Abstract
Background
The clinical high-risk state for psychosis (HRP) is associated with an enhanced probability of developing a psychotic episode over a relatively short period of time. However, the extent to which different diagnostic types of illness develop remains unclear.
Methods
A systematic review was performed to identify studies of HRP participants reporting International Classfication of Diseases/Diagnostic and Statistical Manual of Mental Disorders diagnostic outcomes at follow-up. Demographic, clinical, and methodological variables were extracted from each publication or obtained directly from its authors. A meta-analysis was performed of transition to schizophrenic (SP) or affective psychoses (AP) and to specific diagnostic categories. Statistical heterogeneity and small study bias were assessed, and meta-regressions were performed.
Results
Twenty-three studies were retrieved, including a total of 2182 HRP participants, 560 (26%) of them developed a frank psychotic disorder over the follow-up time (mean = 2.35 y). Among HRP participants who developed psychosis, 73% were diagnosed with SP and only 11% with AP (Risk Ratio, RR = 5.43, 95% CI from 3.35 to 8.83). The specific transition risk to ICD/DSM schizophrenia was of 15.7% (over 2.35y). Heterogeneity was statistically significant and moderate in magnitude. Use of basic symptoms criteria in the baseline clinical assessment was associated with a further increase in the proportion progressing to SP vs AP (RR = 17.1). There was no evidence of publication bias and the sensitivity analysis confirmed robustness of the above results.
Conclusions
The HRP state is heterogeneous in term of longitudinal diagnoses; however, the current HRP diagnostic criteria appear strongly biased toward an identification of early phases of SP rather than AP.
Keywords: psychosis, prodromal, high risk, schizophrenia, ARMS, SIPS, BS, affective psychosis, bipolar
Introduction
Over the last 2 decades, there has been increasingly interest in people presenting with potentially prodromal symptoms of psychosis.1 A substantial body of research data has been reported including several clinical trials that aimed to delay or prevent the onset of psychotic disorder.2 Recently, academic interest has been translated into clinical psychiatry to the extent that there is an ongoing debate as to whether an attenuated psychosis syndrome should be included in Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5; website).3,4,5 This clinical syndrome has been variably termed “Prodromal,” “At-Risk Mental State,” “Ultra High Risk,” or “Clinical High Risk”.6 Individuals in these studies are generally young and referred from clinical care services when distress, dysfunction, and help-seeking behaviors define a disorder rather than a primary risk state. Here, we use the term “clinical high-risk state for psychosis” (HRP) as we evaluate psychosis outcomes. Operationalized criteria have been developed to identify it6 and usually require one of the following presentations: Attenuated Psychotic Symptoms (APS), full-blown psychotic symptoms that are brief and self-limiting (Brief Limited Intermittent Psychotic Episode, BLIP), a significant decrease in functioning in the context of a genetic risk for schizophrenia (Genetic Risk and Deterioration Syndrome, GRD), or basic symptoms (BS),7 alongside with distress, dysfunction and help seeking behavior. A number of recent meta-analyses have confirmed that belonging to one of these HRP groups is associated with significant impairments in neuropsychological performance8 and alterations in brain structure,9,10 function, and neurochemistry.11,12 These abnormalities are associated with a consistent risk of developing a psychotic episode of 18% at 6 months, 22% at 1 year, 29% at 2 years, 32% at 3 years and 36% after 3 years, a risk which is substantially greater than in the general population (1%).6 Although transition risks from an HRP state are comparable across inclusion criteria and prodromal services,6 the specific diagnostic outcomes of the HRP individuals are mostly unknown.
The continuum model of psychosis underlying much HRP research emphasizes similarities across different psychotic diagnostic categories; however, there are also important differences between these disorders, in particular between affective psychoses (AP: depression with psychotic features and bipolar disorder with psychotic features) or schizophrenic psychoses (SP: schizophrenia, schizophreniform disorder, and schizoaffective disorder).13 More importantly, these differences may directly impact on the development of new preventative strategies in the HRP state, in particular on the basis of emerging evidence suggesting specific clinical presentation and needs in the early phases of bipolar14,15 or depressive16 disorders.
It is unclear to what extent current HRP criteria identify developing AP rather than SP. Because of small sample sizes, individual studies have yielded conflicting and uncertain results. To the best of our knowledge, there is no review or meta-analysis quantifying diagnostic outcomes in the HRP samples. We conducted here a new literature search to specifically estimate the mean risk of transition to AP and SP in HRP subjects. We first collected the Diagnostic and Statistical Manual of Mental Disorders (DSM)/International Classfication of Diseases (ICD) longitudinal diagnoses from different prodromal groups worldwide and then we performed a quantitative meta-analysis. We also specifically estimated how the risk of transition varied across the specific diagnoses of psychosis. Finally, we tested the potentially confounding effect of between-center variations in the assessment instruments and diagnostic criteria used, the demographic features of the samples, and the types of treatment they were administered.
Methods
Selection Procedures
Search Strategies.
A systematic search strategy was used to identify relevant studies. Two independent researchers (P.F.P. and I.B.) conducted a two-step literature search and then extracted the data. First, a PubMed and Embase search was performed to identify putative studies reporting diagnostic outcomes in subjects at increased clinical risk (HRP) for psychosis. The search was conducted up to September 2011, with no time span specified for date of publication. The following search terms were used: “psychosis risk,” “ultra high risk,” “prodromal psychosis,” “basic symptoms,” “structured interview for prodromal symptoms,” “at risk mental state,” “psychosis transition.” In a second step, the reference lists of the articles included in the review were manually checked for any studies not identified by the computerized literature search. There was no language restriction, although all the included articles were in English.
Selection Criteria.
Studies were included if they met the following criteria (a) were reported in an original article in a peer-reviewed journal, (b) had involved subjects at HRP for psychosis defined according to established international criteria (see below), and (c) had reported follow-up (DSM or ICD) diagnoses of psychosis (schizophrenia, schizophreniform disorder, schizoaffective disorder, depression with psychotic features, bipolar disorder with psychotic features, delusional disorder, brief psychotic episode, and psychosis NOS). When the inclusion criteria for the HRP group were not clearly defined, the study was excluded. Studies of subjects at genetic risk for psychosis (twins, first- or second-degree relatives) or schizotypal personality disorder were not included. When there were 2 or more studies from the same center, we contacted the authors to clarify whether there was overlap in the respective samples. Duplicating studies were excluded; if several articles dealt with the same population, we selected the article with the largest sample. When studies reported the proportion of transition to psychosis at follow-up irrespective of DSM or ICD diagnosis, we contacted the respective authors to collect the specific diagnoses.
Recorded Variables.
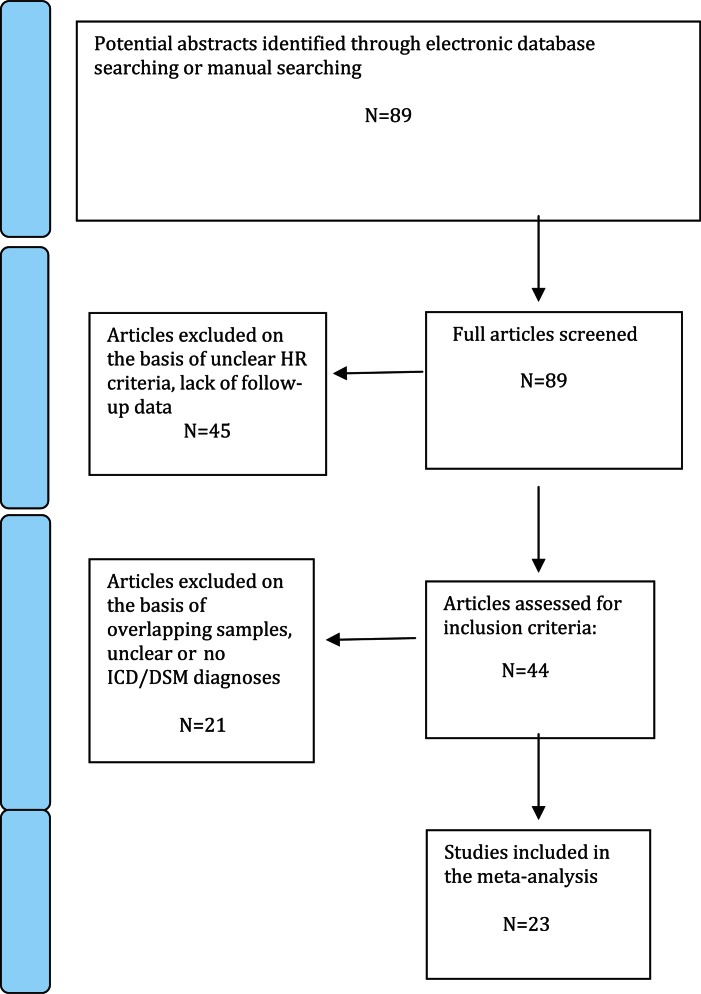
The variables for each article included in the meta-analysis were research center, inclusion criteria for the HRP state, psychometric instruments employed to assess the psychosis risk and transition to psychosis (see online supplementary material), international criteria employed to assess the type of psychosis (DSM/ICD), sample size (HRP at baseline on an intention-to-treat-basis), number of HRP subjects who made transition to psychosis, number of diagnoses in each diagnostic group, duration of follow-up, year of publication, gender (proportion of females), mean age of participants, and exposure to antipsychotics. To achieve a high standard of reporting, we have adopted “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” guidelines17(see Figure 1).
Fig. 1.
PRISMA flow-chart of search strategy used for the inclusion of the studies considered in the current meta-analysis.
Quality Assessment.
We used a simple objective rating system of study quality that coded studies quality on a scale of 0 to 1018 assigning 2 points each for: a description of the sampling method, the presence of clearly stated inclusion criteria, the assessment of ethnic diversity, the assessment of educational diversity, and comprehensive describing of outcome. Because evidence on the validity of quality ratings in observational research is lacking, we employed the MOOSE approach19 with broad initial inclusion of studies and use of sensitivity analysis to determine incremental effects of lower-quality studies.
Statistical Analysis
Data were entered into an electronic database and analyzed with a quantitative meta-analytical approach using Comprehensive Meta-Analysis Software (CMA) version 2 (Biostat, Inc., Englewood, NJ).20 CMA software allows the meta-analysis of risks in a single group using the number of events and the total sample21 and employs the same computational algorithms used by the Cochrane collaborators to weight studies by the inverse variance method.20 Meta-analysis of diagnostic proportions was performed using logit transformation. The primary outcome was transition to development of SP (schizophrenia, schizophreniform disorder, and schizoaffective disorder) vs AP (depression with psychotic features and bipolar disorder with psychotic features). A secondary analysis was performed of the risk of developing specific DSM/ICD diagnoses of schizophrenia, schizophreniform disorder, schizoaffective disorder, depressive disorder with psychotic features, bipolar disorder with psychotic features, delusional disorder, brief psychotic disorder, and psychosis NOS.
To determine whether categorical factors modified the transition risks (inclusion criteria for the HRP state, treatment with antipsychotics, criteria employed to define the psychosis onset), subgroup analyses were performed.18 The influence of continuous moderator variables (age, year of publication, proportion of females, and duration of follow-up) was tested using meta-regression analyses. The slope of meta-regression (β-coefficient: direct [+] or inverse [−]) of the regression line indicates the strength of a relationship between moderator and outcome. To limit risk of false positive (type I) errors arising from multiple comparisons, we adjusted P < .05 by dividing α with the number of meta-regressions.
Heterogeneity among study point estimates was assessed with the Q statistic18 with magnitude of heterogeneity being evaluated with the I 2index.22 Because the studies in this meta-analysis were characterized by consistent statistical heterogeneity, random effects models were used. The possibility of a small study bias, such as publication bias was examined by visual inspection of funnel plots and Egger’s test.23 In addition, we used the fail-safe procedure of Orwin,24which is based on effect sizes that would be considered practically insignificant rather than the traditional null-effect reference. This generated a number of unpublished studies with effects at the estimated population base risk for the development of psychotic disorders25 that would be needed to move estimates to a nonsignificant difference from base risks. To assess the robustness of the results, we performed sensitivity analyses by sequentially removing each study and rerunning the analysis. We also conducted a separate analysis excluding studies with quality ratings in the lowest third to determine if potential methodological weaknesses influenced meta-analytic estimates.
Results
Retrieved Sample
Twenty-three studies published between 2001 and 2011 met the HRP inclusion criteria (figure 1). The overall database comprised 2182 HRP subjects (age range 15–29 y, 44% females) (table 1). Inclusion and transition criteria employed across studies are detailed in the online supplementary material.
Table 1.
HRP Studies Included in the Meta-Analysis
| Author | Year | Research Center | Assessment Instrument | Psychosis Diagnosis | N | Psychosis Risk | Females % | Age | |
| HR | HR-T | ||||||||
| Klosterklotter26 | 2001 | Multicenter (CER) | BSABS | DSM-IV | 110 | 77 | 0.70 | 46.4 | 29.0 |
| Miller27 | 2003 | New Haven (PRIME) | SOPS/SIPS | DSM-IV | 14 | 8 | 0.57 | ? | 18.0 |
| Yung28 | 2004 | Melbourne (PACE) | BPRSa | DSM-IV | 104 | 36 | 0.35 | 51.0 | 19.4 |
| Mason29 | 2004 | Newcastle (PAS) | BPRSa | DSM-IV + OPCRIT | 74 | 37 | 0.50 | 47.3 | 17.3 |
| Lencz30 | 2006 | New York (RAP) | SOPS | DSM-IV | 38 | 12 | 0.31 | 42.0 | 17.0 |
| Schultze-Lutter31 | 2007 | Cologne (FETZ) | SPIA | DSM-IV | 146 | 51 | 0.35 | 30.8 | 24.4 |
| Phillips32 | 2007 | Melbourne (PACE) | BPRSa | DSM-IV | 59 | 22 | 0.37 | 42.3 | 20.0 |
| Cornblatt33 | 2007 | New York (RAP) | SOPS/SIPS | DSM-IV | 48 | 12 | 0.25 | 39.6 | 16.0 |
| Borgwardt34 | 2008 | Basel (FEPSY) | BSIP | ICD-10 + OPCRIT | 20 | 10 | 0.50 | 60.0 | 25.0 |
| Koutsouleris35 | 2009 | Munich (FETZ) | SPIA, SIPS | ICD-10 | 46 | 15 | 0.33 | 37.0 | 25.1 |
| Woodsb , 36 | 2009 | Multicenter (NAPLS) | SIPS | DSM-IV | 377 | 59 | 0.16 | 37.9 | 18.2 |
| Keri37 | 2009 | Hungary | CAARMS | DSM-IV | 67 | 31 | 0.46 | 46.3 | ? |
| Lemos-Giráldez38 | 2009 | Cantabria | SIPS | DSM-IV | 61 | 14 | 0.23 | 34.4 | 22.0 |
| Ruhrmann39 | 2010 | Multicenter (EPOS) | BSABS, SIPS | DSM-IV | 245 | 37 | 0.15 | 44.1 | 23.0 |
| Nelson40 | 2010 | Melbourne (PACE) | CAARMS | DSM-IV | 168 | 15 | 0.09 | 60.7 | 18.3 |
| Sabb41 | 2010 | Los Angeles | SIPS | DSM-IV | 40 | 15 | 0.38 | 30.0 | 17.4 |
| Velthorst42 | 2010 | Amsterdam | SIPS | DSM-IV | 77 | 20 | 0.26 | 33.8 | 19.2 |
| Mittal43 | 2010 | Multicenter (Los Angeles + Atlanta) | SIPS | DSM-IV | 90 | 24 | 0.27 | 32.2 | 16.0 |
| Simon44 | 2010 | Bruderholz Switzerland | SIPS | DSM-IV | 72 | 7 | 0.10 | 40.3 | 20.3 |
| Demjaha45 | 2010 | London (OASIS) | CAARMS | ICD-10 | 122 | 18 | 0.15 | 42.6 | 23.4 |
| Bechdolf46 | 2010 | Melbourne (PACE) | CAARMS | ICD-10 | 92 | 20 | 0.22 | 65.2 | 18.0 |
| Amminger47 c | 2010 | Vienna | CAARMS | DSM-IV | 40 | 11 | 0.28 | 66.7 | 16.4 |
| Ziermans48 | 2011 | Utrecht (DUPS) | BSABS, SIPS/SOPS | DSM-IV | 72 | 9 | 0.13 | 30.5 | 15.3 |
Note: HRP, clinical high risk; APS, Attenuated Psychotic Symptoms; BLIPS, Brief Limited Intermittent Psychotic Symptoms; GRD, Genetic Risk and Deterioration Syndrome; BS, Basic Symptoms; BSABS, Bonn Scale for the Assessment of Basic Symptoms; SOPS/SIPS, Scale Of Prodromal Symptoms and Structured Interview for Prodromal Symptoms; BPRS, Brief Psychiatric Rating Scale; CAARMS, Comprehensive Assessment of the At Risk Mental State; BSIP, Basel Screening Instrument for Psychosis; SPIA, Schizophrenia Proneness Instrument; PACE, Personal Assessment and Crisis Evaluation; PAS, Psychological Assistance Service; PRIME, Prevention Through Risk Identification, Management and Education; FETZ, Early Recognition and Intervention Center for mental crises; EPOS, Prospective European Prediction of Psychosis Study; FEPSY, Früherkennung von Psychosen; NAPLS, North American Prodrome Longitudinal Study; RAP: Recognition And Prevention; CER:Cologne Early Recognition; OASIS, Outreach and Support in South London; DUPS, Dutch Predictor of Psychosis Study; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; ICD-10, International Classification of Diseases, Tenth Edition; OPCRIT, Operational Criteria checklist.
All these studies employed the PACE (At Risk Mental State, ARMS) criteria before the CAARMS was developed and used BPRS or PANSS (Positive and Negative Syndrome Scale) to assess APS, BLIPS, or GRD.
Revised analysis of Cannon et al,49 only subjects with a formal DSM diagnosis were included.
Placebo group only.
Outcomes of the HRP State
The 2182 HRP subjects were followed-up for an average period of 2.35 years and 560 of them (26%, 95% CI from 23% to 36%) developed a frank psychotic episode over time.
Schizophrenic or Affective Psychoses?
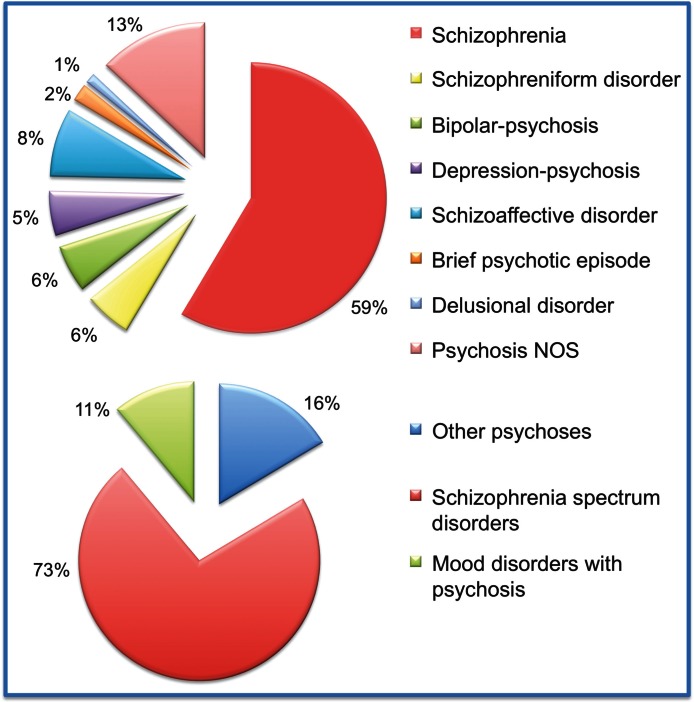
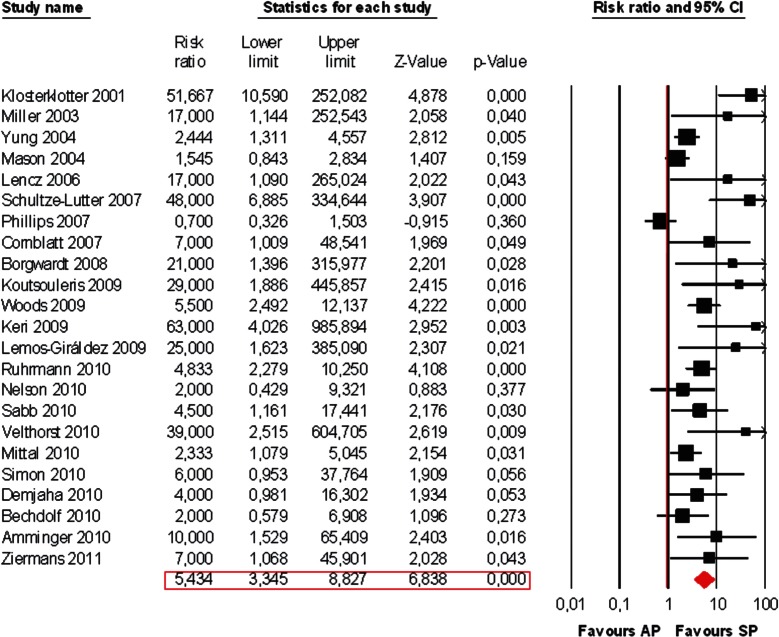
We then formally computed at meta-analytical level the risk ratio toward the development of SP vs AP. Within the HRP who later transited to full psychosis, 73% developed an SP and 11% AP (figure 2). There was a significant risk pattern toward the development of SP vs AP (Risk Ratio, RR = 5.434, 95% CI from 3.345 to 8.827, Z = 6.838, P < .001, figure 3).
Fig. 2.
Descriptive analysis of International Classification of Diseases /Diagnostic and Statistical Manual of Mental Disorders diagnoses in the high-risk subjects who later developed a frank psychotic episode (n = 560). Specific meta-analytical risk estimates across the different diagnostic categories are reported in table 2.
Fig. 3.
Meta-analysis of transition risk ratio in the HRP state: affective psychoses (AP) vs schizophrenic psychoses (SP). Random effect models applied.
The proportion of ICD/DSM diagnoses within the 560 HRP subjects who later developed psychosis is depicted in figure 2. The exact transition risks towards specific DSM/ICD psychotic disorders was formally tested in separate meta-analyses (n = 8). There was a transition risk to schizophrenia of 15.7% over the 2.35 years follow-up time. The transition risks to the other psychotic disorders are summarized in table 2.
Table 2.
Independent Meta-Analyses (n = 8) Addressing the Risk of Developing Specific ICD/DSM Psychotic Disorders From an HRP State Over Follow-up (2.35 y)
| ICD/DSM Diagnosis | HR Sample | Number of Cases | Risk | 95% CI |
| Schizophrenia | 2182 | 328 | 0.157 | 0.103–0.232 |
| Schizophreniform disorder | 2182 | 32 | 0.021 | 0.013–0.032 |
| Schizoaffective disorder | 2182 | 46 | 0.025 | 0.015–0.042 |
| Bipolar disorder with psychotic features | 2182 | 32 | 0.022 | 0.016–0.031 |
| Depressive disorder with psychotic features | 2182 | 30 | 0.018 | 0.010–0.032 |
| Delusional disorder | 2182 | 7 | 0.009 | 0.006–0.015 |
| Brief psychotic disorder | 2182 | 12 | 0.012 | 0.008–0.018 |
| Psychosis NOS | 2182 | 73 | 0.044 | 0.031–0.062 |
Note: Each line reports the meta-analytical risk estimate for a specific psychotic disorder across the included studies (n = 23, sample size = 2182, random effect models applied). DSM, Diagnostic and Statistical Manual of Mental Disorders; ICD, International Classification of Diseases; HRP, clinical high risk state for psychosis.
Moderator Factors
Meta-regressions showed that publication year, and duration of follow-up had no significant impact on the meta-analytical estimates (respectively: β = .091, Z = 1.955, P = .071; β = −.347, Z = −2.505, P = .081). Age of HRP had a small albeit significant effect, with older subjects presenting a trend toward higher rates of SP vs AP (β = .143, 95% CI from 0.041 to 0.231, Z = 2.818, Q = 9.943, P = 0.005). Subgroup analyses showed no significant modulating effect of the diagnostic criteria (ICD vs DSM) or exposure to antipsychotics (treated vs untreated) on the primary outcome measure (P > .05). Conversely, studies employing BS alone or in combination with ultra high risk criteria (i.e. APS+GRD+BLIP) were strongly balanced toward the identification of SP rather than AP (RR SP vs AP in studies using BS = 17.068, 95% CI from 4.61 to 63.27; RR SP vs AP in UHR studies = 3.815, 95% CI from 2.362 to 6.162; between groups Q = 21.108, P < .001). A similar but significant effect was observed for gender: the majority (63%) of HRP subjects who later transited to psychosis (SP + AP) were males (RR males vs females = 1.818, 95% CI from 1.327 to 2.489, Z = 3.725, Q = 11.761, P < .001).
Heterogeneity, Publication Bias, Sensitivity, and Quality Analyses
Heterogeneity across studies was statistically significant and moderate in magnitude (Q = 54.293, P < .001, I 2 = 41.613). Visual inspection of funnel plots revealed no obvious evidence of small study bias, and quantitative evaluation of publication bias, as measured by the Egger intercept, was nonsignificant (P = .569). The Orwin24 fail-safe procedure estimated that 69 unpublished studies would be needed to bring the overall meta-analytic estimate of transition risk to be nonsignificantly different from the base prevalence risk of psychotic disorders in the general population.50 No study affected the meta-analytic estimate by more than 4.7%. Removing studies with quality ratings in the lowest 30% decreased the meta-analytic estimate of transition risk by only 5.7%. The pattern of differences across the subanalyses remained essentially unchanged in direction and magnitude.
Discussion
This is the first meta-analysis to quantitatively measure the DSM/ICD diagnostic outcomes in subjects at enhanced clinical risk for psychosis. In a database of 2182 HRP subjects, we found a greater risk toward the development of SP than AP over a mean follow-up period of 2.35 years (RR = 5.43). A total of 73% of HRP subjects who later transited to full-blown illness developed SP while only 11% developed AP. Specific Transition risk to ICD/DSM schizophrenia was of 15.7%. There was a significant modulator effect for HRP inclusion criteria, gender, and age of participants.
These results are of great relevance to the indicated prevention of psychosis. They confirm that the available HRP criteria (in particular the BS criteria, see below) are strongly biased toward the identification of early prodromal phases of SP rather than AP. A recent study screened a nationally representative sample of 8028 persons for AP and SP and showed the lifetime prevalence for SP is 1.26% and for AP 0.59%.50 However, it is not possible to directly compare our risks with those observed in the general population. To date HRP transition estimates have largely been made in samples of help-seeking subjects who were referred because they were regarded as potentially at risk for psychosis and thus would be expected to have a higher risk of psychosis than those in the general population. Under this scenario, it is usually assumed that the HRP criteria are conceptually biased toward the identification of the prodromal phase of SP rather than AP. However, the present study is the first to formally test this assumption at meta-analytical level. To our best knowledge, our study is also the first one to exactly quantify the risk of developing ICD/DSM schizophrenia in the HRP state (15.7% over 2.35 y). The historical and conceptual developments of current HRP criteria were grounded on research into the putative prodromal phases of schizophrenia-related psychoses, mainly schizophrenia. The term “prodromal” was first introduced by Mayer-Gross in 1932 and further developed by Huber who investigated the BS in prodromal schizophrenia.51 In the late 80s (1989), for the first time, the prodromal symptoms were examined on a representative population. It was shown that the vast majority of schizophrenia cases the disorder began with a prodromal phase, which lasted on average 5 years.51 Consequently, current HRP criteria are conceptually related to positive psychotic symptoms.52 For this reason it can be argued that the psychotic threshold employed by prodromal services is biased toward the identification of positive psychotic episode, and HRP subjects who develop severe negative or affective symptoms (but not severe positive symptoms) may still be categorized as not having made a transition.53 This raises the suspect that the actual prevalence of AP is underestimated within the HRP individuals. However, in all studies included in the present meta-analysis, transition to psychosis was first evaluated clinically within the prodromal team and thereafter confirmed according to established international diagnostic criteria (ICD/DSM), in many cases with the additional support of standardized instruments (ie. Structured Clinical Interview for DSM and Operational Criteria checklist criteria). Our results seem thus truly attributable to the underlying higher power of HRP to detect SP prodrome than AP prodrome. The finding that 73% of HRP will develop an SP and 11% an AP may thus be reliably used to inform future basic research aiming at distinguish the neurobiological correlates of the early phases of the 2 disorders.54
On the other hand, the presence of 11% of HRP subject who will later develop an AP should also impact the future development of HRP criteria. Although current HRP criteria are built toward the identification of SP, their psychopathological boundaries are not so well defined. For example, available GRD criteria admit the presence of relatives of patients diagnosed with AP, coupled with functional decline. If the scope of HRP criteria was to solely identify subjects at risk for SP, such an inclusion criteria may appear somewhat contradictory.55 Further questions arise with respect to the frequent comorbid affective symptoms observed in HRP individuals at the time of the first assessment. Nonspecific and negative or affective symptoms are key features of early psychosis, and one of the largest retrospective studies of prodromal schizophrenia reported that nearly 75% of patients experience these symptoms up to 5 years prior to the onset of positive symptoms.56 A recent study found that nearly 70% of those who were referred to, but subsequently declined prodromal service did engage with other clinical services addressing anxiety and mood dysregulation problems.57 These individuals may present with HRP psychotic features closed to the AP spectrum that are not well addressed by the traditional prodromal services. As current HRP international agreement and practice dictates that clients themselves must be help seeking before any formal intervention can be implemented,58 individuals with more pronounced affective or negative psychotic features are less likely to engage with the prodromal service if no assertive assessment is provided. In line with such a hypothesis, a diagnosis of psychosis was given to 23% of the individuals who were referred to but then declined the prodromal service, a psychosis risk very close to that observed in subjects diagnosed with an HRP who fully engage with the service.57 It is also possible that HRP symptoms may not only indicate a specific risk for SP but also suggest a more general underlying psychopathology that predisposes one to other mental disorders.59 In line with this, a prospective 30-year span study demonstrated that subclinical psychosis generally represents a risk factor for the development of common mental disorders and a liability for cooccurring disorders, including dysthymia, bipolar disorder, social phobia, and obsessive-compulsive disorder59.
Over the past few years, there has been additional evidence indicating affective or negative symptoms may be associated with later increased risk of developing AP and tentative assessment criteria are under development.60 There are also several specific arguments supporting the development of a nonpsychotic bipolar prodrome research and intervention strategy. First, there is a strong genetic vulnerability to bipolar disorder: the lifetime risk is 15%–30% in persons with one first-degree relative with bipolar disorder and up to 75% in persons with 2 affected first-degree relatives.61 Furthermore, there is a long duration from first onset of symptoms to initiation of specific treatment and anxiety, concentration difficulties, antisocial behavior, and substance use are usually present during these early stages predicting an unfavorable course.61 The presence of psychotic features and in particular the number of manic episodes is also correlated with higher relapse rates, more cognitive deficits, and an unfavorable overall course.61 Finally, the response to lithium, atypical antipsychotics, and psychotherapy.is usually greater during the early phases of illness and declines with increasing number of episodes.61 These “Bipolar At Risk” criteria (BAR) comprise the peak age range of the first onset of bipolar disorder (15–25 y) and fulfilling 1 of the 3 groups: subthreshold mania symptoms (group 1), minor depression plus cyclothymic features (group 2), and minor depression plus genetic risk (group 3).15 One study has retrospectively assessed prevalence of BAR criteria within the HRP samples by internal medical-file audit of baseline assessments. Very interestingly, the authors found 12.7% of the HRP subjects also met the BAR criteria.15 This is striking as it is very close to our result of 11% HRP developing an AP, which is the first to provide meta-analytical evidence for the existence of a prodromal phase to affective spectrum disorders. It will be important for future studies to define the baseline psychopathological, neurobiological, and functional characteristics of the HRP-AP vs HRP-SP in order to optimize criteria for AP risk and evaluate interventions for indicated prevention of psychosis. In particular, prospective studies in larger samples, with longer follow-up periods and adequate psychometric measures of transition, are needed to provide further validity of these criteria. Retrospective studies indicated mania and schizophrenia prodrome characteristics overlapped considerably.60 However, subsyndromal unusual ideas seem more likely part of the schizophrenia prodrome, while obsessions/compulsions, suicidality, difficulty thinking/communicating, depressed mood, decreased concentration/memory, tiredness/lack of energy, mood lability, and physical agitation seem more likely part of the mania prodrome.60 The overlap between BAR and HRP criteria is well confirmed by our findings. In fact, with our meta-analysis available, it is clear that the HRP state is heterogeneous in term of diagnosis and underlying features. This may give account for the several inconsistencies across the available literature. For example, there is emerging interest for the potential role played by antidepressant33,62 or mood stabilizers63 in preventing the psychotic onset in HRP cohorts. Identifying the subset of HRP subjects who lay on the AP rather than SP spectrum may definitively improve the risk/benefit ratio for such experimental treatments and support effective interventions to be developed in the field.
Our results were controlled for a number of potential confounders including publication year, age, gender, duration of follow-up, exposure to antipsychotics, ICD/DSM, and HRP inclusion criteria. The latter had strong influence meta-analytical estimates, with studies using BS rather than UHR criteria (i.e. APS+BLIP+GRD) strongly balanced toward the identification of SP rather than AP. This result is in line with the historical development of the BS approach, which was tailored toward the identification of the prodromal phases of schizophrenia. Male gender was also found to be associated with higher risk to psychosis. Age had a small effect, with older HRP more likely to develop SP rather than AP. With the above moderators, we were able to explain 79% of the observed heterogeneity. Overall, our moderator analysis suggests heterogeneity of findings may be reduced in future studies by consensus conferences to standardize the HRP inclusion criteria and the criteria employed to define the psychotic threshold. It should be also noted that the included studies are usually based on clinical referrals to specialized services. Clinicians in such services form supporting relationships with the patients address distress, identify treatment targets such as anxiety, depression, sleep disturbance, and substance abuse, provide a perspective for patient and family, and support the patient in social and role function.64 The observed transition to psychosis risks are therefore not necessarily the natural course of the HRP state. Furthermore, it is important to acknowledge that the ICD/DSM diagnoses are only the initial first-episode psychosis diagnoses and that subsequent diagnostic stability is not warranted. Finally, as noted above here, the HRP data come from help-seeking samples, which are nonepidemiologically representative of the general population, thus sampling biases could have influenced the observed AP vs SP transition risk comparison. chotic bipolar disorder with attenuated positive symptoms detectable by the HRP criteria could have been systematically excluded lowering the AP transition risk on the basis of sampling bias.
Conclusions
The HRP state is heterogeneous in term of longitudinal diagnoses, with 73% of transitions satisfying DSM/ICD criteria for SP and 11% for AP (RR = 5.43). With this finding available, future HRP studies are requested to address the impact of different diagnostic outcomes on baseline psychopathological characteristics, treatments, and development of dedicated services.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
The authors declare no conflicts of interest in relation to the manuscript.
References
- 1.McGorry PD, Nelson B, Amminger GP, et al. Intervention in individuals at ultra-high risk for psychosis: a review and future directions. J Clin Psychiatry. 2009;70:1206–1212. doi: 10.4088/JCP.08r04472. [DOI] [PubMed] [Google Scholar]
- 2.Ruhrmann S, Schultze-Lutter F, Bechdolf A, Klosterkotter J. Intervention in at-risk states for developing psychosis. Eur Arch Psychiatry Clin Neurosci. 2010;260(suppl 2):S90–S94. doi: 10.1007/s00406-010-0139-5. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter WT, van Os J. Should attenuated psychosis syndrome be a DSM-5 diagnosis? Am J Psychiatry. 2011;168:460–463. doi: 10.1176/appi.ajp.2011.10121816. [DOI] [PubMed] [Google Scholar]
- 4.Nelson B, Yung AR. Should a risk syndrome for first episode psychosis be included in the DSM-V? Curr Opin Psychiatry. 2011;24:128–133. doi: 10.1097/YCO.0b013e32834190cd. [DOI] [PubMed] [Google Scholar]
- 5.Fusar-Poli P, Yung A. Should attenuated psychosis syndrome be included in the DSM5? The debate. Lancet. 2012;379:591–592. doi: 10.1016/S0140-6736(11)61507-9. [DOI] [PubMed] [Google Scholar]
- 6.Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: a meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- 7.Yung AR, Nelson B, Stanford C, et al. Validation of “prodromal” criteria to detect individuals at ultra high risk of psychosis: 2 year follow-up. Schizophr Res. 2008;105:10–17. doi: 10.1016/j.schres.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Fusar-Poli P, Deste G, Smieskova R, et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 2012 doi: 10.1001/archgenpsychiatry.2011.1592. In press. [DOI] [PubMed] [Google Scholar]
- 9.Fusar-Poli P, Borgwardt S, Crescini A, et al. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2011;35:1175–1185. doi: 10.1016/j.neubiorev.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. [published online ahead of print November 10, 2011] Schizophr Bull. 2011. doi: 10.1093/schbul/sbr134. doi:10.1093/schbul/sbr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smieskova R, Fusar-Poli P, Allen P, et al. Neuroimaging predictors of transition to psychosis – A systematic review and meta-analysis. Neurosci Biobehav Rev. 2010;34:1207–1222. doi: 10.1016/j.neubiorev.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Fusar-Poli P, Perez J, Broome M, et al. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2007;31:465–484. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Altamura AC, Goikolea JM. Differential diagnoses and management strategies in patients with schizophrenia and bipolar disorder. Neuropsychiatr Dis Treat. 2008;4:311–317. doi: 10.2147/ndt.s2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNamara RK, Nandagopal JJ, Strakowski SM, DelBello MP. Preventative strategies for early-onset bipolar disorder: towards a clinical staging model. CNS Drugs. 2010;24:983–996. doi: 10.2165/11539700-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Bechdolf A, Nelson B, Cotton SM, et al. A preliminary evaluation of the validity of at-risk criteria for bipolar disorders in help-seeking adolescents and young adults. J Affect Disord. 2010;127:316–320. doi: 10.1016/j.jad.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Iacoviello BM, Alloy LB, Abramson LY, Choi JY. The early course of depression: a longitudinal investigation of prodromal symptoms and their relation to the symptomatic course of depressive episodes. J Abnorm Psychol. 2010;119:459–467. doi: 10.1037/a0020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paulson JF, Bazemore SD. Prenatal and postpartum depression in fathers and its association with maternal depression: a meta-analysis. JAMA. 2010;303:1961–1969. doi: 10.1001/jama.2010.605. [DOI] [PubMed] [Google Scholar]
- 19.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 20.Borenstein MHL, Higgins J, Rothstein H. Comprehensive Meta-Analysis Version 2. Englewood, NJ: Biostat; 2005. [Google Scholar]
- 21.Nielssen O, Large M. Rates of homicide during the first episode of psychosis and after treatment: a systematic review and meta-analysis. Schizophr Bull. 2010;36:702–712. doi: 10.1093/schbul/sbn144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipsey M, Wilson D. Practical Meta-Analysis. Thousand Oaks, CA: Sage Publications; 2000. [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orwin R. A fail-safe N for effect size in meta-analysis. J Educ Stat. 1983;8:157–159. [Google Scholar]
- 25.Van Os J, Linscott R, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–195. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- 26.Klosterkotter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- 27.Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- 28.Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67:131–142. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 29.Mason O, Startup M, Halpin S, Schall U, Conrad A, Carr V. Risk factors for transition to first episode psychosis among individuals with “at-risk mental states”. Schizophr Res. 2004;71:227–237. doi: 10.1016/j.schres.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Lencz T, Smith CW, McLaughlin D. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry. 2006;59:863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Schultze-Lutter F, Klosterkötter J, Picker H, Steinmeyer E, Ruhrmann S. Predictin first-episode psychosis by basic symptoms criteria. Clin Neuropsychiatry. 2007;4:11–22. [Google Scholar]
- 32.Phillips LJ, McGorry PD, Yuen HP. Medium term follow-up of a randomized controlled trial of interventions for young people at ultra high risk of psychosis. Schizophr Res. 2007;96:25–33. doi: 10.1016/j.schres.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Cornblatt BA, Lencz T, Smith CW. Can antidepressants be used to treat the schizophrenia prodrome? Results of a prospective, naturalistic treatment study of adolescents. J Clin Psychiatry. 2007;68:546–557. doi: 10.4088/jcp.v68n0410. [DOI] [PubMed] [Google Scholar]
- 34.Borgwardt SJ, McGuire PK, Aston J. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr Res. 2008;106:108–114. doi: 10.1016/j.schres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Koutsouleris N, Schmitt GJ, Gaser C. Neuroanatomical correlates of different vulnerability states for psychosis and their clinical outcomes. Br J Psychiatry. 2009;195:218–226. doi: 10.1192/bjp.bp.108.052068. [DOI] [PubMed] [Google Scholar]
- 36.Woods SW, Addington J, Cadenhead KS. Validity of the prodromal risk syndrome for first psychosis: findings from the North American Prodrome Longitudinal Study. Schizophr Bull. 2009;35:894–908. doi: 10.1093/schbul/sbp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keri S, Kiss I, Kelemen O. Effects of a neuregulin 1 variant on conversion to schizophrenia and schizophreniform disorder in people at high risk for psychosis. Mol Psychiatry. 2009;14:118–119. doi: 10.1038/mp.2008.1. [DOI] [PubMed] [Google Scholar]
- 38.Lemos-Giraldez S, Vallina-Fernandez O, Fernandez-Iglesias P. Symptomatic and functional outcome in youth at ultra-high risk for psychosis: a longitudinal study. Schizophr Res. 2009;115:121–129. doi: 10.1016/j.schres.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Ruhrmann S, Schultze-Lutter F, Salokangas RK. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67:241–251. doi: 10.1001/archgenpsychiatry.2009.206. [DOI] [PubMed] [Google Scholar]
- 40.Nelson B, Yung AR. Can clinicians predict psychosis in an ultra high risk group? Aust N Z J Psychiatry. 2010;44:625–630. doi: 10.3109/00048671003620210. [DOI] [PubMed] [Google Scholar]
- 41.Sabb FW, van Erp TG, Hardt ME. Language network dysfunction as a predictor of outcome in youth at clinical high risk for psychosis. Schizophr Res. 2010;116:173–183. doi: 10.1016/j.schres.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Velthorst E, Nieman DH, Klaassen RM. Three-year course of clinical symptomatology in young people at ultra high risk for transition to psychosis. Acta Psychiatr Scand. 2010;123:36–42. doi: 10.1111/j.1600-0447.2010.01593.x. [DOI] [PubMed] [Google Scholar]
- 43.Mittal VA, Walker EF, Bearden CE. Markers of basal ganglia dysfunction and conversion to psychosis: neurocognitive deficits and dyskinesias in the prodromal period. Biol Psychiatry. 2010;68:93–99. doi: 10.1016/j.biopsych.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon AE, Umbricht D. High remission rates from an initial ultra-high risk state for psychosis. Schizophr Res. 2010;116:168–172. doi: 10.1016/j.schres.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Demjaha A, Valmaggia L, Stahl D, Byrne M, McGuire P. Disorganization/cognitive and negative symptom dimensions in the at-risk mental state predict subsequent transition to psychosis. Schizophr Bull. 2012;38:351–359. doi: 10.1093/schbul/sbq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bechdolf A, Thompson A, Nelson B. Experience of trauma and conversion to psychosis in an ultra-high-risk (prodromal) group. Acta Psychiatr Scand. 2010;121:377–384. doi: 10.1111/j.1600-0447.2010.01542.x. [DOI] [PubMed] [Google Scholar]
- 47.Amminger GP, Schafer MR, Papageorgiou K. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 48.Ziermans T, Schothorst P, Sprong M, Van Engeland E. Transition and remission in adolescents at ultra-high risk for psychosis. Schizophr Res. 2011;126:58–64. doi: 10.1016/j.schres.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 49.Cannon TD, Cadenhead K, Cornblatt B. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perala J, Suvisaari J, Saarni SI, et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry. 2007;64:19–28. doi: 10.1001/archpsyc.64.1.19. [DOI] [PubMed] [Google Scholar]
- 51.Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high risk state: a comprehensive state of the art review. Arch Gen Psychiatry. 2012 doi: 10.1001/jamapsychiatry.2013.269. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fusar-Poli P, Borgwardt S. Integrating the negative psychotic symptoms in the high risk criteria for the prediction of psychosis. Med Hypotheses. 2007;69:959–960. doi: 10.1016/j.mehy.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 53.Yung AR, Nelson B, Thompson A, Wood SJ. The psychosis threshold in ultra high risk (prodromal) research: is it valid? Schizophr Res. 2010;120:1–6. doi: 10.1016/j.schres.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 54.Yu K, Cheung C, Leung M, Li Q, Chua S, McAlonan G. Are bipolar disorder and schizophrenia neuroanatomically distinct? An anatomical likelihood meta-analysis. Front Hum Neurosci. 2010;4:189. doi: 10.3389/fnhum.2010.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kendler KS, McGuire M, Gruenberg AM, O'Hare A, Spellman M, Walsh D. The Roscommon Family Study. IV. Affective illness, anxiety disorders, and alcoholism in relatives. Arch Gen Psychiatry. 1993;50:952–960. doi: 10.1001/archpsyc.1993.01820240036005. [DOI] [PubMed] [Google Scholar]
- 56.Häfner H, Maurer K, Löffler W, et al. The ABC Schizophrenia Study: a preliminary overview of the results. Soc Psychiatry Psychiatr Epidemiol. 1998;33:380–386. doi: 10.1007/s001270050069. [DOI] [PubMed] [Google Scholar]
- 57.Green CE, McGuire PK, Ashworth M, Valmaggia LR. Outreach and Support in South London (OASIS). Outcomes of non-attenders to a service for people at high risk of psychosis: the case for a more assertive approach to assessment. Psychol Med. 2011;41:243–250. doi: 10.1017/S0033291710000723. [DOI] [PubMed] [Google Scholar]
- 58.IEPA IEPAWG. International clinical practice guidelines for early psychosis. Br J Psychiatry. 2005;18(suppl):S120–S124. doi: 10.1192/bjp.187.48.s120. [DOI] [PubMed] [Google Scholar]
- 59.Rossler W, Hengartner MP, Ajdacic-Gross V, Haker H, Gamma A, Angst J. Sub-clinical psychosis symptoms in young adults are risk factors for subsequent common mental disorders. Schizophr Res. 2011;131:18–23. doi: 10.1016/j.schres.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 60.Correll CU, Penzner JB, Frederickson AM, et al. Differentiation in the preonset phases of schizophrenia and mood disorders: evidence in support of a bipolar mania prodrome. Schizophr Bull. 2007;33:703–714. doi: 10.1093/schbul/sbm028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bechdolf A, Ratheesh A, Wood S, et al. Rationale and first results of developing at-risk (prodromal) criteria for bipolar disorder. Curr Pharm Des. 2012;18:358–375. doi: 10.2174/138161212799316226. [DOI] [PubMed] [Google Scholar]
- 62.Fusar-Poli P, Valmaggia L, McGuire P. Can antidepressants prevent psychosis? Lancet. 2007;370:1746–1748. doi: 10.1016/S0140-6736(07)61732-2. [DOI] [PubMed] [Google Scholar]
- 63.Berger G, Wood S, Ross M, et al. Neuroprotective effects of low-dose lithium in individuals at ultra-high risk for psychosis. A longitudinal MRI/MRS study. Curr Pharm Des. 2012;18:570–575. doi: 10.2174/138161212799316163. [DOI] [PubMed] [Google Scholar]
- 64.McGlashan TH, Addington J, Cannon T, et al. Recruitment and treatment practices for help-seeking “prodromal” patients. Schizophr Bull. 2007;33:715–726. doi: 10.1093/schbul/sbm025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.