Abstract
OBJECTIVES
Evaluation of the feasibility, safety and oncological validity of video-assisted thoracic lobectomy (VATS). The VATS study exclusion criteria included T3 or T4 tumours, central hilar tumours, tumours visible on bronchoscopy requiring sleeve resection, hilar lymphadenopathy, N2 disease, history of neoadjuvant chemotherapy or radiation, previous thoracic surgery or pleurodesis.
METHODS
A retrospective study of 410 patients (143 women, mean age 61.5 ± 13.1 years (84–15) treated by VATS lobectomy between 1996 and 2011 was performed at our institution. VATS lobectomy was performed for lung cancer (n = 364, 88.9%), pulmonary metastasis (n = 25, 5.8%) and non-neoplastic diseases (n = 21, 5.1%). In lung cancer, a systematic radical lymph node dissection was performed.
RESULTS
There was no intraoperative death. The conversion rate was 6.1% (n = 25): bleeding (n = 4), extended pleural adhesion (n = 6, 1.4%), technical difficulty (n = 6, 1.4%), tumour extension to the fissure or mediastinum or adenopathy (n = 7, 1.7%) and intolerance to one-lung ventilation (n = 2, 0.4%). The postoperative mortality rate was 1.2% (n = 5). Major complications occurred in 21 patients (5.1%). The mean number of mediastinal nodes removed was 14.6 (5–44) and 42 patients (10.2%) presented N2 disease at the definitive staging. The mean operating time was 152 (85–315) min. The mean drainage duration was 3.2 days (1–15). Mean postoperative length of hospital stay before return at home was 6.8 days (3–75) and 5.5 days in patients without major complications. There was no port site recurrence. Kaplan–Meier 3-year survival rates were 76.5% for Stage I and 87.3% for Stage IA, 58% for Stage II and 61% for Stage III.
CONCLUSIONS
VATS lobectomy is an acceptable alternative and seems equivalent to open lobectomy in terms of complications and oncological value. Our experience prompts us to consider VATS lobectomy for early stage NSCLC as the first surgical approach in view of the improvement in outcome, provided that the procedure is performed by a surgeon with adequate experience with this approach.
Keywords: Lung cancer, Video-assisted thoracic lobectomy
INTRODUCTION
Although video-assisted thoracic lobectomy (VATS) with hilar and mediastinal lymph node dissection has been used for more than a decade in the management of patients with lung cancer, this technique is not widely practiced. A recent meta-analysis [1] has demonstrated the feasibility and safety of VATS lobectomy and its potential application to early lung cancer since its initial description in the early 1990s). Despite obvious advantages (less pain and length of hospital stay), thoracoscopic lobectomy has not achieved the awaited wide acknowledgement observed for other thoracoscopic procedures. VATS remains rarely performed in the field of major pulmonary resection, particularly in the treatment of lung cancer, because of a potential increase in surgical risk and the questioning of its oncological validity. Therefore, controversy persists about the role of VATS lobectomy in the treatment of patients with lung cancer and this is particularly true in Europe, where VATS lobectomy is performed in <10% of academic centres which is significantly lower than in the USA or Asia [2]. The database of the French Society for Thoracic Surgery [3] showed in 2011 that VATS lobectomies accounted for <1% of all lobectomies while they reached 32% in the USA [4]. Confirmation of the oncological effectiveness of VATS lobectomy would require a large, prospective, randomized series, which is not forthcoming. More experience supporting the oncological validity of VATS lobectomy is needed in order to strengthen the widespread agreement on this procedure in usual practice. The following is a retrospective review in VATS lobectomy of a single centre, with special regard to its feasibility, safety and oncological effectiveness, particularly for the treatment of early stage lung cancer.
MATERIALS AND METHODS
Patients selection and preoperative studies
Between January 1996 and December 2010, 2513 patients underwent major pulmonary resections at our institution, and among them 410 underwent VATS lobectomy.
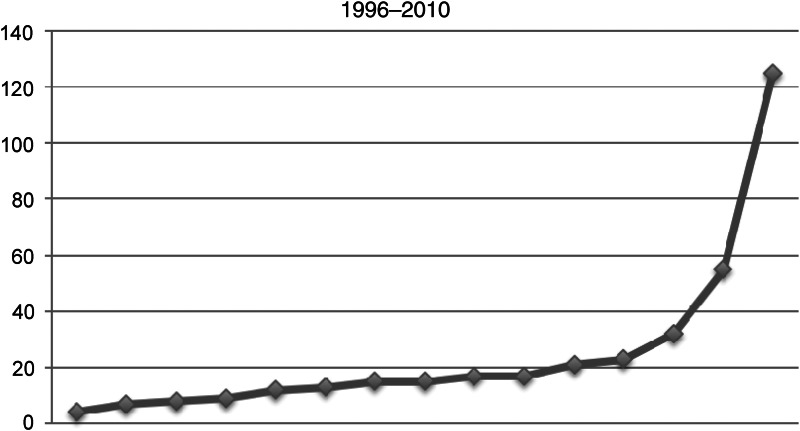
The distribution per year during that interval is reported in Fig. 1. The indication for lobectomy was lung cancer, pulmonary metastasis, infectious disease and benign pulmonary tumours or congenital disease.
Figure 1:
VATS lobectomy year distribution.
Patients were included in the study data if the preoperative surgical goal was thoracoscopic lobectomy, regardless of whether the surgery was completed thoracoscopically.
All patients underwent fibreoptic bronchoscopy and non-invasive staging with thoraco-abdominal and cerebral computed tomography (CT) scan to assess the mediastinum and verify the absence of multiple pulmonary lesions and hepatic, adrenal or brain metastases. Since 2005, preoperative work-up has included positron emission tomography (PET) scan. Enlarged mediastinal nodes >10 mm in the short axis or hyperfixation on PET scan required invasive mediastinal staging with nodal biopsy through echo-endoscopy, mediastinoscopy or VATS to rule out N2 disease in patients with lung cancer.
Criteria for inclusion in the study were Stage I disease, tumour distant from hilar vessels, without extension to the interlobar fissures, no history of previous thoracic surgery or pleurodesis. The VATS study exclusion criteria included T3 or T4 tumours, central hilar tumours, tumours visible on bronchoscopy requiring sleeve resection, hilar lymphadenopathy, N2 disease, history of neoadjuvant chemotherapy or radiation, previous thoracic surgery or pleurodesis and inability to achieve single-lung ventilation.
In pulmonary metastasis, criteria were single centrolobar metastasis, or multiple metastases, that could be completely resected by lobectomy with or without ipsilateral or contralateral wedge resection.
Standard functional tests were used for assessing pulmonary reserve. In patients with limited pulmonary function, ventilation–perfusion scintigraphy was used to predict postoperative pulmonary function. Postoperative forced expiratory volume (FEV) >40% was required to schedule the surgical procedure.
Surgical procedures
All patients had an anatomical lobectomy. Patients who underwent a lesser resection or a more extensive operation were excluded. All surgical procedures were performed under general anaesthesia, with the use of single-lung ventilation through a double-lumen endotracheal tube (Mallinckrodt, Covidien, Mansfield, MA, USA).
All patients underwent complete anatomical lobectomy, with mediastinal lymph node dissection in those with lung cancer. The bronchial stump was systematically analysed. The procedure was quite similar to the operation performed in open surgery.
In our practice, VATS used two techniques:
With only thoracoscopic instruments and no utility thoracotomy which was performed for specimen extraction when the lobectomy was achieved.
With open instrumentation via utility thoracotomy (5–7 cm) without rib spreading and thoracoscopic instruments through two ports.
After surgery, all patients recovered in the postoperative anaesthesia care unit and were transferred subsequently to a monitored care setting.
Criteria for discharge included: absence of complication, removal of the drains for 24 h, control chest X-ray without atelectasis, pleural effusion or residual pleural space, no chest pain or pain controlled without major analgesia.
Data prospectively collected and retrospectively analysed, included: physical status score (ASA score), histopathological diagnosis, pathological stage in patients with lung cancer, operating time, number of nodes in the nodal dissection, drainage duration, complications, mortality, length of hospital stay, level pain, postoperative treatment and survival.
Operative mortality included all patients who died within 30 days of surgical intervention or within the same hospitalization.
Major complications included: respiratory failure, fistula and empyema, atrial fibrillation (AF) requiring cardioversion or anticoagulation and reoperation.
Minor complications involved prolonged air leak, AF not requiring cardioversion and anticoagulation, pneumonia without respiratory failure, pleural effusion, recurrent nerve palsy, wound infection. Patients were considered to have AF if the episode lasted >5 min, as determined by means of heart rate monitoring. Most cases of AF were confirmed by using 12-lead electrocardiographic analysis. Transient AF not requiring medical treatment was not recorded. Atelectasis was considered a complication if it required bronchoscopy. A prolonged air leak was defined as an air leak present on postoperative day 5. Bleeding was considered a complication if reoperation was required. Pneumonia was defined as meeting three of five characteristics: fever, leucocytosis, chest radiographic scan with infiltrate, positive culture from sputum or treatment with antibiotics.
Postoperative pain control
Postoperative pain relief was provided by continuous epidural administration of fentanyl and bupivacaine and/or intravenous opioid administration. Patients evaluated their pain levels on a scale of 0–9 (0 no pain, 1–3 mild pain, 4–6 moderate pain and 7–9 severe pain) at the first postoperative follow-up visit at 1 month post-surgery. The use of medications for pain relief as none, non-steroidal anti-inflammatory agents, opiates or both was recorded.
Follow-up
The clinical surveillance approach included a physical examination and an imaging study (CT scan) every 6 months for 2 years and then annually.
Statistical analysis
These data were collected retrospectively on the basis of chart review, postoperative follow-up visits, discharge summaries and patient or physicians interviews. All data are expressed as mean standard error. Statistical analysis was performed with StatView version 5 (SAS Institute, Inc., Cary, NC, USA). Continuous and categorical variables were analysed with the Student's t-test and Fisher's exact test, respectively. Postoperative survival was plotted according to the Kaplan–Meier method, and any difference in survival between the groups was evaluated with the log-rank test.
RESULTS
There were 267 males and 143 females (sex ratio 1.8). The mean age was 61.5 ± 13.1 years (84–15).
The indication for lobectomy was lung cancer in 364 patients (88.9%), pulmonary metastasis in 25 (5.8%), infectious disease (bronchiectasis. pulmonary abcess, tuberculosis and aspergilloma) in 17 (4.1%) and benign pulmonary tumours (hamartoma) or congenital disease (adenomatous malformation) in 4 (1.2%). Our favourable initial experience has prompted us to consider VATS lobectomy for early-stage non-small-cell lung cancer (NSCLC) as the primary mode of surgical intervention. This policy is demonstrated by the dramatic shift in our surgical approach for early-stage NSCLC (Fig. 1).
The ASA score was 1 in 38 patients (9.2%), 2 in 221 (53.9%), 3 in 145 (35.3%) and 4 in 6 (1.4%).
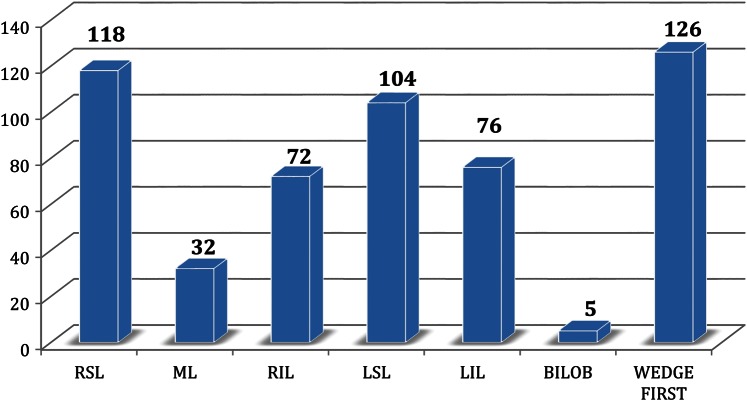
A summary of resections performed by anatomical location is shown in Fig. 2. The most frequent lobectomies were right superior lobectomy (118) and left superior lobectomy (104). Wedge resection and frozen section were done first in 126 patients (30.7%), when the preoperative diagnosis was unknown and the tumour was located in a peripheral position.
Figure 2:
Summary of resections performed by anatomic location. RSL: right superior lobe; ML: middle lobe; RIL: right inferior lobe; LSL: left superior lobe; LIL: left inferior lobe; BILOB; bilobectomy.
Details on pathological diagnosis in lung cancer are reported in Table 1. Adenocarcinoma was the most frequently occurring pathological form (250 patients, 68.7%) followed by squamous cell carcinoma (74 patients, 20.3%).
Table 1:
Histological subtypes in patients with lung cancer
| Adenocarcinoma | 250 (68.7%) |
| Squamous carcinoma | 74 (20.3%) |
| Carcinoid | 21 (5.7%) |
| Others | 19 (5.3%) |
| Large cell carcinoma | |
| Adenosquamous carcinoma | |
| Mucoepidermoid carcinoma | |
| Poorly differentiated carcinoma | |
| Total | 364 (100%) |
The majority of lobectomies was performed for pathological stage I lung cancer (276 patients, 67.3%). The details on the pathological stages in the group of patients with lung cancer are reported in Table 2.
Table 2:
Pathological staging in patients with lung cancer
| Ia | 183 | 50.3% |
| Ib | 90 | 24.7% |
| IIa | 10 | 2.7% |
| IIb | 29 | 8% |
| IIIa | 42 | 11.5% |
| IIIb | 6 | 1.7% |
| IV | 4 | 1.1% |
| Total | 364 | 100% |
Lymph node dissection was systematically performed in all patients with lung cancer. The mean number of lymph nodes removed was 14.6 (5–44) and the mean number of mediastinal station dissected was 3.4.
Forty-two patients (10.2%) presented N2 disease at the definitive pathological staging. In these patients, preoperative work-up (CT scan and/or PET scan) failed to detect mediastinal node extension requiring invasive mediastinal staging according to our operative criteria (short-axis diameter >10 mm significant increase in the fludeoxyglucose (FDG) uptake on PET). Thirty-two patients (76%) presented one mediastinal nodal station involvement, 10 presented two nodal stations involvement and 4 presented three nodal stations involvement. The most frequent sites involved were the lower right paratracheal area 4R (16), subcarinal 7 (16) and para-aortic 6 (10). All patients but 3 who presented serious postoperative complications were treated with adjuvant chemotherapy; radiotherapy in 32 patients, chemotherapy in 6, and radiotherapy alone in 1. The chemotherapy regimen was platinum-based doublet.
Conversion to open thoracotomy was required in 25 patients (6.1%), because of bleeding in 4 patients, extended pleural adhesion in 6, technical difficulty in 6, tumour extension to the fissure or mediastinum or adenopathy in 7, and intolerance to one-lung ventilation in 2 patients (Table 3). Technical complexity was related to the volume of the tumour, resulting in difficulty in lobar mobilization and exposure and in case of fissureless lung anatomy or inflammatory adherent nodes, making dissection in the fissure or the hilum risky. There was no intraoperative catastrophic event defined as an event that resulted in an additional unplanned major surgical procedure other than the planned lobectomy. There were 3 cases of pulmonary artery and 1 of truncus artery injury at the time of the hilar dissection requiring thoracotomy for control and repair but without leading to angioplasty or pneumonectomy.
Table 3:
Conversion to thoracotomy
| Bleeding | 4 (0.9%) |
| Pleural adhesions | 6 (1.4%) |
| Tumour extension | 7 (1.7%) |
| Technical difficulty | 6 (1.4%) |
| Intolerance to one-lung ventilation | 2 (0.4%) |
| Total | 25 (6.1%) |
The mean operating time was 152 min (85–315). The mean operating time was longer at the initial phase. Our learning curve was significantly longer compared with that of the 2 last years (205 vs 135 min, P = 0.004).
Mortality
There was no intraoperative death. Postoperative mortality (Day 30) was 1.2%. Five patients died in the postoperative course: death was related to extensive pneumonia and acute respiratory distress syndrome (ADRS) (3), massive pulmonary embolus (1) and massive haemoptysis related to bronchopleural fistula (1). In 372 patients (90.7%), the postoperative course was uneventful or experienced minor complications.
Morbidity
Details on minor and major complications are reported in Table 4. Sixty-three patients (15.3%) experienced minor complications. The most common minor complication was prolonged air leaks (>8 days) occurring in 23 patients (5.6%). Transient AF that did not require medical treatment was not recorded. Twenty-one patients (5.1%) presented major complications. All patients with major complications experienced a prolonged stay in the intensive care unit (4–75 days). Five patients presented postoperative pneumonia that did not require reintubation, with favourable outcome. Four patients presented acute respiratory failure managed with iterative bronchoscopy and non-invasive ventilation. Three patients suffered ADRS requiring prolonged assisted ventilation and intensive care unit stay. Three patients presented postoperative haemothorax requiring reoperation through a thoracoscopic approach in 2 patients. One patient with an oesophago-pleural fistula resulting in calcified subcarinal lymph nodes dissection required reoperation (direct suture covered with omental flap).
Table 4:
Details on minor and major complications
| Minor complications |
Major complications |
||
|---|---|---|---|
| Prolonged air leaks >7 days | 23 (5.6%) | Pneumonia | 5 (1.2%) |
| Atelectasis | 13 (3.1%) | Acute respiratory failure | 4 (0.9%) |
| Atrial fibrillation | 10 (2.5%) | Haemothorax | 3 (0.7%) |
| Pleural effusion | 7 (1.7%) | Pulmonary embolus | 2 (0.4%) |
| Recurrent nerve palsy | 6 (1.4%) | ADRS | 3 (0.7%) |
| Wound infection | 2 (0.4%) | Bronchial fistula | 2 (0.4%° |
| Minor chylothorax | 2 (0.4%) | Oesophageal fistula | 1 (0.2%) |
| Perforated ulcus | 1 (0.2%) | ||
| Total | 63 (15.4%) | 21 (5.1%) | |
Length of hospital stay
The mean drainage duration was 3.2 days (1–15). The mean postoperative hospital stay was 6.8 days (3–75) and 5.5 days in 389 patients (95%) without major complication. The postoperative length of stay was longer in the group that required surgical conversion (10.8 days).
Pain
At the first postoperative follow-up visit at a median time of 4 weeks (mean 4.4 weeks) post-surgery, patients were asked to assess their level of pain on a scale of 0–9. The mean pain score based on this scale was 3.2 (median 0; range 0–7). Patients reported the medications that they were using for pain relief. At this median follow-up period, 43% of patients were not using any pain medication, 45% were taking NSAIDs and 12% reported some narcotic use.
Survival and recurrence
There was no port-site recurrence. There was a parietal recurrence in 1 patient, located in the anterior part of the chest after left superior lobectomy, remote from port sites and related to parietal seeding after CT scan needle biopsy at the preoperative work-up. Recurrence occurred in 26 patients (9.6%) with Stage I: local, 11; distant, 15. Recurrence occurred in 10 patients (26.3%) with Stage II: local, 4; distant, 6. Recurrence occurred in 11 patients (25%) with Stage III: local, 5; distant, 6.
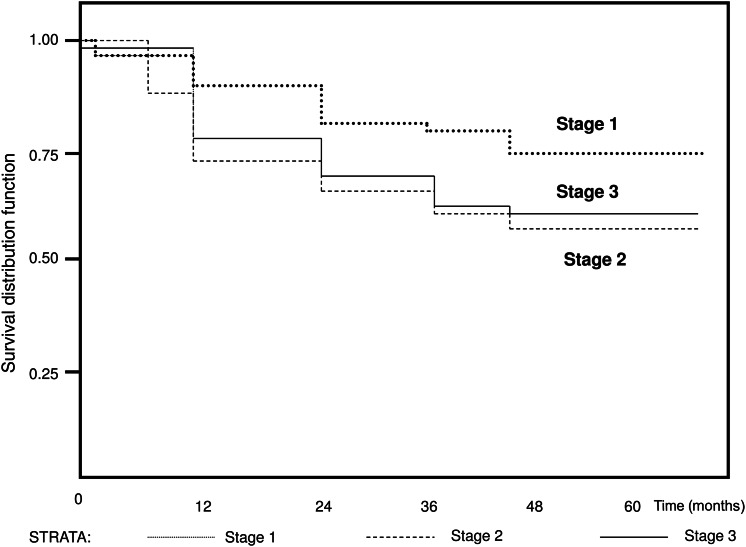
Kaplan–Meier 3-year survival rates were 76.5% for Stage I and 87.3% for stage IA, 58% for Stage II and 61% for Stage III (Fig. 3). The statistical analysis did not disclose any time effect bias.
Figure 3:
Kaplan–Meier survival curve showing the overall survival after VATS lobectomy for NSCLC.
DISCUSSION
VATS lobectomy has increased during the past 10 years and has begun to be used to treat patients with early-stage NSCLC such as clinical stage I disease. The expected benefits of VATS lobectomy include less pain and complications, shorter hospital stay and faster patient recovery. However, the routine use of VATS lobectomy for the treatment of resectable NSCLC remains controversial. Despite large series emphasizing the feasibility and the safety of this procedure [2, 5–7], some doubts persist regarding its oncological value in patients with NSCLC. Preliminary reports of VATS lobectomy have led to many discussions and criticism because of the potential compromise of the principle of oncological surgery and patient safety during a major pulmonary resection performed through a thoracoscopic approach. Therefore, VATS lobectomy remains relatively unused and accounts for >10% of thoracic surgeons in Europe, whereas this procedure increases to 32% of all lobectomies in the USA as reported in the Society of Thoracic Surgeons database published recently [4]. Our favourable initial experience has prompted us to consider VATS lobectomy for early-stage NSCLC the primary mode of surgical intervention. This policy is demonstrated by the dramatic shift in our surgical approach for early-stage NSCLC (Fig. 1).
Mortality and morbidity
Recently, the Society of Thoracic Surgeons published data on open lobectomy in 5957 patients with a morbidity rate of 32% and 30-day mortality of 2% [4]. In the largest series of patients undergoing lobectomy by thoracotomy, morbidity ranged from 28 to 38% and mortality ranged from 1.2 to 2.9% [8–10].
Prospective [2] and large retrospective studies [5–7, 11–16] have shown that VATS lobectomy compares favourably with thoracotomy, with morbidity rates ranging from 7.7 to 24.1% and perioperative mortality ranging from 0.8 to 2.5% (Table 5).
Table 5:
Summary of reports on VATS lobectomy and overall results
| Reference | No. of cases | Morbidity (%) | Mortality (%) | Survival (Stage I) | Conversion (%) | LOS |
|---|---|---|---|---|---|---|
| Flores et al. [2] | 398 | 24.1 | 0.2 | 79%, 5 years | 17.5 | 5 |
| McKenna et al. [5] | 1100 | 15.3 | 0.8 | 80%, 5 years | 2.5 | 4.78 |
| Onaitis et al. [7] | 500 | 23.2 | 1 | 80%, 2 years | 1.6 | 3 |
| Roviaro et al. [12] | 259 | 7.7 | 0.84 | 63%, 5 years | 23 | 5 |
| Kim et al. [13] | 555 | 9.1 | 1.3 | 87%, 3 years | 3.9 | 6 |
| Gonzalez et al. [14] | 200 | 21 | 2.5 | 85%, 3 years | 14.5 | 4 |
| Yim and Liu [16] | 214 | 22 | 1.8 | 93%, 2 years | 19 | 6.8 |
| Present series | 410 | 20.5 | 1.2 | 86.3%, 5 years | 6.1 | 6.5 |
LOS: length of stay.
McKenna et al. [5] reported the largest single-institution series of VATS lobectomy (1100 patients) with a 0.8% mortality rate and a 15.3% morbidity rate. In a systematic review including 39 studies with 3256 thoracotomy and 3114 VATS patients, Whitson et al. [15] showed that, compared with thoracotomy, VATS lobectomy was associated with shorter chest tube duration and shorter length of hospital stay, and lower morbidity improved survival. Our results correlate with the results reported in other retrospective studies with regard to a low perioperative mortality (1.2%) and morbidity (20.6%). Prolonged air leaks were the most common complication in our experience and were related to the technical difficulty in achieving a complete pneumostasis through a thoracoscopic approach. Prolonged air leaks were more frequent at the beginning of our experience and were significantly reduced by avoiding dissection of the fissure and by performing lobectomy after initial ligation of the hilar structures and completion of the fissure at the end of the procedure. There is a low rate of AF in our experience (2.5%) because continuous heart rate monitoring was not routinely used. Only persistent symptomatic arrhythmias requiring treatment were recorded. Our perioperative mortality rate (1.2%) is similar to the mortality rate reported in previous large published studies, ranging from 0.8 to 2.5% [2, 4, 5, 7, 13, 16] and is comparable with the mortality rate of open lobectomy in our practice (1.4%) and in the literature (1.2–2.9%) [9, 10]. In our experience, there were 5 postoperative deaths. Causes of death were ADRS in 3 patients; all these patients had a history of severe chronic obstructive pulmonary disease (COPD) with heavy smoking and were classified ASA3.
Safety
There is serious concern regarding the intraoperative safety of VATS lobectomy and the potential occurrence of catastrophic intraoperative complications. Flores et al. [17] have recently demonstrated that these events are uncommon. They reported only 13 major intraoperative complications in a series of 633 VATS lobectomies during an 8-year experience (1%). For Flores et al., a catastrophic complication was defined as an event that resulted in an additional unplanned major surgical procedure other than the planned lobectomy. These complications included mainly vascular injuries (pulmonary artery and vein), transection of main pulmonary artery or bronchus and injury to bronchi or broncho-oesophageal fistula. In our experience, massive intraoperative bleeding related to pulmonary artery injury required, in 3 patients, emergent conversion to achieve vessel control and repair, but without requiring more extensive resection.
Oncological validity
This report supports the claim that VATS lobectomy is an equivalent oncological resection to open lobectomy allowing anatomical resection with individual hilar ligation, and a complete lymph node dissection and providing a number of dissected lymph nodes similar to that of open surgery. Concerns about the oncological validity of VATS lobectomy arise from the possibility of insufficient lymph node dissection [18]. Suspicions regarding the effectiveness of thoracoscopic mediastinal lymph node dissection are unjustified. Japanese authors [18, 19] have shown that a standard lobectomy with radical nodal dissection is technically feasible and can be performed safely through a thoracoscopic approach. Kondo et al. [19] reported that the posterior approach to utility thoracotomy is more suitable for lymph node dissection of the subcarinal nodes, but also for the paratracheal on the right side. In our experience, the same lymph node mediastinal dissection as in the open procedure, was performed in all patients with lung cancer and included radical dissection in 2R, 4R, 6, 7 stations and systematic sampling in 4L, 5, 8, 9 stations. The mean number of nodes removed through VATS lobectomy was 14.6 and was equivalent to the median number of nodes removed through an open procedure. The Eastern Cooperative Oncology Group in the ECOG 3590 study [20] defined lymphadenectomy as the removal of ≥10 lymph nodes from at least two or more mediastinal lymph nodes stations. Denliger et al. [21] evaluating lymph node dissection, sampled fewer lymph nodes with VATS lobectomy compared with open surgery, but without survival difference. The authors reported fewer level 7 nodes for left-side resections, particularly upper lobe resection in the VATS lobectomy vs thoracotomy, because mobilization of lower lobes for VATS lobectomy provides good exposure of the subcarinal area, making Station 7 dissection easier; on the other hand, exposure of the subcarinal space is not essential in upper lobe lobectomy, making dissection of the subcarinal 7 lymph node technically more challenging than others stations. Increase of the tidal volume up to 500–600 ml by the anaesthesiologist may help to facilitate the nodal dissection in this area by elevating the mediastinum. Concerns regarding the effectiveness of the thoracoscopic mediastinal lymph node dissection are unfounded.
There is no difference between VATS and open procedures in 5-year survival rates for surgically resected early-stage lung carcinoma and the results of VATS lobectomy compare favourably with most recent studies of patients undergoing open lobectomy [22]. In a multicenter review performed in Japan [20], the 5-year survival rate for patients with early-stage lung cancer is not statistically significant between the two procedures. In our study, the 5-year survival in patients with early-stage lung cancer (86%) was quite similar to the survival in other large series reporting the use of VATS lobectomy ([2, 5, 7, 12–14, 16], Table 5). Some studies [20] demonstrated that 10–25% of patients with T1 tumours had N+ disease at the definitive staging despite careful preoperative work-up. In our study, nodal involvement was not identified at the preoperative work-up and was present in 63 patients at the definitive staging. Twenty-one patients (5%) presented N1 disease and 42 patients N2 disease (11.5%). Previous studies showed favourable outcomes in patients staged N0 at the clinical staging and who presented lymph node involvement during surgery or at the definitive pathological examination [23]. Kim et al. [13] reported, for patients with pathological N1 or N2 disease after VATS lobectomy, a survival comparable with that after open lobectomy. The 3-year overall survival rates were 98% for patients with N1 disease and 89% for those with N2 disease. Even if lymph node metastasis is unexpectedly detected during video-assisted thoracic surgery lobectomy for clinical stage I disease, the authors do not recommend converting to conventional thoracotomy. In our study, outcomes of unexpected N1 and N2 disease after VATS lobectomy for clinical stage I NSCLC were also favourable, and the 5-year overall survival were, respectively, 58 and 61%. Flores et al. [24] investigated the patterns of recurrence rates for VATS lobectomy in patients with lung cancer and concluded that they are at least equivalent to those for thoracotomy. A limitation should be taken into account: we report only mid-term results on disease-free survival. Long-term results on disease-free survival are available only for a few patients. This is due to the relatively recent widespread use of this technique by our team.
A limitation to the widespread use of VATS lobectomy is the supposed complexity in learning the operation that young thoracic surgeons face. The learning curve of the attending surgeons will be dependent on their training in open thoracic surgery and thoracoscopic surgery in less complex procedures. Specific training for this procedure has been given in dedicated courses, but providing this training during residency remains difficult. Reed proposed a model of teaching with a stepwise introduction of the specific thoracoscopic techniques during open surgery until progression to complete lobectomy: initially achieve exposure and take down the pulmonary ligament, then dissect the hilum, first venous and bronchus and finally the pulmonary artery. VATS lobectomy is a safe, feasible and oncologically valuable procedure for the treatment of early-stage lung cancer in terms of mortality, complications and survival and should be considered at least equivalent to open lobectomy. On the other hand, VATS lobectomy offers some potential advantages such as decreased pain, shorter length of stay, cosmetic and lower overall cost. In our series, the data confirm the results obtained in other retrospective series with regard to low mortality, morbidity and oncological results. Furthermore, this minimally invasive method allowed to the maintenance of lobectomy as the gold standard in high-surgical-risk patients (36.8% of patients in this series) instead of alternative therapy as radiofrequency ablation or stereotactic body radiotherapy. Confirmation of the oncological effectiveness of VATS lobectomy would require a large, prospective, randomized series, which is not forthcoming. Our additional experience supporting the oncological validity of VATS lobectomy may be useful to strengthen the widespread agreement on this procedure in usual practice.
Our experience prompts us to consider VATS lobectomy for early-stage NSCLC as the primary mode of surgical intervention. VATS lobectomy should be considered the standard of care for early lung cancer in view of the improvement in outcome, provided that the procedure is performed by a surgeon with adequate experience with this approach.
Conflict of interest: none declared.
REFERENCES
- 1.Yan TD, Black D, Bannon PG, McCaughan BC. Systematic review and meta-analysis of randomized and non-randomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-call lung cancer. J Clin Oncol. 2009;27:2553–62. doi: 10.1200/JCO.2008.18.2733. doi:10.1200/JCO.2008.18.2733. [DOI] [PubMed] [Google Scholar]
- 2.Flores RM, Park BJ, Dycoco J, Aronova A, Hirth Y, Rizk NP, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg. 2009;138:11–8. doi: 10.1016/j.jtcvs.2009.03.030. doi:10.1016/j.jtcvs.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 3.Rivera C. Video-assisted thoracoscopic surgery, 20 years later: state of the art in France. 65th Congress of the French Society of Thoracic and Cardiovascular Surgery. French Society for Thoracic and Cardiovascular Surgery; Nice, France: 2012. [Google Scholar]
- 4.Boffa DJ, Allen MS, Grab JD. Data from the Society of Thoracic Surgeons General Thoracic Surgery Database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg. 2008;135:247–54. doi: 10.1016/j.jtcvs.2007.07.060. doi:10.1016/j.jtcvs.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 5.McKenna RJ, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg. 2006;81:421–6. doi: 10.1016/j.athoracsur.2005.07.078. doi:10.1016/j.athoracsur.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 6.Daniels LJ, Balderson SS, Onaitis MW, D'Amico TA. Thoracoscopic lobectomy: a safe and effective strategy for patients with stage I lung cancer. Ann Thorac Surg. 2002;74:860–4. doi: 10.1016/s0003-4975(02)03764-5. doi:10.1016/S0003-4975(02)03764-5. [DOI] [PubMed] [Google Scholar]
- 7.Onaitis MW, Petersen RP, Balderson SS, Toloza E, Burfeind WR, Harpole DH, Jr, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg. 2006;244:420–5. doi: 10.1097/01.sla.0000234892.79056.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen MS, Darling GE, Pechet TT, Mitchell JD, Herndon JE, Landreneau RJ, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg. 2006;81:1013–9. doi: 10.1016/j.athoracsur.2005.06.066. doi:10.1016/j.athoracsur.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 9.Deslauriers J, Ginsberg RJ, Piantadosi S, Fournier B. Prospective assessment of 30-day operative morbidity for surgical resections in lung cancer. Chest. 1994;106:329–30. doi: 10.1378/chest.106.6_supplement.329s. doi:10.1378/chest.106.2.329. [DOI] [PubMed] [Google Scholar]
- 10.Wada H, Nakamura T, Nakamoto K, Maeda M, Watanabe Y. Thirty-day operative mortality for thoracotomy in lung cancer. J Thorac Cardiovasc Surg. 1998;115:70–3. doi: 10.1016/s0022-5223(98)70444-1. doi:10.1016/S0022-5223(98)70444-1. [DOI] [PubMed] [Google Scholar]
- 11.Sugi K, Kaneda Y, Esato K. Video-assisted thoracoscopic lobectomy achieves a satisfactory long-term prognosis in patients with clinical stage IA lung cancer. World J Surg. 2000;24:27–31. doi: 10.1007/s002689910006. doi:10.1007/s002689910006. [DOI] [PubMed] [Google Scholar]
- 12.Roviaro G, Varoli F, Vergani C, Nucca O, Maciocco M, Grignani F. Long-term survival after videothoracoscopic lobectomy for stage I lung cancer. Chest. 2004;126:725–32. doi: 10.1378/chest.126.3.725. doi:10.1378/chest.126.3.725. [DOI] [PubMed] [Google Scholar]
- 13.Kim HK, Choi YS, Kim J, Shim YM, Kim K. Outcomes of unexpected pathologic N1 and N2 disease after video-assisted thoracic surgery lobectomy for clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2010;140:1288–93. doi: 10.1016/j.jtcvs.2010.06.011. doi:10.1016/j.jtcvs.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez D, de la Torre M, Paradela M, Fernandez R, Delgado M, Garcia J, et al. Video-assisted thoracic surgery lobectomy: 3-year initial experience with 200 cases. Eur J Cardiothorac Surg. 2011;40:21–8. doi: 10.1016/j.ejcts.2011.02.051. doi:10.1016/j.ejcts.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 15.Whitson BA, Andrade RS, Boettcher A, Bardales R, Kratzke RA, Dahlberg PS, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2007;83:1965–70. doi: 10.1016/j.athoracsur.2007.01.049. doi:10.1016/j.athoracsur.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 16.Yim AP, Liu HP. Thoracoscopic major lung resection: indications, technique, and early results : experience from two centers in Asia. Surg Laparosc Endosc. 1997;7:241–4. doi:10.1097/00019509-199706000-00013. [PubMed] [Google Scholar]
- 17.Flores RJ, Ihekweazu U, Dycoco J. Video-assisted thoracoscopic surgery (VATS) lobectomy: catastrophic intraoperative complications. J Thorac Cardiovasc Surg. 2011;142:1412–7. doi: 10.1016/j.jtcvs.2011.09.028. doi:10.1016/j.jtcvs.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 18.Sagawa M, Sato M, Sakurada A, Matsumura Y, Endo C, Handa M, et al. A prospective trial of systematic nodal dissection for lung cancer by videoassisted thoracic surgery: can it be perfect? Ann Thorac Surg. 2002;73:900–4. doi: 10.1016/s0003-4975(01)03409-9. doi:10.1016/S0003-4975(01)03409-9. [DOI] [PubMed] [Google Scholar]
- 19.Kondo T, Sagawa M, Tanita T, Sato M, Ono S, Matsumura Y, et al. Is complete systematic nodal dissection by thoracoscopic surgery possible? A prospective trial of video-assisted lobectomy for cancer of the right lung. J Thorac Cardiovasc Surg. 1998;116:651. doi: 10.1016/S0022-5223(98)70175-8. doi:10.1016/S0022-5223(98)70175-8. [DOI] [PubMed] [Google Scholar]
- 20.Tahara RW, Lackner RP, Graver LM. Is there a role for routine mediastinoscopy in patients with peripheral T1 lung cancers? Am J Surg. 2000;180:488–91. doi: 10.1016/s0002-9610(00)00509-2. doi:10.1016/S0002-9610(00)00509-2. [DOI] [PubMed] [Google Scholar]
- 21.Denliger CE, Fernandez F, Meyers BF, Pratt W, Zoole JB, Patterson GA, et al. Lymph node evaluation in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Ann Thorac Surg. 2010;89:1730–6. doi: 10.1016/j.athoracsur.2010.02.094. doi:10.1016/j.athoracsur.2010.02.094. [DOI] [PubMed] [Google Scholar]
- 22.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IALSC Lung Cancer Staging Poject: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;8:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. doi:10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 23.Inoue M, Sawabata N, Takeda S, Ohta M, Ohno Y, Maeda H. Results of surgical intervention for p-stage IIIA (N2) non-small cell lung cancer: acceptable prognosis predicted by complete resection in patients with single N2 disease with primary tumor in the upper lobe. J Thorac Cardiovasc Surg. 2004;127:1100–6. doi: 10.1016/j.jtcvs.2003.09.012. doi:10.1016/j.jtcvs.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Flores RM, Ihekweazu UN, Rizk N, Dycoco J, Bains MS, Downey RJ, et al. Patterns of recurrence and incidence of second primary tumors after lobectomy by means of video-assisted thoracoscopic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg. 2011;141:59–64. doi: 10.1016/j.jtcvs.2010.08.062. doi:10.1016/j.jtcvs.2010.08.062. [DOI] [PubMed] [Google Scholar]