Abstract
Disruption of visual percepts by a subsequent stimulus (ie, backward masking) has been consistently noted in schizophrenia, with some evidence that this fragility in early perception is present in people with genetic liability for the disorder. Given the potential of backward masking paradigms to mark neural processes that confer risk for schizophrenia, it is important to test the diagnostic specificity of abnormalities in visual perception. To more fully assess whether masking visual stimuli reveals a marker of genetic liability (ie, endophenotype) specific to schizophrenia, we tested 44 people with the disorder, 29 people with bipolar disorder, 56 first-degree biological relatives of people with schizophrenia, 26 first-degree biological relatives of people with bipolar disorder, and 43 nonpsychiatric control participants using a magnocellular-biased visual backward masking procedure that included target-to-mask onset asynchronies ranging from 0 to 80 ms. Relatives of people with schizophrenia who were without schizophrenia spectrum disorders exhibited impaired performance compared with nonpsychiatric control participants and relatives of people with bipolar disorder when a visual mask interrupted early perception (eg, 27 ms). A similar vulnerability of early processes was noted in people with schizophrenia, yet they also had impaired performance when masks occurred at later time points (ie, 80 ms). Performance deficits were not attributable to intellectual function, measures of attention and memory, symptomatology, or medication dosage. Bipolar patients and their relatives failed to exhibit deficits on the backward masking task. Fragility of early visual percepts appears to mark genetic liability specific to schizophrenia and may serve as an endophenotype for the disorder.
Keywords: backward masking, magno-cellular, bipolar disorder, endophenotype, unaffected relatives
Introduction
Abnormalities in the early processing of visual stimuli may mark genetic risk for schizophrenia.1 Individuals with schizophrenia exhibit a protracted interruption in perceiving a visual stimulus when it is followed by a visual “mask.”2 Because the mask obstructs perception of an object that occurred before it, the phenomenon is termed “backward masking.” Studies have shown the deficit to be stable over time in people with schizophrenia3,4 and not an epiphenomenon of antipsychotic medications.5–7 Researchers have also found both remitted schizophrenia patients7 and healthy first-degree biological relatives of schizophrenia patients8–11 to have visual percepts that are unusually vulnerable to a subsequent masking stimulus.
Although backward masking anomalies are present in individuals with schizophrenia who are symptom-free as well as potential genetic carriers for the disorder, an important requirement for a marker of genetic risk specific to a disorder (ie, endophenotype12) is that the abnormality is absent in individuals with genetic liability for other mental disorders.13,14 Because of a failure to find specific points of genetic variation reliably associated with clinically defined mental disorders (see Gershon et al15 for commentary), there has been a renewed suggestion that endophenotypes (ie, intermediate phenotypes) are important to dissecting the genetic contributions to specific disorders.16 To more completely examine early visual processing anomalies as markers of genetic liability for schizophrenia, we assessed backward masking performance in clinically stable outpatients with schizophrenia and bipolar disorder, first-degree biological relatives of individuals with schizophrenia or bipolar disorder, and nonpsychiatric control participants. Although a few studies have contrasted backward masking effects across schizophrenia and bipolar disorder,11,17,18 no published study has included diagnostic group comparisons for both patients and relatives to more fully test a visual perceptual abnormality against endophenotype criteria.
Researchers have used masking paradigms in an attempt to determine whether the magnocellular (M) or parvocellular (P) division of the visual processing stream underlies backward masking anomalies in schizophrenia (for a review, see Green et al19). Cells of the M system transiently fire and generally function to identify the location of objects. Objects that are moving and of low-spatial frequency and contrast preferentially activate M cells while cells of the P system generally subserve the analysis of object features.20 P cells fire in a relatively sustained manner and are preferentially activated by high-spatial frequency, high contrast, stationary, and chromatically colored objects.
To date, evidence appears to point to M-biased tasks yielding visual masking anomalies associated with genetic liability for schizophrenia. Three studies of unaffected first-degree relatives of schizophrenia patients revealed greater abnormalities with M-biased tasks (ie, location and blurred-target identifications) as compared with P-biased tasks (simple identification).8,9,11 Importantly, 2 of the studies8,11 revealed performance deficits in unaffected siblings only for short delay periods between the target stimulus and mask (ie, less than 60 ms) suggesting that a fragility of M-biased early perceptual processes is associated with genetic risk for schizophrenia. Also, Bedwell et al10 used a red background to suppress the M system and contrasted performance with a gray background on location and identification tasks. They showed performance of controls was lower when a red background was used, but that there was no difference for relatives of schizophrenia patients. Although there was no group difference in backward masking, the authors suggested the data were consistent with M cell hyperactivity in the relatives. The one study failing to show an effect in first-degree biological relatives of individuals with schizophrenia failed to use a location condition (ie, M-biased) in their task.21 One study contrasting schizophrenia, depressed, and healthy individuals revealed that schizophrenia was associated with a location task-masking deficit purportedly independent of “intellectual decline,” while such a deficit was absent in depression.22
Individuals with bipolar disorder and their biological relatives appear to have mostly intact performance on M-biased visual masking tasks. In the only published study contrasting relatives of schizophrenia and relatives of bipolar patients, Keri et al11 used both location (M-biased) and identification (P-biased) masking tasks and found normal performance in relatives of bipolar patients while demonstrating greater performance deficits on the location than on the identification task in relatives of schizophrenia patients. Similarly, 2 other studies have shown an absence of visual backward masking abnormalities in offspring of individuals with bipolar disorder.23,24 Most investigations revealing backward masking dysfunction in bipolar patients employed paradigms requiring the participant to determine the identity of objects (ie, a P-system–biased task)25,26 or studied bipolar disorder participants with ongoing psychotic symptomatology.27,28 When M-biased tasks have been used, different patterns of masking for bipolar disorder and schizophrenia patients are evident.17,29 Specifically, bipolar disorder patients with mild symptomatology generally fail to show a backward masking dysfunction,30,31 and instead lifetime history of psychosis in bipolar disorder appears predictive of increased errors on backward masking paradigms.23
We carried out the present study to directly test the specificity of backward-masking elicited perceptual deficits to schizophrenia. A marker specific to the genetic vulnerability for schizophrenia may be valuable in identifying genes relevant to schizophrenia and not bipolar disorder and ideally point the way toward etiologic mechanisms unique to schizophrenia. For this study, we used an M-biased backward-masking task provided by Green et al32 that required participants to identify the location of a masked object. We hypothesized that schizophrenia patients and their relatives would exhibit backward-masking dysfunction, particularly at brief target-mask stimulus onset asynchronies (SOA’s), while bipolar patients and relatives of bipolar patients would exhibit near normal performance.
Methods
Participants
As part of a larger family study of severe mental disorders, 44 schizophrenia patients, 29 bipolar disorder patients, 56 first-degree biological relatives of schizophrenia patients, 26 first-degree biological relatives of bipolar patients, and 43 nonpsychiatric control participants completed a visual backward masking procedure. Table 1 presents the characteristics of participants. We recruited schizophrenia and bipolar participants from the outpatient clinics of the Minneapolis VA Medical Center, community support programs for the mentally ill, and a county mental health clinic. Research staff identified first-degree biological relatives of probands by completing a pedigree from the patient’s report. Study staff identified potential nonpsychiatric control participants through posted announcements at community libraries, fitness centers, the Minneapolis VA Medical Center, and in newsletters for veterans and fraternal organizations. All participants completed an informed consent process and the Minneapolis VA Medical Center and University of Minnesota Institutional Review Boards approved the study protocol. Diagnoses and symptomatology were assessed with standardized structured interviews and questionnaires that are described in the online supplementa ry material and have been reported elsewhere.33,34
Table 1.
Characteristics of Participants
| Variable | Schizophrenia Patients | Bipolar Disorder Patients | Relatives of Schizophrenia Patients | Relatives of Bipolar Disorder Patients | Nonpsychiatric Controls | Statistic | P Value |
| n = 42 | n = 36 | n = 53 | n = 35 | n = 43 | |||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Age (years) | 44.7 (10.5) | 42.9 (9.5) | 48.3 (10.0) | 46.7 (13.5) | 48.0 (15.1) | F 4,204 = 1.5 | ns |
| Percent female | 21a , b , c | 28a , b , c | 60 | 49 | 53 | χ2(4) = 20.2 | <.00051 |
| Year of education | 14.1 (2.8) | 14.9 (2.2) | 14.8 (2.4) | 14.4 (2.1) | 15.7 (4.6) | F 4,204 = 1.7 | ns |
| Estimated IQ | 99.4 (12.1)a , b , c , d | 109.3 (16.5) | 107.2 (13.9) | 109.0 (13.0) | 111.1 (10.9) | F 4,204 = 4.9 | .001 |
| BPRS total score | 41.4 (11.5)d | 38.4 (9.8) | NA | NA | NA | F 1,73 = 1.5 | ns |
| Reality distortion | 2.01 (1.5)d | 0.69 (1.20) | NA | NA | NA | F 1,75 = 18.5 | <.0005 |
| Formal thought disorder | 1.10 (1.11) | 1.08 (1.18) | NA | NA | NA | F 1,75 = 0.0 | ns |
| Negative symptoms | 1.64 (0.90)d | 0.76 (0.69) | NA | NA | NA | F 1,75 = 22.4 | <.0005 |
| Cluster A symptoms | NA | NA | 1.2 (1.9)c | 0.7 (1.8) | 0.3 (0.6) | F 2,126 = 4.2 | .017 |
| SPQ total score | NA | NA | 15.4 (9.6)c | 11.4 (11.9) | 9.5 (6.2) | F 2,122 = 4.7 | .010 |
| Perceptual aberration | NA | NA | 1.6 (1.9) | 1.4 (1.6) | 1.0 (1.2) | F 2,122 = 1.7 | ns |
| Magical ideation | NA | NA | 2.8 (2.5) | 2.5 (2.8) | 2.7 (2.4) | F 2,122 = 0.9 | ns |
| Physical anhedonia | NA | NA | 12.9 (5.5) | 13.4 (6.0)c | 10.9 (4.6) | F 2,122 = 2.5 | .088 |
| Social anhedonia | NA | NA | 8.9 (6.0)c | 7.2 (5.8) | 6.1 (4.7) | F 2,122 = 3.0 | .054 |
Note: Four relatives of schizophrenia patients had schizophrenia spectrum conditions (1 with schizoaffective disorder, 2 with schizoid personality disorder, and 1 with schizophrenia) and 2 relatives of bipolar disorder patients exhibited these conditions (1 with delusional disorder and 1 with paranoid personality disorder). ns, not significant; IQ, intelligence quotient; Estimated IQ was derived from Block Design and Vocabulary subtests of the Wechsler Adult Intelligence Scale - Third Edition (WAIS-III). BPRS, Brief Psychiatric Rating Scale; NA, not assessed. The Scale for the Assessment of Negatives Symptoms and Scale for the Assessment of Positive Symptoms were used to derive scores on symptom factors of Reality Distortion (average global rating for Delusions and Hallucinations), Positive Formal Thought Disorder (global rating), and Negative Symptoms (average global rating for affective blunting, alogia, anhedonia-asociality, and avolition-apathy). Cluster A symptoms were assessed using the Structured Interview for Schizotypy. SPQ, Schizotypal Personality Questionnaire. Perceptual Aberration, Magical Ideation, Physical Anhedonia, and Social Anhedonia were assessed through self-report questionnaires. lDenotes significance level for chi-square test.
Different from Relatives of Schizophrenia Group mean, P < .05.
Different from Relatives of Bipolar Group mean, P < .05.
Different from Control Group mean, P < .05.
Different from Bipolar Group mean, P < .05.
Visual Backward Masking Procedure
The present study employed a computerized visual backward masking procedure that has been used by others to reveal masking deficits in schizophrenia. Please see Green et al32 for a detailed description of the masking procedure. Stimuli were presented on a 17″ NEC 150 Hz monitor that was positioned 1 m from the participants’ eyes. Room lighting was constant across subjects, measured using a photographic light meter, and provided indirectly by a lamp with its fixture pointed toward the ceiling. Participants had corrected acuity of at least 20/32; however, 1 schizophrenia patient, 2 relatives of bipolar patients, and 1 relative of a schizophrenia patient had 20/40 acuity, and 1 control had 20/50 acuity. Target stimuli were dark gray and appeared on a white background and consisted of a square with a gap in the middle of one side.
Participants first completed a procedure to determine the critical stimulus intensity (CSI) for the masking task. The CSI procedure consisted of participants having to verbally identify the side of the square that had a gap in it (top, side and bottom). During the CSI procedure, stimuli were presented for 13 ms and without a mask. The gray scale of the target was adjusted until a gray scale level where the participant would obtain about 84% correct. This threshold was determined by computing the average of 6 gray scale adjustment reversals (eg, progressively lighter to progressively darker grays). Six participants failed to establish a CSI and did not proceed onto the backward masking task due to poor performance or inconsistent responding. The CSI procedure guaranteed that all participants could accurately perceive unmasked stimuli.
After establishing a participant’s CSI, staff administered a target location task with a high-energy visual mask. A trial consisted of a 300 ms fixation cross, 100 ms blank screen, 13 ms target, a variable posttarget period, and then a 26 ms mask. The participant’s task was to identify the location of the target in terms of being to the upper left, upper right, lower left, or lower right of fixation (1 visual degree displacement from fixation). No fixation cross was displayed during the target, mask, or target-mask intervening period. Participants verbally reported the perceived location of the target to the experimenter who pressed the appropriate key and then administered the next trial when the participant was prepared. A chance level of performance was 25%. The time between the onset of the target and the onset of the mask (ie, SOA) varied between 0, 13, 27, 40, 53, 67, and 80 ms, and 12 trials per condition were presented. A select set of attention, memory, and other cognitive tests were also administered to participants and are described in the online supplementa ry materials.
Results
Preliminary Analyses
Participant characteristics are reported in table 1. The 5 participant groups were of similar age and education. Individuals with schizophrenia had lower estimated intelligence by about a SD, as compared with the other groups. Both patient groups had a lower percentage of female participants, which is indicative of the sample largely deriving from a Veterans Affairs medical center (Male and female participants failed to differ on any measure of performance for the backward masking task. When gender was included as a factor in analyses, group effects were essentially identical to when gender was excluded from analyses.). The 2 patient groups predominantly consisted of stable outpatients and did not differ in terms of severity of overall symptomatology as measured by the Brief Psychiatric Rating Scale, while individuals with schizophrenia had more reality distortion (hallucinations and delusions) and negative symptomatology than bipolar disorder participants.
The first-degree biological relatives of schizophrenia patients showed elevated rates of Cluster A personality disorder symptoms as rated on interview and self-reported schizotypal characteristics as measured by the Schizotypal Personality Questionnaire (SPQ) and Social Anhedonia scales. The first-degree biological relatives of bipolar disorder patients had greater self-reported physical anhedonia than nonpsychiatric control participants.
The psychophysical control procedure that individualized contrast thresholds generated similar CSI values across groups (see table 2) indicating that no one group had a disadvantage in viewing target stimuli.
Table 2.
Performance on Visual Perceptual, Attention, and Memory Tasks by Group
| Variable | Schizophrenia Patients | Bipolar Disorder Patients | Relatives of Schizophrenia Patients | Relatives of Bipolar Disorder Patients | Nonpsychiatric Controls | ANOVA Statistic | P Value |
| n = 42 | n = 36 | n = 53 | n = 35 | n = 43 | |||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| CSI | 22.5 (8.2) | 18.3 (8.7) | 20.5 (9.7) | 21.9 (10.3) | 21.1 (10.5) | F 4,204 = 1.1 | ns |
| Visual perception: backward masking (% correct) | |||||||
| Mean all SOA’s | 34.8 (10.9)a , b , c , d | 42.5 (10.8) | 40.7 (11.8) | 41.0 (12.5) | 43.0 (12.0) | F 4,204 = 3.3 | .012 |
| 27 ms SOA | 29.0 (11.9)b , c , d | 36.8 (16.5) | 30.8 (14.8)b , d | 38.6 (14.0) | 35.7 (13.6) | F 4,204 = 3.5 | .009 |
| Sustained visual attention: DS-CPT | |||||||
| Perceptual sens(d′) | 2.45 (0.88) | 2.25 (0.95) | 2.58 (1.00) | 2.26 (0.93) | 2.56 (1.21) | F 4,197 = 0.9 | ns |
| Threshold (lnβ) | .74 (.78) | .91 (.77) | .67 (.99) | .93 (.73) | .74 (.99) | F 4,197 = 0.7 | ns |
| Visual search: SPAN (% correct) | |||||||
| 3-Item array | 92.6 (7.9)a , b , c | 94.0 (5.1) | 96.3 (3.7) | 96.0 (3.9) | 95.7 (5.0) | F 4,187 = 3.5 | .009 |
| 12-Item array | 78.8 (9.9)a | 82.3 (9.0) | 83.0 (8.3) | 80.9 (6.7) | 80.5 (7.5) | F 4,187 = 1.6 | ns |
| Working memory/attention | |||||||
| Digit span | 17.0 (8.1)c | 18.1 (6.7) | 16.4 (3.5) | 17.1 (4.1) | 19.0 (4.6) | F 4,202 = 1.6 | ns |
| Letter number sequence | 9.4 (2.2)a , b , c | 10.3 ( 2.7)c | 10.5 ( 2.6)c | 11.3 (1.9) | 11.9 (2.7) | F 4,188 = 6.3 | <.0005 |
| Episodic verbal memory | |||||||
| CVLT list A | 42.7 (12.8)a , b , c , d | 51.2 (10.4) | 49.4 (11.9)b , c | 55.8 (7.8) | 54.3 (9.0) | F 4,188 = 8.8 | <.0005 |
Note: ns, not significant; CSI, Critical Stimulus Intensity level (which reflected the level of contrast to ensure similar performance on unmasked stimuli across participants); SOA, interval between onsets of target and mask; DS-CPT, Degraded-Stimulus Continuous Performance; SPAN, Span of Apprehension Test; CVLT List A, Total is the total items recalled from list A over 5 trials of the California Verbal Learning Test.
Different from Relatives of Schizophrenia Group mean, P < .05.
Different from Relatives of Bipolar Group mean, P < .05.
Different from Control Group mean, P < .05.
Different from Bipolar Group mean, P < .05.
Backward Masking Task Performance
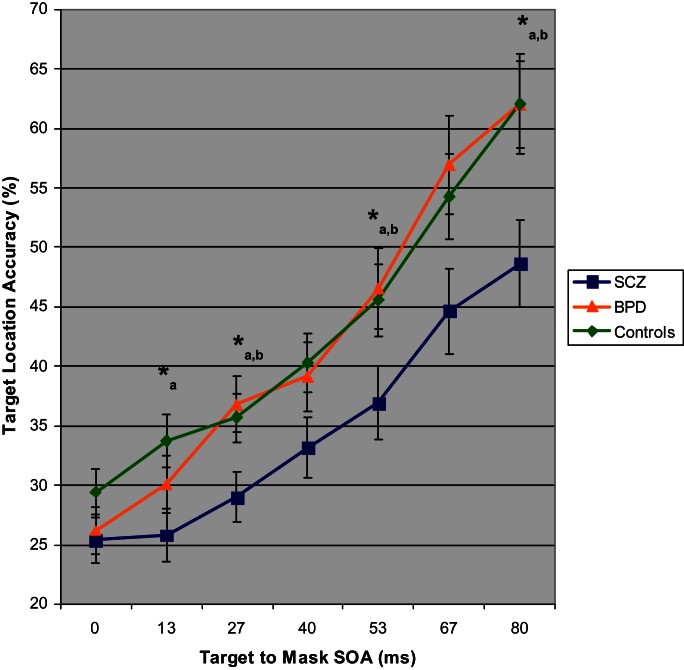
To test whether schizophrenia as compared with bipolar disorder was associated with a disproportionately large impairment in identifying the location of backwardly masked stimuli, we carried out a MANOVA of target location accuracy in people with schizophrenia, bipolar disorder, and nonpsychiatric control participants. The analysis of patients allowed testing of whether backward masking performance deficits were specific to the clinical disorder of schizophrenia. Figure 1 depicts performance on the backward masking task for people with schizophrenia, bipolar disorder, and nonpsychiatric control participants. The analysis revealed a main effect of group, F 2,118 = 6.89, P = .001, and SOA, Wilk’s Lambda = .44, F 6,113 = 26.64, P < .0005, with performance improving from SOA 13–27 ms and thereafter to longer SOA’s. Follow-up ANOVA’s showed that the 3 groups differed at SOA’s of 13, 27, 53, and 80 ms. Paired comparisons for each of these SOA’s revealed that schizophrenia patients had worse target location accuracy than controls at 13, 27, 53, and 80 ms. People with schizophrenia also had lower performance than bipolar disorder individuals at 27, 53, and 80 ms. These findings were indicative of schizophrenia patients failing to maintain the masked visual percept to the same degree as bipolar patients. Bipolar patients showed no target location deficits across the SOA’s (all P’s > .22).
Fig. 1.
Target location mean accuracy with SEs for schizophrenia, bipolar disorder, and nonpsychiatric control participants as a function of stimulus onset asynchrony (SOA) between target and visual mask. Three-group ANOVA for each SOA: *P < .05. Follow-up paired comparisons with P < .05: a = schizophrenia group lower accuracy than control group and b = schizophrenia group lower accuracy than bipolar disorder group.
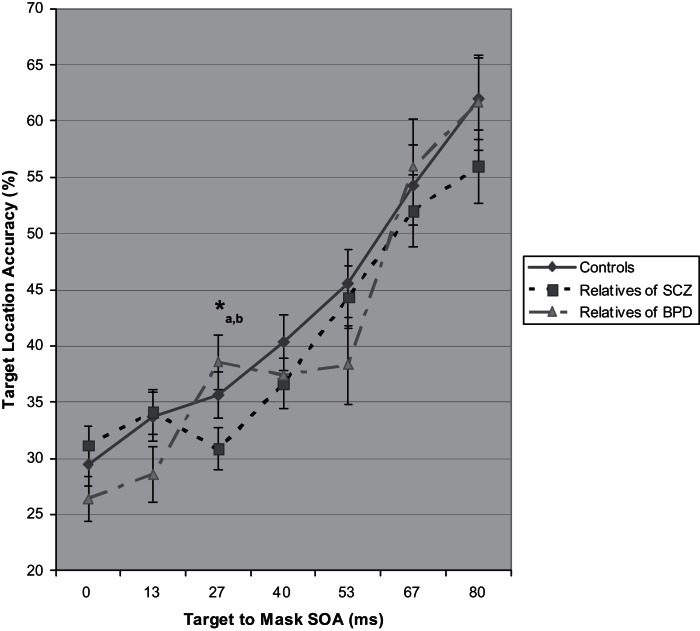
To examine whether genetic liability for schizophrenia was associated with impaired performance by a backward mask, we conducted a MANOVA of target location accuracy in first-degree biological relatives of schizophrenia patients, first-degree biological relatives of bipolar disorder patients, and control participants. This analysis of relatives allowed testing of whether backward masking performance deficits were specific to genetic liability for schizophrenia. Figure 2 depicts performance on the backward masking task for the groups of relatives and nonpsychiatric control participants. The analysis yielded a main effect of SOA, Wilk’s Lambda = .39, F 6,123 = 31.57, P < .0005, and an interaction of group and SOA, Wilk’s Lambda = .84, F 12, 246 = 1.87, P = .04, but no main effect of group. The main effect of SOA reflected that performance improved across every increase in SOA (0–13 ms, 13–27 ms, and so on). The interaction of group and SOA was specifically evident for performance from 13–27 ms, F 2,128 = 6.29, P = .003, which indicated the failure of one group to escape from masking effects like others. Follow-up ANOVA’s revealed a group effect for the 27 ms SOA (F 2,131 = 3.36, P = .04) but no other SOA, with relatives of schizophrenia patients showing a trend toward worse performance than controls (P < .10) and significantly worse performance than relatives of bipolar patients (P = .01). Importantly, when 6 relatives with schizophrenia spectrum diagnoses were excluded, the difference between relatives of schizophrenia patients and controls became significant (P = .03) and all previously reported effects for relatives remained the same (Four individuals with spectrum disorders were relatives of schizophrenia patients, while 2 were relatives of bipolar patients. The spectrum relatives of schizophrenia patients had slightly higher backward masking performance than the spectrum relatives of bipolar patients, and thus their exclusion moved the group difference at the 27 ms SOA from a trend to significant.). Therefore, impaired performance was independent of schizophrenia-spectrum disorders in the relatives. Note in figure 2 that mean location accuracy values decreased from 13–27 ms SOA for relatives of schizophrenia patients, while accuracy improved for relatives of bipolar patients. Thus, the effects of the mask on location identification were the strongest at 27 ms for the relatives of schizophrenia patients, with performance nearly equaling that of individuals with schizophrenia (30.8 and 29.0%, respectively) as compared with the nonpsychiatric controls, relatives of individuals with bipolar disorder, and bipolar disorder patients (35.7, 38.6, and 36.8, respectively). Table 2 presents results of paired comparisons for overall masking performance and at the 27 ms SOA.
Fig. 2.
Target location mean accuracy with SEs for first-degree biological relatives of people with schizophrenia, first-degree biological relatives of people with bipolar disorder, and nonpsychiatric control participants as a function of stimulus onset asynchrony (SOA) between target and visual mask. Three-group ANOVA for each SOA: *P < .05. Follow-up paired comparisons: a = P < .10 for relatives of schizophrenia with lower accuracy than controls and b = P < .05 for relatives of schizophrenia with lower accuracy than relatives of bipolar disorder. When relatives affected by schizophrenia-spectrum conditions were excluded, the difference between relatives of schizophrenia patients and control participants became significant (P = .034) and the difference between the 2 groups of relatives remained (P = .012).
Other Measures of Visual Attention and Cognition
To understand how abnormalities in early visual perception may be associated with other visual attention functions35,36 and cognitive deficits, we examined the performance of participants on measures of sustained visual attention, visual search, and working and episodic memory. Table 2 presents means, SDs, and participant group comparisons for the attention and cognitive indices. Interestingly, no deficits were evident on measures of sustained visual attention (d′ [perceptual sensitivity] and lnβ [response threshold]) and the more demanding visual search condition (SPAN 12-item array) (An absence of deficit in schizophrenia patients on the degraded-stimulus continuous performance test (DS-CPT) may reflect the selection of schizophrenia probands of similar levels of educational attainment to controls, as compared with studies (eg, Kumar et al37) of people with schizophrenia having more marked educational and intelligence deficits.). Backward masking task performance in the schizophrenia patients and their relatives was minimally associated with sustained visual attention and visual search performance with only a modest association between mean masking task performance with d′ from the DS-CPT, r(95) = .22, P = .03. Thus, early visual perception abnormalities in schizophrenia patients and their relatives revealed through backward masking were not secondary to other attentional processes. Individuals with schizophrenia as well as their first-degree biological relatives were impaired in working memory that required manipulation of material in memory (Letter Number Sequencing) and episodic verbal memory; however, neither of these measures were associated with backward masking task performance consistent with visual perception abnormalities being independent of higher level cognitive functions. Additionally, estimated intelligence was generally uncorrelated with backward masking task performance in relatives of people with schizophrenia (all r’s < .19) and those with the disorder (all r’s < .28, except r(42) =.37 at SOA 40 ms).
Symptomatology and Clinical Correlates
To determine whether performance deficits on the backward masking task were associated with symptomatology or other clinical factors, we examined a variety of measures of psychopathology. For schizophrenia patients, masking task performance was unrelated to measures of overall symptomatology or dimensions of psychotic symptomatology (all r’s < .30). For relatives of individuals with schizophrenia, worse performance at the 27 ms SOA was associated with lower levels of schizotypal signs as measured by the SPQ, r(50) = .32, P = .03, and a lower number of cluster A personality disorder symptoms, r(52) = .28, P = .04. Although in the unexpected direction, the association indicates that poor masking task performance in the relatives of schizophrenia patients was not secondary to schizotypal symptomatology. Finally, for the 28 schizophrenia and bipolar patients who were taking antipsychotic medication and had dosage information available, there was no association between chlorpromazine equivalents and performance on the backward masking task (all r’s < .31).
Discussion
This first direct test contrasting performance of schizophrenia and bipolar disorder patients, as well as their first-degree biological relatives, on a backward masking paradigm provided evidence that an early visual perceptual deficit is an indicator of genetic liability specific to schizophrenia. When a visual masking stimulus with a 27 ms SOA interrupted early perceptual processes, biological relatives of schizophrenia patients without schizophrenia spectrum disorders exhibited poor identification of target locations compared with nonpsychiatric control participants and relatives of bipolar patients. A similar failure of early visual perception to escape the effects of a mask was noted in schizophrenia probands when compared with bipolar probands and control participants; however, individuals with schizophrenia also had impaired target location identification when masks occurred at the longest SOA (ie, 80 ms). The vulnerability of early percepts to a visual mask was not accounted for by misperception of the target because the groups showed no difference in the contrast level required to discern the target (ie, CSI). Additionally, groups were well matched on demographic characteristics. Ways in which the patient and relative groups deviated from the control group (gender composition, estimated IQ, select attention and memory measures, symptomatology, and medications) failed to be associated with target location performance during early visual masks. Thus, findings point to the fragility of early visual percepts marking genetic liability for schizophrenia and possibly serving as an endophenotype specific to the disorder.
Although a magnocellular(M)-biased task was selectively employed (ie, target location, not identity), the presence of deficits only at short SOA’s in relatives of schizophrenia patients argues for abnormal M-type processes within early visual areas as most reflective of genetic liability for schizophrenia. The early perceptual deficit in relatives is most evident at the first SOA where the target and mask do not overlap in time (ie, 27 ms). At 0 and 13 ms SOAs, the target and mask images overlap and may well be processed as a single stimulus. The first point where the mask is a separable event in time is at 27 ms when there is a 14 ms gap between the offset of the target and the onset of the mask. It is then that the masking performance deficit is present in the relatives of schizophrenia patients.
Previous investigations that included both M-biased and P-biased masking tasks have yielded evidence that individuals who carry genetic risk for schizophrenia primarily exhibit deficits on tasks tapping magnocellular rather than parvocellular processes.8,11 Results of electrophysiological studies suggest that aberrations in gamma range oscillations noted in individuals with schizophrenia during masking paradigms may reflect an aspect of the neural underpinnings of impaired performance seen in the disorder.38 Also, functional magnetic resonance imaging (MRI) has revealed that people with schizophrenia fail to activate the lateral occipital complex during masking paradigms39 and that the region appears to interact less richly with other aspects of the neural architecture supporting visual object perception.40 Interestingly, when transcranial magnetic stimulation is used to interrupt processes of the occipital lobe, similar impairments in task performance are seen in schizophrenia patients and healthy control participants.41 It may be that anomalies in gamma oscillations and lateral occipital hemodynamic response reflect the more generalized impairment in perceiving masked visual stimuli observed in schizophrenia rather than the specific early perceptual abnormalities noted in the present study and by other researchers.8,11 Indeed, abnormal lateral occipital complex function during masking paradigms appears not to mark genetic liability for the disorder.42
In summary, a direct comparison of people with schizophrenia, bipolar disorder, and their first-degree biological relatives on a magnocellular-biased visual masking task showed that deficient performance was specific to schizophrenia and genetic liability for the disorder. People with bipolar disorder or genetic liability for bipolar disorder showed intact visual perception during masking. The early perceptual deficit shared by people with schizophrenia and their biological relatives appears not to result from other aspects of the disorder because performance generally failed to be associated with levels of symptomatology, impaired attention and memory, and medication dosage. Given recent concern with traditional approaches to classifying mental disorders, quantitative markers of genetic liability for severe psychopathology may serve to guide development of a more etiologically valid classification system. In this way, visual backward masking may be a promising marker for schizophrenia, but it is presently of questionable value in individual differential diagnostic assessment. Nonetheless, the findings from the present study suggest that fragile early percepts mark genetic liability for schizophrenia and that the neural processes, which account for these abnormalities may lie in early visual areas.
Funding
This work was supported by grants from the Department of Veterans Affairs Clinical Science Research Service (I01CX000227) and the Department of Energy for Mental Illness and Neuroscience Discovery (MIND) Institute (DE-FG02-99ER62764), and the Mental Health Patient Service Line at the Veterans Affairs Medical Center, Minneapolis, Minnesota.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
We thank Michael F. Green, PhD and his staff for provision of the backward masking computer program and consultation during study implementation. We also thank participants of the study, research assistants for their work, as well as Cheryl A. Olman, PhD for review of this report. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Nuechterlein KH, Dawson ME. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- 2.Braff DL, Saccuzzo DP. The time course of information-processing deficits in schizophrenia. Am J Psychiatry. 1985;142:170–174. doi: 10.1176/ajp.142.2.170. [DOI] [PubMed] [Google Scholar]
- 3.Rund BR, Landro NI, Orbeck AL. Stability in backward masking performance in schizophrenics, affectively disturbed patients, and normal subjects. J Nerv Ment Dis. 1993;181:233–237. doi: 10.1097/00005053-199304000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Lee J, Nuechterlein KH, Subotnik KL, et al. Stability of visual masking performance in recent-onset schizophrenia: an 18-month longitudinal study. Schizophr Res. 2008;103:266–274. doi: 10.1016/j.schres.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braff DL, Saccuzzo DP. Effect of antipsychotic medication on speed of information processing in schizophrenic patients. Am J Psychiatry. 1982;139:1127–1130. doi: 10.1176/ajp.139.9.1127. [DOI] [PubMed] [Google Scholar]
- 6.Butler PD, Harkavy-Friedman JM, Amador XF, Gorman JM. Backward masking in schizophrenia: relationship to medication status, neuropsychological functioning, and dopamine metabolism. Biol Psychiatry. 1996;40:295–298. doi: 10.1016/0006-3223(96)00007-8. [DOI] [PubMed] [Google Scholar]
- 7.Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Backward masking in unmedicated schizophrenic patients in psychotic remission: possible reflection of aberrant cortical oscillation. Am J Psychiatry. 1999;156:1367–1373. doi: 10.1176/ajp.156.9.1367. [DOI] [PubMed] [Google Scholar]
- 8.Green MF, Nuechterlein KH, Breitmeyer B. Backward masking performance in unaffected siblings of schizophrenic patients. Evidence for a vulnerability indicator. Arch Gen Psychiatry. 1997;54:465–472. doi: 10.1001/archpsyc.1997.01830170091012. [DOI] [PubMed] [Google Scholar]
- 9.Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Forward and backward visual masking in unaffected siblings of schizophrenic patients. Biol Psychiatry. 2006;59:446–451. doi: 10.1016/j.biopsych.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 10.Bedwell JS, Brown JM, Miller LS. The magnocellular visual system and schizophrenia: what can the color red tell us? Schizophr Res. 2003;63:273–284. doi: 10.1016/s0920-9964(02)00356-0. [DOI] [PubMed] [Google Scholar]
- 11.Keri S, Kelemen O, Benedek G, Janka Z. Different trait markers for schizophrenia and bipolar disorder: a neurocognitive approach. Psychol Med. Jul 2001;31:915–922. doi: 10.1017/s0033291701004068. [DOI] [PubMed] [Google Scholar]
- 12.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 13.Faraone SV, Kremen WS, Lyons MJ, Pepple JR, Seidman LJ, Tsuang MT. Diagnostic accuracy and linkage analysis: how useful are schizophrenia spectrum phenotypes? Am J Psychiatry. 1995;152:1286–1290. doi: 10.1176/ajp.152.9.1286. [DOI] [PubMed] [Google Scholar]
- 14.Iacono WG. Identifying psychophysiological risk for psychopathology: examples from substance abuse and schizophrenia research. Psychophysiology. 1998;35:621–637. [PubMed] [Google Scholar]
- 15.Gershon ES, Alliey-Rodriguez N, Liu C. After GWAS: searching for genetic risk for schizophrenia and bipolar disorder. Am J Psychiatry. 2011;168:253–256. doi: 10.1176/appi.ajp.2010.10091340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivleva EI, Morris DW, Moates AF, Suppes T, Thaker GK, Tamminga CA. Genetics and intermediate phenotypes of the schizophrenia–bipolar disorder boundary. Neurosci Biobehav Rev. 2010;34:897–921. doi: 10.1016/j.neubiorev.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. II. Specifying the visual channels. Arch Gen Psychiatry. 1994;51:945–951. doi: 10.1001/archpsyc.1994.03950120017004. [DOI] [PubMed] [Google Scholar]
- 18.Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. I. Specifying a mechanism. Arch Gen Psychiatry. 1994;51:939–944. doi: 10.1001/archpsyc.1994.03950120011003. [DOI] [PubMed] [Google Scholar]
- 19.Green MF, Lee J, Wynn JK, Mathis KI. Visual masking in schizophrenia: overview and theoretical implications. Schizophr Bull. 2011;37:700–708. doi: 10.1093/schbul/sbr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breitmeyer BG, Ogmen H. Recent models and findings in visual backward masking: a comparison, review, and update. Percept Psychophys. 2000;62:1572–1595. doi: 10.3758/bf03212157. [DOI] [PubMed] [Google Scholar]
- 21.Lieb K, Denz E, Hess R, Schuttler R, Kornhuber HH, Schreiber H. Preattentive information processing as measured by backward masking and texton detection tasks in adolescents at high genetic risk for schizophrenia. Schizophr Res. 1996;21:171–182. doi: 10.1016/0920-9964(96)00025-4. [DOI] [PubMed] [Google Scholar]
- 22.Koelkebeck K, Ohrmann P, Hetzel G, Arolt V, Suslow T. Visual backward masking: deficits in locating targets are specific to schizophrenia and not related to intellectual decline. Schizophr Res. 2005;78:261–268. doi: 10.1016/j.schres.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Duffy A, Hajek T, Alda M, Grof P, Milin R, MacQueen G. Neurocognitive functioning in the early stages of bipolar disorder: visual backward masking performance in high risk subjects. Eur Arch Psychiatry Clin Neurosci. 2009;259:263–269. doi: 10.1007/s00406-008-0862-3. [DOI] [PubMed] [Google Scholar]
- 24.MacQueen GM, Grof P, Alda M, Marriott M, Young LT, Duffy A. A pilot study of visual backward masking performance among affected versus unaffected offspring of parents with bipolar disorder. Bipolar Disord. 2004;6:374–378. doi: 10.1111/j.1399-5618.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 25.Fleming K, Green MF. Backward masking performance during and after manic episodes. J Abnorm Psychol. 1995;104:63–68. doi: 10.1037//0021-843x.104.1.63. [DOI] [PubMed] [Google Scholar]
- 26.Tam WC, Sewell KW, Deng HC. Information processing in schizophrenia and bipolar disorder: a discriminant analysis. J Nerv Ment Dis. 1998;186:597–603. doi: 10.1097/00005053-199810000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Schubert DL, Saccuzzo DP, Braff DL. Information processing in borderline patients. J Nerv Ment Dis. 1985;173:26–31. doi: 10.1097/00005053-198501000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Saccuzzo DP, Braff DL. Information-processing abnormalities: trait- and state-dependent components. Schizophr Bull. 1986;12:447–459. doi: 10.1093/schbul/12.3.447. [DOI] [PubMed] [Google Scholar]
- 29.Goghari VM, Sponheim SR. Divergent backward masking performance in schizophrenia and bipolar disorder: association with COMT. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:223–227. doi: 10.1002/ajmg.b.30583. [DOI] [PubMed] [Google Scholar]
- 30.Saccuzzo DP, Braff DL. Early information processing deficit in schizophrenia. New findings using schizophrenic subgroups and manic control subjects. Arch Gen Psychiatry. 1981;38:175–179. doi: 10.1001/archpsyc.1981.01780270061008. [DOI] [PubMed] [Google Scholar]
- 31.Green M, Walker E. Attentional performance in positive- and negative-symptom schizophrenia. J Nerv Ment Dis. 1986;174:208–213. doi: 10.1097/00005053-198604000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Green MF, Nuechterlein KH, Breitmeyer B. Development of a computerized assessment for visual masking. Int J Methods Psychiatr Res. 2002;11:83–89. doi: 10.1002/mpr.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venables NC, Bernat EM, Sponheim SR. Genetic and disorder-specific aspects of resting state EEG abnormalities in schizophrenia. Schizophr Bull. 2009;35:826–839. doi: 10.1093/schbul/sbn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Docherty AR, Sponheim SR. Anhedonia as a phenotype for the Val158Met COMT polymorphism in relatives of patients with schizophrenia. J Abnorm Psychol. 2008;117:788–798. doi: 10.1037/a0013745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rassovsky Y, Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Modulation of attention during visual masking in schizophrenia. Am J Psychiatry. 2005;162:1533–1535. doi: 10.1176/appi.ajp.162.8.1533. [DOI] [PubMed] [Google Scholar]
- 36.Granholm E, Verney SP. Pupillary responses and attentional allocation problems on the backward masking task in schizophrenia. Int J Psychophysiol. 2004;52:37–51. doi: 10.1016/j.ijpsycho.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Kumar CT, Christodoulou T, Vyas NS, et al. Deficits in visual sustained attention differentiate genetic liability and disease expression for schizophrenia from bipolar disorder. Schizophr Res. 2010;124:152–160. doi: 10.1016/j.schres.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Wynn JK, Light GA, Breitmeyer B, Nuechterlein KH, Green MF. Event-related gamma activity in schizophrenia patients during a visual backward-masking task. Am J Psychiatry. 2005;162:2330–2336. doi: 10.1176/appi.ajp.162.12.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green MF, Lee J, Cohen MS, et al. Functional neuroanatomy of visual masking deficits in schizophrenia. Arch Gen Psychiatry. 2009;66:1295–1303. doi: 10.1001/archgenpsychiatry.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harvey PO, Lee J, Cohen MS, et al. Altered dynamic coupling of lateral occipital complex during visual perception in schizophrenia. Neuroimage. 2011;55:1219–1226. doi: 10.1016/j.neuroimage.2010.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luber B, Stanford AD, Malaspina D, Lisanby SH. Revisiting the backward masking deficit in schizophrenia: individual differences in performance and modeling with transcranial magnetic stimulation. Biol Psychiatry. 2007;62:793–799. doi: 10.1016/j.biopsych.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee J, Cohen MS, Engel SA, et al. Regional brain activity during early visual perception in unaffected siblings of schizophrenia patients. Biol Psychiatry. 2010;68:78–85. doi: 10.1016/j.biopsych.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.