Abstract
Psychotic disorders are associated with neurocognitive alterations that aggregate in unaffected family members, suggesting that genetic vulnerability to psychotic disorder impacts neurocognition. The aim of the present study was to investigate whether selected schizophrenia candidate single nucleotide polymorphisms (SNPs) are associated with (1) neurocognitive functioning across populations at different genetic risk for psychosis (2) and psychotic disorder. The association between 152 SNPs in 43 candidate genes and a composite measure of neurocognitive functioning was examined in 718 patients with psychotic disorder. Follow-up analyses were carried out in 750 unaffected siblings and 389 healthy comparison subjects. In the patients, 13 associations between SNPs and cognitive functioning were significant at P < .05, situated in DRD1, DRD3, SLC6A3, BDNF, FGF2, SLC18A2, FKBP5, and DNMT3B. Follow-up of these SNPs revealed a significant and directionally similar association for SLC18A2 (alternatively VMAT2) rs363227 in siblings (B = −0.13, P = .04) and a trend association in control subjects (B = −0.10, P = .12). This association was accompanied by a significantly increased risk for psychotic disorder associated with the T allele (linear OR = 1.51, 95% CI 1.10–2.07, P = .01), which was reduced when covarying for cognitive performance (OR = 1.29, 95% CI 0.92–1.81, P = .14), suggesting mediation. Genetic variation in VMAT2 may be linked to alterations in cognitive functioning underlying psychotic disorder, possibly through altered transport of monoamines into synaptic vesicles.
Keywords: cognition, single nucleotide polymorphism, psychosis, schizophrenia, siblings, vesicular monoamine transporter 2 (VMAT2)
Introduction
Generalized cognitive alterations, extending across most domains of cognitive functioning, present themselves as a stable trait-related aspect of schizophrenia that appears to be present before the onset of the disorder. Similar, though attenuated, cognitive alterations have been reported in unaffected biological relatives of schizophrenia patients, suggesting these changes may reflect the expression of genetic vulnerability for schizophrenia.1,2 Several studies have provided evidence for significant heritability of cognitive functioning in patients with schizophrenia3 and in healthy controls,4 in particular for general cognitive functioning rather than specific cognitive skills.5 Furthermore, Toulopoulou and colleagues6 reported shared genetic variance accounting for 92% of the covariance between intelligence and schizophrenia. However, despite substantial evidence for cognitive functioning as intermediate phenotype for psychotic disorder, little is known about the underlying molecular-genetic variation. For example, the COMT Val158Met polymorphism has been implicated in both risk for schizophrenia and cognitive functioning, but the evidence for association with neurocognition is weak.7 Another putative risk allele that has been implicated in psychosis susceptibility by genome-wide association studies and is located in the Zinc Finger Protein 804A gene (ZNF804A) has been associated with relatively spared cognitive ability.8 A recent meta-analysis suggests that another gene previously linked to psychosis, DTNBP1, influences general cognitive ability in healthy subjects.9
The aim of the present study was to examine common molecular-genetic variation underlying general cognitive functioning associated with psychotic disorder. That is, if cognitive functioning truly represents an intermediate phenotype, the genes associated with cognitive alterations in patients would be expected to be identical to the genetic determinants of general cognitive ability in healthy control subjects.10 This is in agreement with the liability-threshold model describing genetic risk for schizophrenia as the sum of possibly thousands of common single nucleotide polymorphisms (SNPs), each of them having individually small effects.11,12 This model also states that, despite the fact that underlying genetic risk for schizophrenia is the linear additive function of individual SNPs, the actual phenotype only becomes expressed once a certain threshold of risk (when interconnected systems can no longer compensate the effects of individual SNPs) is reached. Following this reasoning, it may be argued that the examination of associations between cognition and individual SNPs can be most efficiently explored against a background of above-average genetic risk. Thus, SNPs were first tested for association with cognitive performance alterations in a large sample of patients, recruited as part of the Genetic Risk and OUtcome of Psychosis (GROUP) study. Significantly associated SNPs were subsequently reexamined for their effects on cognition in unaffected siblings of these patients and in healthy control subjects. Their association with psychotic disorder was studied as well, in order to further investigate whether these SNPs truly increase risk for psychotic disorder by altering cognitive performance across populations at different genetic risk.
Methods
Sample and Measures
The full GROUP sample consisted of 1120 patients with nonaffective psychotic disorder, 1057 siblings of these 1120 patients, 919 parents of the patients and their siblings, and 590 unrelated control subjects. Inclusion criteria were: (1) age range 16–50 years, (2) diagnosis of nonaffective psychotic disorder, and (3) good command of Dutch language. Control subjects had no first- or second-degree relative with a psychotic disorder as established by the Family Interview for Genetic Studies13 with the control subject as the informant. Diagnosis was based on the Diagnostic and Statistical Manual of Mental Disorder, Fourth Edition (DSM-IV) criteria,14 assessed with the Comprehensive Assessment of Symptoms and History (CASH) interview15 or Schedules for Clinical Assessment for Neuropsychiatry (SCAN 2.1).16 DSM-IV diagnoses of the patients were schizophrenia and related disorders (DSM-IV 295.x; n = 945, 84%), other psychotic disorders (DSM-IV 297/298; n = 149, 13%), and psychotic illness in the context of substance abuse or somatic illness (n = 9; 1%) (see GROUP17 for further details).
Cognitive Functioning
All subjects were assessed with a comprehensive neurocognitive test battery containing the following tasks (intended cognitive domains of focus are placed between brackets): Wechsler Adult Intelligence Scale (WAIS)-III Digit Symbol—Coding (processing speed),18 Continuous Performance Test-HQ (attention/vigilance),19,20 Word Learning Task (verbal learning and memory),21 WAIS-III Arithmetic (working memory),18 WAIS-III Block Design (reasoning and problem solving),18 Response-Shifting Task (set shifting), which is a modified version of the Competing Programs Task,22,23 and WAIS-III Information (verbal comprehension).18 To calculate a measure of global cognitive functioning, raw test scores were converted into standardized z scores against the means and SDs of the healthy control group. Z scores were calculated for each cognitive domain and were recoded if necessary such that more negative z scores reflected worse performance for all measures. For verbal learning and memory, the z score was based on the mean value of the z scores for immediate recall and retention rate. For attention/vigilance, the z score was based on the mean value of the z scores for reaction time and for a sensitivity index defined as the number of correct detections of targets minus the number of false alarms for nontarget Q stimuli. For set shifting, the z score used in the analyses was based on the decrement in accuracy during a reversal response rule condition compared with an imitation response rule condition.23,24 The final composite measure of neurocognition was based on the mean of the 7 domain scores (speed of information processing, attention/vigilance, verbal learning and memory, working memory, reasoning and problem solving, set shifting, and verbal comprehension), while allowing for 4 missing values.25 The coefficient alpha for this composite cognition measure was adequate (α = .76 for patient sample; α = .74 for overall sample).
Genotyping
A previous study in the GROUP sample, which examined molecular-genetic interactions with cannabis, selected a total of 179 SNPs.26 Gene selection in this study was based on previous evidence of association with schizophrenia, involvement in dopamine or endocannabinoid signaling or an involvement in the regulation of environmental influences including epigenetic mechanisms (see online supplementary table S1). Because plausible hypotheses with regard to effects on cognition are possible for most of these SNPs and reporting bias (selective reporting of interesting or significant findings) is a considerable concern in psychiatric genetics, the entire set of SNPs was also selected for the present study. These SNPs were selectively determined by Sequenom (Hamburg, Germany) using the Sequenom MassARRAY iPLEX platform at the facilities of the manufacturer; SNPs, therefore, were not selected from a larger set of genome-wide markers. In accordance with a priori quality control criteria of the GROUP study, SNPs with more than 10% genotyping errors were excluded, as were SNPs in marked Hardy-Weinberg disequilibrium (P < .001). Of the 179 SNPs originally included, 23 SNPs were excluded because they had more than 10% genotyping errors, and an additional 3 SNPs were excluded because they were in marked Hardy-Weinberg disequilibrium in the control subjects; no variation was found for 1 variant, leaving a final set of 152 SNPs in 43 genes suitable for analysis.
Statistical Analysis
Associations between SNPs and cognition in patients were examined using linear regression analyses. SNP genotypes were coded 0, 1, or 2 and modeled as linear effects, such that a positive regression coefficient indicated that the minor allele was associated with better cognitive performance. Linear models were used because this method can deal with different genotype distributions, including distributions with a low minor allele frequency, avoiding stratification into small subgroups.27 Given the fact that some families contributed more than one subject, hierarchical clustering of data was taken into account by including a family random effect in the model, using the multilevel random regression XTREG routine in STATA, version 11.28
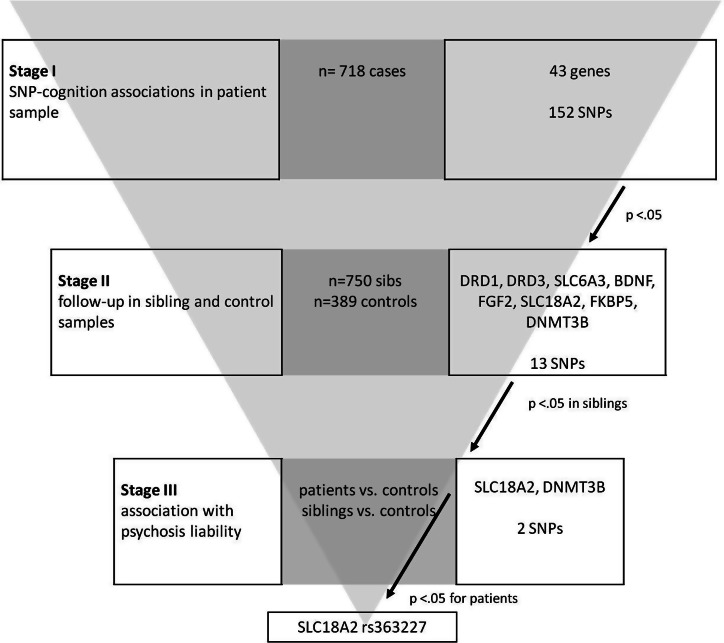
Given the fact that 152 SNPs were tested; a strategy to deal with multiple testing is necessary. The Bonferroni-adjusted significance level for the number of SNPs tested would result in a corrected alpha of α = .05/152 = .0003. Power analysis, however, revealed only a 17.6%, 57.6%, and 85.6% power to detect an effect of, respectively, r 2 = .01, .02, and .03 at this alpha (Quanto program version 1.2.4, http://hydra.usc.edu/GxE), indicating that the efforts to reduce the chance for type I error would, for SNPs that are expected to individually have small effects, also result in a substantial risk for type II error. Therefore, consistency of associations across the different samples and statistical approaches was used to reduce the risk for type I error rather than stringent Bonferroni correction. Thus, the analyses of SNPs with a P value < .05 in the patient group were repeated in the sample of unaffected siblings of these patients as well as in the control sample. Associations that were replicated in the unaffected sibling and control samples where further explored using logistic regression, to test for potential enrichment of the allele associated with worse cognitive functioning in patients with psychotic disorder, or their unaffected siblings, in comparison with control subjects. See figure 1 for an overview of the multistage design. Age and sex were a priori entered into all of the regression analyses as covariates. Subjects with more than 10% genotyping errors were excluded from the analyses.
Fig. 1.
Study design. Overview and outcome of multistage study design utilized, including all samples and single nucleotide polymorphisms (SNPs) analyzed in each stage.
Results
Patients
Genetic data were available for 801 patients, of which 43 (5.4%) had more than 10% genotyping errors. Of the 758 remaining subjects, composite cognition scores were available for 718 patients (stage I in figure 1). Patients had significantly lower composite cognition scores than control subjects (table 1).
Table 1.
Demographic and Clinical Characteristics of Patients, Siblings, and Control Subjects For Whom Both Genetic and Cognitive Data Were Available
| Patients | Siblings | Controls | Between-Group Comparisons | P | |
| n = 718 | n = 750 | n = 389 | Test Statistic | ||
| Age (SD) | 28.0 (8.2) | 27.4 (8.0) | 30.0 (10.4) | F = 12.22 | <.001 |
| Sex (% male) | 75.8 | 46.4 | 44.5 | χ2 = 162.7 | <.001 |
| IQ (SD) | 95.4 (16.5) | 102.6 (15.7) | 109.9 (14.8) | F = 112.8 | <.001 |
| Composite cognition score | −0.60 | −0.20 | 0.01 | F = 133.5 | <.001 |
| Age at onset of psychosis (SD) | 22.5 (6.8) | ||||
| Duration of illness in years (SD) | 4.4 (4.2) | ||||
| Recent onset psychosis (<1 y) | 17.1% | ||||
| Psychotic episodes (number) | 1.73 (1.12) | ||||
| PANSS score | |||||
| Positive | 1.81 (.77) | ||||
| Negative | 2.03 (.84) | ||||
| General | 1.76 (5.3) | ||||
| Current antipsychotics | 79.0% | ||||
| Mean dose a | 5.49 (20.44) | ||||
Note: PANSS, Positive and Negative Syndrome Scale.
Mean dose of antipsychotics in milligram per day haloperidol equivalents.
Nominal associations with cognitive functioning at P < .05 were found for 13 SNPs (8.7%) in DRD1, DRD3, SLC6A3, BDNF, FGF2, SLC18A2, FKBP5, and DNMT3B (see table 2). Therefore, further analyses were conducted with these SNPs in the sibling and control samples (stage II in figure 1). Notably, 8 of the 13 associations identified in stage I were situated in genes that encode proteins implicated in the regulation of synaptic dopamine (DRD1, DRD3, VMAT2, and DAT), out of 32 SNPs tested (in the following genes: COMT, DRD1, DRD2, DRD3, VMAT2, and DAT), significantly more than expected by chance (χ2 (df 1) = 4.3, P < .039).
Table 2.
Associations (P < .05) Between Genetic Polymorphisms and Cognitive Functioning in Patients With Psychotic Disorder
| Single Nucleotide Polymorphism | Gene | Chromosome | Functional Relevance | Risk Allelea | B | P |
| rs265981 | DRD1 | 5 | 5′ UTR | G | 0.11 | .010 |
| rs6280 | DRD3 | 3 | Exonic missense | C | −0.09 | .031 |
| rs456082 | SLC6A3 | 5 | Intronic | C | −0.09 | .047 |
| rs463379 | SLC6A3 | 5 | Intronic | G | −0.09 | .047 |
| rs464049 | SLC6A3 | 5 | Intronic | C | −0.09 | .028 |
| rs988748 | BDNF | 11 | Intronic | G | −0.10 | .027 |
| rs7700205 | FGF2 | 4 | Intronic | C | 0.11 | .025 |
| rs363393 | SLC18A2 | 10 | Intronic | A | 0.13 | .011 |
| rs363338 | SLC18A2 | 10 | Intronic | C | −0.11 | .008 |
| rs363227 | SLC18A2 | 10 | Intronic | T | −0.13 | .017 |
| rs1334894 | FKBP5 | 6 | Intronic | G | −0.13 | .005 |
| rs2424913 | DNMT3B | 20 | Intronic | T | −0.08 | .035 |
| rs406193 | DNMT3B | 20 | 3′ UTR | C | 0.14 | .017 |
Allele associated with worse cognitive performance in patients.
Follow-Up in Sibling and Control Samples
Genetic data were available for 813 siblings, of which 44 (5.4%) had more than 10% genotyping errors. Of these 769 subjects, composite cognition scores were available for 750 siblings. For control subjects, genetic data were available for 413 individuals, of which 19 (4.6%) had more than 10% genotyping errors. Of these 394 subjects, composite cognition scores were available for 389 control subjects. Siblings had significantly lower composite cognition scores than control subjects (table 1).
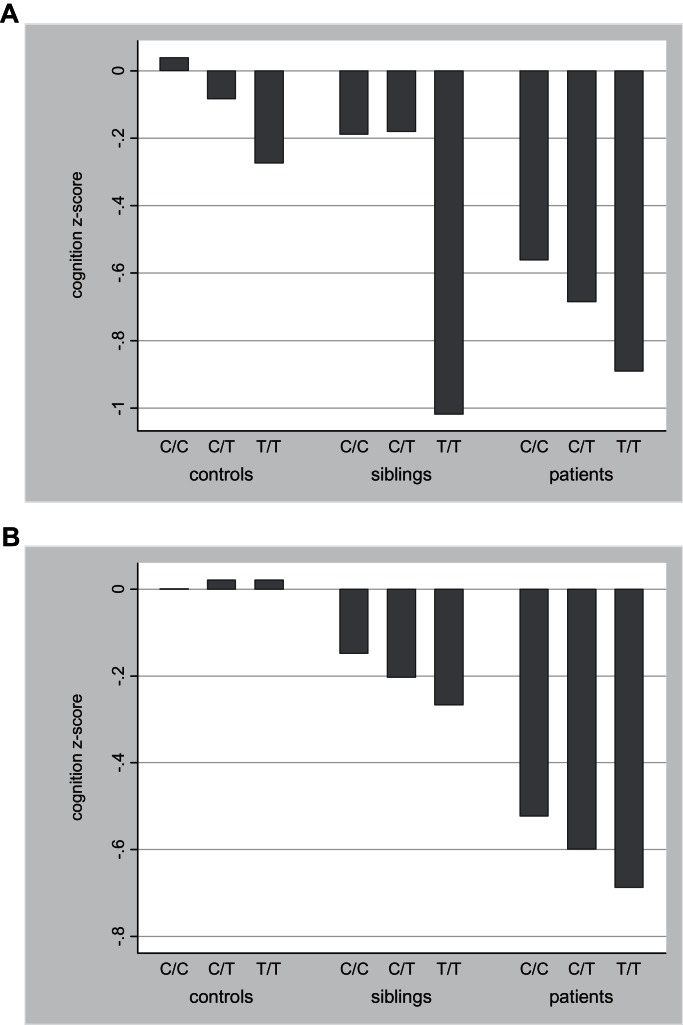
In siblings, there was a significant association between SNP rs363227 in SLC18A2 and cognitive functioning, with the thymine (T) allele conferring risk for poorer cognitive functioning (B = −0.10, P = .04), parallel to the findings in the patient group (B = −0.13, table 2). In controls, the results were suggestive of an additive effect of the T allele resulting in poorer cognitive functioning, although the results were not statistically significant (B = −0.10, P = .12). No other associations were found for the 13 SNPs in siblings and controls, except for a trend association in siblings between SNP rs2424913 in DNMT3B and cognitive functioning (B = −0.06, P = .06), parallel to the findings in the patient group (B = −0.08, table 2). Figure 2 displays the mean composite cognition scores for SLC18A2 rs363227 and DNMT3B rs2424913 genotypes in patients, sibling, and controls. To estimate the proportion of the variance in neurocognitive composites scores explained by these genotypes individually, effect size estimates (partial eta2) were calculated based on linear regression models allowing for the use of observations that are not independent within clusters (families) and adjusted for age and sex. These estimates suggested that SLC18A2 rs363227 as well as DNMT3B rs2424913 explained just under 1% of the variation in cognitive performance, both in the patient sample and in the combined sample with group entered as covariate in the model.
Fig. 2.
Association between cognitive functioning and (a) SLC18A2 rs363227 (controls: C/C n = 316, C/T n = 70, T/T n = 3; siblings: C/C n = 594, C/T n = 144, T/T n = 11; patients: C/C n = 544, C/T, n = 159, T/T n = 15) and (b) DNMT3B rs2424913 (controls: C/C n = 118, C/T n = 192, T/T, n = 78; siblings: C/C n = 218, C/T n = 318, T/T n = 148; patients: C/C n = 210, C/T n = 360, T/T n = 147) (a) SLC18A2 rs363227 (b) DNMT3B rs2424913.
Since the sibling sample is not independent of the patient sample, sensitivity analyses were run for the sibling group, with genotype of the affected family member as a covariate. These analyses yielded similar results for SLC18A2 rs363227 (B = −0.12, P = .04) while the association for DNMT3B rs2424913 became larger and statistically significant when entering genotype of affected family member as a covariate into the regression (B = −0.10, P = .01).
Association With Psychotic Disorder
To investigate whether SLC18A2 rs363277 was also associated with psychosis liability, logistic regression was used to calculate ORs for the frequency of the T allele for the patient and sibling groups compared with the control group (stage III in figure 1). Patients had higher T allele rates than control subjects (P = .01) (table 3). Siblings did not differ in frequency of the T allele compared with control subjects (P = .38). Furthermore, when entering neurocognitive composite scores into the logistic regression analysis, the T allele was no longer significantly enriched in patients (OR = 1.29, 95% CI 0.92–1.81, P = .14), suggesting that the association between SLC18A2 rs363227 and psychotic disorder was mediated by cognitive functioning.
Table 3.
SLC18A2 rs363227 Allele and Genotype Frequency Per Subject Group
| Group | Allele, n (%) | Genotype, n (%) | OR | 95% CI | |||
| C | T | C/C | C/T | T/T | |||
| Patients | 1247 (86.8) | 189 (13.2) | 544 (75.8) | 159 (22.1) | 15 (2.1) | 1.51a | 1.10–2.07 |
| Siblings | 1332 (88.9) | 166 (11.1) | 594 (79.3) | 144 (19.2) | 11 (1.5) | 1.15a | 0.84–1.57 |
| Controls | 702 (90.2) | 76 (9.8) | 316 (81.2) | 70 (18.0) | 3 (0.8) | ||
Comparing group with control subjects (logistic regression adjusted for age and sex).
DNMT3B rs2424913, on the other hand, did not show evidence for association with psychosis liability, as the risk of having a T allele was not higher in either the patient (OR = 1.06, 95% CI 0.81–1.41, P = .66) or the sibling group (OR = 1.05, 95% CI = 0.80–1.38, P = .71).
Discussion
In a large sample of patients with psychotic disorder, a range of associations between SNPs and cognitive functioning was identified at the P < .05 level. The association between cognitive functioning and one of these SNPs, rs363227, located in the SLC18A2 gene, replicated in unaffected siblings of the patients, and a trend for association was also found in healthy control subjects. Furthermore, the risk (T) allele of this SNP was also associated with risk for psychotic disorder, an association that was mediated by its effects on cognition. These findings suggest that SLC18A2 may impart an increased risk for psychosis through its effect on cognitive functioning.
Solute carrier family 18, member 2 (SLC18A2), also called vesicular monoamine transporter 2 (VMAT2), encodes for the VMAT protein that is located in the membranes of presynaptic vesicles of monoamine neurons where it mediates the reuptake of monoamines into the synaptic vesicle of the presynaptic neuron.29 There are very few studies analyzing VMAT in psychoses or in relation to cognitive functioning. Although 2 studies have failed to find a direct link between VMAT2 gene and susceptibility for schizophrenia spectrum disorders,30,31 Gutiérrez and colleagues32 found evidence for a risk haplotype for schizophrenia and bipolar disorder in this gene. Furthermore, Zubieta and colleagues33 reported higher ventral brainstem VMAT binding in patients with schizophrenia and patients with bipolar disorder compared with healthy control subjects. In another study, Zubieta and colleagues34 found that VMAT binding in the thalamus and ventral brainstem of euthymic bipolar patients not only was higher than the expression of VMAT in healthy controls but that VMAT concentrations in these regions also correlated with performance on tests of frontal executive functioning. The findings by Talkowski and colleagues35 indicate that a network of dopaminergic gene variations appears to be implicated in schizophrenia, variants at VMAT2 individually and jointly with other dopaminergic genes conferring the risk for schizophrenia. These studies suggest that VMAT2 may be a candidate gene for psychotic disorder. The present study not only adds further support to this suggestion but also suggests that the VMAT2 gene may exert its impact on psychosis liability through its impact on brain processes affecting cognitive functioning. It may be speculated that VMAT2 expression may exert is effects on cognitive functioning through its impact on the functional integrity of the striatal presynaptic dopamine system that has been shown to be compromised before the onset of schizophrenia36 and may be associated with cognitive mechanisms.36,37 However, increased levels of VMAT2 in schizophrenia have been found in the ventral brainstem,33 but not in the striatum,38 nor did VMAT2 expression correlate with clinical data such as duration of illness, number of hospitalizations, and positive or negative symptoms.38 Furthermore, given that the proportion of variance in cognitive performance explained by rs363227 in VMAT2 was small, and epistatic interactions likely exist,35 further examination at the level of the implicated pathways is justified. The results of this study suggest that pathway-based analysis of genes coding for proteins that regulate synaptic dopamine concentrations may be fruitful, as the number of reported associations in this pathway significantly exceeded chance level expectations.
A second SNP, rs2424913 in DNMT3B, that was associated with cognitive functioning at nominal significance level in patients, replicated in siblings at trend level. In contrast, there was no association between DNMT3B rs2424913 and cognitive functioning in control subjects. Furthermore, the T allele of DNMT3B rs2424913, which corresponded to worse cognitive functioning in schizophrenia patients, was not enriched in the patient sample compared with the control sample. These findings suggest that, whereas VMAT2 may increase the risk for psychotic disorder by impacting on intermediate cognitive processes, DNMT3B may not be directly associated with risk for psychotic disorder through mediation of altered cognitive functioning. It should be noted that functional effects of the 2 intronic SNPs in VMAT2 and DNMT3B have not been elucidated and that they could be tagging a functional variant within the gene. Additional efforts will be needed to identify the putative functional loci.
The most studied hypothesis-driven candidate genes (COMT, DRD3, DRD2, HTR2A, NRG1, BDNF, DTNBP1, and SLC6A4) did not show evidence of reliable association with altered cognitive performance across populations at different genetic risk for psychosis. This is in agreement with a recent study reporting no support that these genes are enriched for common genetic variation in schizophrenia,39 arguing against a notable contribution of these genes to risk for psychosis.
Strengths and Limitations
The present study is unique in that it assessed a large sample of patients with psychotic disorder and their unaffected siblings, using a comprehensive list of a priori candidate SNPs. Nevertheless, some limitations need to be taken into account.
Not all subjects had complete cognitive test scores, which was predominantly caused by computer errors. However, patient status was associated with number of missing test results, patients having significantly more missing values than control subjects. Patients who failed to complete the whole battery may have been more compromised cognitively than those without missing values. The affected individuals in the present sample will constitute a healthier group than the population of patients with a psychotic disorder as a whole, as they were able to participate in the study. This selection is common and will apply to most studies focusing on cognitive functioning in patients with psychotic disorder, likely shifting results toward the null.
Second, the use of SDs of the healthy control subject group to generate z scores for the cognitive domains in the patient group may spuriously inflate z scores if the SDs in the control subject group are smaller than those in the patient group.40 Larger between- and within-subject variation on cognitive tests has often been observed in patient groups. Although this may have impact on the magnitude of the impairments comparing patients with control subjects, it is hard to see how this could have affected associations with SNPs within-subject groups.
The effects of genetic variation on cognitive functioning were studied across the schizophrenia disorder spectrum with most patients currently taking antipsychotic medication, thereby raising the possibility that the observed effects in the patient sample may be confounded by severity of schizophrenia spectrum disorder or medication effects. However, the finding of similar results in the sibling and control samples for the SNP in the VMAT2 gene suggest that the association between genetic variation in VMAT2 and cognitive functioning in patients is not solely related to illness-related factors that do not confer psychosis vulnerability. Furthermore, post hoc analyses revealed that mean dose of antipsychotic medication (B = 0.25, P = .75) and symptoms (positive: B = −0.32, P = .60, negative: B = 0.10, P = .13) were not significantly associated with the VMAT2 SNP, and covarying for antipsychotic dose and positive and negative symptoms in the linear regression model did not attenuate the effect of rs363227 on cognitive functioning in patients (B = −13, P = .02).
Selection of molecular-genetic variation was based on published literature prior to the major genome-wide association studies of schizophrenia. Nevertheless, the selected SNPs are a fair and comprehensive representation of the most widely studied candidate genes for schizophrenia.
Although the sibling and control samples provided replication of the findings of the patient sample, the replication in siblings was not strictly independent as siblings shared 50% of their genetic variation with the patient sample. However, a sensitivity analysis in siblings, covarying for SNP genotype in the affected family member, yielded similar results. Furthermore, the control sample did provide a directional replication independent of the findings in the patient sample.
In conclusion, an association was found between VMAT2 and general cognitive ability in patients with psychotic disorder and in their unaffected siblings, that replicated directionally at trend level in an independent cohort of healthy control subjects, suggests that the hypothesis that VMAT2 genotype influences variation in cognitive performance merits further investigation. Although the results suggest the hypothesis that VMAT2 may be a candidate gene for cognitive alterations encountered in psychotic disorder, the proportion of explained variance in cognitive performance was small.
Funding
The GROUP study was supported by the Geestkracht program of the Dutch Health Research Council (ZON-MW, grant number 10-000-1002) and matching funds from participating universities and mental health care organizations (Site Amsterdam: Academic Psychiatric Centre AMC, Ingeest, Arkin, Dijk en Duin, Rivierduinen, Erasmus MC, GGZ Noord Holland Noord; Site Utrecht: University Medical Centre Utrecht, Altrecht, Symfora, Meerkanten, Riagg Amersfoort, Delta; Site Groningen: University Medical Center Groningen, Lentis, GGZ Friesland,GGZ Drenthe, Dimence, Mediant, GGZ De Grote Rivieren, and Parnassia psycho-medical centre; Site Maastricht: Maastricht University Medical Center, GGZ Eindhoven en de Kempen, GGZ Midden-Brabant, GGZ Oost-Brabant, GGZ Noord- Midden Limburg, Mondriaan Zorggroep, Prins Clauscentrum Sittard, RIAGG Roermond, Universitair Centrum Sint-Jozef Kortenberg, CAPRI University of Antwerp, PC Ziekeren Sint-Truiden, PZ Sancta Maria Sint-Truiden, GGZ Overpelt, OPZ Rekem). The research leading to these results has received funding from the European Community's Seventh Framework Program (grant agreement No. HEALTH-F2-2009-241909, Project EU-GEI). The analyses were supported by unrestricted grants from Jansen-Cilag, Eli Lilly and Company, Astra-Zeneca, and Lundbeck.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
We would like to thank the families for making time and lending an effort to make the GROUP project possible.
Appendix.
Genetic Risk and Outcome of Psychosis (GROUP) investigators:
René S. Kahn, MD PhD and Wiepke Cahn, MD PhD, Department of Psychiatry, Rudolf Magnus Institute of Neuroscience, University Medical Center Utrecht, Utrecht, The Netherlands.
Don H. Linszen, MD PhD and Lieuwe de Haan, MD, PhD, Department of Psychiatry, Academic Medical Centre, University of Amsterdam, Amsterdam, The Netherlands.
Jim van Os, MD PhD, Department of Psychiatry and Psychology, School for Mental Health and Neuroscience, European Graduate School of Neuroscience (EURON), South Limburg Mental Health Research and Teaching Network (SEARCH), Maastricht University Medical Centre, Maastricht, The Netherlands; Department of Psychosis Studies, Institute of Psychiatry, King's College London, King's Health Partners, London, UK.
Durk Wiersma, PhD and Richard Bruggeman, MD PhD, Department of Psychiatry, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Lydia Krabbendam, MSc PhD and Inez Myin-Germeys, MSc PhD, Department of Psychiatry and Psychology, School for Mental Health and Neuroscience, European Graduate School of Neuroscience (EURON), South Limburg Mental Health Research and Teaching Network (SEARCH), Maastricht University Medical Centre, Maastricht, The Netherlands.
References
- 1.Sitskoorn MM, Aleman A, Ebisch SJH, Appels MCM, Kahn RS. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophr Res. 2004;71:285–295. doi: 10.1016/j.schres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Snitz B, MacDonald A, Carter C. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gur RE, Nimgaonkar VL, Almasy L, et al. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry. 2007;164:813–819. doi: 10.1176/ajp.2007.164.5.813. [DOI] [PubMed] [Google Scholar]
- 4.Plomin R, Craig I. Genetics, environment and cognitive abilities: review and work in progress towards a genome scan for quantitative trait locus associations using DNA pooling. Br J Psychiatry Suppl. 2001;178:s41–s48. doi: 10.1192/bjp.178.40.s41. [DOI] [PubMed] [Google Scholar]
- 5.Plomin R, DeFries JC. The genetics of cognitive abilities and disabilities. Sci Am. 1998;278:62–69. doi: 10.1038/scientificamerican0598-62. [DOI] [PubMed] [Google Scholar]
- 6.Toulopoulou T, Picchioni M, Rijsdijk F, et al. Substantial genetic overlap between neurocognition and schizophrenia: genetic modeling in twin samples. Arch Gen Psychiatry. 2007;64:1348–1355. doi: 10.1001/archpsyc.64.12.1348. [DOI] [PubMed] [Google Scholar]
- 7.Barnett JH, Scoriels L, Munafò MR. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol Psychiatry. 2008;64:137–144. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Walters JTR, Corvin A, Owen MJ, et al. Psychosis susceptibility gene ZNF804A and cognitive performance in schizophrenia. Arch Gen Psychiatry. 2010;67:692–700. doi: 10.1001/archgenpsychiatry.2010.81. [DOI] [PubMed] [Google Scholar]
- 9.Zhang JP, Burdick KE, Lencz T, Malhotra AK. Meta-analysis of genetic variation in DTNBP1 and general cognitive ability. Biol Psychiatry. 2010;68:1126–1133. doi: 10.1016/j.biopsych.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Need AC, Attix DK, McEvoy JM, et al. A genome-wide study of common SNPs and CNVs in cognitive performance in the CANTAB. Hum Mol Genet. 2009;18:4650–4661. doi: 10.1093/hmg/ddp413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wray NR, Visscher PM. Narrowing the boundaries of the genetic architecture of schizophrenia. Schizophr Bull. 2010;36:14–23. doi: 10.1093/schbul/sbp137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NIMH.Genetics.Initiative. Family Interview for Genetic Studies (FIGS) Rockville, MD: National Institute of Mental Health; 1992. [Google Scholar]
- 14.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, revisied. Washington, DC: American Psychiatric Association; 2000: 1992. [Google Scholar]
- 15.Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- 16.Wing JK, Babor T, Brugha T, et al. SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry. 1990;47:589–593. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PubMed] [Google Scholar]
- 17.Genetic Risk and Outcome in Psychosis (GROUP) Investigators. Evidence that familial liability for psychosis is expressed as differential sensitivity to cannabis: an analysis of patient-sibling and sibling-control pairs. Arch Gen Psychiatry. doi: 10.1001/archgenpsychiatry.2010.132. 2011;68:138–147. [DOI] [PubMed] [Google Scholar]
- 18.Wechsler D. WAIS-III: Wechsler Adult Intelligence Scale. Administration and Scoring Manual. 3rd ed. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 19.Nuechterlein KHR, Dawson ME. Information processing and attentional functioning in the developmental course of schizophrenics disorders. Schizophr Bull. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- 20.Wohlberg GW, Kornetsky C. Sustained attention in remitted schizophrenics. Arch Gen Psychiatry. 1973;28:533–537. doi: 10.1001/archpsyc.1973.01750340065011. [DOI] [PubMed] [Google Scholar]
- 21.Van der Elst W, Boxtel MPJ, Van Breukelen GJP, Jolles J. Rey's verbal learning test: normative data for 1,855 healthy participants aged 24–81 years and the influence of age, sex, education, and mode of presentation. J Int Neuropsycholog Soc. 2005;11:290–302. doi: 10.1017/S1355617705050344. [DOI] [PubMed] [Google Scholar]
- 22.Bilder RM, Turkel E, Lipschutz-Broch L, Lieberman JA. Antipsychotic medication effects on neuropsychological functions. Psychopharmacol Bull. 1992;28:353–366. [PubMed] [Google Scholar]
- 23.Nolan KA, Bilder RM, Lachman HM, Volavka J. Catechol O-methyltransferase Val158Met polymorphism in schizophrenia: differential effects of Val and Met alleles on cognitive stability and flexibility. Am J Psychiatry. 2004;161:359–361. doi: 10.1176/appi.ajp.161.2.359. [DOI] [PubMed] [Google Scholar]
- 24.Meiran N, Levine J, Meiran N, Henik A. Task set switching in schizophrenia. Neuropsychology. 2000;14:471–482. doi: 10.1037//0894-4105.14.3.471. [DOI] [PubMed] [Google Scholar]
- 25.Quee PJ, van der Meer L, Bruggeman R, et al. Insight in psychosis: relationship with neurocognition, social cognition and clinical symptoms depends on phase of illness. Schizophr Bull. 2011;37:29–37. doi: 10.1093/schbul/sbq133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Winkel R Genetic Risk Outcome of Psychosis Investigators. Family-based analysis of genetic variation underlying psychosis-inducing effects of cannabis: sibling analysis and proband follow-up. Arch Gen Psychiatry. 2011;68:148–157. doi: 10.1001/archgenpsychiatry.2010.152. [DOI] [PubMed] [Google Scholar]
- 27.Cordell HJ, Clayton DG. Genetic association studies. Lancet. 2005;366:1121–1131. doi: 10.1016/S0140-6736(05)67424-7. [DOI] [PubMed] [Google Scholar]
- 28.StataCorp. Stata/SE Statistical Software, Release 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- 29.Henry J-P, Scherman D. Radioligands of the vesicular monoamine transporter and their use as markers of monoamine storage vesicles. Biochem Pharmacol. 1989;38:2395–2404. doi: 10.1016/0006-2952(89)90082-8. [DOI] [PubMed] [Google Scholar]
- 30.Kunugi H, Ishida S, Akahane A, Nanko S. Exon/intron boundaries, novel polymorphisms, and association analysis with schizophrenia of the human synaptic vesicle monoamine transporter (SVMT) gene. Mol Psychiatry. 2001;6:456–460. doi: 10.1038/sj.mp.4000895. [DOI] [PubMed] [Google Scholar]
- 31.Persico A, Wang Z, Black D, et al. Exclusion of close linkage of the dopamine transporter gene with schizophrenia spectrum disorders. Am J Psychiatry. 1995;152:134–136. doi: 10.1176/ajp.152.1.134. [DOI] [PubMed] [Google Scholar]
- 32.Gutiérrez B, Rosa A, Papiol S, et al. Identification of two risk haplotypes for schizophrenia and bipolar disorder in the synaptic vesicle monoamine transporter gene (SVMT) Am J Med Genet B Neuropsychiatr Genet. 2007;144B:502–507. doi: 10.1002/ajmg.b.30499. [DOI] [PubMed] [Google Scholar]
- 33.Zubieta J-K, Taylor SF, Huguelet P, et al. Vesicular monoamine transporter concentrations in bipolar disorder type I, schizophrenia, and healthy subjects. Biol Psychiatry. 2001;49:110–116. doi: 10.1016/s0006-3223(00)00981-1. [DOI] [PubMed] [Google Scholar]
- 34.Zubieta JK, Huguelet P, Ohl LE, et al. High vesicular monoamine transporter binding in asymptomatic bipolar I disorder: sex differences and cognitive correlates. Am J Psychiatry. 2000;157:1619–1628. doi: 10.1176/appi.ajp.157.10.1619. [DOI] [PubMed] [Google Scholar]
- 35.Talkowski ME, Kirov G, Bamne M, et al. A network of dopaminergic gene variations implicated as risk factors for schizophrenia. Hum Mol Genet. 2008;17:747–758. doi: 10.1093/hmg/ddm347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howes OD, Montgomery AJ, Asselin M-C, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 37.O'Daly OG, Joyce D, Stephan KE, Murray RM, Shergill SS. Functional magnetic resonance imaging investigation of the amphetamine sensitization model of schizophrenia in healthy male volunteers. Arch Gen Psychiatry. 2011;68:545–554. doi: 10.1001/archgenpsychiatry.2011.3. [DOI] [PubMed] [Google Scholar]
- 38.Taylor SF, Koeppe RA, Tandon R, Zubieta JK, Frey KA. In vivo measurement of the vesicular monoamine transporter in schizophrenia. Neuropsychopharmacology. 2000;23:667–675. doi: 10.1016/S0893-133X(00)00165-2. [DOI] [PubMed] [Google Scholar]
- 39.Collins AL, Kim Y, Sklar P, O'Donovan MC, Sullivan PF. Hypothesis-driven candidate genes for schizophrenia compared to genome-wide association results. Psychol Med. 2012 doi: 10.1017/S0033291711001607. 42:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychol Rev. 2003;13:43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.