Abstract
Background: The psychosis phenotype appears to exist in the population as a continuum, but it is not clear if subclinical psychotic symptoms and psychotic disorders share the same neurobiology. We investigated whether the dopaminergic dysfunction seen in psychotic disorders is also present in healthy, well-functioning people with hallucinations.
Methods: We compared dopamine synthesis capacity (using 6-[18F]fluoro-L-DOPA [[18F]-DOPA] positron emission tomography imaging) in 16 healthy individuals with frequent persistent auditory verbal hallucinations (hallucinating group) with that in 16 matched controls. Results: There was no significant difference in dopamine synthesis capacity in the striatum, or its functional subdivisions, between groups and no relationship between subclinical psychotic symptom severity or schizotypal traits and dopamine synthesis capacity in the hallucinating group. Conclusions: Altered dopamine synthesis capacity is unlikely to underlie subclinical hallucinations, suggesting that although there may be a phenomenological psychosis continuum, there are distinctions at the neurobiological level.
Keywords: hallucination, schizophrenia, aetiology, neurochemistry, subclinical, PET, symptom, dopamine, psychosis continuum
Introduction
The psychosis phenotype is present in the population as a continuum from relatively common transient psychotic experiences at one end, through subclinical symptoms to relatively rare clinical psychotic disorders at the other end (see review and meta-analysis1). Within this putative continuum, there is a group of people who are not functionally impaired, yet report persistent auditory verbal hallucinations.2,3 The hallucinations they report are of psychotic intensity and are quite similar at the phenomenological level to those in patients with schizophrenia and related psychotic disorders.4 However, subjects with these symptoms do not meet diagnostic criteria for a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) psychotic disorder because the hallucinations are not associated with distress and subjects do not show functional impairment.3
It is thought that dopaminergic dysfunction underlies the development of psychosis.5,6 Supporting this, 15 molecular imaging studies suggest increased dopamine release and elevated dopamine synthesis capacity in patients with schizophrenia, including those in their first psychotic episode (see Abi-Dargham,7 Howes et al,8 and Meisenzahl et al9 for reviews). While these abnormalities have not been found in studies of patients with nonpsychotic mania, depression and anxiety disorders (see Howes et al5 for review), increased dopamine synthesis capacity has also been reported in people with psychosis due to temporal lobe epilepsy.10 This suggests that elevated dopamine synthesis capacity may underlie the expression of psychosis more generally rather than being specific for schizophrenia. If this is the case, then one would expect elevated dopamine synthesis capacity to be present in individuals with persistent subclinical psychotic symptoms.
Kapur and colleagues have proposed an influential model that links dopaminergic dysfunction to the phenomenology of psychosis.11,12 They hypothesized that dopaminergic dysfunction leads to the aberrant assignment of salience to objects and associations. This leads to the attribution of salience to irrelevant stimuli. In an attempt to make sense of these confusing experiences, the individual makes unwarranted connections between the overvalued irrelevant stimuli, which results in the formation of a delusion. Psychosis, however, consists of several symptom domains: delusions, hallucinations, catatonia, and formal thought disorder. While the aberrant salience model provides a good explanation of how dopaminergic dysfunction could lead to delusions, it is less clear how it could explain the occurrence of hallucinations. The vast majority of patients with psychosis present with delusions and hallucinations (and some degree of formal thought disorder).13 Since very few schizophrenia patients experience hallucinations in the complete absence of other psychotic symptoms,13 the specific association between dopaminergic dysfunction and hallucinations has never been assessed. One strategy to investigate the specific relation between hallucinations and dopamine synthesis is the study of well individuals who experience this symptom in relative isolation.
If healthy subjects with hallucinations show a similar increase in dopamine synthesis capacity to psychotic patients, then hallucinations are likely to be related to, and possibly the result of, dopaminergic dysfunction. On the other hand, if dopamine synthesis capacity in the nonclinical hallucinating subjects is similar to that in the controls, then it may be concluded that hallucinations in isolation are not associated with altered dopamine synthesis capacity. We sought to test the hypothesis that increased dopamine synthesis capacity is associated with hallucinations in a sample of healthy subject reporting persistent auditory verbal hallucinations.
Methods
The study was approved by the local research ethics committees in London and Utrecht. Following complete description of the study, all subjects gave written informed consent to participate.
Subjects
Exclusion criteria for all subjects were (1) presence of any significant current physical disorder or treatment, (2) history of any disorder or treatment linked to psychiatric symptoms (such as systemic steroid treatment, endocrine disorders, antimalarial treatment), (3) contraindications to positron emission tomography (PET) scanning (pregnancy/breast-feeding/participation in other PET studies), and (4) history of current or past neurological disorder or head injury resulting in loss of consciousness. All subjects provided urine samples to screen for drugs of abuse and, in women, for pregnancy. Subjects were excluded if either test was positive prior to the scan. Smoking data were collected for both groups using a semi-structured interview (see Howes et al14).
Hallucinating Group
Subjects with auditory verbal hallucinations (hallucinating group) were recruited with the help of a website (“Explore your mind”—http://www.verkenuwgeest.nl). On this Web site, potential subjects were asked to complete a self-report questionnaire based on the Launay and Slade Hallucinations Scale designed to quantify the tendency to hallucinate in healthy individuals. People with high scores were invited to further assessment, including an interview administered by a trained psychiatrist to determine whether they met criteria for a psychiatric disorder using the Comprehensive Assessment of Symptoms and History (CASH) and the Structured Clinical Interview for Personality Disorders (SCIDP).15,16 Subjects who met the following inclusion criteria were invited to participate in the study: (1) persistent auditory verbal hallucinations experienced at least once a month for over 1 year, (2) no Axis I or II diagnosis of current or past psychiatric disorder or substance dependence other than past depressive or anxiety disorder in complete remission or nicotine dependence, (3) no alcohol or drug abuse for at least 3 months, and (4) no history of drug or alcohol abuse preceding the onset of the auditory verbal hallucinations. The subjects did not meet DSM-IV criteria for psychosis not otherwise specified despite having persistent auditory verbal hallucinations because the symptoms were not associated with occupational, social, or psychological dysfunction. They completed the following assessments administered by experienced psychiatrists trained in their use: Peter’s Delusion Inventory (PDI), Schizotypal Personality Questionnaire (SPQ), and Psyrats Auditory Hallucinations Rating Scale (AHRS) modified as previously described3 and the Global Assessment of Function scale.3,17–19
Sixteen subjects with persistent auditory hallucinations participated in the study (11 females; mean [SD] age/y at scanning = 43.9 [8.9], 5 smokers, cigarettes smoked/d: mean [SD] = 6.6 [4.9] and median Interquartile Range [IQR] = 5 [7]). Five had a family history of a psychotic disorder. Their clinical characteristics are reported in table 1.
Table 1.
Clinical Characteristics of the Hallucinating Group
| Mean (SD) | Range of Scores | Approximate Description of the Group Mean Score | |
| Age of onset of hallucinations (y) | 13.3 (11.3) | 4–44 | — |
| Voice frequency rating | 3.6 (1.1) | 2–6 | Voices occur at least once/hour |
| Voice duration rating | 1.9 (0.8) | 1–4 | Voices last for several minutes at a time |
| Voice location rating | 2.0 (1.1) | 1–4 | Voices sound outside the head but close to ears or head |
| Voice loudness rating | 1.9 (0.6) | 1–3 | About the same loudness as own voice |
| Voice origin rating | 3.3 (0.9) | 1–4 | Holds >50% (but<100%) conviction that voices originate from external causes |
| Voice distress rating | 0.2 (0.4) | 0–3 | Voices not distressing at all |
| Voice disruption rating | 0.06 (0.3) | 0–1 | No disruption to life, able to maintain family and social relationships |
| SPQ score | 26.4 (11.4) | 12–54 | Similar to general population mean score (26.9)17 |
| PDI conviction | 15.4 (7.1) | 6–37 | Lower than general population mean score (20.4)18 |
| PDI distress | 4.0 (8.2) | 0–34 | Lower than general population mean score (15.5)18 |
| PDI preoccupation | 16.3 (7.5) | 6–30 | Slightly higher than general population mean score (15.4)18 |
| GAF score | 83.4 (7.2) | 70–95 | Good functioning in all areas, no more than everyday problems |
Note: SPQ, Schizotypal Personality Questionnaire; PDI, Peters Delusion Inventory; GAF, Global Assessment of Function.
Healthy Control Group
Healthy controls (control group) were recruited via advertisement and were matched to the hallucinating group on the basis of age (within 5 years). Prior to inclusion, they received a psychiatric interview using the CASH and SCIDP to determine if they met the following inclusion criteria: (1) no history of current or past hallucinations in any form or any other psychotic symptom; (2) no diagnosis of current or past psychiatric disorder, personality disorder, or substance dependence other than past depressive or anxiety disorder in complete remission or nicotine dependence; (3) no alcohol or drug abuse for at least three months; and (4) no known family history of schizophrenia or other psychotic disorder in a first or second degree relative (determined using the Family Interview for Genetic Studies—http://www.nimhgenetics.org/interviews/figs/). Sixteen matched controls participated in the study (mean [SD] age/y = 42.8 [10.9]; 10 females, 4 smokers, cigarettes smoked/d: mean [SD] = 7.4 [3.9] and median [IQR] = 8 [7.4]).
Positron Emission Tomography
All subjects received a PET scan using an ECAT HR+ 962 PET scanner (CTI/Seimens) in 3D mode, with an axial field of view of 15.5 cm. Head movement was monitored and minimized using a light head strap. A 10-minute transmission scan was performed prior to radiotracer injection using a 150-MBq cesium-137 rotating point source in order to correct for attenuation and scatter. All subjects were required to fast and abstain from smoking from midnight on the day of the scan and received 150 mg carbidopa and 400 mg entacapone orally 1 hour prior to scanning to reduce the formation of radiolabeled metabolites, of which [18F]-O-methyl-fluorodopa crosses the blood-brain barrier.21 Subjects were positioned with the orbitomeatal line parallel to the transaxial plane of the tomograph. Head position was marked and monitored via laser crosshairs and a camera. The radiotracer was synthesized as previously described20 by 18F-fluorine reaction with the L-3,4-dihydroxyphenylalanine (l -DOPA) precursor (4,5-Di-BOC-N-ormyl-2-trimethyl-L-phenylalanine ethyl ester). 18F-fluorine was produced by the 18O(p,n) 18F reaction, bombarding enriched 18O-oxygen with 19 MeV protons at 40 mA for 30–45 minutes followed by a second bombardment of 19 MeV protons on 0.2% fluorine in argon, during which time the 18F bound to the target walls is isotopically exchanged. A sample from each synthesis was taken for quality assurance and analyzed using reverse phase high-pressure liquid chromatography. To proceed with injection, the radiochemical purity of the synthesis was required to be 95.0% or higher.
Approximately 180 MBq of 6-[18F]fluoro-l-DOPA ([18F]-DOPA) was administered by bolus intravenous injection 30 seconds after the start of the emission scan which lasted 95 minutes. The injected dose and specific activity (data missing for 2 subjects in the hallucinating group) were recorded.
Image Analysis
A frame-by-frame realignment and denoising method was employed to correct for head movement in the scanner.21 Non-attenuation–corrected images were used for the realignment algorithm because they include a significant scalp signal in comparison to attenuation-corrected images.22 Frames were realigned to a single, “reference” frame acquired 7 minutes postinjection using a mutual information algorithm,23 and the transformation parameters were then applied to the corresponding attenuation-corrected dynamic images.
These realigned frames were then summated, creating a movement-corrected dynamic image to be used in the analysis. Standardized regions in Montreal Neurologic Institute space were defined in the cerebellum (as it contains few, if any, dopaminergic terminals, it is used to provide data on nonspecific uptake24) using a probabilistic atlas25 and in the whole striatum delineated as previously described to create a Region of Interest (ROI) map.26 SPM5 (http://www.fil.ion.ucl.ac.uk/spm) was then used to normalize the ROI map together with the tracer specific ([18F]-DOPA) template (the template aids normalization) to each individual PET summation image. This nonlinear transformation procedure allowed ROIs to be placed automatically on individual [18F]-DOPA PET dynamic images. Striatal subdivisions were delineated as previously described to generate limbic (LS), associative (AST), and sensorimotor (SMST) subregions of the whole striatal ROI which have been shown to have high reproducibility.26 These “functional” subdivisions reflect the topographical arrangement of corticostriatal projections. Projections to the LS are from limbic areas such as the hippocampus and amygdala, projections to the AST originate in associative areas such as the dorsolateral prefrontal cortex, and projections to the SMST come from motor and related areas such as primary motor cortex, premotor cortex, and supplementary motor cortex.27 Influx constants (Ki cer values, denoted Ki in some previous publications28) for the whole striatal ROI and the functional subdivisions were calculated relative to uptake in the reference region for left and right sides combined using a graphical approach.29
Voxel-based statistical image analyses were performed to confirm the results obtained from the conventional ROI analysis. Wavelet-based kinetic modeling was used to produce maps of the uptake constant for [18F]-DOPA using the cerebellum as a reference region. It has been documented that wavelet-based methodology increases the signal-to-noise ratio of Ki cer maps for [18F]-DOPA by a factor of 3 without significant loss of resolution.30 Statistical analyses of parametric images were performed using SPM5 (Wellcome Department of Cognitive Neuroscience, London, UK) and Matlab6.5 (Mathworks, Natick, MA). An explicit anatomical mask confining the analyses to striatal areas was used. The results of the voxel-based analysis were analyzed corrected for multiple comparisons (P < .05, family-wise error rate) and using a liberal threshold value of P < .001 (uncorrected).
Statistical Analysis
The data were normally distributed as assessed using the Kolmogorov-Smirnov test. After confirming homogeneity of variance with Levene’s test, independent t tests were used to compare demographic and PET variables and striatal dopamine synthesis capacity between groups, and a Mann–Witney U test was used to compare nonparametric variables (cigarettes smoked/day and gender) between groups. Where there was a significant difference in the sociodemographic or PET variables, this was used as a covariate in a univariate analysis with striatal dopamine synthesis capacity as the dependent variable and group as the fixed factor. The relationship between Ki cer values and subclinical symptom scores or injected dose was explored using Pearson’s correlation coefficient, and cook’s distance and centred leverage plots were used to identify influential or outlying data points. A two-tailed significance level of P = .05 was used throughout.
Results
Subject Characteristics
There was no significant difference in the age (t = 0.3, df = 30, P = .8), cigarettes smoked/day (Z = 0.6, P = .5), or gender distribution between the two groups (Z = 0.4, P = .7) or in the specific activity of [18F]-DOPA administered (mean [SD] specific activity/GBq/μmol, in the hallucinating group = 0.034 [0.02] and 0.035 [0.02] in the control group; t = 0.7, df = 28, P = .9). By chance, the hallucinating group received a small but significantly greater injected dose (mean [SD] injected dose /MBq in the hallucinating group = 184 [4.6] and in the control group = 179 [4.3]; t = 3.1, df = 29, P = .005). However, there was no relationship between injected dose and striatal dopamine synthesis capacity in either group (r < .2 and P > .5 in both groups).
Striatal Dopamine Synthesis Capacity
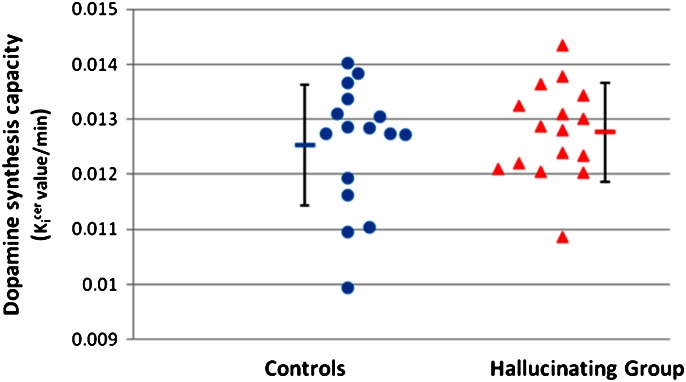
There was no significant difference in the dopamine synthesis capacity between groups in either the whole striatum (figure 1) or its functional subdivisions (table 2). There was no effect of group on striatal dopamine synthesis capacity in the univariate analysis with injected dose as a covariate (F = 0.2, df = 1,28, P = .6). The parametric analysis showed no difference between groups even at the liberal threshold of P < .01 uncorrected. In further sensitivity analyses, we repeated the analysis after excluding the subject in the hallucinating group with the lowest dopamine synthesis capacity and after excluding the subjects with a family history of a psychotic disorder (n = 5) and found that there was still no difference between groups in either case (F = 0.8, df = 1,28, P = .4 and F = 1.1, df = 1,24, P = 0.4 respectively). In case there was an effect of gender, we repeated the analysis with gender as a covariate and found there was no main effect of gender on whole striatal Ki cer (F = 0.2, df = 1,28; P = 0.6) or gender × group interaction (F < 0.1, df = 1,28, P = .99).
Fig. 1.
Striatal dopamine synthesis capacity (Ki cer value/min) for subjects and group means (error bars = SD).
Table 2.
Striatal Dopamine Synthesis Capacity (Mean [SD] Ki cer value/min) in the Hallucinating and Control Groups
| Controls | Hallucinating Group | Statistical Test | 95% Confidence Interval | |
| Whole striatum | 0.0125 (0.0011) | 0.0128 (0.0009) | t = 0.68, df = 30, P = .5 | −0.001 to 0.0005 |
| Associative striatum | 0.0124 (0.0012) | 0.0126 (0.0008) | t = 0.36, df = 30, P = .7 | −0.0009 to 0.0006 |
| Limbic Striatum | 0.0133 (0.0012) | 0.0135 (0.0010) | t = 0.34, df = 30, P = .7 | −0.0009 to 0.0007 |
| Sensorimotor striatum | 0.0140 (0.0013) | 0.0143 (0.0013) | t = 0.57, df = 30, P = .6 | −0.0012 to 0.0007 |
In the hallucinating group, there was no relationship between whole striatal dopamine synthesis capacity and total scores on the Psyrat AHRS (figure 2a, r = −0.15, P = .6), PDI (figure 2b, r = −.14, P = .6), or SPQ (figure 2c, r = .13, P = .63) ratings, or their sub-scales, or with age of onset of the hallucinations (r = .07, P = .8). The cook’s distance-centred leverage plots identified influential data associated with one case. However, the correlations remained nonsignificant after excluding this case.
Fig. 2.
Showing the relationship between striatal dopamine synthesis capacity (Ki cer value/min) and clinical ratings in the hallucinating group. (a) Total Psyrats Auditory Hallucinations Rating Scale score. There was no significant relationship between the 2 variables (r = −.15, P = .6). (b) Peter’s Delusion Inventory Score. There was no significant relationship between the 2 variables (r = −.14, P = .6). (c) Total Schizotypal Personality Questionnaire Score. There was no significant relationship between the 2 variables (r = .13, P = .63).
Discussion
We observe no difference in dopamine synthesis capacity in people with persistent auditory hallucinations compared with matched healthy controls and no relationship between the clinical ratings of their psychotic-like experiences and dopamine synthesis capacity. These findings indicate that striatal dopaminergic dysfunction, as indexed by [18F]-DOPA PET, does not underlie auditory verbal hallucinations in nonclinical individuals. Previous findings in subjects with nonclinical persistent auditory verbal hallucinations have found strong similarities in the phenomenology of auditory verbal hallucinations (AVH) between these nonpsychotic individuals and schizophrenia patients.4 A recent functional magnetic resonance imaging study revealed the same pattern of cortical neural responses during hallucinations in these nonpsychotic individuals as those seen during hallucinations in patients with psychotic disorders.31 Taken with our findings, this suggests that cortical abnormalities, rather than alterations in striatal dopamine synthesis capacity, are associated with hallucinations in nonclinical subjects, although alterations in other aspects of the dopaminergic system remain possible.
Methodological Considerations
The possibility of a type II error must be considered. However, the study has over 98% power to detect an elevation in dopamine synthesis capacity of the size we have previously found in schizophrenia and over 80% power to detect an effect size similar to that reported in people with schizotypal personality disorder (power calculations for two-tailed independent t tests with alpha = .05 using effect sizes from our previous studies).28,32 Our hallucinating group includes subjects with a family history of a psychotic disorder, and this, or other sources of heterogeneity in the sample, could potentially confound our findings. However, family history was not associated with altered dopamine synthesis capacity in a twin study,33 and although it was associated with elevated dopamine synthesis capacity in a sibling study,34 excluding those with a positive family history made no difference to our findings. It could be argued that the auditory hallucinations in our hallucinating group are a different phenomenon to those in psychotic disorders. However, while there are de facto differences between our hallucinating group and patients, most notably in the lack of distress or disruption caused by the voices in the hallucinating group, the number of voices, loudness, location, and familiarity of the voices are similar to those seen in patients with psychotic disorders.4 Some of the hallucinating group reported that the hallucinations began in early childhood, and early age of onset of hallucinations has been found to differentiate nonpsychotic hallucinating individuals from patients with psychotic disorders.4 While this suggests that age of onset is an important factor to consider before extrapolating findings in nonpsychotic hallucinating subjects to psychosis, people who go on to develop schizophreniform disorders also report hallucinations in childhood,35 albeit less frequently, indicating that this is not a categorical distinction between clinical and nonclinical subjects. Furthermore, we did not find a relationship between age of onset and dopamine synthesis capacity.
Interpretation of the Findings
The absence of delusional thinking or conceptual disorganization and functional impairment differentiates the nonclinical subjects with hallucinations in our study from patients with psychotic disorders and those with schizotypal personality disorder in whom increased dopamine synthesis capacity and/or release has been reported in previous studies.10,28,36,37 Taken with our findings, it could be speculated that in the psychotic and schizotypal patients, it is the additional presence of dopaminergic dysregulation that leads to delusions (possibly secondary to hallucinations) and a consequent change in behavior and functioning that results in a clinically significant disorder. Finally, while our study shows hallucinations can occur in the absence of altered dopamine synthesis capacity, it remains possible that different mechanisms underlie hallucinations in our hallucinating group and patients with schizophrenia. Thus, a study of patients who only experience hallucinations would be needed to categorically exclude the possibility that dopaminergic dysfunction underlies hallucinations in schizophrenia.
Our findings are also consistent with recent evidence indicating that whilst dopamine synthesis capacity was elevated in people at clinical risk of psychosis who presented with subclinical psychotic symptoms,28 the elevation was specific to those who subsequently went on to develop a psychotic disorder and increased further with the onset of the disorder.14,38 In this previous work, there was no alteration in those subjects who did not go on to develop a psychotic disorder, although they had subclinical psychotic symptoms including hallucinatory experiences.14 Our findings extend these findings by showing that dopamine synthesis capacity is also unaltered in people who are healthy and show no clinically significant impairment or distress, yet experience persistent hallucinations. These findings thus suggest that elevated dopamine synthesis capacity is specific to the development of a clinically significant psychotic disorder, rather than underlying single subclinical psychotic experiences in general, at least in terms of hallucinations.
Conclusions
Dopamine synthesis capacity is unaltered in healthy people with auditory verbal hallucinations and not related to the severity of subclinical psychotic experiences in these individuals. This suggests that alterations in striatal dopaminergic function, as indexed by [18F]-DOPA, do not underlie all aspects of the psychosis continuum and other abnormalities underlie hallucinations.
Funding
Medical Research Council UK (MC_US_A655_002_2135_0000); the Biomedical Research Council and by the Dutch Brain Foundation (Hersenstichting Nederland).
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subjects of this study. Drs Howes and Shotbolt contributed equally to the study and share first authorship.
References
- 1.van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–195. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- 2.Tien AY. Distributions of hallucinations in the population. Soc Psychiatry Psychiatr Epidemiol. 1991;26:287–292. doi: 10.1007/BF00789221. [DOI] [PubMed] [Google Scholar]
- 3.Sommer IE, Daalman K, Rietkerk T, et al. Healthy individuals with auditory verbal hallucinations; who are they? Psychiatric assessments of a selected sample of 103 subjects. Schizophr Bull. 2010;36:633–641. doi: 10.1093/schbul/sbn130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daalman K, Boks MP, Diederen KM, et al. The same or different? A phenomenological comparison of auditory verbal hallucinations in healthy and psychotic individuals. J Clin Psychiatry. 2011;72:320–325. doi: 10.4088/JCP.09m05797yel. [DOI] [PubMed] [Google Scholar]
- 5.Howes OD, Montgomery AJ, Asselin MC, Murray RM, Grasby PM, McGuire PK. Molecular imaging studies of the striatal dopaminergic system in psychosis and predictions for the prodromal phase of psychosis. Br J Psychiatry Suppl. 2007;51:s13–s18. doi: 10.1192/bjp.191.51.s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13:358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- 7.Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol. 2004;7(suppl 1):S1–S5. doi: 10.1017/S1461145704004110. [DOI] [PubMed] [Google Scholar]
- 8.Howes OD, Egerton A, Allan V, McGuire P, Stokes P, Kapur S. Mechanisms underlying psychosis and antipsychotic treatment response in schizophrenia: insights from PET and SPECT imaging. Curr Pharm Des. 2009;15:2550–2559. doi: 10.2174/138161209788957528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meisenzahl EM, Schmitt GJ, Scheuerecker J, Moller HJ. The role of dopamine for the pathophysiology of schizophrenia. Int Rev Psychiatry. 2007;19:337–345. doi: 10.1080/09540260701502468. [DOI] [PubMed] [Google Scholar]
- 10.Reith J, Benkelfat C, Sherwin A, et al. Elevated dopa decarboxylase activity in living brain of patients with psychosis. Proc Natl Acad Sci USA. 1994;91:11651–11654. doi: 10.1073/pnas.91.24.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapur S, Mizrahi R, Li M. From dopamine to salience to psychosis–linking biology, pharmacology and phenomenology of psychosis. Schizophr Res. 2005;79:59–68. doi: 10.1016/j.schres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–645. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- 14.Howes OD, Bose S, Valli I, et al. Dopamine synthesis capacity before onset of psychosis: a prospective [18]-DOPA PET imaging study. Am J Psychiatry. 2011;168(12):1311–7. doi: 10.1176/appi.ajp.2011.11010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- 16.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: history, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 17.Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- 18.Peters E, Joseph S, Day S, Garety P. Measuring delusional ideation: the 21-item Peters et al. Delusions Inventory (PDI) Schizophr Bull. 2004;30:1005–1022. doi: 10.1093/oxfordjournals.schbul.a007116. [DOI] [PubMed] [Google Scholar]
- 19.Haddock G, McCarron J, Tarrier N, Faragher EB. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS) Psychol Med. 1999;29:879–889. doi: 10.1017/s0033291799008661. [DOI] [PubMed] [Google Scholar]
- 20.Namavari M, Bishop A, Satyamurthy N, Bida G, Barrio JR. Regioselective radiofluorodestannylation with [18F]F2 and [18F]CH3COOF: a high yield synthesis of 6-[18F]Fluoro-L-DOPA. Int J Radiat Appl Instr A. 1992;43:989–996. doi: 10.1016/0883-2889(92)90217-3. [DOI] [PubMed] [Google Scholar]
- 21.Turkheimer FE, Brett M, Visvikis D, Cunningham VJ. Multiresolution analysis of emission tomography images in the wavelet domain. J Cereb Blood Flow Metab. 1999;19:1189–1208. doi: 10.1097/00004647-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Bose SK, Turkheimer FE, Howes OD, et al. Classification of schizophrenic patients and healthy controls using [18F] fluorodopa PET imaging. Schizophr Res. 2008;106:148–155. doi: 10.1016/j.schres.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Studholme C, Hill DL, Hawkes DJ. Automated three-dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Med Phys. 1997;24:25–35. doi: 10.1118/1.598130. [DOI] [PubMed] [Google Scholar]
- 24.Kumakura Y, Cumming P. PET studies of cerebral levodopa metabolism: a review of clinical findings and modeling approaches. Neuroscientist. 2009;15:635–650. doi: 10.1177/1073858409338217. [DOI] [PubMed] [Google Scholar]
- 25.Hammers A, Allom R, Koepp MJ, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19:224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egerton A, Demjaha A, McGuire P, Mehta MA, Howes OD. The test-retest reliability of 18F-DOPA PET in assessing striatal and extrastriatal presynaptic dopaminergic function. Neuroimage. 2009;50:524–531. doi: 10.1016/j.neuroimage.2009.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 29.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- 30.Turkheimer FE, Aston JA, Asselin MC, Hinz R. Multi-resolution Bayesian regression in PET dynamic studies using wavelets. Neuroimage. 2006;32:111–121. doi: 10.1016/j.neuroimage.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Diederen KM, Daalman K, de Weijer AD, et al. Auditory hallucinations elicit similar brain activation in psychotic and nonpsychotic individuals. [published online ahead of print April 28, 2011] Schizophr Bull. 2011 doi: 10.1093/schbul/sbr033. doi:10.1093/schbul/sbr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGowan S, Lawrence AD, Sales T, Quested D, Grasby P. Presynaptic dopaminergic dysfunction in schizophrenia: a positron emission tomographic [18F]fluorodopa study. Arch Gen Psychiatry. 2004;61:134–142. doi: 10.1001/archpsyc.61.2.134. [DOI] [PubMed] [Google Scholar]
- 33.Shotbolt P, Stokes PR, Owens SF, et al. Striatal dopamine synthesis capacity in twins discordant for schizophrenia. Psychol Med. 2011;41:2331–2338. doi: 10.1017/S0033291711000341. [DOI] [PubMed] [Google Scholar]
- 34.Huttunen J, Heinimaa M, Svirskis T, et al. Striatal dopamine synthesis in first-degree relatives of patients with schizophrenia. Biol Psychiatry. 2007;63:114–117. doi: 10.1016/j.biopsych.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 35.Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H. Children's self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry. 2000;57:1053–1058. doi: 10.1001/archpsyc.57.11.1053. [DOI] [PubMed] [Google Scholar]
- 36.Hietala J, Syvalahti E, Vuorio K, et al. Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet. 1995;346:1130–1131. doi: 10.1016/s0140-6736(95)91801-9. [DOI] [PubMed] [Google Scholar]
- 37.Abi-Dargham A, Kegeles LS, Zea-Ponce Y, et al. Striatal amphetamine-induced dopamine release in patients with schizotypal personality disorder studied with single photon emission computed tomography and [123I]iodobenzamide. Biol Psychiatry. 2004;55:1001–1006. doi: 10.1016/j.biopsych.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 38.Howes O, Bose S, Turkheimer F, et al. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Mol Psychiatry. 2011;16:885–886. doi: 10.1038/mp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]