Abstract
OBJECTIVES
Mitral annular calcification is associated with significant morbidity and mortality at the time of mitral valve surgery. However, few data are available describing the impact of mitral annular calcification on early and late outcomes following mitral valve repair in the current era.
METHODS
Between 2001 and 2011, 625 patients were referred for mitral valve repair of severe mitral regurgitation due to myxomatous degeneration. The mean patient age was 63.9 ± 12.7 years and 164 (26%) were female. Concomitant coronary artery bypass grafting was performed in 91 (15%) and 24 (4%) had previous cardiac surgery. Calcification of the mitral annulus was observed in 119 patients (19%), of whom complete debridement and extensive annulus reconstruction were performed in 14. The mean follow-up was for 2.4 ± 2.3 years.
RESULTS
There were no deaths within 30 days of surgery. Risk factors associated with mitral annular calcification included older age (odds ratio 1.05 ± 0.02 per increasing year), female gender (odds ratio 1.88 ± 0.42) and larger preoperative left atrial size (odds ratio 1.04 ± 0.03 per increasing mm) (all P<0.01). Severe renal impairment defined as a creatinine clearance <30 mL/min was observed in 9 patients, all of whom had mitral annular calcification. Intraoperative conversion to mitral valve replacement was performed in 19 patients (97% repair rate), 5 of whom had mitral annular calcification. Extension of mitral annular calcification into one or more leaflet scallops was observed for all patients who required conversion to valve replacement. Five-year survival, freedom from recurrent mitral regurgitation ≥2+ and freedom from recurrent mitral regurgitation ≥3+ was 88.1 ± 2.4, 89.6 ± 2.3 and 97.8 ± 0.8%, respectively. Mitral annular calcification was not associated with survival or recurrent mitral regurgitation.
CONCLUSIONS
Risk factors for mitral annular calcification in patients with myxomatous degeneration and severe mitral regurgitation include older age, female gender, severe renal dysfunction and larger preoperative left atrial size. Nevertheless, favourable early and late results can be achieved with mitral valve repair in this population.
Keywords: Follow-up studies, Mitral regurgitation, Mitral valve repair, Survival, Valves
INTRODUCTION
Mitral annular calcification (MAC) represents a technical challenge at the time of mitral valve surgery [1–3]. Calcification of the mitral annulus has been associated with disruption of the atrioventricular groove, operative haemorrhage, coronary ischaemia and death at the time of valve replacement [1–3]. Furthermore, MAC also complicates mitral valve repair since MAC may extend into the leaflets, papillary muscle or left ventricle, which may limit leaflet resection or chordal repair strategies [1].
Pathological studies have shown that MAC has a prevalence of 10–15% and is typically limited to the mitral annulus [1, 4, 5]. The posterior annulus is more commonly involved than the anterior annulus although circumferential MAC is rare [1]. Age and female gender are described risk factors for MAC [1, 5].
Although MAC is common and associated with significant surgical risk, the literature describing operative management of MAC is limited. Published studies have involved mixed cohorts of patients with different causes of mitral regurgitation (MR) [6–12]. Furthermore, most series have described outcomes following en bloc resection alone, which may not be necessary in the majority of patients with MAC.
We therefore performed a longitudinal follow-up study of a large, contemporary, cohort of patients with myxomatous degeneration and MR. Our objectives were to describe: (i) the incidence of MAC in patients undergoing mitral valve surgery, (ii) our surgical approach and technique in addressing MAC and (iii) the long-term outcomes following mitral valve repair in this population.
METHODS
Ethics approval
The University of Ottawa Heart Institute has existing ethics approval from its institutional research ethics board to anonymously publish data that are prospectively collected before and after heart valve surgery. As such, individual patient consent was waived.
Patients and follow-up
Between 2001 and 2011, 625 patients with myxomatous degeneration were referred for mitral valve repair. Of these, 119 (19%) had MAC. The calcification involved more than one scallop of the mitral annulus in 46 patients (39%), and at least half the mitral annulus in 27 (23%). The mitral valve was repaired in 606 patients, in whom MAC was observed in 114. Intraoperative conversion to mitral valve replacement was performed for 19 patients (97.0% repair rate for patients with myxomatous degeneration), in whom MAC was observed in 5, and involved more than half of the mitral annulus in 2. The overall mitral valve repair rate in patients with MAC was 96.0% (114 of 119 patients). The aetiology of MR was determined preoperatively via established echocardiographic criteria [13] and confirmed intraoperatively by the operating surgeon. These data were recorded prospectively as part of the Mitral Valve Clinic at the University of Ottawa.
Patients were assessed regularly in a dedicated mitral valve clinic. Clinic visits consisted of a history and physical examination, electrocardiogram, chest radiograph, complete blood count, serum chemistries and international normalized ratio (when applicable). Echocardiographic follow-up was also performed on all mitral valve repair patients postoperatively at 1, 3, 6 and 12 months. After 1 year, echocardiograms were performed every 1–3 years or when clinically indicated. Mean follow-up was 2.4 ± 2.3 years (maximum 8.6 years, total 251 years).
Operative technique and surgical management of mitral annular calcifications
All mitral valve repairs were performed with median sternotomy, cardiopulmonary bypass and cardioplegic arrest with cold blood cardioplegia. Mitral valve annuloplasty was performed in 593 patients with the Medtronic Futureband in 490 (Medtronic, Inc., Minneapolis, MN, USA), Duran Ancore Band in 63 (Medtronic, Inc.), Carpentier-Edwards Physio Annuloplasty Ring in 37 (Baxter Healthcare Corp., Irvine, CA, USA) and Cosgrove-Edwards Annuloplasty Band in 3 (Baxter Healthcare Corp., Irvine, CA, USA). Notably, all patients with MAC who underwent annuloplasty received the Medtronic Futureband (Medtronic, Inc.).
In patients with posterior leaflet prolapse and calcification limited to the prolapsing segment, resection of the calcified, prolapsing scallop was followed by annulus debridement, reattachment of the reconstructed leaflet to the annulus and annuloplasty placement. When the annular calcification and the prolapsing segment involved either commissure, the annulus was debrided and a commissuroplasty was performed. Annular calcification that was limited to a non-prolapsing area of the valve was ignored, and the annuloplasty band was rotated whenever possible to avoid placing annular sutures in calcium. Insertion of a C-shaped band precluded the need for placement of annuloplasty sutures in the anterior portion of the annulus between the trigones. In general, extensive annulus debridement was avoided considering advanced age, circumferential MAC, MAC extending into the ventricle or papillary muscles, a small mitral valve annulus or the need for multiple concomitant cardiac procedures. Consequently, 13 of the 27 patients with extensive calcification of the mitral annulus did not undergo annuloplasty band placement. In these patients, a simpler technique involving an edge-to-edge leaflet repair was employed in 6 (central edge-to-edge in 3, and an edge-to-edge repair towards either commissure in 3) and polytetrafluoroethylene artificial chord placement in 7. Of the 7 patients who underwent artificial chord placement, native chordal transfer was also performed in 2.
Conversely, en bloc resection of MAC was performed, followed by reconstruction of the annulus (autologous pericardium in 6 and bovine pericardium in 8) and annuloplasty band placement. Notably, in 2 of these 14 patients, the MAC was circumferential, but only the posterior aspect of the annulus was debrided and reconstructed. Bioglue (CryoLife, Atlanta, GA, USA) was used to reinforce the atrioventricular groove in 4 patients. In 3 patients, calcification of the posterior annulus extended to the mid-basal portion of the reconstructed posterior mitral leaflet. Therefore, the patch reconstruction of the annulus was extended to the base of the leaflet to allow a posterior leaflet height of ∼1 cm.
In all patients who underwent mitral valve repair, MR >1+ or a mean mitral gradient >5 mmHg, determined intraoperatively, were indications for further mitral valve intervention. There were 5 patients with severe MAC who underwent intraoperative conversion to mitral valve replacement. Mitral valve repair was attempted in all of these patients. En bloc resection of the annulus and leaflet calcification was performed in one, but residual 2+ MR was noted due to posterior leaflet restriction. In fact, posterior leaflet restriction and residual MR were also the causes of two other valve replacements, whereas lack of anterior leaflet mobility was noted in the remaining 2 patients.
Statistical analysis
Data were imported and analysed in Stata 11.2 (College Station, TX, USA). Continuous variables were expressed as a mean ± standard deviation and compared with separate Wilcoxon signed-rank tests. Categorical variables were described as a percentage of the total and compared with either a χ2 test or Fisher's exact test, when the frequency was <5. Risk factors associated with MAC and intraoperative conversion to mitral valve replacement were determined using multivariable logistic regression. The Kaplan–Meier method was used to assess survival, freedom from recurrent MR (≥2+), freedom from recurrent MR (≥3+) and freedom from mitral valve reoperation. Risk factors associated with these outcomes were determined by using separate semi-parametric Cox proportional hazards models. The proportional hazards assumption was verified with Schoenfeld residuals.
RESULTS
Population characteristics
The mean age of this cohort was 63.9 ± 12.7 years and 164 (26%) were female. Severe renal impairment defined as a creatinine clearance <30 ml/min was observed in 9 (1%), diabetes mellitus in 33 (5%) and hypertension in 242 (39%). One hundred and forty (22%) had either permanent or paroxysmal atrial fibrillation prior to surgery and 24 had previous cardiac surgery.
Five hundred and ninety patients (94%) had 3+ or 4+ MR preoperatively, whereas the remainder had 2+ MR with another indication for cardiac surgery [14]. The preoperative left atrial diameter for patients in this cohort was 50.4 ± 8.0 mm. Concomitant coronary artery bypass grafting was performed in 91 (15%), tricuspid valve repair in 59 (9%) and maze procedure in 100 (16%). En bloc resection of MAC was performed in 14 patients who underwent mitral valve repair (Table 1). Patients in whom en bloc resection was performed were younger (69.7 ± 11.8 vs 78.5 ± 6.7 years, P = 0.003) and had a longer cardiac ischaemic time at surgery (90.6 ± 21.8 vs 53.9 ± 36.9 min, P = 0.005) compared with those in whom complete resection and annuloplasty were not performed (Table 1). Of note, concomitant coronary artery bypass grafting was performed in 2 patients in each group. Of the 14 patients who underwent annulus debridement and reconstruction, 6 had a concomitant Maze procedure and 2 underwent concomitant tricuspid valve repair (Table 1).
Table 1:
Characteristics of patients with severe mitral annular calcification with and without debridement and annuloplasty at the time of mitral valve repair
| Decalcification (n = 14) | No decalcification and no annuloplasty (n = 13) | P-value | |
|---|---|---|---|
| Patient characteristics | |||
| Age (years) | 69.7 ± 11.8 | 78.5 ± 6.7 | 0.003a |
| Atrial fibrillationb | 7 (50%) | 5 (38%) | 0.4c |
| Female gender | 5 (36%) | 2 (15%) | 0.2c |
| Preoperative echocardiographic characteristics | |||
| Left atrial diameter (mm) | 54.9 ± 7.0 | 46.7 ± 6.9 | 0.02a |
| Left ventricle ejection fraction ≥50% | 12 (86%) | 12 (92%) | 0.7c |
| Operative characteristics | |||
| Ischaemic time (min) | 90.6 ± 21.8 | 53.9 ± 36.9 | 0.005a |
| Concomitant coronary artery bypass grafting | 2 (14%) | 2 (15%) | 0.9c |
| Concomitant aortic valve replacement | 0 (0%) | 3 (23%) | 0.07c |
| Concomitant maze procedured | 6 (43%) | 3 (23%) | 0.2c |
| Concomitant tricuspid valve repair | 2 (14%) | 0 (0%) | 0.1c |
aCompared with a non-parametric Wilcoxon signed-rank test.
bIncludes permanent and known paroxysmal atrial fibrillation preoperatively.
cCompared with either a χ2 test or Fisher's exact test, when the frequency was <5.
dAblation performed with the Medtronic Cardioblate irrigated unipolar radiofrequency system (Medtronic, Inc.).
Risk factors associated with mitral annular calcification
Age and female gender were associated with MAC (Table 2). Notably, preoperative left atrial diameter was also associated with MAC (odds ratio 1.04 ± 0.02 per increasing mm, P = 0.01). Severe renal impairment defined as a creatinine clearance <30 ml/min was observed in 9 patients, all of whom had MAC.
Table 2:
Risk factors associated with mitral annular calcification
| Odds ratio (95% confidence interval) | P-value | |
|---|---|---|
| Age (years) | 1.05 (1.02–1.07) | <0.001 |
| Atrial fibrillationa | 0.86 (0.48–1.52) | 0.6 |
| Diabetes mellitus | 0.63 (0.20–2.01) | 0.4 |
| Female gender | 1.88 (1.13–3.12) | 0.01 |
| Hypertension | 0.86 (0.48–1.51) | 0.6 |
| Preoperative left atrial diameter (per increasing mm) | 1.04 (1.01–1.08) | 0.01 |
| Severe renal dysfunctionb | – | – |
aIncludes permanent and known paroxysmal atrial fibrillation preoperatively.
bEstimated creatinine clearance <30 ml/min. All patients with renal dysfunction had mitral annular calcification; therefore, it could not be entered into the multivariable model.
Risk factors associated with intraoperative conversion to mitral valve replacement
Although older patients and women were more likely to have MAC, they were not more likely to require an intraoperative conversion to mitral valve replacement (Table 3). Importantly, all 5 patients with MAC who required valve replacement had either anterior or posterior leaflet calcification extending beyond the body of at least one leaflet scallop. The presence of MAC, in the absence of extensive leaflet calcification, was not associated with the need for valve replacement (Table 3).
Table 3:
Risk factors associated with intraoperative conversion to mitral valve replacement
| Odds ratio (95% confidence interval) | P-value | |
|---|---|---|
| Age (per increasing year) | 1.01 (0.96–1.07) | 0.7 |
| Concomitant CABG | 0.98 (0.19–5.16) | 0.9 |
| Female gender | 0.58 (0.11–3.13) | 0.5 |
| Mitral annular calcification | 1.56 (0.45–5.35) | 0.5 |
| Preoperative left atrial diameter (per increasing mm) | 1.00 (0.92–1.09) | 0.9 |
| Preoperative left ventricular ejection fraction <35% | 8.84 (2.17–36.07) | 0.002 |
CABG: coronary artery bypass grafting.
Survival
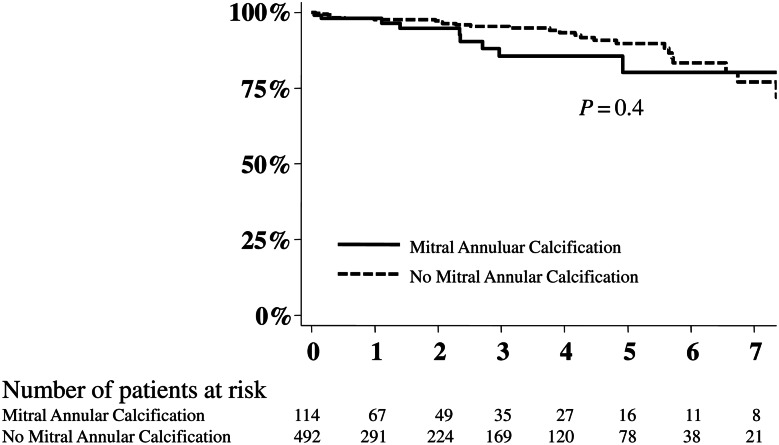
There were no deaths within 30 days of surgery. Five-year actuarial survival following mitral valve repair was 88.1 ± 2.4% and not different between patients with and without MAC (P = 0.4) (Fig. 1).
Figure 1:
Impact of mitral annular calcification on survival following mitral valve repair of myxomatous degeneration. No deaths occurred within 30 days of surgery. After a mean clinical follow-up of 2.4 ± 2.3 years, an additional 38 patients died.
Freedom from recurrent mitral regurgitation and mitral valve reoperation
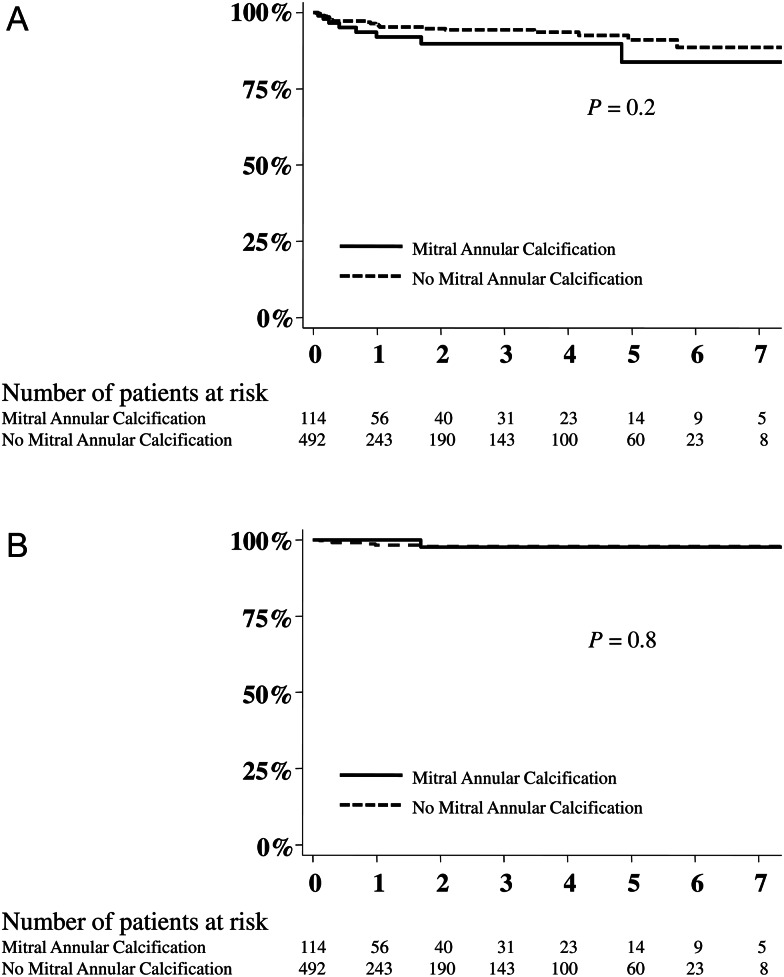
At follow-up, 29 patients, of the entire cohort of myxomatous degeneration who underwent mitral valve repair, had recurrent MR (≥2+), of whom 6 had 3+ or 4+ MR. Overall, 5-year freedom from recurrent MR (≥2+) was 83.8 ± 6.8% and 91.1 ± 2.4% for patients with and without MAC, respectively (P = 0.2) (Fig. 2). Five-year freedom from recurrent 3+ or 4+ MR was 97.8 ± 0.8% for the entire cohort.
Figure 2:
Impact of mitral annular calcification on recurrent mitral regurgitation following mitral valve repair of myxomatous degeneration. (A) Freedom from recurrent mitral regurgitation (MR) ≥2+. (B) Freedom from recurrent MR ≥3+. Echocardiographic follow-up was also performed on all mitral valve repair patients postoperatively at 1, 3, 6 and 12 months. After 1 year, echocardiograms were performed every 1–3 years or when clinically indicated. Recurrent mitral regurgitation (≥2+) was observed in 29 patients, of whom 6 had 3+ or 4+ regurgitation at follow-up. The mean echocardiographic follow-up was 2.0 ± 2.1 years.
Mitral valve reoperation was subsequently performed in 9 patients, in whom 7 had recurrent 3+ or 4+ MR. One patient underwent reoperation due to recurrent moderate–severe mitral stenosis detected only on exercise testing. Another patient had recurrent moderate mitral stenosis, but also developed severe tricuspid regurgitation and coronary artery disease requiring surgical intervention. Two of the 9 patients who underwent mitral valve reoperation had MAC noted at the time of their first mitral valve repair. Overall, successful valve re-repair was performed in 5. Of the 5 patients who underwent successful valve re-repair, 3 separate patients had new prolapse of an adjacent scallop that previously did not require repair. One patient had an elongated polytetrafluoroethylene artificial chord to P2, which was subsequently replaced and reinforced with an additional artificial chord. The last patient had a successful mitral repair 5 years previously, but developed mitral stenosis secondary to pannus formation around the annuloplasty ring. Overall, 5-year freedom from mitral valve reoperation was 97.0 ± 1.2%.
DISCUSSION
In this study, we evaluated MAC in a large, contemporary cohort of patients with myxomatous degeneration who were referred for mitral valve repair.
Overall, MAC was observed in 119 (19%) patients, which is consistent with previously published data [1, 4, 5]. MAC was more frequently observed in older patients, women, patients with severe renal impairment [5] and also in patients with a larger preoperative left atrium. This last observation may relate to the extent and duration of mitral valve disease, although we have no data confirming this proposed mechanism. Successful mitral valve repair was performed in 97% of patients and compares favourably to those in the published literature [14, 15]. Notably, leaflet calcification and mobility, but not the presence of MAC itself, were associated with failed mitral valve repair. In patients with MAC extending into the body of the posterior leaflet, we previously resected the calcified scallop and re-approximated the remaining leaflet tissue, likely causing restriction and/or tension of the remaining scallops. Our current approach is to extend the patch reconstruction of the annulus into the leaflet itself. We feel that the patch augmentation of the leaflet reduces tension on the remaining leaflet tissue and allows for a more physiological height of coaptation. En bloc resection of MAC was required in a smaller subset of patients with annular calcification and was less often performed in older patients. In these patients, our approach was more conservative, involving an edge-to-edge repair without radical MAC resection. We achieved acceptable results with this approach, which lends support for the use of an edge-to-edge repair, whether applied centrally or towards either commissure, in this population. This last finding is interesting since these data can perhaps be extended to justify a percutaneous mitral valve repair strategy for high-risk or inoperable patients.
There were no early deaths in this study. Previous reports describing mitral valve surgery in patients with MAC have reported an operative mortality ranging between 3 and 14% [6–12]. However, comparison with the literature is limited since these published studies have included mixed etiologies of MR with varying surgical approaches. The available data describing outcomes following mitral surgery in patients with MAC have focused predominantly on patients who underwent complete annular debridement and reconstruction alone [6–12]. It has been our experience that en bloc resection and reconstruction is required in only a subset of patients. Therefore, the real-world approach to MAC may be under-represented in the published literature. With the described approach, which may seem conservative since radical MAC resection was performed in approximately half of all patients with severe MAC, we had no incidences of atrioventricular groove rupture, early death or early/late ring dehiscence. Naturally, careful intraoperative evaluation is required prior to deciding upon annulus debridement and reconstruction. Severe MAC, although intimidating, may be reasonably simple to address provided that it does not extend too far into the leaflet body or into the ventricular wall. If MAC is confined to the annulus and can be removed as a homogeneous calcium bar, the reconstruction can be relatively easy. The operation can be more challenging if the decalcification can only be performed in limited pieces. In a few patients with extensive debridement and annular patch reconstruction, we have also used biological glue to reinforce the atrioventricular groove.
Overall 5-year survival, freedom from recurrent (≥3+) and freedom from mitral valve reoperation were 88, 98 and 97%, respectively. These findings therefore support our surgical assertions that: (i) MAC does not necessarily interfere with the durability of mitral valve repair, (ii) a tailored approach can be used to address severe MAC. Considering patient and operative factors a conservative approach without radical MAC resection can offer favourable early and mid-term results and (iii) the lack of decalcification of moderate MAC does not preclude the placement of an annuloplasty device in the majority of patients.
Limitations
This is a retrospective study describing our institutional approach to MAC at the time of mitral surgery. No attempts were made to compare patients with and without en bloc resection of MAC in a randomized setting. Long-term conclusions regarding the durability of mitral valve repair are also unknown given the limited duration of follow-up.
CONCLUSION
Despite the limitations mentioned above, this study comprises a large cohort of patients with myxomatous degeneration who underwent detailed serial follow-up after mitral valve repair. Based on these data, MAC appears common in patients presenting to mitral valve surgery with myxomatous degeneration. However, favourable early and mid-term results can be achieved with mitral valve repair in this population.
Funding
Financial support for this study was provided by the Division of Cardiac Surgery at the University of Ottawa Heart Institute.
Conflict of interest: Marc Ruel is a proctor for minimally invasive coronary bypass surgery with Medtronic.
APPENDIX. CONFERENCE DISCUSSION
Dr R. Dion (Genk, Belgium): The authors have reviewed the outcome of 119 patients with mitral annulus calcification in myxomatous degenerative disease operated on between 2001 and 2011, so it is a quite recent cohort of patients. This represented about 19% of all the mitral valve repairs that they performed in the same time interval. The authors propose the conclusion of their work is that mitral annulus calcification is not associated with survival or recurrent mitral regurgitation, and they have to be commended for their excellent outcome, and particularly no deaths.
Although they wished to comment on long-term results, the mean follow-up is only 2.4 years, and of these 119 mitral annulus calcification patients, only 46 have more than half of the posterior annulus involved, and I personally reckon that only these patients may pose a technical issue. Of these patients, 13 received no annuloplasty but were treated either with an edge-to-edge repair or with neo-chordae PTFE; only 14 had a total debridement of the posterior annulus plus reconstruction with pericardium, and five ended up with mitral valve replacement because of a leaflet restriction problem after the repair. Mitral annulus calcification of a non-prolapsing area was ignored and an incomplete annuloplasty band was rotated in order to avoid the calcified area.
I have a few questions. First, in myxomatous disease, in my experience, the vast majority of mitral annulus calcifications are easily removed because the calcium does not invade the left ventricle. In your series, debridement was only attempted in 50% of the patients with extensive mitral annuloplasty; in the other 50% no annuloplasty was attempted, and you recommend edge-to-edge repair or PTFE, or even suggest in your manuscript the use of a MitraClip. My problem is that even Ottavio Alfieri doesn't find it a good idea to use the edge-to-edge repair in the presence of annular calcification precluding the use of an annuloplasty. So I would like to hear from you the outcome of this particular group of patients in whom you performed only an edge-to-edge without annuloplasty or used PTFE chords. What is the outcome of these cases in terms of recurrent mitral regurgitation, for instance?
Dr Mesana: You have rightly mentioned that we have 27 patients with more severe calcification. In this cohort of patients, we actually converted only two. Five patients is the overall cohort for the whole mitral annular calcification population, which is 119 patients. Therefore, we had two out of 27 that were converted. These are patients that were converted in the OR because of recurrent MR with some degree of restriction of the valve, and maybe in these patients we should have performed annular reconstruction and possibly have avoided conversion. One of them had it, actually. So regarding your question, I was actually surprised that we could have reasonable results with low recurrence of MR with the edge-to-edge technique, because Alfieri doesn't show that. Moreover, MitraClip is not recommended with a calcified annulus.
These patients were really old patients. Actually the young patients with mitral annulus calcification with a Barlow I think are easier to repair, as you rightly mentioned. Older patients may have calcifications going down into the LV, you don't want to extend the surgery, and edge-to-edge is a bailout procedure, preferable to a replacement. We have not had any reoperations in these patients so far. We found some of them had some moderate recurrent MR, but again, most of them are older and sicker patients and we believe it is an acceptable result.
Dr Dion: Personally in this type of situation I would augment one of the leaflets and have the same result as with an annuloplasty, because you increase the tissue within the annulus. I have a second question. In other patients with mitral annulus calcification in a non-prolapsing area, the annuloplasty band was rotated. And what about the anterior annulus then? How do you place the band? Do you resect a part of it?
Dr Mesana: As I showed in the slide, we basically abandoned full rings in 2004. We exclusively use a posterior semi-rigid ring, which actually covers two-thirds of the annulus. So we don't have the problem in the anterior leaflet because we don't put the ring there. It is a posterior band.
Dr Dion: But if you rotate the band, most of the opening is on the calcium.
Dr Mesana: Well, we rotate the band when there is a commissural calcification of the commissures, so we avoid the area of calcification.
Dr Dion: And my last question is that five of the 14 total debridements have led to mitral valve replacement because of a "restrictive leaflet" motion. How can it happen in a myxomatous disease, because you have excess tissue? How do you get two restrictive leaflets?
Dr Mesana: Again, I'm sorry, but that is not the case. It is two out of the 27, and one of them out of the 14 reconstructions. I believe the reason was that with some cases of posterior leaflet calcification we were left with not enough height of the posterior leaflet and probably have not extended the patch sufficiently. So maybe in this particular case that had to be converted, there was a technical problem. But I concur with your very good suggestion, to use more the patch augmentation for these patients. We should probably have done that for this particular case. That is actually only one patient.
REFERENCES
- 1.Carpentier AF, Pellerin M, Fuzellier JF, Relland JY. Extensive calcification of the mitral valve anulus: pathology and surgical management. J Thorac Cardiovasc Surg. 1996;111:718–29. doi: 10.1016/s0022-5223(96)70332-x. discussion doi:10.1016/S0022-5223(96)70332-X 729–30. [DOI] [PubMed] [Google Scholar]
- 2.MacVaugh H, Joyner CR, Johnson J. Unusual complications during mitral valve replacement in the presence of calcification of the annulus. Ann Thorac Surg. 1971;11:336–42. doi: 10.1016/s0003-4975(10)65459-8. doi:10.1016/S0003-4975(10)65459-8. [DOI] [PubMed] [Google Scholar]
- 3.Deniz H, Sokullu O, Sanioglu S, Sargin M, Ozay B, Ayoglu U, et al. Risk factos for posterior ventricular rupture after mitral valve replacement: results of 2560 patients. Eur J Cardiothorac Surg. 2008;34:780–4. doi: 10.1016/j.ejcts.2008.06.009. doi:10.1016/j.ejcts.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Pomerance A. Pathological and clinical study of calcification of the mitral valve ring. J Clin Pathol. 1987;23:354–61. doi: 10.1136/jcp.23.4.354. doi:10.1136/jcp.23.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox CS, Vasan RS, Parise H, Levy D, O'Donnell CJ, D'Agostino RB, et al. Framingham Heart Study. Mitral annular calcification predicts cardiovascular morbidity and mortality: the Framingham Heart Study. Circulation. 2003;107:1492–6. doi: 10.1161/01.cir.0000058168.26163.bc. doi:10.1161/01.CIR.0000058168.26163.BC. [DOI] [PubMed] [Google Scholar]
- 6.Feindel CM, Tufail Z, David TE, Ivanov J, Armstrong S. Mitral valve surgery in patients with extensive calcification of the mitral annulus. J Thorac Cardiovasc Surg. 2003;126:777–82. doi: 10.1016/s0022-5223(03)00081-3. doi:10.1016/S0022-5223(03)00081-3. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich M, Doss M, Aybek T, Martens S, Scherer M, Wimmer-Greinecker G, et al. Decalcification of the mitral annulus: surgical experience in 81 patients. Thorac Cardiovasc Surg. 2006;54:464–7. doi: 10.1055/s-2006-924438. doi:10.1055/s-2006-924438. [DOI] [PubMed] [Google Scholar]
- 8.Papadopoulos N, Dietrich M, Christodoulou T, Moritz A, Doss M. Midterm survival after decalcification of the mitral annulus. Ann Thorac Surg. 2009;87:1143–7. doi: 10.1016/j.athoracsur.2008.12.041. doi:10.1016/j.athoracsur.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 9.David TE, Feindel CM, Armstrong S, Sun Z. Reconstruction of the mitral anulus. A ten-year experience. J Thorac Cardiovasc Surg. 1995;110:1323–32. doi: 10.1016/S0022-5223(95)70055-2. doi:10.1016/S0022-5223(95)70055-2. [DOI] [PubMed] [Google Scholar]
- 10.d'Alessandro C, Vistarini N, Aubert S, Jault F, Acar C, Pavie A, et al. Mitral annulus calcification: determinants of repair feasibility, early and late surgical outcome. Eur J Cardiothorac Surg. 2007;32:596–603. doi: 10.1016/j.ejcts.2007.06.044. doi:10.1016/j.ejcts.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 11.Fasol R, Mahdjoobian K, Joubert-Hubner E. Mitral repair in patients with severely calcified annulus: feasibility, surgery and results. J Heart Valve Dis. 2002;11:153–9. [PubMed] [Google Scholar]
- 12.Ng CK, Punzengruber C, Pachinger O, Nesser J, Auer H, Franke H, et al. Valve repair in mitral regurgitation complicated by severe annulus calcification. Ann Thorac Surg. 2000;70:53–8. doi: 10.1016/s0003-4975(00)01347-3. doi:10.1016/S0003-4975(00)01347-3. [DOI] [PubMed] [Google Scholar]
- 13.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, et al. American Society of Echocardiography. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. doi:10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 14.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients with Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e1–142. doi: 10.1016/j.jacc.2008.05.007. doi:10.1016/j.jacc.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Castillo JG, Anyanwu AC, Fuster V, Adams DH. A near 100% repair rate for mitral valve prolapse is achievable in a reference center: implications for future guidelines. J Thorac Cardiovasc Surg. 2012;144:308–12. doi: 10.1016/j.jtcvs.2011.12.054. doi:10.1016/j.jtcvs.2011.12.054. [DOI] [PubMed] [Google Scholar]