Abstract
OBJECTIVES
Prior studies have found that cardiac surgery patients receiving blood transfusions are at risk for increased mortality and morbidity following surgery. It is not clear whether this increased risk occurs across all haematocrit (HCT) levels. The goal of this study was to compare operative mortality in propensity-matched cardiac surgery patients based on stratification of the preoperative HCT levels.
METHODS
Between 1 August 2004 and 30 June 2011, 3516 patients had cardiac surgery. One thousand nine hundred and twenty-two (54.5%) required blood transfusion during or after surgery. A propensity score for transfusion was developed based on 22 baseline variables. One thousand seven hundred and fourteen patients were matched: 857 in the transfusion group (TG) and 857 in the non-transfused control group (CG). Univariate analyses demonstrated that, after propensity matching, the groups did not differ on any baseline factors included in the propensity model. Operative mortality was defined as death within 30 days of surgery. Preoperative HCT was stratified into four groups: <36, 36–39, 40–42 and ≥43.
RESULTS
For HCT <36%, 30-day mortality was higher in the TG than that in the CG (3.0 vs 0.0%). For HCT 36–39, operative mortality was similar between TG (1.1%, N = 180) and CG (0.8%, N = 361; P = 0.748). For HCT 40–42, operative mortality was significantly higher in the TG compared with that in the CG (1.9 vs 0%, N = 108 and 218, respectively; P = 0.044). For HCT of ≥43, there was a trend towards higher operative mortality in the TG vs the CG (2.0 vs 0%, N = 102 and 152, respectively; P = 0.083). Other surgical complications followed the same pattern with higher rates found in the transfused group at higher presurgery HCT levels. HCT at discharge for the eight groups were similar, with an average of 29.1 ± 1.1% (P = 0.117).
CONCLUSIONS
Our study indicates that a broad application of blood products shows no discernible benefits. Furthermore, patients who receive blood at all HCT levels may be placed at an increased risk of operative mortality and/or other surgical complications. Paradoxically, even though patients with low HCTs theoretically should benefit the most, transfusion was still associated with a higher complication and mortality rate in these patients. Our results indicate that blood transfusion should be used judiciously in cardiac surgery patients.
Keywords: Blood transfusion, 30-day mortality, Postoperative anaemia, Haematocrit
INTRODUCTION
Twenty percent of blood transfusions in America are associated with cardiovascular surgery, with 13% of them going to patients undergoing some combination of coronary artery bypass grafting (CABG) and valve surgery [1, 2]. Blood transfusion in cardiac surgery has been shown to be associated with decreased survival rates, some citing as high as a 70% increase in late mortality [3, 4]. Even small amounts of blood products can be detrimental. Risk of low-output heart failure is increased by 27%, and both short- and long-term survival are decreased when patients under the age of 80 receive just 1–2 units of blood [3, 5]. In cardiac patients over 80, the use of blood products was shown to be detrimental, although a recent study did not find this association [6]. Even in patients who were considered low-risk preoperatively, the transfusion of blood added significant risks for cardiac events, infection and morbidity postoperatively [7].
Other hazards associated with transfusions are transfusion-transmitted infections (such as human immunodeficiency virus (HIV) or hepatitis and the newly discovered Creutzfeld-Jacob disease and the dengue virus) and non-infectious complications [4, 8]. Non-infectious complications include acute transfusion reactions (such as allergic, haemolytic, hypotensive and febrile reactions), transfusion-related acute lung injury (TRALI), and transfusion-associated circulatory overload (TACO) among others. It is believed that the incidence of non-infectious complications is under-diagnosed and under-reported [4].
Traditionally, the ‘10/30 Rule’ has been used to guide transfusions. The 10/30 refers to haemoglobin (HMG) levels <10 g/dl or haematocrit (HCT) levels <30%; however, this standard has fallen into disfavour for more contemporary, evidence-based guidelines [9]. The current Society of Thoracic Surgeons (STS) and The Society of Cardiovascular Anesthesiologists (SCA) clinical practice guidelines indicate red blood cell (RBC) transfusion in patients with uncontrolled blood loss or who have lost >1500 ml or ≥30% of the total blood volume regardless of HMG levels. Patients with chronic cardiovascular/pulmonary disease or HBG ≤7.0 g/dl (≤6.0 g/dl if on cardiopulmonary bypass) should also receive packed RBC. Patients with HBG ≥10 g/dl actually do worse when given blood products. There are no clear benefits or consequences of transfusing patients with HBG levels between 7 and 10 g/dl [9, 10]. By their own admission, these STS/SCA ‘transfusion triggers’ are based on very limited studies and require further objective investigation [9, 10]. There is no randomized prospective trial in cardiac surgery, but there is one randomized trial in critical care patients conducted by Hebert et al. [11]. This study found that transfusion was associated with a higher rate of in-hospital mortality. In addition, they found that using a transfusion trigger of <7 g/dl was associated with increased survival over HMG <10 g/dl.
A recent study has demonstrated a marginal change in transfusion rates since the publication of the guidelines [12]. Furthermore, Varghese and colleagues found that, in a recent survey of several American and Canadian anesthesia and perfusion societies, only 22% of anaesthesiologists and 33% of perfusionists had read the guidelines. Sadly, only 20% of institutions had acknowledged the guidelines [13]. These facts likely explain the variation with regards to blood utilization, which may vary significantly across different institutions. For example, the transfusion rates in CABG patients span from 7.8 to 92.8% for packed RBCs, 0 to 97.5% for fresh-frozen plasma and 0.4 to 90.4% for platelets in the USA [14]. Studies have shown that patients are more likely to be transfused during a combination of CABG and valvular surgery or when the procedure involves the use of cardiopulmonary bypass compared with those undergoing an isolated procedure or an off-pump operation. However, it is important to note that increased complication and mortality rates were seen in all transfused patients regardless of the type of surgery [3].
The purpose of this study was to evaluate the impact of blood transfusion use during hospitalization on in-hospital and 30-day mortalities in propensity-matched cardiac surgery patients stratified by the preoperative HCT levels.
MATERIALS AND METHODS
Patient demographics and propensity matching
From July 2007 to June 2011, 3516 consecutive patients underwent cardiac surgery at The Valley Columbia Heart Center. There were no exclusion criteria. A retrospective analysis determined that 1922 patients received blood products during the time between the initiation of surgery to discharge (typically 5–7 days) and were included in the cohort. Patients receiving blood products were found to be older, with higher rates of diabetes, peripheral vascular disease (PVD), myocardial infarction (MI) history, renal failure, New York Heart Association (NYHA) Class III–IV heart failure, more severe coronary disease, ejection fraction <35%, more likely to be having a reoperation and more urgent presentation (all significant at P < 0.001 level).
Propensity matching was selected as a method to provide a more valid comparison between the groups. Propensity scores representing the estimated probabilities of patients receiving blood transfusion were developed from a logistic regression model. This model was based on 22 observed baseline covariates that included gender, age, body mass index, diabetes, smoking, hypertension, dyslipidaemia, number of diseased coronary vessels, PVD, presence of heart failure, NYHA Class, history of stroke, chronic lung disease, MI history, renal failure, left main disease, left ventricular ejection fraction, creatinine, cardiogenic shock presentation, type of surgery, urgent status and reoperation surgery. Most of these variables are factors that have been used by the STS in risk models for mortality and complications. A logistic regression model with these factors as covariates and the transfusion status as the dependent variable was performed. The nearest-neighbor-matching algorithm with Greedy 5-1 Digit Matching was employed to find as many 1:1 matches between the transfusion and non-transfusion groups based on the propensity scores to produce two balanced patient cohorts. Of the original cohort of 3519 patients, 1714 were matched: 857 in the transfused group (TG) and 857 in the non-transfused control group (CG). Univariate analyses demonstrated that, after propensity matching, the groups did not differ on any baseline factors (Table 1) or surgical procedures performed (Table 2) aside from the CG having a significantly higher incidence of off-pump surgeries. In addition, the STS mortality risk score was calculated for each group and was not statistically different (TG = 2.7 vs CG = 2.5%; P = 0.107). The transfused and non-transfused patients presented with similar admission medications (Table 3). There were no statistically significant differences between the groups. Patients discontinued platelet inhibition therapy prior to surgery. Platelet function was evaluated using the VerifyNow P2Y12 assay (Accumetrics, San Diego, CA, USA) to determine the timing of procedure. At our institution, we use a platelet inhibition <20% as the safe threshold for surgery unless the procedure is a surgical emergency.
Table 1:
STS demographics and clinical characteristics
| TG (N = 857) | CG (N = 857) | P-value | |
|---|---|---|---|
| Gender (female %) | 265 (30.9%) | 293 (34.2%) | 0.149 |
| Age | 69 ± 11 | 68 ± 11 | 0.114 |
| BMI | 28.4 ± 5 | 28.7 ± 5 | 0.223 |
| Smoker | 481 (56.1%) | 463 (54.0%) | 0.382 |
| Smoking current | 119 (13.9%) | 130 (15.2%) | 0.369 |
| Diabetes | 255 (29.8%) | 266 (31.0%) | 0.564 |
| Dyslipidaemia | 635 (74.1%) | 670 (78.2%) | 0.325 |
| Renal failure | 41 (4.8%) | 43 (5.0%) | 0.823 |
| Dialysis | 7 (0.8%) | 1 (0.1%) | 0.070 |
| Hypertension | 677 (79.0%) | 720 (84.0%) | 0.067 |
| CVA | 56 (6.5%) | 37 (4.3%) | 0.103 |
| CVD | 111 (13.0%) | 79 (9.2%) | 0.214 |
| Endocarditis | 17 (2.0%) | 14 (1.6%) | 0.863 |
| Chronic lung disease | 103 (12.0%) | 89 (8.9%) | 0.559 |
| Immunosuppressed | 24 (2.8%) | 24 (2.8%) | 1.000 |
| PVD | 100 (11.7%) | 82 (9.6%) | 0.369 |
| Pacemaker | 40 (4.7%) | 40 (4.7%) | 1.000 |
| MI | 292 (34.1%) | 297 (34.7%) | 0.799 |
| MI within 7 days | 141 (16.5%) | 142 (16.6%) | 0.912 |
| Unstable angina | 156 (18.2%) | 164 (19.1%) | 0.342 |
| History of arrhythmia | 150 (17.5%) | 177 (20.7%) | 0.252 |
| Heart failure Class III/IV | 240 (28.0%) | 205 (23.9%) | 0.061 |
| Triple vessel disease | 455 (53.1%) | 463 (54.0%) | 0.605 |
| Left main disease | 242 (28.2%) | 245 (29.6%) | 0.872 |
| Ejection fraction | 52.9 ± 12 | 52.4 ± 12 | 0.451 |
| Creatinine | 1.11 ± 0.6 | 1.05 ± 0.6 | 0.067 |
All demographics are in accordance with the national STS database.
BMI: body mass index; CVA: cardiovascular accident; CVD: cardiovascular disease; PVD: peripheral vascular disease; MI: myocardial infarction.
Table 2:
Surgery and blood products used
| TG (N = 857) | CG (N = 857) | P-value | |
|---|---|---|---|
| Urgent/emergent operation | 469 (54.8%) | 458 (53.5%) | 0.739 |
| Reoperation | 57 (6.7%) | 62 (7.2%) | 0.635 |
| Isolated CABG | 450 (52.5%) | 423 (49.4%) | 0.192 |
| Valve | 120 (14.0%) | 125 (14.6%) | 0.730 |
| CABG + valve | 78 (9.1%) | 79 (9.2%) | 0.933 |
| Other surgery | 209 (24.4%) | 230 (26.8%) | 0.245 |
CABG: coronary artery bypass grafting.
Table 3:
Admission medications
| TG (N = 857) | CG (N = 857) | |
|---|---|---|
| Beta blockers | 649 (75.7%) | 668 (77.9%) |
| Angiotensin-converting-enzyme inhibitors | 372 (43.3%) | 348 (40.6%) |
| Dyslipidaemia medication | 600 (70.0%) | 631 (73.6%) |
| Nitrates IV | 69 (9.1%) | 79 (9.2%) |
| Anticoagulants | 219 (25.6%) | 223 (26.0%) |
| Coumadin | 23 (2.7%) | 21 (2.5%) |
| Inotropes | 9 (1.1%) | 8 (0.9%) |
| Steroids | 36 (4.2%) | 34 (4.0%) |
| Aspirin | 419 (48.9%) | 421 (49.1%) |
| Adenosine diphosphate receptor inhibitors | 87 (10.2%) | 72 (8.4%) |
| Glycoprotein IIb/IIIa inhibitor | 14 (1.6%) | 5 (0.6%) |
| Dual antiplatelet therapy | 434 (50.6%) | 435 (50.8%) |
Data collection and statistical analysis
Data were collected and stored in a database certified by the STS. All variables were defined and coded using standards endorsed by the STS. Mortality was defined using the STS standard definition of operative mortality as death within 30 days of surgery. Pre- and postoperative HCTs were compiled for both the transfused and non-transfused patients. Patients were stratified by the HCT level into four groups: <36, 36–39, 40–42 and ≥43%. The use of HCT and these groupings was based on the work of DeFoe et al. [15].
Categorical data are expressed as proportions. Univariate statistical tests for continuous data included tests of mean differences using the Student's t-test. Categorical variables were analysed using the χ2 test. Multivariate logistic regression was used to evaluate the association between baseline characteristics, transfusion, HCT levels, cross-clamp time pump use and 30-day mortality. A P-value <0.05 was used to determine the statistical significance of all tests used. Analyses were performed using the SPSS statistical software package version 19.0 (IBM/SPSS, Inc., Chicago, IL, USA).
RESULTS
After propensity matching for baseline risk characteristics, patients receiving transfusions had a higher rate of all postoperative complications with the exception of deep sternal wound infection (Table 4). To address the potential confounding effect of reoperation for bleeding, the results were reanalysed removing those 57 patients obtaining similar patterns that were also statistically significant. When postoperative complications were assessed by the four-level HCT grouping, the lowest level (<36) TG patients had significantly more postoperative complications and 30-day mortality than CG patients (Table 5). In the 36–39 HCT range, there were no differences in postoperative complications, comparing TG and CG patients. Operative mortality was similar between TG (1.1%, N = 180) and CG (0.8%, N = 361) patients (P = 0.748). All deaths occurred in the postoperative period except for one. In the case of the intraoperative death, the patient arrived at the hospital with an advanced ruptured Type A aortic dissection with malperfusion syndrome. There were no other intraoperative deaths in the TG or CG groups. For presurgery HCT levels between 40 and 42, there were more complications in the TG group and a significantly higher rate of mortality (1.9 vs 0.0%; P = 0.044) compared with CG patients. For HCT of ≥43, there was a significantly higher rate of perioperative MI and stroke in the TG group and a trend towards higher operative mortality compared with the CG (2.0 vs 0%; P = 0.083). Discharge HCT (Table 6) was remarkably similar across all groups. Figure 1 shows the relationship between different HCT groups and the rates of transfusion CG and TG. There was a significant difference between CABG patients when stratified by cardiopulmonary bypass status, with more off-pump CABGs comprising the CG than TG (49.2 vs 34.5%, P < 0.0001). Overall, of the total isolated CABG, 82% were performed off pump, and 41% of all off-pump CABGs were transfused. Separate analyses of outcomes for on-pump and off-pump groups were consistent with the overall results. Otherwise, the remaining groups were within 2% of each other.
Table 4:
In-hospital and 30-day outcomes
| TG (N = 857) | CG (N = 857) | P-value | |
|---|---|---|---|
| Reoperation for bleeding | 54 (6.3%) | 3 (0.4%) | 0.0001 |
| Perioperative MI | 19 (2.2%) | 4 (0.5%) | 0.002 |
| Deep sternal wound infection | 3 (0.4%) | 0 (0.0%) | 0.083 |
| Permanent stroke | 17 (2.0%) | 5 (0.6%) | 0.010 |
| Prolonged ventilation | 103 (12.0%) | 34 (4.0%) | 0.0001 |
| Postoperative renal failure | 36 (4.2%) | 10 (1.2%) | 0.0001 |
| Postoperative atrial fibrillation | 200 (23.3%) | 161 (18.8%) | 0.021 |
| 30-day mortality | 20 (2.3%) | 3 (0.4%) | 0.0001 |
Table 5:
Outcomes by haematocrit group
| TG <36% | CG <36% | TG (36–39%) | CG (36–39%) | TG (40–42%) | CG (40–42%) | TG >42% | CG >42% | |
|---|---|---|---|---|---|---|---|---|
| No. of patients | 468 | 124 | 180 | 363 | 107 | 218 | 102 | 152 |
| Perioperative MI | 1.5% | 0.0% | 1.7% | 0.8% | 3.7%* | 0.0% | 4.9%* | 0.0% |
| Deep sternal wound infection | 0.2% | 0.0% | 0.6% | 0.0% | 0.9% | 0.0% | 0.0% | 0.0% |
| Permanent stroke | 1.7% | 1.6% | 1.7% | 0.6% | 2.8%† | 0.5% | 2.9%* | 0.0% |
| Postoperative renal failure | 4.5%* | 0.8% | 2.8% | 1.1% | 3.7%* | 0.5% | 5.9% | 2.6% |
| Postoperative atrial fibrillation | 23.9%* | 12.9% | 25.6% | 19.8% | 21.5% | 20.5% | 18.6% | 19.1% |
| 30-day mortality | 3.0%* | 0.0% | 1.1% | 0.8% | 1.9%* | 0.0% | 2.0%† | 0.0% |
*Significant difference between rates comparing TG and CG, P < 0.05.
†Trend between TG and CG groups, P = 0.083.
Table 6:
Discharge haematocrit
| TG <36% | CG <36% | TG (36–39%) | CG (36–39%) | TG (40–42%) | CG (40–42%) | TG >42% | CG >42% | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| Discharge HCT | 29.2 | 27.4 | 28.6 | 29.1 | 28.5 | 29 | 29.5 | 31.2 | 0.177 |
| Discharge HCT without reoperation for bleeding | 29.1 | 27.4 | 28.5 | 29.1 | 28.5 | 29 | 29.4 | 31.3 | 0.087 |
Figure 1:
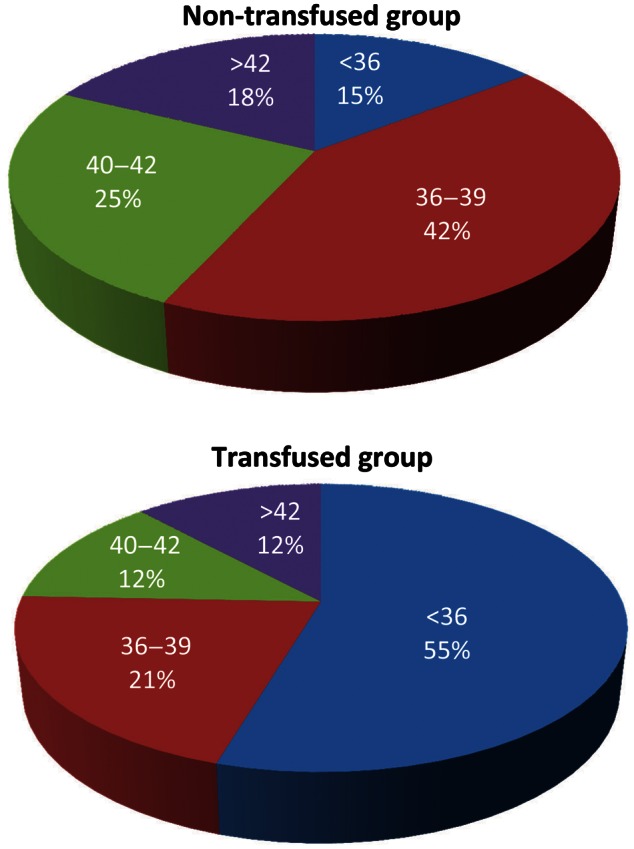
Breakdown of transfusion status by preoperative haematocrit.
The logistic regression analysis (Table 7) showed several factors to be associated with an increased risk of death: renal failure (25-fold), transfusion (7-fold), cross-clamp time >89 min (5-fold), NYHA Class III/IV (5-fold) and preoperative HCT >43 (2.5-fold). Non-significant variables in the model included age, gender, left ventricle ejection fraction, surgical acuity, type of surgery, off pump, left main disease, smoking, preoperative elevated lipids, reoperative status and body mass index.
Table 7:
Logistic regression analysis
| Adjusted odds ratio | 95% Confidence interval | P-value | |
|---|---|---|---|
| History of renal failure | 25.30 | 3.80–168.41 | <0.0001 |
| Blood transfusion | 7.24 | 2.94–13.62 | 0.008 |
| Cross-clamp time >89 min | 5.54 | 1.11–38.11 | 0.035 |
| NYHA Class III/IV | 5.28 | 1.04–26.81 | 0.038 |
| Preoperative HCT >43 | 2.53 | 1.01–21.76 | 0.047 |
DISCUSSION
In our study, we could not find any benefits associated with a broad use of blood transfusion. In fact, we reaffirmed the negative effects associated with the administration of blood products. Compared with a recent paper by Bhaskar et al. [16], both studies showed that transfusions were associated with increased short-term mortality, postoperative MI, new onset arrhythmias, stroke and organ failure. Uniquely, the current study found that there was an association between the use of blood products with complications and operative mortality that existed across higher HCT levels. Furthermore, the Bhaskar et al. [16] paper focuses primarily on long-term follow-up, which is not the focus of our current study. Our analysis showed that transfusing patients with preoperative HCT >42 placed them at a 2.5-fold increase risk of mortality independent of other factors. For HCT 36–39, operative mortality was similar between TG and CG, whereas for HCT 40–42 operative mortality was significantly higher in the TG compared with that in the CG. For HCT of ≥43, there was a trend towards higher operative mortality in the TG vs the CG. Other surgical complications followed the same pattern, with higher rates found in the transfused group at higher preoperative HCT levels.
It is indisputable that, in some situations such as haemorrhagic shock, a transfusion can be the difference between life and death. However, most transfused patients in cardiac surgery are generally not in haemorrhagic shock. Transfusions are commonly given to patients to treat post-surgical anaemia with the expectation that it will help improve patient recovery [16]. However, the body is remarkably well-adapted to handle anaemia.
A tolerance of anaemia varies greatly among patients and is dependent on a multitude of variables [4]. In healthy patients, HMG levels of 6–7 g/dl and HCTs of 15–20% are sufficient to maintain proper tissue oxygenation [17]. In fact, only at HMG levels ≤5 g/dl was there an appreciable increase in mortality for cardiac patients. Furthermore, HCT values ≤14% for low-risk and ≤17% for high-risk patients were shown to predict mortality [18].
Physiologically, the average HMG level for anaemia-induced mortality is 2.5 g/dl; however, it is likely higher in cardiovascular patients [19]. This ability to survive, despite low HMG levels, is likely due to the existence of many different anaemia-coping mechanisms in the human body [4]. In low-oxygen situations, the cells promote increased erythropoiesis, angiogenesis and anaerobic metabolism. The lungs also release more nitric oxide, increase the respiration rate in order to raise the partial pressure of blood oxygen and therefore increase HMG oxygen (Hb-O2) saturation. Furthermore, the body increases cardiac output, blood viscosity, systemic vasodilatation and increased venous return to promote tissue oxygenation. Oxygen extraction increases in the most vital organs such as the brain and the heart. Through these compensatory mechanisms, the human body is remarkably well equipped to handle anaemia. The higher rates of complications and mortality seen in our TG patients could be, in part, related to the transfused blood products interfering with the body's natural mechanisms for handling anaemia.
Recently, a panel of experts evaluated a series of common hypothetical clinical scenarios using evidence from published literature, concluding that the transfusion of blood products is either uncertain or unlikely to improve outcomes in a large number of common hypothetical clinical scenarios [20]. Strict blood-conservation programmes have been shown to successfully reduce the use of blood products and lower complication rates [1, 13, 21, 22]. The likelihood of transfusion is decreased when there is a surgical team (when compared with a single surgeon) and when the surgeon adheres to the STS/SCA transfusion guidelines. Furthermore, studies involving Jehovah's Witnesses show that these patients can safely undergo cardiac surgery without an increased risk of complications or mortality [23]. Although RBC administration is the fasted method to raise HMG levels, the effects of transfusions on muscle oxygenation and microvascular activity are inconsistent and unpredictable [4, 24].
We were surprised to discover that the average patient was discharged with a HCT between 27 and 30%, regardless of preoperative HCT. This suggests that physicians at our institution are transfusing patients partially based on what they perceived to be an acceptable target HCT level. This highlights how the decision to use blood products can be influenced by a physician's preconceived notions and attitudes as much as the patient's medical condition. Furthermore, at our institution, the surgeon, cardiologist and intensivist can all prescribe a blood transfusion for a patient, so there is the potential for three different strategies to manage anaemia throughout the patient's recovery. Given the well-documented risks of transfusion, doctors must decide if the patient's natural processes are enough to compensate for the anaemia, or if the patient truly needs blood products.
On a larger scale, the surgical community may want to reconsider the strategy for treating postoperative anaemia. While there is a mounting amount of published observational data linking transfusion to adverse outcomes, the only prospective randomized trial for transfusion for anaemia was published in 1999 by Hebert et al. [11]. This study only looked at patients in a critical care setting, so there are no randomized trials in the realm of surgery, let alone cardiac surgery. This study is not an attempt to change the blood transfusion guidelines; what we are trying to do with this study is to shed light on the potential complications associated with blood transfusions. It is our hope that these data demonstrate a definite need for a contemporary study delineating the risks and benefits of transfusing patients.
Given the successes seen with institutional blood-conservation programmes and in the Jehovah's Witness community, there is overwhelming data showing that cardiac operations can be safely carried out with strict transfusion guidelines. By using the STS guidelines as guide and implementing a universal point-of-care testing using VerifyNow when patients are on antiplatelet therapy, we have significantly reduced the rate of blood transfusion at our institution in the last 5 years. To further enhance the reduction in blood utilization, we are in the process of implementing a unified approach under single clinical leadership to reduce variance in blood transfusion. There will always be situations (e.g. haemorrhagic shock) that may require immediate transfusions. Given the results of our study in combination with the vast amount of literature on the risks associated with transfusion, physicians should be more fastidious when considering whether to administer blood products in patients undergoing cardiac surgery.
Limitations
In this study, we were limited in our ability to control for unexpected intraoperative events that could have increased complications at the time of the surgical procedure. Clearly, these have a direct effect on patient outcomes and in many instances, are accompanied by the need for blood transfusions. Furthermore, this study was conducted at a single medium-sized hospital. Results may have manifested differently in larger or smaller institutions, but steps were taken (e.g. large sample size, propensity matching) to minimize bias without compromising the goal or integrity of the study. The triggers for transfusion at our institution are in line with STS guidelines, which call for blood transfusion with HMG <7, haemorrhagic shock, >1500 cc blood loss and haemodynamic instability. At our institution, blood transfusion management is tightly controlled by our intensivists while our patients are in the ICU, which is usually for <24 h. After this period, management of the patient is shared by Cardiology and Cardiac Surgery, which could lead to some variability in the criteria used to indicate the need for blood transfusion. Our institution is working on approaches to better standardize this process.
Recently, Dixon et al. [25] published the data that showed chest tube output to be the strongest predictor of postoperative mortality. Furthermore, this study only used chest tube output from the first 24 h post-surgery. Our project looks at all blood products administered in the intra- and postoperative period; therefore, if we followed the Dixon model, we would be presenting a variable that only covers a small window into the time period at which we are looking. In addition, we did not set out to examine the need for transfusion, but to investigate the shot-term effects of transfusion.
Our study, like most other published in the literature, simply describes the association in our cohort between blood transfusion and unfavourable outcomes. This does not imply a direct causation effect between transfusion and poor outcomes. Causation can only be determined through the design of a randomized prospective trial due to the multitude of factors influencing patient outcomes and the motivation behind the use of blood transfusion.
CONCLUSIONS
Patients who receive blood at higher HCT levels may be placed at the increased risk of operative mortality and other surgical complications. This finding is true even considering other patient characteristics and the complexity of the procedure being done, as demonstrated by our multivariate analysis. Patients with preoperative HCT >42 who received blood are placed at a 2.5-fold increase risk of mortality independent of other factors.
Our results indicate that there was a lack of benefit of transfusing patients at all HCT levels. Furthermore, the administration of blood products may increase a patient's risk of operative mortality and/or other surgical complications. Even though patients with low HCTs theoretically should benefit the most from a blood transfusion, transfusion was still associated with a higher complication and mortality rate in these patients. Better patient outcomes may be achieved by the judicious use of blood transfusion and carefully managing cardiac surgical patients using a conservative approach.
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr M. Petricevic (Zagreb, Croatia): A propensity score for transfusion was developed based on 22 baseline variables. The univariate analysis demonstrated that after propensity matching, the groups did not differ on any baseline factors or surgical procedures performed, aside from the control group having a significantly higher incidence of off-pump surgeries. The use of cardiopulmonary bypass certainly affects clinical as well as transfusion outcomes, especially in the group of patients with low values of preoperative haematocrit. Therefore, it would be interesting to evaluate whether exclusion of patients undergoing OPCAB procedures would influence the results.
The second point, the data concerning bleeding extent assessment, such as postoperative chest tube output, are lacking. The fact that chest tube output may in itself be harmful should be considered. Excessive bleeding is inevitably associated with blood product transfusion and may, by itself, influence clinical outcome. So the inclusion of chest tube output in propensity matching would probably bring clearer evidence on the relationship between blood transfusion and clinical outcomes.
And finally I have a few short questions. Were antiplatelets discontinued prior to surgery, or were they continued up to the date of surgery? And were some patients preoperatively exposed to dual antiplatelet therapy? Concerning transfusion, do you have transfusion triggers for each blood component therapy? And in your opinion, does the number of packed red blood cell units transfused make a difference regarding clinical outcomes rather than transfusion of packed red blood cells in the form of binary variables?
And, finally, I agree with you that blood component transfusion should be used judiciously in cardiac surgery, but in my opinion, the restrictive transfusion triggers are probably not enough to improve clinical outcome.
Dr Grau: I appreciate your many questions, which I hope I am going to remember. I am going to start by answering the last question; I will then try to deal with the other ones in order.
The answer to the last question, whether the patients were on dual antiplatelet therapy prior to the operation, is, yes, many of them were. The dual antiplatelet therapy was discontinued prior to the operation in all cases. In our institution we use a point-of-care testing called VerifyNow, so the patients only go to the operating room when platelet inhibition is at or below 20%. So we do wait, but we do not wait a specific number of days. We wait, based on the VerifyNow results, to make the decision to operate in order to better time the surgical intervention. So probably that answers your last question.
I believe the fifth question was whether the chest tube output would have improved the quality of this study. There is no question that is true, but the manpower that is required to do that, because it is not recorded easily in our system, was not there. But we did something else, to answer your question; we looked at the patients who went back for surgery for bleeding. I think we all can agree that if the patient has to go back to the operating room for bleeding, it is likely that your pleural bags are going to be full of blood, right? So there were 57 patients out of an entire cohort of 1700 cases that required to go back to the operating room. That is around 3%. We removed those 57 patients from the propensity match analysis and the results were identical. I think that answers your third or fourth question.
Then I am going to go to the off-pump/on-pump question. That is a very important question. The off-pump was not included in the initial design of the logistic regression analysis, but I did the analysis afterwards because I knew that was going to be a question that the reviewer would raise, and these are the numbers: 82% of the cases were done off-pump, and within that group of 82%, 40-something percent of the patients were transfused. Then we did the multivariate logistic regression analysis. Off-pump was introduced into the model to see if, by a multivariate analysis, that had an impact, and it did not have any impact. So I think that answers the second question. And to be honest with you, I forgot the first question.
Dr Petricevic: Yes. I have got one more question regarding transfusion. Do you have a specific transfusion treatment?
Dr Grau: Yes. This is the problem. We do have, and I think Dr Whitman - I do not know if he is here? You are there, yes. Everything is very tightly controlled at our institution throughout the intraoperative and immediately postoperative period until the patient leaves the ICU. The patient stays in our ICU for no more than 24 h; actually, it is less than 24 h in most cases. And then there is this situation where cardiology and cardiac surgery share the beauty of managing the patients postoperatively. And there is where I think we could control the decision to transfuse in a better way because I think there is a lack of communication, and I am very interested in speaking with Dr Whitman about this after this talk.
And then I think you asked something else that I wanted to answer.
Dr Petricevic: Do you use thromboelastography for transfusion?
Dr Grau: No. We use the VerifyNow. We do not use the thromboelastogram, no.
Dr Petricevic: Only for preoperative assessment of platelet function or intraoperatively?
Dr Grau: Correct. No, we do not use the thromboelastogram in the operating room. We just use the VerifyNow preoperatively. And the trigger for us in the ICU, I think that is the answer to your question, is a haemoglobin of 7 or below for transfusion.
REFERENCES
- 1.Shander A, Moskowitz D, Rijhwani TS. The safety and efficacy of ‘bloodless’ cardiac surgery. Semin Cardiothorac Vasc Anesth. 2005;9:53–63. doi: 10.1177/108925320500900106. [DOI] [PubMed] [Google Scholar]
- 2.Stover EP, Siegel LC, Parks R, Levin J, Body SC, Maddi R, et al. Variability in transfusion practice for coronary artery bypass surgery persists despite national consensus guidelines: a 24-institution study. Institutions of the Multicenter Study of Perioperative Ischemia Research Group. Anesthesiology. 1998;88:327–33. doi: 10.1097/00000542-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Surgenor SD, Kramer RS, Olmstead EM, Ross CS, Sellke FW, Likosky DS, et al. The association of perioperative red blood cell transfusions and decreased long-term survival after cardiac surgery. Anesth Analg. 2009;108:1741–6. doi: 10.1213/ane.0b013e3181a2a696. [DOI] [PubMed] [Google Scholar]
- 4.Shander A, Javidroozi M, Ozawa S, Hare GM. What is really dangerous: anaemia or transfusion? Br J Anaesth. 2011;107(Suppl 1):i41–59. doi: 10.1093/bja/aer350. [DOI] [PubMed] [Google Scholar]
- 5.Surgenor SD, DeFoe GR, Fillinger MP, Likosky DS, Groom RC, Clark C, et al. Intraoperative red blood cell transfusion during coronary artery bypass graft surgery increases the risk of postoperative low-output heart failure. Circulation. 2006;114:I43–8. doi: 10.1161/CIRCULATIONAHA.105.001271. [DOI] [PubMed] [Google Scholar]
- 6.Yun JJ, Helm RE, Kramer RS, Leavitt BJ, Surgenor SD, DiScipio AW, et al. Limited blood transfusion does not impact survival in octogenarians undergoing cardiac operations. Ann Thorac Surg. 2012;94:2038–45. doi: 10.1016/j.athoracsur.2012.06.059. [DOI] [PubMed] [Google Scholar]
- 7.Mohnle P, Snyder-Ramos SA, Miao Y, Kulier A, Bottiger BW, Levin J, et al. Postoperative red blood cell transfusion and morbid outcome in uncomplicated cardiac surgery patients. Intensive Care Med. 2011;37:97–109. doi: 10.1007/s00134-010-2017-z. [DOI] [PubMed] [Google Scholar]
- 8.Squires JE. Risks of transfusion. South Med J. 2011;104:762–9. doi: 10.1097/SMJ.0b013e31823213b6. [DOI] [PubMed] [Google Scholar]
- 9.Ferraris VA, Ferraris SP, Saha SP, Hessel EA, 2nd, Haan CK, Royston BD, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007;83:S27–86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
- 10.Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944–82. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 11.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 12.Bennett-Guerrero E, Song HK, Zhao Y, Ferguson TB, Jr, Gammie JS, Peterson ED, et al. Temporal changes in the use of blood products for coronary artery bypass graft surgery in North America: an analysis of the Society of Thoracic Surgeons Adult Cardiac Database. J Cardiothorac Vasc Anesth. 2010;24:814–6. doi: 10.1053/j.jvca.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Varghese R, Myers ML. Blood conservation in cardiac surgery: let's get restrictive. Semin Thorac Cardiovasc Surg. 2010;22:121–6. doi: 10.1053/j.semtcvs.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Bennett-Guerrero E, Zhao Y, O'Brien SM, Ferguson TB, Jr, Peterson ED, Gammie JS, et al. Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA. 2010;304:1568–75. doi: 10.1001/jama.2010.1406. [DOI] [PubMed] [Google Scholar]
- 15.DeFoe GR, Ross CS, Olmstead EM, Surgenor SD, Fillinger MP, Groom RC, et al. Lowest hematocrit on bypass and adverse outcomes associated with coronary artery bypass grafting. Northern New England Cardiovascular Disease Study Group. Ann Thorac Surg. 2001;71:769–76. doi: 10.1016/s0003-4975(00)02393-6. [DOI] [PubMed] [Google Scholar]
- 16.Bhaskar B, Dulhunty J, Mullany DV, Fraser JF. Impact of blood product transfusion on short and long-term survival after cardiac surgery: more evidence. Ann Thorac Surg. 2012;94:460–7. doi: 10.1016/j.athoracsur.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Doak GJ, Hall RI. Does hemoglobin concentration affect perioperative myocardial lactate flux in patients undergoing coronary artery bypass surgery? Anesth Analg. 1995;80:910–6. doi: 10.1097/00000539-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Fang WC, Helm RE, Krieger KH, Rosengart TK, DuBois WJ, Sason C, et al. Impact of minimum hematocrit during cardiopulmonary bypass on mortality in patients undergoing coronary artery surgery. Circulation. 1997;96:II-194–9. [PubMed] [Google Scholar]
- 19.Weiskopf RB. Emergency transfusion for acute severe anemia: a calculated risk. Anesth Analg. 2010;111:1088–92. doi: 10.1213/ANE.0b013e3181f5ba2b. [DOI] [PubMed] [Google Scholar]
- 20.Shander AFA, Javidroozi M, Erhard J, Farmer SL, Corwin H, Goodnough LT, et al. International Consensus Conference on Transfusion Outcomes Group. Appropriateness of allogeneic red blood cell transfusion: the international consensus conference on transfusion outcomes. Transfus Med Rev. 2011;25:232–46. doi: 10.1016/j.tmrv.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Moskowitz DM, McCullough JN, Shander A, Klein JJ, Bodian CA, Goldweit RS, et al. The impact of blood conservation on outcomes in cardiac surgery: is it safe and effective? Ann Thorac Surg. 2010;90:451–8. doi: 10.1016/j.athoracsur.2010.04.089. [DOI] [PubMed] [Google Scholar]
- 22.Weltert L, Nardella S, Rondinelli MB, Pierelli L, De Paulis R. Reduction of allogeneic red blood cell usage during cardiac surgery by an integrated intra- and postoperative blood salvage strategy: results of a randomized comparison. Transfusion. 2012 doi: 10.1111/j.1537-2995.2012.03836.x. doi:10.1111/j.1537-2995.2012.03836.x. [DOI] [PubMed] [Google Scholar]
- 23.Stamou SC, White T, Barnett S, Boyce SW, Corso PJ, Lefrak EA. Comparisons of cardiac surgery outcomes in Jehovah's versus Non-Jehovah's Witnesses. Am J Cardiol. 2006;98:1223–5. doi: 10.1016/j.amjcard.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 24.Creteur J, Neves AP, Vincent JL. Near-infrared spectroscopy technique to evaluate the effects of red blood cell transfusion on tissue oxygenation. Crit Care. 2009;13(Suppl 5):S11. doi: 10.1186/cc8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixon B, Santamaria JD, Reid D, Collins M, Rechnitzer T, Newcomb AE, et al. The association of blood transfusion with mortality after cardiac surgery: cause or confounding? Transfusion. 2012;53:19–27. doi: 10.1111/j.1537-2995.2012.03697.x. [DOI] [PubMed] [Google Scholar]


