Abstract
Clinical evidence and structural neuroimaging studies linked cerebellar deficits to cognitive-related symptoms in schizophrenia. Yet, in functional neuroimaging literature to date, the role of the cerebellum in schizophrenia was not explored in a systematic fashion. Here, we reviewed 234 functional magnetic resonance imaging studies indexed by PubMed and published in 1997–2010 that had at least one group of schizophrenia patients, used blood oxygenation level dependent contrast and the general linear model to assess neuronal activity. We quantified presence/absence of cerebellar findings and the frequency of hypo- and hyperactivations (ie, less or more activity in patients relative to healthy controls). We used peaks of activations reported in these studies to build a topographical representation of group differences on a cerebellar map. Cerebellar activity was reported in patients in 41.02% of the articles, with more than 80% of these dedicated to cognitive, emotional, and executive processes in schizophrenia. Almost two-thirds of group comparisons resulted in cerebellar hypoactivation, with a frequency that presented an inverted U shape across different age categories. The majority of the hypoactivation foci were located in the medial portion of the anterior lobe and the lateral hemispheres (lobules IV–V) of the cerebellum. Even though most experimental manipulations did not target explicitly the cerebellum’s functions in schizophrenia, the cerebellar findings are frequent and cerebellar hypoactivations predominant. Therefore, although the cerebellum seems to play an important functional role in schizophrenia, the lack of reporting and interpretation of these data may hamper the full understanding of the disorder.
Keywords: schizophrenia, cerebellum, fMRI
Introduction
Over the years, a plethora of studies has been dedicated to investigating the functional role of the cerebellum, even leading to the foundation, in 2002, of a journal dedicated exclusively to this brain structure (The Cerebellum). Traditionally associated with the motor system, specifically with maintaining the balance, motor timing, controlling fine voluntary movement and motor skill learning,1 the cerebellum is now seen to also be involved in higher level cognitive functions, such as attention,2 cognitive learning,3 and language.4 In addition to these studies that used various tests and tasks to measure cerebellar functions indirectly, recent functional magnetic resonance imaging (fMRI) studies in healthy subjects provided direct evidence of cerebellar activity in higher order cognitive functions, such as language.5 Moreover, cognition and emotional regulation disorders have been reported in patients with vascular or degenerative diseases of the cerebellum,6 suggesting a role for this structure in emotion and cognition. The involvement of the cerebellum in a wide range of cognitive, emotional, and motor functions reflects its ability to process information coming from several functionally heterogeneous cortical regions, such as the prefrontal, parietal, sensory, motor, and premotor cortex,7 through what is referred to as the cortico-cerebellar network. Considering that this functional and anatomical connectivity may be disrupted by several psychiatric disorders, such as abnormal mood regulation, psychosis, depression, and mania, the systematic investigation of association between cerebellar dysfunctions and a variety of psychiatric symptoms becomes warranted.6 In schizophrenia, in particular, the characterization of 2 classes of clinical symptoms, “neurological soft signs” (NSS) and “cognitive dysmetria” (CD), suggests an important place for cerebellum in this disorder.
The NSS describe a class of clinical symptoms reflecting minor neurological abnormalities in sensory and motor performance in the absence of any obvious localized pathological lesion underlying these signs.8 They are known to be especially predominant in untreated first-episode patients9–11 and a variety of symptoms included in NSS, such as those related to posture, various motor tasks,12 and sensory prediction,13 have been linked to a cerebellar dysfunction. Even more interestingly, schizophrenia patients with NSS of cerebellar origin have more negative symptoms, poorer premorbid social functioning, and a smaller total cerebellar volume than those without cerebellar NSS.10,14 Despite a still limited number of studies dealing explicitly with the association between NSS and cerebellum, these results suggest that cerebellar deficits may play a central role in the interplay between psychiatric and neurological symptomatology related to sensory-motor integration in schizophrenia.
The other class of clinical symptoms linked to impairments in cerebellar activity in schizophrenia is based on evidence indicating a strong relation between the functioning of the cerebellum and cognition in schizophrenia patients. Since Dr Nancy Andreasen’s proposal that a disruption in the cortico-cerebello-thalamo-cortical network could explain a variety of psychotic symptoms, CD has become well-known neurocognitive model of the disease,15 which is supported by many studies. For example, the cerebellum appears to play some role in global cognition in schizophrenia, as indicated by the presence of a correlation between the global IQ and larger cerebellar volume in healthy subjects and female patients and the lack of such relationship in male schizophrenia patients.16 More specifically, when patients’ attentional capacities were compared with those of healthy subjects, an increase in cerebellar activation was seen during visual oddball detection tasks,17 while a decrease in such activation was observed during auditory oddball detection tasks.18 The increase in activity during the visual task could be explained by a greater demand on a defective attentional network,19 whereas the decrease in activity during the auditory task might indicate that the cerebellum plays different roles depending on the sensory modality. There were also numerous studies suggesting cerebellum’s involvement in memory per se. Although the results on nondeclarative20 and procedural memory21 are inconclusive for the time being, other research teams have reported cerebellar hypoactivation in working memory tasks.22 Regarding episodic memory, increases in cerebellar activation during word encoding23 and recognition tasks have been reported both in patients24 and their relatives.25
Interestingly, a third class of symptoms, those related to emotion and language, seems to also be related to cerebellar dysfunctions in schizophrenia. For instance, when investigating the phenomenon of auditory hallucinations in schizophrenia patients using an inner speech control task and fMRI, a team of researchers found less hemodynamic activation in patients than in healthy subjects (hypoactivation) in a cortico-subcortical network that included the posterior cerebellar cortex, among other structures.26 A similar discovery of hypoactivation in the cerebellum was made in 2 fMRI studies of formal thought disorder (the symptoms of which include delirium and impoverished, incoherent language).27,28 As well, during emotion recognition tasks, activations related to the task and hypoactivation have been reported in different studies involving schizophrenia patients with blunted affect.15,29 It is important to note that despite finding changes of activity in the cerebellum, the interpretation of results reported in these articles put the cerebellum in a secondary place, as articles’ authors were mainly interested in other brain areas. Nevertheless, all of these results suggest that impairments in cerebellar activity may be associated with language-related deficits and processing of emotionally laden information in schizophrenia.
To summarize, theories of schizophrenia that allude or include the cerebellar functions suggested a poor cerebellar function associated with the disease. However, the neuroimaging evidence was ambiguous with many studies finding hypoactivation in patients relative to controls, but with some experiments providing evidence of the opposite pattern. To date, it is difficult to gage the direction of cerebellar dysfunctionality. It is therefore peculiar that, in spite of all these arguments for the cerebellum’s role in the symptomatology of schizophrenia, there are only a small number of functional neuroimaging studies in this clinical population, which explicitly seek out to investigate it. As we mentioned before, in most fMRI articles in schizophrenia research, the main focus was on other brain regions (ie, parahippocampal gyrus, parts of prefrontal cortex, etc.) and cerebellar findings, when reported, were often “buried” in tables or supplementary material. In consequence, the cerebellum’s involvement in the disease’s symptoms is possibly underrated and underreported. In the current quantitative review, we set out to assess the incidence of cerebellar findings in functional neuroimaging studies in schizophrenia research, to evaluate the nature of the cerebellar activation in patients relative to healthy controls (hypo- vs hyperactivation), and, finally, to develop an anatomical map of the cerebellar activations reported therein. To this end, our work is complementary to previous reviews dealing with the role of cerebellum in schizophrenia19,30 for several reasons: first, we reviewed all fMRI studies with schizophrenia patients in the chosen time period, regardless of whether the authors targeted explicitly the cerebellar functions or not; second, we did not confine our search to a particular model of the functional role of cerebellum in schizophrenia, instead we considered data from all types of tasks; and third, we opted to increase the homogeneity of the data collected, by focusing only on functional data (as opposed to structural neuroanatomical data) obtained using the same neuroimaging technique and analyzed using the same statistical model.
Methods
In order to homogenize the studies from methodological and analysis viewpoints, we sought to select only the articles using fMRI as their brain scanning method, blood oxygen level dependent (BOLD) signal as their dependent measure, and the general linear model (GLM) as their main statistical analysis technique. We performed successive bibliographic searches in the abstracts of articles indexed in PubMed between 1997 and August 2010. At first, the keywords “schizophrenia” AND “imaging” were used to create an initial set of 4623 articles. A second pass using the keywords “functional” OR “fMRI” limited the set to 1785 articles. An additional reduction was achieved based on a thorough analysis of all abstracts in this database by excluding theoretical and review articles, studies using other neuroimaging techniques, and articles not written in English. The remaining 472 articles were then divided equally among the coauthors, who extracted the relevant information and further eliminated studies that either (1) did not report the details of the imaging acquisition, (2) did not use the BOLD signal as measure of neuronal activity, (3) did not employ the GLM to extract the activation foci, or (4) excluded the cerebellum from the data acquisition (eg, by possibly using masks or not scanning the whole brain) or explicitly from data analysis. Studies with no schizophrenia patients or with fewer than 6 subjects per group were also excluded. In the end, 234 articles were retained for the analyses presented below.
For each of these articles, we searched the main text, figures, tables, and supplementary materials for reports of cerebellar activation specific to schizophrenia patients, either as a general indicator of cerebellum’s activity, defined by a peak of activation that was statistically significant in a functional contrast (eg, condition A > baseline, condition A > condition B, etc.), or by a group comparison (eg, patients > healthy or viceversa). Further, the tasks used in each study were classified into 6 categories based on the skills targeted in each selected article (perceptual, motor, cognitive, emotional, executive, and linguistic), according to the explicit designation of the authors of the original paper. We used these categories both to evaluate the incidence of cerebellar findings as well as to assess group differences (patients relative to healthy controls) specific to each task type. Chi-square statistical tests were employed to compare the frequency of cerebellar findings across different tasks categories.
Next, cerebellar activation emerging from the group comparisons (between patients and healthy subjects) were subdivided into hyperactivations—whenever the schizophrenia patients showed greater cerebellar activity for a given task than the control subjects and hypoactivations—in the opposite case. Then, the proportions of hypo- and hyperactivation loci were calculated for each task type and each age group in schizophrenia patients across all studies (determined according to the quartile distribution of the variable “age,” in order to retain an equal number of studies in each age group).
Finally, the coordinates of cerebellar activation revealed by group comparisons were used to identify and display the anatomical distributions of hypo- and hyperactivation loci as a function of task type. The identification was done using the “MRI atlas of the human cerebellum,”31 and the display of the loci of hypo- and hyperactivation was accomplished by placing different markers on a flattened representation of the cerebellum comprising of an “unrolled” superior view and placing the vermis on a 2-dimensional plane. In this view, the middle-upper part of map corresponds to the dorsal portion and the extreme upper and the lower parts would correspond to the ventral portion of the cerebellum. The map represents the cerebellum as if one unwraps it starting from the point where the vermis connects to the pons, near the fourth ventricle.
Whenever we compared proportions or frequencies (eg, the difference in the frequency of cerebellar activation between 2 types of tasks), we employed chi-square statistical tests; whenever we compared means (eg, the difference in Positive and Negative Syndrome Scale (PANSS) symptom scores between the studies reporting cerebellum and those which did not), we used independent sample t tests.
Results
The 234 fMRI studies retained for analysis covered the period from 1997 until August 2010, with 76.4% of them published in the last 5 years. It was difficult to establish a clear clinical profile of schizophrenia patients in these studies due to the lack of reporting any information pertaining to the clinical symptoms in 62 articles (26.49% of all articles) and the uneven use of various clinical tests for the remaining articles. Specifically, scores on the Brief Psychiatric Rating Scale were reported in 24 articles (10.26%), Scales for the Assessment of Positive/Negative Symptoms in 31 (13.25%) articles, and PANSS in 117 (50.00%) articles. However, considering the assessment of only the positive and negative symptoms using PANSS, the average values obtained in all studies for these subscales were 14.59 ± 5.30 (mean ± SD) for PANSS positive symptoms score and 16.59 ± 5.54 for PANSS negative symptoms score. There were no significant differences when comparing the PANSS symptom scores for the articles reporting the cerebellar findings with those that did not report them (t 115 = 0.72, P = .47 for PANSS positive; t 115 = 0.99, P = .32 for PANSS negative). In 26 articles, it was reported that the patients were investigated soon after having had the first episode, and in 179—that patients were chronic, with the remaining 29 articles not reporting this information. In more than 80% of the cases, patients were under multiple antipsychotic medications at the time of the investigation. Across all studies, patients’ average age was 32.89 ± 6.62, ranging from 17 to 59 years of age.
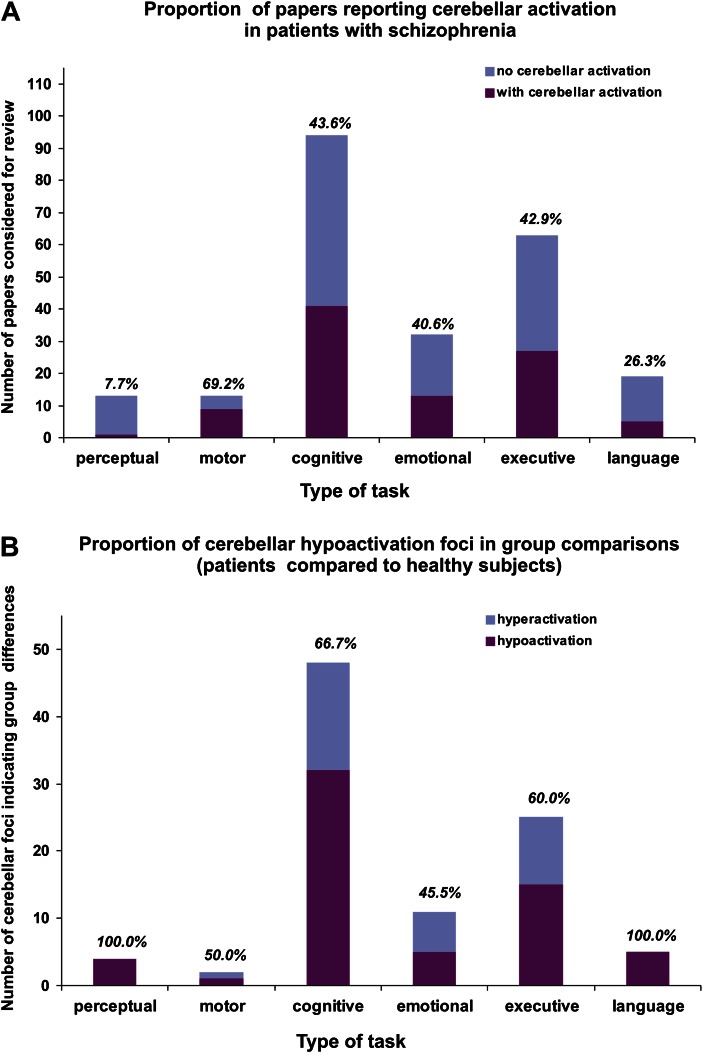
Among all 234 articles, 96 reported at least one activation foci in the cerebellum in patients with schizophrenia, in a functional or group contrast, for an overall incidence of 41.02%. Figure 1A shows the total number of articles for each type of task, as well as the percentage of those reporting cerebellar activation for each task category. Overall, regardless of reporting or not cerebellar findings, 5.5% of the articles used perceptual tasks, 6% motor tasks, 40% cognitive tasks, 13.6% emotional tasks, 26.8% executive tasks, and 8.1% linguistic tasks. We found that the percent of studies that reported cerebellar activation varied by task type. Close to 70% of the articles using motor tasks reported cerebellar activity, while such activation is reported in only 7.7% and 26.3% of those using perceptual and linguistic tasks, respectively. The studies that explored the emotional, cognitive, and executive domains (which constitute more than 80% of the total number of articles) showed a rate of cerebellar activation identical to the overall rate (40.6%, 43.6%, and 42.9%, respectively).
Fig. 1.
(A) The number of articles considered for review that fall in each task category (count) and the incidence of cerebellar findings (percentage) for each type of task. (B) The number of cerebellar activation loci indicating significant differences between the schizophrenia patients and the healthy controls. The percentages represent the proportion of activation loci corresponding to hypoactivation (eg, less activation for patients than for controls).
Considering the traditional role of the cerebellum in motor function, it is not surprising to observe a greater rate of cerebellar activity for motor tasks and a lesser rate for perceptual tasks. Nevertheless, bearing in mind earlier studies that emphasized the role of cerebellum in language,4 our results appear somewhat surprising. Indeed, we observed a lower rate of cerebellar activation in linguistic tasks than in cognitive, emotional, or executive tasks. Chi-square tests showed that the frequency of activation in the cerebellum is significantly greater for cognitive (χ2 = 13.10; df = 1, P < .01) and executive (χ2 = 7.99; df = 1, P < .01) tasks than for linguistic tasks.
Moreover, when all the contrasts yielding activation foci in the cerebellum in the schizophrenia patients were taken into account (n = 211) (ie, a study may report more than one activation foci in cerebellum indicating differences between conditions or groups), 48% of them were aimed to test group differences between subjects with schizophrenia and healthy subjects (with the remaining contrasts tested differences between experimental conditions). Here, 65.3% of these group contrasts showed hypoactivation (patients < healthy) and 34.7% hyperactivation (patients > healthy) in cerebellum, which constitutes a significant bias toward hypoactivation, as revealed by a chi-square test (χ2 = 8.85; df = 1, P < .01). Figure 1B illustrates the distribution of group differences across task types as well as the proportion of hypoactivation for each one. Despite the small number of contrasts reported for perceptual and linguistic tasks, 100% of them show hypoactivation. For the remaining categories, the chi-square tests showed a proportion of hypoactivation significantly greater than 50% in the cognitive category alone (χ2 = 5.33; df = 1, P < .05), whereas the other categories demonstrated distributions that did not differ significantly from the cutoff of 50%. In online supplementary table S1, we present the list of all articles which presented cerebellar findings, outlining those which reported group differences (patients vs. healthy controls) and those which reported only differences between experimental conditions in schizophrenia patients.
Given the small number of articles which included first-episode patients as their target population (26 articles out of 234) and the even smaller number reporting any cerebellar activation in these patients (9 articles), it was hard to properly compare their findings, using statistical tests, with those coming from the articles dealing with chronic patients. However, regarding the first-episode patients, we found that only 1 article of the 9 reported hyperactivation, with the remaining reporting hypo-activation (88.8%), thus suggesting that at the onset of the disease, the cerebellar hypoactivation may be more frequent.
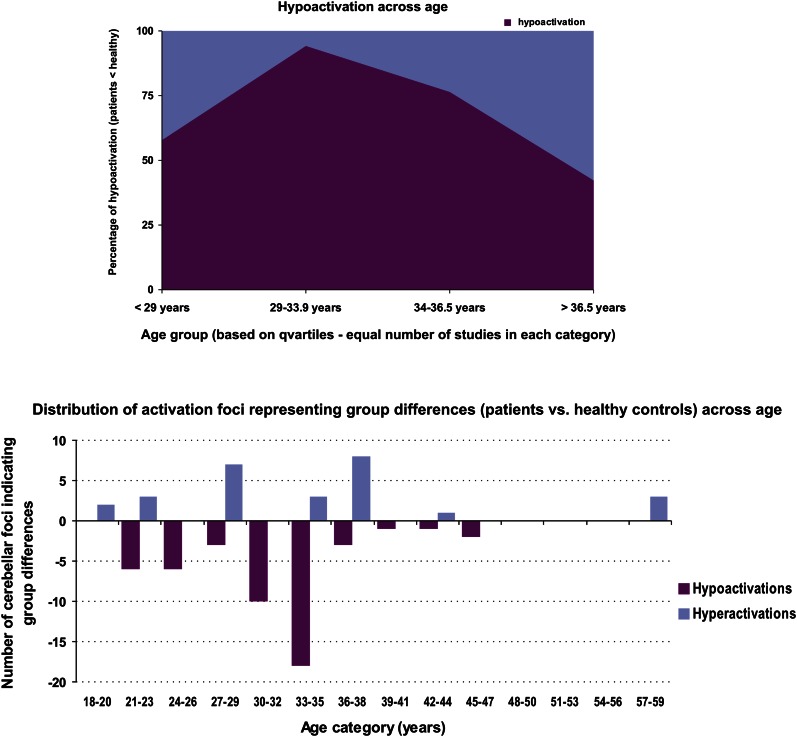
Figure 2 illustrates the distribution of proportion of hypoactivation as a function of patients’ age category. The chi-square test showed a significant predominance of hypoactivation (ie, significantly greater than 50%) for the 2 middle age groups (χ2 = 13.23; df = 1, P < .01 for the group aged 29–33.9 y old and χ2 = 4.76; df = 1, P < .05 for the group aged 34–36.5 y old). In addition, chi-square tests indicated that the proportion of hypoactivation in the second age group was significantly higher than that of the groups at the 2 ends of the scale (<29 and >36.5 y), whereas the proportion of hypoactivation in the third group was greater than that in the oldest group (>36.5 y) (P < .01 for all comparisons). We have to note here that repeating the same analysis, but only for articles, which used executive and cognitive tasks, we obtained the same reversed U shape for the proportion of hypoactivation and the same statistical results.
Fig. 2.
Upper panel: the percentage of hypoactivation across age categories in studies reporting differences in cerebellar activation (patients < healthy controls). The age categories were based on the quartile distribution of the studies, such as to have an equal number of studies in each category. Lower panel: the number of hypo- and hyperactivation loci across age (in slots of 3 y) found in cerebellum whenever schizophrenia patients were compared with their healthy counterparts.
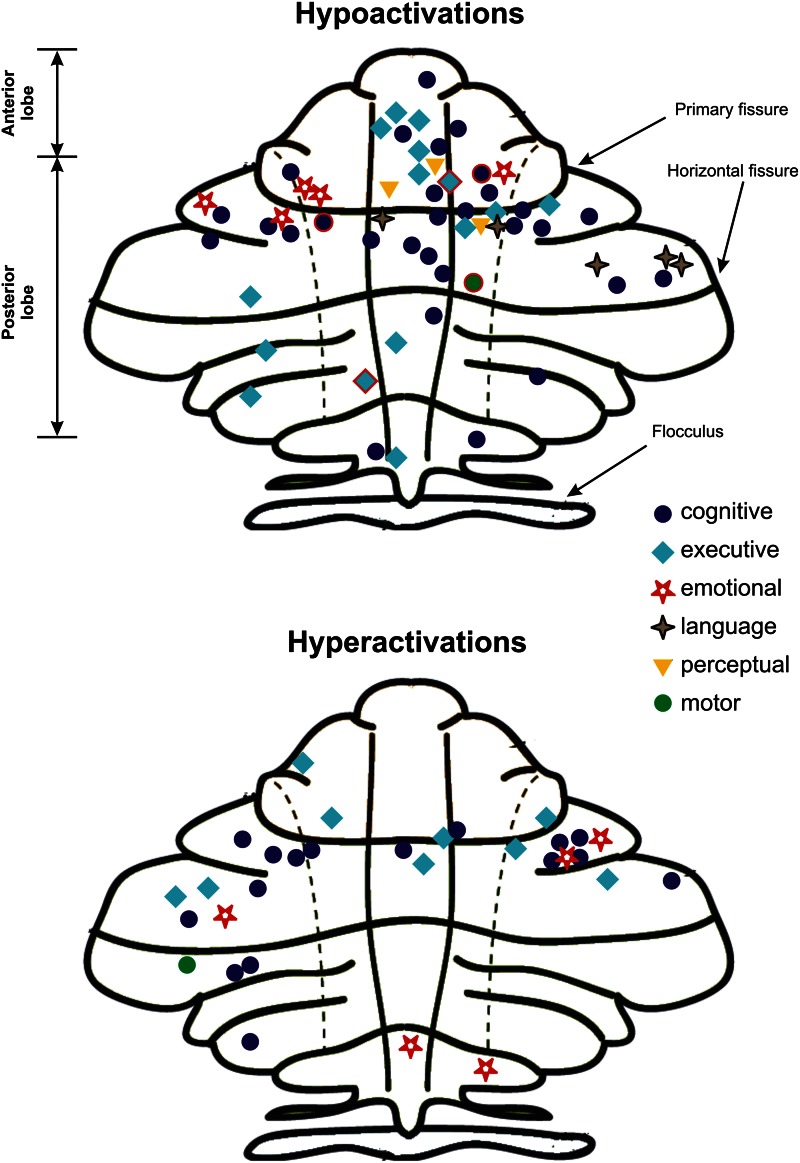
Figure 3 shows the anatomical distribution of hypo- and hyperactivations for the 6 task types. The hypoactivations appeared to predominate in the medial portion of the anterior lobe and in lobules IV and V bilaterally. With the exception of 4 contrasts, the rest of the hyperactivations were observed in the cerebellar cortex and not in the cerebellar nuclei. While the hypoactivation foci in executive tasks were mainly located medially, in the paleocerebellum, where sensory input from all modalities is integrated, the hypoactivation foci in the cognitive tasks were located not only medially but also laterally in the neocerebellum, which receives inputs exclusively from the cerebral cortex.
Fig. 3.
The spatial localization of foci of activation for contrasts reporting hypoactivations (top) or hyperactivations (bottom) in cerebellum, as a function of task type. The symbols surrounded by a red contour (only 4 of them) are activations that were localized in cerebellar nuclei; all the others were found in the cerebellar cortex.
Discussion
The results of the current study highlighted a relative predominance of cerebellar activity in studies using motor tasks in schizophrenia in comparison to those using other types of tasks, in accordance with the motor role that has classically been attributed to the cerebellum. However, one has to take into account that, overall, there were far fewer studies employing pure motor paradigm. On the other hand, activation of the cerebellum was less frequently reported in studies that used linguistic tasks than in those that used cognitive, emotional, or executive tasks. This result runs counter to the current trend, supported by studies of both normal and pathological functioning, that suggests a “linguistic” role for the cerebellum.4 That being said, the exact contribution of the cerebellum in this field has not yet been clearly determined, as certain authors point out,4 and the number of articles exploring the specific role of the cerebellum in schizophrenia patients’ language disorders is still quite low for review, see ref.5
Close to half of the total number of cerebellar activations was aimed at testing group differences and they mainly indicated hypoactivation of the cerebellum in subjects with schizophrenia relative to their healthy counterparts. Moreover, we should point out that all of the group differences revealed in linguistic and perceptual experimental paradigm resulted in hypoactivation, despite the small number of articles using such tasks. This is consistent with studies that reported a decrease in cerebellar activity in schizophrenia patients compared with healthy subjects during the performance of a visual object processing task32 or an audiovisual stimulus perception task.33 Given that language is based on processing and integration of audiovisual information, a disturbance in this area could affect language function; therefore, what seems to be the cerebellar implication in language could be, in fact, a reflection of its role in the integration of audiovisual information.
Cerebellar hypoactivation was most marked in cognitive tasks (present in more than 60% of group contrasts); it is important to also note that these tasks represented the ones most frequently studied (more than 40% of the total articles selected). These results again confirm the role of the cerebellum in the pathophysiology of the cognitive disorders present in schizophrenia, as Bleuer34 originally proposed in 1924 and as modern neuroimaging techniques have recently corroborated. Moreover, they lead us to reconsider the “true” role of the cerebellum, which has traditionally been considered to be a “motor” structure. In fact, we now know that cerebellum is a very complex structure, with various parts playing different roles, hence, it is likely that it plays a multifaceted role in schizophrenia, as well. Given that some of the foci of cerebellar hypoactivation were observed laterally, where input from cerebral cortex is integrated, it remains to be determined whether cerebellar hypoactivation in schizophrenia is merely a consequence of the hypoactivation of other brain regions (ie, it appears after that in the evolution of the disease), such as the frontal cortex in particular or whether it is observed simultaneously with it. Clearly, we cannot answer this question on the basis of our analyses, and both hypotheses are potentially valid. We know, for example, that NSS, including mild sensory and motor deficits, may be present in schizophrenia patients who present with a first-psychotic episode and that they correlate with reduced density of gray and white matter in the frontal regions and the cerebellum.35 This suggests that a general deterioration affects the brain globally and simultaneously in schizophrenia. On the other hand, studies dating from the 1970s suggest that the resting anterior-to-posterior blood flow gradient, which normally results from a greater flow in frontal than in posterior regions, declines and may even be reversed in schizophrenia patients as compared with healthy subjects.36 This hypofrontality has been reported in several studies that used tasks activating the frontal lobe in schizophrenia patients (eg, attention, working memory, cognitive, and executive control, etc.).37 Yet, regardless of whether the cerebellar hypoactivation observed in our study occurs simultaneously with or subsequently to the hypofrontality, it is nevertheless associated with disrupted cognitive functions in schizophrenia.
Probably, the most interesting result of this study concerns the changes in the frequency of cerebellar hypoactivation with age. The 2 middle age groups (29–36 y old) appear to show the greatest hypoactivation of the cerebellum in relation to healthy subjects. These results suggest that hypoactivation progresses over time, taking the shape of an inverted U. This observation could support the hypothesis that cerebellar hypoactivation occurs subsequently to hypofrontality, and that the dysfunction progresses with time from the anterior brain regions to the more posterior regions, reaching the cerebellum later in the course of the acute phase of the disease. The reduced hypoactivation in the oldest age group could be due to a potential age-related dysfunction of the cerebellum in healthy subjects.38 We are aware that this type of analysis regarding the effect of patients’ age would need to take into consideration the confounding effects of the age of disease onset, its duration, as well as the temporal length of antipsychotic medication. Unfortunately, we found no consistency in reporting this additional information in the studies that we reviewed, as many failed to mention the actual duration of the disease. Nevertheless, our results still appear to be consistent with earlier research on NSS, which attribute to the cerebellum a predominant role in the neurophysiological bases of these signs and show that they increase during the acute phase of the disease, followed by a remission when patients are stabilized with medication.9 That being said, no matter what the explanation of cerebellar hypoactivation over the lifespan of schizophrenia patients may turn out to be, our results suggest not only that the change over time takes the shape of an inverted U but also that it may be primarily related to changes in cognitive functioning.
Our analysis is the first to reveal the functional map of hypo- and hyperactivation of the cerebellum in schizophrenia on the basis of fMRI studies. It is interesting to note not only the predominance of hypoactivation but also the fact that it is essentially located in the cerebellar hemispheres, the anterior lobe, and in lobules IV and V bilaterally. This indicates that the neocerebellum (ie, the most recent part that developed across species, philogenetically) is the part most affected in schizophrenia, particularly in cognitive and executive tasks. This finding is not surprising, given that, in nonhuman primates, the neocerebellum receives connections from various associative areas of the cerebral cortex, some of which appear to be primarily involved in cognitive tasks.7 Moreover, there is evidence that, like the prefrontal cortex, the cerebellum matures quite late in the course of child development, and, in fact, the development of the 2 structures seems to be interrelated.39 In addition, recent findings demonstrate cerebellar pathology as early as the first episodes in schizophrenia.40
Dr Nancy Andreasen and her colleagues15 have stated that the fundamental deficit in schizophrenia could be conceptualized as a sort of CD characterized by impairments in coordinating the perception, encoding, retrieval, and prioritarization of experience and information. CD is, in fact, a neurological metaphor. Dysmetria denotes voluntary movement dysfunctions often encountered in cerebellar syndromes. The term refers first and foremost to spatial coordination defects that consist essentially of an exaggeration in the amplitude of a movement, which exceeds its goal but maintains its direction. The interesting thing about the neurological metaphor employed by Andreasen and colleagues15 is that these voluntary movement dysfunctions are found in a major neurological disorder called ataxia. Ataxia, too, has been used as a cerebellar metaphor to describe a psychiatric semiology of schizophrenia; Stransky, a contemporary of Kraeplin, described schizophrenia (precox dementia) as an example of loss of psychic cohesion, which he called “intrapsychic ataxia,” meaning a dissociation in the functional connection between the noopsyche (cognition) and thymopsyche.41 Our analysis confirms that the role of cerebellum is not any more a neurological metaphor (ie, CD) but an actual perturbation of cognitive functions in schizophrenia.
In conclusion, the results of our analyses suggest that the cerebellum plays an important functional role in schizophrenia, especially in the cognitive and executive domains. We believe that cerebellar hypoactivation, defined similarly as the hypofrontality, is a reality that needs to be taken into account to a greater extent in the development of drug therapy in schizophrenia, especially that involving antipsychotics. For instance, dopamine (D2) receptor occupancy in cerebellum is often taken as a reference measure in single-photon emission computed tomography pharmacological research using iodobenzamide as radioactive tracer in order to assess the efficiency of a given molecule. However, this approach may need to be reconsidered given that cerebellum itself may be affected by the disease. We thereby emphasize the importance of systematically including and especially reporting the findings about this structure in future fMRI studies of schizophrenia, which would help in building a model of this disease that will include the cerebellar dysfunctions, with important consequence on therapy.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Postdoctoral research grant from the Ministère du Développement Économique, de l'Innovation et de l'Exportation (MDEIE) du Québec (to O.L.). MD-M.Sc. training award, Fonds de la recherche en santé du Québec (FRSQ) (to S.P.). Doctoral training award, Fonds de la recherche en santé du Québec (FRSQ) (to K.D.). Postdoctoral research grant from the Canadian Institutes of Health Research (CIHR) (D.L.). Eli Lilly Research Chair of schizophrenia of University of Montreal (to E.S.).
Supplementary Material
Acknowledgments
All authors state that they do not have conflict of interests.
References
- 1.Paulin MG. The role of the cerebellum in motor control and perception. Brain Behav Evol. 1993;41:39–50. doi: 10.1159/000113822. [DOI] [PubMed] [Google Scholar]
- 2.Courchesne E, Allen G. Prediction and preparation, fundamental functions of the cerebellum. Learn Mem. 1997;4:1–35. doi: 10.1101/lm.4.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Ravizza SM, McCormick CA, Schlerf JE, Justus T, Ivry RB, Fiez JA. Cerebellar damage produces selective deficits in verbal working memory. Brain. 2006;129(pt 2):306–320. doi: 10.1093/brain/awh685. [DOI] [PubMed] [Google Scholar]
- 4.De Smet HJ, Baillieux H, De Deyn PP, Marien P, Paquier P. The cerebellum and language: the story so far. Folia Phoniatr Logop. 2007;59:165–170. doi: 10.1159/000102927. [DOI] [PubMed] [Google Scholar]
- 5.Walter N, Joanette Y. The unnoticed contributions of the cerebellum to language. Folia Phoniatr Logop. 2007;59:171–176. doi: 10.1159/000102928. [DOI] [PubMed] [Google Scholar]
- 6.Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- 7.Brodal P, Bjaalie JG. Salient anatomic features of the cortico-ponto-cerebellar pathway. Prog Brain Res. 1997;114:227–249. doi: 10.1016/s0079-6123(08)63367-1. [DOI] [PubMed] [Google Scholar]
- 8.Heinrichs DW, Buchanan RW. Significance and meaning of neurological signs in schizophrenia. Am J Psychiatry. 1988;145:11–18. doi: 10.1176/ajp.145.1.11. [DOI] [PubMed] [Google Scholar]
- 9.Bachmann S, Bottmer C, Schroder J. Neurological soft signs in first-episode schizophrenia: a follow-up study. Am J Psychiatry. 2005;162:2337–2343. doi: 10.1176/appi.ajp.162.12.2337. [DOI] [PubMed] [Google Scholar]
- 10.Bottmer C, Bachmann S, Pantel J, et al. Reduced cerebellar volume and neurological soft signs in first-episode schizophrenia. Psychiatry Res. 2005;140:239–250. doi: 10.1016/j.pscychresns.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Thomann PA, Roebel M, Dos Santos V, Bachmann S, Essig M, Schroder J. Cerebellar substructures and neurological soft signs in first-episode schizophrenia. Psychiatry Res. 2009;173:83–87. doi: 10.1016/j.pscychresns.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald PB, Brown TL, Daskalakis ZJ, Kulkarni J. A transcranial magnetic stimulation study of inhibitory deficits in the motor cortex in patients with schizophrenia. Psychiatry Res. 2002;114:11–22. doi: 10.1016/s0925-4927(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 13.Shergill SS, Samson G, Bays PM, Frith CD, Wolpert DM. Evidence for sensory prediction deficits in schizophrenia. Am J Psychiatry. 2005;162:2384–2386. doi: 10.1176/appi.ajp.162.12.2384. [DOI] [PubMed] [Google Scholar]
- 14.Ho BC, Mola C, Andreasen NC. Cerebellar dysfunction in neuroleptic naive schizophrenia patients: clinical, cognitive, and neuroanatomic correlates of cerebellar neurologic signs. Biol Psychiatry. 2004;55:1146–1153. doi: 10.1016/j.biopsych.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 15.Andreasen NC, Paradiso S, O'Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 16.Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr Res. 2004;70:117–145. doi: 10.1016/j.schres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Eyler LT, Olsen RK, Jeste DV, Brown GG. Abnormal brain response of chronic schizophrenia patients despite normal performance during a visual vigilance task. Psychiatry Res. 2004;130:245–257. doi: 10.1016/j.pscychresns.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Kiehl KA, Stevens MC, Celone K, Kurtz M, Krystal JH. Abnormal hemodynamics in schizophrenia during an auditory oddball task. Biol Psychiatry. 2005;57:1029–1040. doi: 10.1016/j.biopsych.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picard H, Amado I, Mouchet-Mages S, Olie JP, Krebs MO. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr Bull. 2008;34:155–172. doi: 10.1093/schbul/sbm049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofer E, Doby D, Anderer P, Dantendorfer K. Impaired conditional discrimination learning in schizophrenia. Schizophr Res. 2001;51:127–136. doi: 10.1016/s0920-9964(00)00118-3. [DOI] [PubMed] [Google Scholar]
- 21.Bigelow NO, Turner BM, Andreasen NC, Paulsen JS, O'Leary DS, Ho BC. Prism adaptation in schizophrenia. Brain Cogn. 2006;61:235–242. doi: 10.1016/j.bandc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Mendrek A, Kiehl KA, Smith AM, Irwin D, Forster BB, Liddle PF. Dysfunction of a distributed neural circuitry in schizophrenia patients during a working-memory performance. Psychol Med. 2005;35:187–196. doi: 10.1017/s0033291704003228. [DOI] [PubMed] [Google Scholar]
- 23.Ragland JD, Gur RC, Valdez JN, et al. Levels-of-processing effect on frontotemporal function in schizophrenia during word encoding and recognition. Am J Psychiatry. 2005;162:1840–1848. doi: 10.1176/appi.ajp.162.10.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crespo-Facorro B, Wiser AK, Andreasen NC, et al. Neural basis of novel and well-learned recognition memory in schizophrenia: a positron emission tomography study. Hum Brain Mapp. 2001;12:219–231. doi: 10.1002/1097-0193(200104)12:4<219::AID-HBM1017>3.0.CO;2-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whyte MC, Whalley HC, Simonotto E, et al. Event-related fMRI of word classification and successful word recognition in subjects at genetically enhanced risk of schizophrenia. Psychol Med. 2006;36:1427–1439. doi: 10.1017/S0033291706008178. [DOI] [PubMed] [Google Scholar]
- 26.Shergill SS, Brammer MJ, Fukuda R, Williams SC, Murray RM, McGuire PK. Engagement of brain areas implicated in processing inner speech in people with auditory hallucinations. Br J Psychiatry. 2003;182:525–531. doi: 10.1192/bjp.182.6.525. [DOI] [PubMed] [Google Scholar]
- 27.Kircher TT, Liddle PF, Brammer MJ, Williams SC, Murray RM, McGuire PK. Neural correlates of formal thought disorder in schizophrenia: preliminary findings from a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:769–774. doi: 10.1001/archpsyc.58.8.769. [DOI] [PubMed] [Google Scholar]
- 28.Kircher TT, Bulimore ET, Brammer MJ, et al. Differential activation of temporal cortex during sentence completion in schizophrenic patients with and without formal thought disorder. Schizophr Res. 2001;50:27– 40. doi: 10.1016/s0920-9964(00)00042-6. [DOI] [PubMed] [Google Scholar]
- 29.Stip E, Fahim C, Liddle P, et al. Neural correlates of sad feelings in schizophrenia with and without blunted affect. Can J Psychiatry. 2005;50:909–917. doi: 10.1177/070674370505001405. [DOI] [PubMed] [Google Scholar]
- 30.Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmahmann J, Doyon J, Toga A, Petrides M, Evans A. MRI Atlas of Human Cerebellum. London, UK: Academic Press; 2000. [Google Scholar]
- 32.Sehatpour P, Dias EC, Butler PD, et al. Impaired visual object processing across an occipital-frontal-hippocampal brain network in schizophrenia: an integrated neuroimaging study. Arch Gen Psychiatry. 2010;67:772–782. doi: 10.1001/archgenpsychiatry.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surguladze SA, Calvert GA, Brammer MJ, et al. Audio-visual speech perception in schizophrenia: an fMRI study. Psychiatry Res. 2001;106:1–14. doi: 10.1016/s0925-4927(00)00081-0. [DOI] [PubMed] [Google Scholar]
- 34.Bleuer E. Textbook of Psychiatry (Published Originally in 1911) New York, NY: Maximilian Co.; 1924. [Google Scholar]
- 35.Thomann PA, Wustenberg T, Santos VD, Bachmann S, Essig M, Schroder J. Neurological soft signs and brain morphology in first-episode schizophrenia. Psychol Med. 2009;39:371–379. doi: 10.1017/S0033291708003656. [DOI] [PubMed] [Google Scholar]
- 36.Ingvar DH, Franzen G. Distribution of cerebral activity in chronic schizophrenia. Lancet. 1974;2:1484 –1486. doi: 10.1016/s0140-6736(74)90221-9. [DOI] [PubMed] [Google Scholar]
- 37.Ragland JD, Yoon J, Minzenberg MJ, Carter CS. Neuroimaging of cognitive disability in schizophrenia: search for a pathophysiological mechanism. Int Rev Psychiatry. 2007;19:417–427. doi: 10.1080/09540260701486365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reuter-Lorenz P. New visions of the aging mind and brain. Trends Cogn Sci. 2002;6:394. doi: 10.1016/s1364-6613(02)01957-5. [DOI] [PubMed] [Google Scholar]
- 39.Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 2000;71:44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- 40.Rasser PE, Schall U, Peck G, et al. Cerebellar grey matter deficits in first-episode schizophrenia mapped using cortical pattern matching. Neuroimage. 2010;53:1175–1180. doi: 10.1016/j.neuroimage.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 41.Stip E. From intrapsychic ataxia to cognitive dysmetria: from Stransky to Andreasen. Can J Psychiatry. 1997;42:777. doi: 10.1177/070674379704200716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.