9. [DOI] [PubMed] [Google Scholar]
- 29.Karabinos I, Tafralis D, Papadopoulos A. Heart leiomyoma extending from the inferior vena cava to the right heart. Hellenic J Cardiol. 2008;49:186–7. [PubMed] [Google Scholar]
- 30.Peng HJ, Zhao B, Yao QW, Qi HT, Xu ZD, Liu C. Intravenous leiomyomatosis: CT findings. Abdom Imaging. 2012;37:628–31. doi: 10.1007/s00261-011-9798-6. doi:10.1007/s00261-011-9798-6. [DOI] [PubMed] [Google Scholar]
- 31.Nili M, Liban E, Levy MJ. Tricuspid stenosis due to intravenous leiomyomatosis—a call for caution: case report and review of the literature. Tex Heart Inst J. 1982;9:231–5. [PMC free article] [PubMed] [Google Scholar]
- 32.Roman DA, Mirchandani H. Intravenous leiomyoma with intracardiac extension causing sudden death. Arch Pathol Lab Med. 1987;111:1176–8. [PubMed] [Google Scholar]
- 33.Ricci MA, Cloutier LM, Mount S, Welander C, Leavitt BJ. Intravenous leiomyomatosis with intracardiac extension. Cardiovasc Surg. 1995;3:693–6. doi: 10.1016/0967-2109(96)82871-7. doi:10.1016/0967-2109(96)82871-7. [DOI] [PubMed] [Google Scholar]
- 34.Rajaii-Khorasani A, Kahrom M, Hashemzadeh M, Tayebi S, Ghazi M, Hamedanchi A. Pulmonary artery extension of uterine leiomyoma. J Card Surg. 2012;27:466–9. doi: 10.1111/j.1540-8191.2012.01469.x. doi:10.1111/j.1540-8191.2012.01469.x. [DOI] [PubMed] [Google Scholar]
- 35.Burke M, Opeskin K. Death due to intravenous leiomyomatosis extending to the right pulmonary artery. Pathology. 2004;36:202–3. doi: 10.1080/00313020410001672075. doi:10.1080/00313020410001672075. [DOI] [PubMed] [Google Scholar]
- 36.Harris LM, Karakousis CP. Intravenous leiomyomatosis with cardiac extension: tumor thrombectomy through an abdominal approach. J Vasc Surg. 2000;31:1046–51. doi: 10.1067/mva.2000.104601. doi:10.1067/mva.2000.104601. [DOI] [PubMed] [Google Scholar]
- 37.Derubertis BG, Clair D, Faries P, Kapur S, Park K, Kent KC. Resection of an intravenous leiomyoma with intracardiac extension with use of endovascular techniques. J Vasc Surg. 2004;40:554–8. doi: 10.1016/j.jvs.2004.05.024. doi:10.1016/j.jvs.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 38.Ozer N, Engin H, Akgül E, Sahiner L, Atalar E, Aksöyek S, et al. An unusual case of recurrent mass in the right atrium: intravenous leiomyomatosis. Echocardiography. 2005;22:514–6. doi: 10.1111/j.1540-8175.2005.04053.x. doi:10.1111/j.1540-8175.2005.04053.x. [DOI] [PubMed] [Google Scholar]
- 39.Luciani N, Anselmi A, Glieca F, Martinelli L, Possati G. Diagnostic and surgical issues in emergency presentation of a pelvic leiomyoma in the right heart. Ann Thorac Surg. 2009;87:1589–92. doi: 10.1016/j.athoracsur.2008.09.077. doi:10.1016/j.athoracsur.2008.09.077. [DOI] [PubMed] [Google Scholar]
- 40.Lee S, Kim DK, Narm KS, Cho SH. Pulmonary artery embolization of intravenous leiomyomatosis extending into the right atrium. Korean J Thorac Cardiovasc Surg. 2011;44:243–6. doi: 10.5090/kjtcs.2011.44.3.243. doi:10.5090/kjtcs.2011.44.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anichini C, Calamai G, Pedemonte E, Moroni M, Tozzini S, Nesi G. Intravenous leiomyoma with cardiac involvement. Int Angiol. 2001;20:345–7. [PubMed] [Google Scholar]
- 42.Butler MW, Sanders A. Obstructive shock in a 47 year old female with a deep venous thrombosis due to intravascular leiomyomatosis: a case report. Cases J. 2009;2:8159. doi: 10.4076/1757-1626-2-8159. doi:10.4076/1757-1626-2-8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang LQ, Zhang B, Liu BG, Liu FH. Diagnosis of intravenous leiomyomatosis extending to heart with emphasis on magnetic resonance imaging. Chin Med J (Engl) 2012;125:33–7. [PubMed] [Google Scholar]
- 44.Virzì G, Ragazzi S, Bussichella F, D'Agati P, Caputo S, Scaravilli F, et al. Intravenous leiomyomatosis extending from the inferior caval vein to the pulmonary artery. J Thorac Cardiovasc Surg. 2007;133:831–2. doi: 10.1016/j.jtcvs.2006.10.050. doi:10.1016/j.jtcvs.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 45.Matsuo K, Fleischman F, Ghattas CS, Gabrielyan AS, Ballard CA, Roman LD, et al. Successful extraction of cardiac-extending intravenous leiomyomatosis through gonadal vein. Fertil Steril. 2012;98:1341–5.e1. doi: 10.1016/j.fertnstert.2012.07.1121. doi:10.1016/j.fertnstert.2012.07.1121. [DOI] [PubMed] [Google Scholar]
- 46.Liu B, Liu C, Guan H, Li Y, Song X, Shen K, et al. Intravenous leiomyomatosis with inferior vena cava and heart extension. J Vasc Surg. 2009;50:897–902. doi: 10.1016/j.jvs.2009.04.037. doi:10.1016/j.jvs.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 47.Altinok D, Yildiz YT, Tacal T, Karapinar K, Eryilmaz M. MRI of intravascular leiomyomatosis extending to the heart. Eur Radiol. 2000;10:871. doi: 10.1007/s003300051023. doi:10.1007/s003300051023. [DOI] [PubMed] [Google Scholar]
- 48.Vural C, Özen Ö, Demirhan B. Intravenous lipoleiomyomatosis of uterus with cardiac extension: a case report. Pathol Res Pract. 2011;207:131–4. doi: 10.1016/j.prp.2010.10.004. doi:10.1016/j.prp.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Stolf NA, Santos GG, Haddad VL, Simões RM, Avelar SF, Jr, Ferreira FH, Jr, et al. Successful one-stage resection of intravenous leiomyomatosis of the uterus with extension into the heart. Cardiovasc Surg. 1999;7:661–4. doi: 10.1016/s0967-2109(99)00003-4. doi:10.1016/S0967-2109(99)00003-4. [DOI] [PubMed] [Google Scholar]
- 50.Biri A, Korucuoglu U, Zumrutbas N, Tiras B, Guner H. Intravenous leiomyomatosis treated with aromatase inhibitor therapy. Int J Gynaecol Obstet. 2008;101:299–300. doi: 10.1016/j.ijgo.2007.12.002. doi:10.1016/j.ijgo.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Politzer F, Kronzon I, Wieczorek R, Feiner H, De Marco LE, Weintraub PR, et al. Intracardiac leiomyomatosis: diagnosis and treatment. J Am Coll Cardiol. 1984;4:629–34. doi: 10.1016/s0735-1097(84)80113-8. doi:10.1016/S0735-1097(84)80113-8. [DOI] [PubMed] [Google Scholar]
- 52.Mazzola A, Gregorini R, Procaccini B, Moretti V, Lucantoni R, Lorenzi W, et al. Intracaval and intracardiac leiomyomatosis of uterine origin. Ann Vasc Surg. 1986;1:134–8. doi: 10.1016/S0890-5096(06)60715-2. [DOI] [PubMed] [Google Scholar]
- 53.Rosenberg JM, Marvasti MA, Obeid A, Johnson LW, Bonaventura M. Intravenous leiomyomatosis: a rare cause of right sided cardiac obstruction. Eur J Cardiothorac Surg. 1988;2:58–60. doi: 10.1016/1010-7940(88)90099-1. doi:10.1016/1010-7940(88)90099-1. [DOI] [PubMed] [Google Scholar]
- 54.Shida T, Yoshimura M, Chihara H, Nakamura K. Intravenous leiomyomatosis of the pelvis with reextension into the heart. Ann Thorac Surg. 1986;42:104–6. doi: 10.1016/s0003-4975(10)61850-4. doi:10.1016/S0003-4975(10)61850-4. [DOI] [PubMed] [Google Scholar]
- 55.Miranda-Guardiola F, Josa M, Valls V, Azqueta M, Anguera I, Paré C. A case of uterine leiomyomatosis extending into the right heart with an unusual echocardiographic appearance. Echocardiography. 1997;14:149–52. doi: 10.1111/j.1540-8175.1997.tb00702.x. doi:10.1111/j.1540-8175.1997.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 56.Bahary CM, Gorodeski IG, Nilly M, Neri A, Avidor I, Garti IJ. Intravascular leiomyomatosis. Obstet Gynecol. 1982;59:73S–7S. [PubMed] [Google Scholar]
- 57.Lo KW, Lau TK. Intracardiac leiomyomatosis. Case report and literature review. Arch Gynecol Obstet. 2001;264:209–10. doi: 10.1007/s004040000115. doi:10.1007/s004040000115. [DOI] [PubMed] [Google Scholar]
- 58.Subramaniam B, Pawlowski J, Gross BA, Kim YB, LoGerfo FW. TEE-guided one-stage excision of intravenous leiomyomatosis with cardiac extension through an abdominal approach. J Cardiothorac Vasc Anesth. 2006;20:94–5. doi: 10.1053/j.jvca.2004.11.049. doi:10.1053/j.jvca.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 59.Kocica MJ, Vranes MR, Kostic D, Kovacevic-Kostic N, Lackovic V, Bozic-Mihajlovic V, et al. Intravenous leiomyomatosis with extension to the heart: rare or underestimated? J Thorac Cardiovasc Surg. 2005;130:1724–6. doi: 10.1016/j.jtcvs.2005.08.021. doi:10.1016/j.jtcvs.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 60.Anselmi A, Tsiopoulos V, Perri G, Palladino M, Ferrante A, Glieca F. Case series of resection of pelvic leiomyoma extending into the right heart: surgical safeguards and clinical follow-up. J Cardiovasc Med (Hagerstown) 2010;11:583–6. doi: 10.2459/JCM.0b013e328337d856. doi:10.2459/JCM.0b013e328337d856. [DOI] [PubMed] [Google Scholar]
- 61.Okamoto H, Itoh T, Morita S, Matsuura A, Yasuura K. Intravenous leiomyomatosis extending into the right ventricle: one-stage radical excision during hypothermic circulatory arrest. Thorac Cardiovasc Surg. 1994;42:361–3. doi: 10.1055/s-2007-1016525. doi:10.1055/s-2007-1016525. [DOI] [PubMed] [Google Scholar]
- 62.Garcia FA, Villanueva RA, Narciso FV, Aventura AP. Intravenous leiomyomatosis of the uterus and pelvis presenting as a cardiac tumor. Ann Thorac Surg. 1986;42:S41–3. doi: 10.1016/s0003-4975(10)64641-3. doi:10.1016/S0003-4975(10)64641-3. [DOI] [PubMed] [Google Scholar]
- 63.Gan HL, Zhang JQ, Bo P. The classification and surgical strategy of intracardiac leiomyomatosis. Asian J Surg. 2009;32:129–36. doi: 10.1016/S1015-9584(09)60383-3. doi:10.1016/S1015-9584(09)60383-3. [DOI] [PubMed] [Google Scholar]
- 64.Zhang H, Miao Q, Liu J, Wang C, Zhang C, Zhai H. Giant intravenous leiomyomatosis with intracardiac extension. Ann Thorac Surg. 2012;94:1013. doi: 10.1016/j.athoracsur.2012.02.047. doi:10.1016/j.athoracsur.2012.02.047. [DOI] [PubMed] [Google Scholar]
- 65.Kocaoglu M, Bulakbasi N, Ugurel MS, Ors F, Tayfun C, Ucoz T. Value of magnetic resonance imaging in the depiction of intravenous leiomyomatosis extending to the heart. J Comput Assist Tomogr. 2003;27:630–3. doi: 10.1097/00004728-200307000-00033. doi:10.1097/00004728-200307000-00033. [DOI] [PubMed] [Google Scholar]
- 66.Peh WC, Cheung DL, Ngan H. Smooth muscle tumors of the inferior vena cava and right heart. Clin Imaging. 1993;17:117–23. doi: 10.1016/0899-7071(93)90050-w. doi:10.1016/0899-7071(93)90050-W. [DOI] [PubMed] [Google Scholar]
- 67.Esmaeilzadeh M, Tavakolli A, Safaei A. Recurrent intracardiac leiomyomatosis. Can J Cardiol. 2007;23:1085–6. doi: 10.1016/s0828-282x(07)70879-6. doi:10.1016/S0828-282X(07)70879-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanaka YO, Jikuya T, Iijima T, Sakakibara Y, Itai Y. Intravenous leiomyomatosis diagnosed by plain radiographs. Clin Radiol. 2002;57:1037–40. doi: 10.1053/crad.2002.1078. doi:10.1053/crad.2002.1078. [DOI] [PubMed] [Google Scholar]
- 69.Yuan SM, Shinfeld A, Lavee J, Kuperstein R, Haizler R, Raanani E. Imaging morphology of cardiac tumours. Cardiol J. 2009;16:26–35. [PubMed] [Google Scholar]
- 70.Wang JG, Li YJ, Liu H, Li NN, Zhao J, Xing XM. Clinicopathologic analysis of cardiac myxomas: seven years’ experience with 61 patients. J Thorac Dis. 2012;4:272–83. doi: 10.3978/j.issn.2072-1439.2012.05.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steger CM, Hager T, Ruttmann E. Primary cardiac tumours: a single-center 41-year experience. ISRN Cardiol. doi: 10.5402/2012/906109. 2012; DOI:10.5402/2012/906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pacini D, Careddu L, Pantaleo A, Berretta P, Leone O, Marinelli G, et al. Primary benign cardiac tumours: long-term results. Eur J Cardiothorac Surg. 2012;41:812–9. doi: 10.1093/ejcts/ezr067. doi:10.1093/ejcts/ezr067. [DOI] [PubMed] [Google Scholar]
- 73.Castelli P, Caronno R, Piffaretti G, Tozzi M. Intravenous uterine leiomyomatosis with right heart extension: successful two-stage surgical removal. Ann Vasc Surg. 2006;20:405–7. doi: 10.1007/s10016-006-9024-0. doi:10.1007/s10016-006-9024-0. [DOI] [PubMed] [Google Scholar]
- 74.Roques F, Sanchez B, Bucher B, Larivière J. Role of pre-operative assessment in the surgical management of leiomyoma extended to the right heart chambers: a compendium of information from isolated reports. Eur J Cardiothorac Surg. 2001;19:522–4. doi: 10.1016/s1010-7940(01)00604-2. doi:10.1016/S1010-7940(01)00604-2. [DOI] [PubMed] [Google Scholar]
- 75.Fujiwara K, Haba M, Noguchi Y, Yamamoto S, Iwasaki M. Successful one-stage surgical removal of intravenous uterine leiomyomatosis with right heart extension. Jpn J Thorac Cardiovasc Surg. 2003;51:462–5. doi: 10.1007/BF02719605. doi:10.1007/BF02719605. [DOI] [PubMed] [Google Scholar]
- 76.Oehler MK, Scopacasa L, Brown M, Kumar G, Edwards J. Intravenous uterine leiomyomatosis extending into the right heart. Aust N Z J Obstet Gynaecol. 2011;51:92–4. doi: 10.1111/j.1479-828X.2010.01249.x. doi:10.1111/j.1479-828X.2010.01249.x. [DOI] [PubMed] [Google Scholar]
- 77.Li B, Li RY, Chen X, Xu LJ, You QH, Ni YM, et al. One-stage complete removal of intracardiac leiomyomatosis without cardiac arrest. Thorac Cardiovasc Surg. 2013;61:88–90. doi: 10.1055/s-0032-1324712. doi:10.1055/s-0032-1324712. [DOI] [PubMed] [Google Scholar]
- 78.Stancanelli B, Seminara G, Vita A, Pantò A, Romano M. Extension of a pelvic tumor into the right atrium. N Engl J Med. 1995;333:1013–4. doi: 10.1056/NEJM199510123331519. doi:10.1056/NEJM199510123331519. [DOI] [PubMed] [Google Scholar]
- 79.Tasdelen A, Mercan AS, Sezgin A, Karapinar K, Yaveri A, Aslamaci S. Two discrete masses of leiomyomatosis in a patient, one extending to the right atrium. Thorac Cardiovasc Surg. 2000;48:161–3. doi: 10.1055/s-2000-9634. doi:10.1055/s-2000-9634. [DOI] [PubMed] [Google Scholar]
- 80.Brescia RJ, Tazelaar HD, Hobbs J, Miller AW. Intravascular lipoleiomyomatosis: a report of two cases. Hum Pathol. 1989;20:252–6. doi: 10.1016/0046-8177(89)90132-9. doi:10.1016/0046-8177(89)90132-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.