Abstract
Background:
White matter (WM) abnormalities have been implicated in schizophrenia, yet the mechanisms underlying these abnormalities are not fully understood. Several lines of evidence suggest that polyunsaturated fatty acids (PUFAs) play a role in myelination, and there is substantial evidence documenting decreased PUFA concentrations in schizophrenia. We therefore hypothe sized that lower membrane PUFA concentrations may be related to reduced WM integrity in schizophrenia and related disorders. Methods: In 30 male patients with a recent-onset psychotic disorder, erythrocyte membrane PUFA concentrations were assessed and diffusion tensor imaging was performed with voxelwise analysis. Results: Lower total PUFA concentration was associated with lower fractional anisotropy (FA) throughout the corpus callosum and bilateral parietal, occipital, temporal and frontal WM (P < .05, corrected). Of the individual PUFAs, lower arachidonic acid concentration, and to a lesser extent, lower nervonic acid, linoleic acid, and docosapentaenoic acid concentration were significantly associated with lower FA. PUFA concentrations were inversely associated with radial diffusivity but showed little association with axial diffusivity. Greater severity of negative symptoms was associated with lower nervonic acid concentration and lower FA values. Conclusions: Membrane PUFA concentrations appear to be robustly related to brain WM integrity in early phase psychosis. These findings may provide a basis for studies to investigate the effects of PUFA supplementation on WM integrity and associated symptomatology in early psychosis.
Key words: schizophrenia, first-episode psychosis, diffusion tensor imaging, myelin, white matter, polyunsaturated fatty acids
Introduction
White matter (WM) pathology has been implicated in the pathophysiology of schizophrenia. Genetic association studies have repeatedly found that single nucleotide polymorphisms in genes that are involved in WM integrity are associated with schizophrenia.1,2 Postmortem studies have identified reductions in the expression of myelin- and oligodendrocyte-related genes and reduced number of oligodendrocytes in schizophrenia3,4. Finally, a robust body of evidence from diffusion tensor imaging (DTI), a putative magnetic resonance imaging (MRI) measure of WM integrity, suggests compromised WM in patients at high risk for psychosis and first-episode schizophrenia patients including neuroleptic-naive patients.5 These data suggest that abnormalities in myelination may represent a critical node of dysfunction in schizophrenia.
Several lines of evidence suggest that polyunsaturated fatty acids (PUFAs) play a role in myelination. Unsaturated fatty acids are essential constituents of all cell membranes, and the myelin sheaths around the axons are formed from the membranes of oligodendrocytes. Bourre et al. 6 determined in rats that docosahexaenoic acid (DHA, an omega-3 fatty acid) constituted 5.8% of myelin and 5.1% of oligodendrocytes. In rats with PUFA deficiency, morphological myelin changes were found. 7 In experimental allergic encephalomyelitis, a model of acute multiple sclerosis, supplementation with a source of linoleic acid (LA, an omega-6 fatty acid) has shown a marked protective effect.8 Moreover, dietary supplementation with omega-3 fatty acids has been reported to stimulate the expression of myelin proteins in rat brain,9 decrease the occurrence of WM abnormalities in elderly humans,10 and decrease exacerbation rate and disability in multiple sclerosis patients.11
A large body of evidence suggests that PUFA concentrations are decreased in schizophrenia. In particular arachidonic acid (AA, an omega-6 fatty acid) and DHA are found reduced in peripheral blood measures of schizophrenia patients in the early phase of the illness including never-medicated patients,12–16 as well as in postmortem samples.17 Other PUFAs found reduced in schizophrenia include docosapentaenoic acid (DPA, an omega-3 fatty acid),12–15 LA,15,17 and nervonic acid (NA, a mono-unsaturated omega-9 fatty acid).12,13 Intriguingly, a recent placebo-controlled clinical trial that administered omega-3 PUFAs to patients at clinical high risk for psychosis found that omega-3 supplements decreased the risk of transition to psychosis.18
Because of the relationship between PUFAs and myelination, it is plausible that the relationship between PUFAs and schizophrenia may be mediated by effects on WM integrity. To date, there has been little study of PUFAs and WM integrity in patients with schizophrenia. In a preliminary DTI study in 12 patients with recent-onset schizophrenia or related disorder, we found evidence for a relationship between total PUFA concentration in erythrocyte membranes and WM integrity, using a region-of-interest approach.19 We therefore hypothesized that alterations in erythrocyte PUFA concentrations, which reflect membrane phospholipid metabolism in the brain,20,21 may be associated with DTI abnormalities in early phase schizophrenia and related disorders. In the present study, we examined in an independent larger sample at high-field MRI whether (1) there is an effect of specific PUFAs on WM integrity and (2) specific brain regions are implicated, by applying a stringent voxelwise analysis of the cerebral WM tracts.
Methods
Participants
Patients with a diagnosis of a psychotic disorder according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, were recruited from the Department of Early Psychosis of the Academic Medical Center, University of Amsterdam, the Netherlands. Diagnoses were made after assessment with a structured diagnostic interview, history taken from a significant other, all other available clinical information, and a consensus staff meeting with 2 clinical psychiatrists. To exclude confounding effects of sex on WM structure, we included only male patients in our study.22 Exclusion criteria were mental retardation according to DSM-IV criteria, an endocrine or neurological illness that may affect brain structure or fatty acid concentrations, history of head trauma with loss of consciousness for more than 15min, and standard MRI exclusion criteria. After complete description of the study, written informed consent was obtained. This study was approved by the Medical Ethics Committee of the Academic Medical Center, Amsterdam, and by the Institutional Review Board of the Feinstein Institute for Medical Research, Manhasset, NY.
Diffusion Tensor Imaging
All participants were scanned with a 3.0 Tesla MRI system (Philips Medical Systems, Best, the Netherlands). DTI data were acquired using multislice spin echo single-shot echo-planar imaging with the following parameters: TE/TR = 94/5506ms; diffusion sensitivities of b = 0 and b = 1000 s/mm2; 32 diffusion gradient directions, 1 nondiffusion-weighted volume; contiguous (no interslice gap) slices, slice thickness 3mm; field of view 230 × 230mm2; acquisition matrix 112×112; reconstruction matrix 256×256. DTI scan time was approximately 5min.
All data were anonymized prior to analysis. Preprocessing of the DTI data was performed using in-house developed software, written in Matlab (the MathWorks, Natick, MA), on the Dutch Grid (www.biggrid.nl).23 Head motion and deformations induced by eddy currents were corrected by an affine registration of the diffusion-weighted images (DWIs) to the nondiffusion-weighted image. The gradient directions were corrected by the rotation component of the transformation. The DWIs were resampled isotropically. Rician noise in the DWIs was reduced by an adaptive noise-filtering method.24 Nonbrain tissue was removed with an automated algorithm and then manually edited when necessary. From the diffusion tensors, fractional anisotropy (FA) and diffusivity maps were computed. All images were visually inspected to rule out gross artifacts.
Voxelwise analyses of the FA images were carried out using Tract-Based Spatial Statistics (TBSS; www.fmrib.ox.ac.uk/FSL/).25 All subjects’ FA data were registered to the FMRIB58 FA template in MNI standard space through nonlinear registration, using a b-spline representation of the registration warp field. Next, the mean FA image was created and thinned to create a mean FA skeleton that represents the centers of all tracts common to the group. The FA threshold for the mean FA skeleton was set at 0.25. Each subject’s aligned FA data were then projected onto this skeleton and the resulting data were fed into voxelwise cross-subject statistics. To test for local correlations between FA values and PUFA concentrations, permutation-based testing was done with 5000 random permutations across the image. Inference on the statistic maps was carried out using threshold-free cluster enhancement. The final t-statistic image was then thresholded at P < .05, which is fully corrected for multiple comparisons across space (ie, family-wise error). Anatomical location of significant WM clusters was determined with the probabilistic cortical and subcortical atlases and probabilistic WM tractography atlas provided in the FMRIB software library (www.fmrib.ox.ac.uk/FSL/).
PUFA Measurements
Venous blood samples were collected at the Academic Medical Center. Median time between MRI scanning and collection of the blood samples was 68.5 days (range 1–419). Plasma was separated within 4h of collection, and washed erythrocytes were counted and subsequently stored at −80°C until analysis (less than 1 week). Poly- and mono-unsaturated fatty acids in erythrocytes (ie, omega-3 [C18:3n3, C18:4n3, C20:5n3, C22:5n3, C22:6n3], omega-5 [C14:1n5], omega-6 [C18:2n6, C18:3n6, C20:2n6, C20:3n6, C20:4n6, C22:2n6, C22:4n6, C22:5n6], omega-7 [C16:1n7, C18:1n7, C20:1n7], and omega-9 [C16:1n9, C18:1n9, C20:1n9, C20:3n9, C22:1n9, C24:1n9]) were analyzed by capillary gas chromatography as their methyl esters, as described previously.26 Briefly, a 50µl sample of erythrocyte hemolysate was added to 1ml of a 3M methanolic HCl solution, and the lipids were hydrolyzed at 90°C for 4h, achieving simultaneous methylation of the liberated fatty acids. After cooling, the fatty acid methyl esters were extracted with 2ml hexane. Following evaporation of the solvent, the fatty acid methyl esters were separated on a capillary free fatty acid phase column. All concentrations (expressed as pmol/10e6 cells) were calculated with reference to the internal standard 18-methylnonadecanoic acid. Primary fatty acid measures of interest were: total (poly)unsaturated fatty acid concentration (ie, total concentration of the poly- and mono-unsaturated fatty acids described above; referred to next as (P)UFAs), AA, and DHA. Other (P)UFAs included in the analysis were DPA, NA, and LA.
Statistical Analysis
Total (P)UFA concentration was analyzed for voxelwise correlations with FA values of the cerebral WM tracts using TBSS, as described above. In addition, to assess which individual (P)UFAs were driving observed associations, mean FA was extracted from each significant cluster and correlated with AA, DHA, DPA, NA, and LA concentrations (Spearman’s rho, 2 tailed). Furthermore, AA, DHA, DPA, NA, and LA concentrations were each analyzed separately for voxelwise correlations with FA values using TBSS.
To examine which diffusion parameters were driving significant FA findings, radial diffusivity (RD) and axial diffusivity (AD) values were extracted from each significant cluster and correlated with the respective (P)UFA concentration (Spearman’s rho, 2 tailed). While FA is a sensitive, yet nonspecific marker of WM integrity,27 RD and AD may provide some indication to the underlying substrate of observed FA changes, because these DTI indices have been related to histopathological evidence of demyelination and axonal damage, respectively.28
Because one-third of patients had used cannabis in the month prior to blood collection (see below), and considering the impact of cannabis on lipid-arachidonic pathways,29 analyses were repeated in the subsample of patients without cannabis use prior to blood collection. To further explore potential confounders, partial correlations were conducted between the mean FA values of the significant clusters and the (P)UFA concentrations adjusting for age, duration of illness, dose of antipsychotic medication, smoking (daily number of cigarettes),30 and time between DTI scanning and collection of the blood samples.
To test for associations with psychopathology, (P)UFA concentrations and FA values were also correlated with positive and negative symptom scores as assessed with the Positive and Negative Syndrome Scale (PANSS) (Spearman’s rho, 2 tailed).31
Results
Sample Characteristics
Subjects were all male, 68% Caucasian, with a mean total IQ of 91±13 (table 1). Twenty-six patients experienced a first psychotic episode, 2 patients a second episode, and 2 patients a third episode. Twenty-five patients used antipsychotic medication; 2 patients were antipsychotic drug-free at time of blood collection (unknown for 3 patients). Current antipsychotic medication of patients was as follows: risperidone (n = 5; one of whom also used aripiprazole), quetiapine (n = 1), olanzapine (n = 12), clozapine (n = 2; one of whom also used haloperidol), haloperidol (n = 2), and aripiprazole (n = 3) (data missing for 3 patients).
Table 1.
Sample Characteristics (n = 30)
| Diagnosis, n (%) | |
| Schizophrenia | 15 (50%) |
| Schizoaffective disorder | 6 (20%) |
| Schizophreniform disorder | 1 (3.3%) |
| Psychotic disorder, not otherwise specified | 7 (23.3%) |
| Psychotic disorder, amphetamine induced | 1 (3.3%) |
| Age, mean (SD) | 22.7 (3.0) |
| Right handedness, n (%) | 24 (80) |
| Illness duration, years, median (range)1 | 1.4 (0.2–8.2) |
| Age of onset first psychosis, mean (SD)1 | 19.6 (3.1) |
| Number of psychotic episodes, mean (SD) | 1.2 (0.6) |
| Antipsychotic medication, n, yes/no1 | 25/2 |
| Dose of antipsychotic medication, mean (SD)1 , 2 | 3.1 (1.9) |
| Smoking, n, yes/no | 22/8 |
| PANSS positive symptom score, mean (SD)3 | 13.7 (6.5) |
| PANSS negative symptom score, mean (SD)3 | 17.9 (7.3) |
| Total (poly)unsaturated fatty acid concentration in erythrocyte membranes, pmol/10e6 cells, mean (SD) | 296.6 (30.7) |
| Arachidonic acid (C20:4n6) | 75.4 (10.0) |
| Docosahexaenoic acid (C22:6n3) | 14.7 (5.2) |
| Docosapentaenoic acid (C22:5n3) | 9.8 (1.9) |
| Nervonic acid (C24:1n9) | 17.5 (4.1) |
| Linoleic acid (C18:2n6) | 61.2 (9.1) |
More than half of patients (59%) had used alcohol in the past month, with a median of 2 glasses per week (range 0–42). One-third of patients (33%) had used cannabis in the past month. Use of other illicit drugs in the past month was limited (13% of patients).
Correlations Between (P)UFA Concentrations and DTI Indices
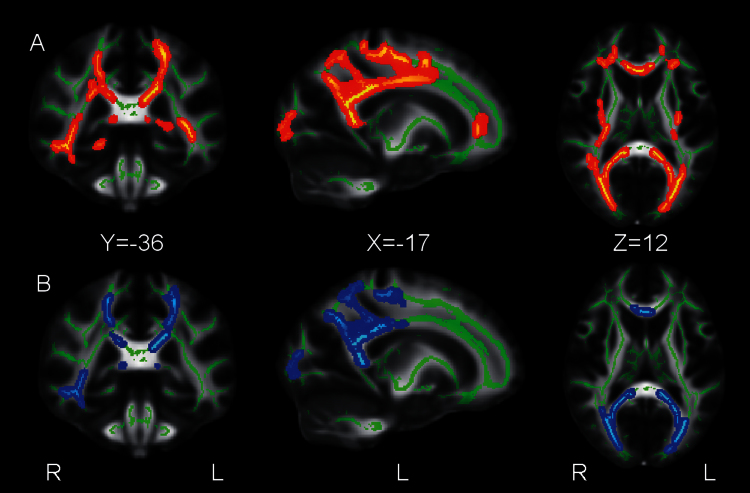
Total (P)UFA concentration showed significant positive correlations with FA throughout the corpus callosum (CC) and in bilateral parietal, occipital, temporal and frontal WM (see table 2 and figures 1A and 2A). Mean FA extracted from the largest cluster comprising the CC and bilateral parietal, temporal, occipital and frontal WM correlated significantly with AA (r s = 0.521, P = .003), NA (r s = 0.440, P = .015), and LA (r s = 0.376, P = .041), and this was a trend for DPA (r s = 0.346, P = .061) and DHA (r s = 0.319 P = .085). Mean FA extracted from the second largest cluster comprising left frontal WM correlated significantly with DPA (r s = 0.368, P = .046), and this was a trend for AA (r s = 0.357, P = .053) and NA (r s = 0.302, P = .104). Mean FA extracted from the cluster comprising right temporal WM correlated significantly with AA (r s = 0.405, P = .026). Mean FA extracted from the cluster comprising right frontal WM correlated significantly with NA (r s = 0.448, P = .013), and this was a trend for DHA (r s = 0.351, P = .057), AA (r s = 0.335, P = .071), and DPA (r s = 0.308, P = .098).
Table 2.
Clusters Showing Significant Positive Correlations Between (Poly)Unsaturated Fatty Acid Concentrations in Erythrocyte Membranes and White Matter Fractional Anisotropy in 30 Patients With a Recent-Onset Psychotic Disorder
| Cluster | Number of Voxels | WM Area | WM Tract(s) | MNI x, y, z Peak Coordinate (mm) | Peak P-value (corrected) | Correlation With Diffusivity Measures1 | |
| RD | AD | ||||||
| Total (poly)unsaturated fatty acids | |||||||
| 1 | 16,441 | Parietal & temporal & occipital, L; frontal, L; parietal & temporal & occipital, R; frontal, R | CC body & splenium, AF, EC, CST, CR, Fx/ST, F-maj., ILF, IFOF, PTR; CC genu, CST, CR; AF, EC, CST, CR, F-maj., ILF, IFOF, PTR; ATR, CC genu, CR, F- min., IFOF, UF | −3, −10, 25 | 0.013 | −0.571** | NS |
| 2 | 453 | Frontal, L | ATR, IFOF, F-min., UF | −25, 34, −2 | 0.047 | −0.480** | NS |
| 3 | 99 | Temporal, R | CG (hippocampus) | 23, −38, −7 | 0.048 | −0.484** | NS |
| 4 | 71 | Frontal, R | (SFG/SMA) | 16, 5, 51 | 0.047 | −0.452* | NS |
| Arachidonic acid | |||||||
| 1 | 5,950 | Parietal & occipital, L; frontal, L | CC body & splenium, CR, CST, F-maj., IFOF, ILF, PTR; CC genu & body, CST, CR | −14, −42, 24 | 0.015 | −0.473** | NS |
| 2 | 3,454 | Parietal & occipital, R; frontal, R | CC splenium & body, CGp, CR, CST, F-maj., IFOF, ILF, PTR; CST | 29, −65, 20 | 0.012 | −0.491** | 0.391* |
| 3 | 246 | Temporal, R | IFOF, ILF, SLF | 50, −36, −9 | 0.046 | −0.404* | NS |
| 4 | 82 | Temporal, L | IFOF, PLEC | −32, −21, 0 | 0.046 | −0.484** | NS |
| 5 | 42 | Parietal, R | — | 18, −51, 55 | 0.048 | −0.423* | 0.392* |
| 6 | 29 | Temporal, R | — | 39, −48, −14 | 0.048 | NS | NS |
| 7 | 20 | Temporal, R | ILF | 45, −21, −17 | 0.050 | NS | 0.422* |
| 8 | 13 | Temporal, L | SLF | −48, −42, −5 | 0.049 | −0.515** | NS |
Note: AF, arcuate fasciculus; ATR, anterior thalamic radiation; CC, corpus callosum; CG, cingulum; CR, corona radiata; CST, corticospinal tract; EC, external capsule; Fx/ST, fornix/stria terminalis; F-maj., forceps major; F-min., forceps minor; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; L, left; MNI, Montreal Neurological Institute; NS, nonsignificant; p, posterior; PCG, precentral gyrus; PLEC, posterior limb of the external capsule; PTR, posterior thalamic radiation; R, right; SFG, superior frontal gyrus; SLF, superior longitudinal fasciculus; SMA, supplementary motor area; UF, uncinate fasciculus. Only clusters comprising >10 voxels are reported.
1Spearman’s rank correlations with radial diffusivity and axial diffusivity of the clusters that showed significant correlations with fractional anisotropy.
*p < 0.05, **p < 0.01.
Fig. 1.
Clusters showing significant positive correlations between (poly)unsaturated acid concentrations in erythrocyte membranes and white matter (WM) fractional anisotropy in 30 patients with a recent-onset psychotic disorder.
Panel A: Results for total (poly)unsaturated fatty acids. Panel B: Results for arachidonic acid. Significant voxels (P < 0.05, corrected) are colored, “thickened” by filling them out into the local tracts, and overlaid on the mean WM skeleton (green) and the FMRIB58 FA template, for display purposes.
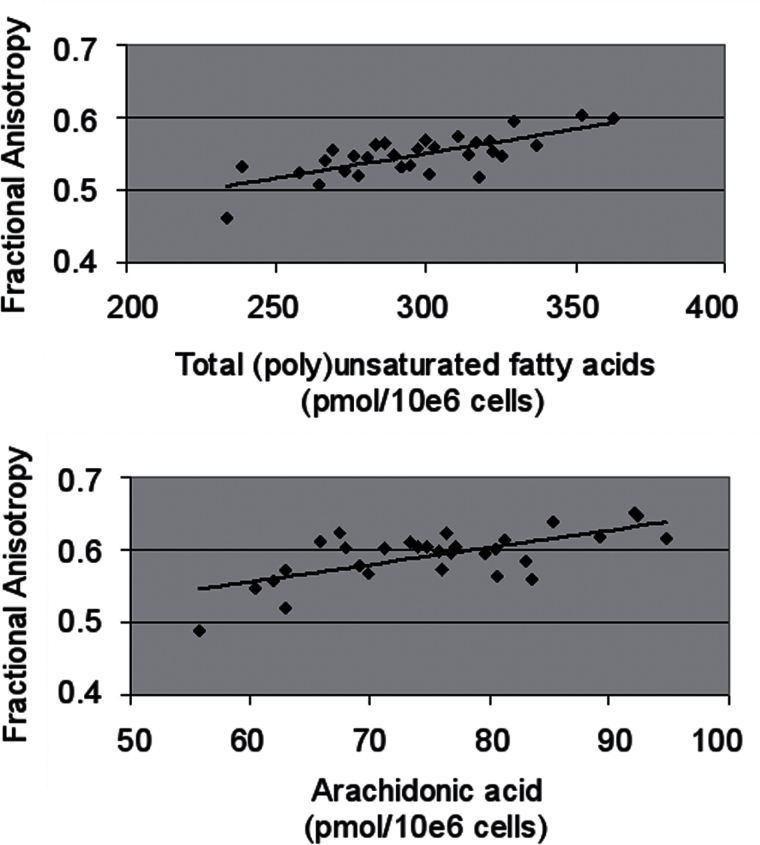
Fig. 2.
Correlations between (poly)unsaturated fatty acid concentrations in erythrocyte membranes and white matter (WM) fractional anisotropy in 30 patients with a recent-onset psychotic disorder.
Top panel: Significant correlation between total (poly)unsaturated fatty acid concentration and fractional anisotropy in a large WM cluster comprised of 16 441 voxels, centered in the corpus callosum and extending into parietal, occipital, temporal and frontal WM (r s = 0.636, P < 0.001).
Bottom panel: Significant correlation between arachidonic acid concentration and fractional anisotropy in a large WM cluster comprised of 5 950 voxels, centered in the corpus callosum and extending into parietal, occipital, temporal and frontal WM (r s = 0.544, P = 0.002).
We also examined the strength of intercorrelations between the individual (P)UFAs to assess their potential effects. AA correlated significantly with DPA and NA (r s = 0.472 and 0.567, respectively) but not with DHA or LA. DHA was significantly correlated with DPA (r s = 0.474) and NA (r s = 0.498) but not with AA or LA (trend for association with LA: r s = 0.322, P = .082). LA was not significantly correlated with any of the other (P)UFAs. DPA correlated highly with NA (r s = 0.788).
In the voxelwise analyses of the individual (P)UFAs, AA showed significant positive correlations with FA in WM regions that overlapped with the main cluster significant for total (P)UFA concentration, comprising the CC and bilateral parietal, temporal, occipital and frontal WM (see table 2 and figures 1B and 2B). DHA did not significantly correlate with FA (P = .22 for positive correlations and P = .86 for negative correlations). DPA, NA, and LA showed trend-level positive correlations with FA in WM regions that overlapped with the WM clusters significant for total (P)UFA concentration or AA concentration (P = .17, P = .14 and P = .11, respectively). There were no significant or trend-level negative correlations between any of the (P)UFAs and FA. To assess in more detail the direction and extent of the correlations between the (P)UFAs and FA, the statistical images uncorrected for multiple comparisons were inspected. All (P)UFAs showed significant positive correlations with FA throughout the WM (P < .05, uncorrected), and no significant negative correlations were observed.
RD values extracted from the FA clusters significant for total (P)UFA concentration showed significant negative correlations with total (P)UFA concentration, whereas AD values did not significantly correlate with total (P)UFA concentration (see table 2). RD values extracted from the FA clusters significant for AA concentration also showed significant negative correlations with AA concentration, except for two of the smaller clusters in the right temporal WM (clusters 6 and 7 in table 2); AD values showed significant positive correlations with AA concentration in 3 clusters comprising right parietal, temporal, occipital and frontal WM (clusters 2, 5, and 7 in table 2).
The voxelwise analyses in patients without cannabis use prior to blood collection produced significant positive correlations between FA and total (P)UFA concentration or AA concentration. The clusters overlapped with the WM clusters significant for total (P)UFA concentration or AA concentration in the total sample, yet with some notable differences. For total (P)UFAs, the WM clusters in the right hemisphere were no longer significant except in the CC; in the left hemisphere, the clusters in frontal WM were no longer significant except for the genu of the CC. For AA, results were similar as for the total sample. Of note, the correlations between total (P)UFA or AA concentration and FA were similar with or without the 2 subjects with heavy alcohol use (>21 drinks per week).
The association between total (P)UFA concentration and mean FA of the cluster in the right temporal lobe (cluster 3 in table 2) was attenuated to trend level when adjusting for dose of antipsychotic medication (r = 0.421, P = .057). The association between AA concentration and mean FA of the cluster in the right temporal lobe (cluster 7 in table 2) was attenuated to trend level when adjusting for duration of illness (r = 0.369, P = .064). Correlations between total (P)UFA or AA concentration and mean FA of significant clusters remained significant when adjusting for age, smoking, or time between DTI scanning and collection of the blood samples.
Negative symptom rating on the PANSS showed a significant negative correlation with NA concentration (r s = −0.432, P = .022). Negative symptom rating also showed a significant negative correlation with mean FA of the largest cluster significant for total (P)UFAs comprising the CC and bilateral parietal, temporal, occipital and frontal WM (r s = −0.379, P = .047), and with mean FA of the largest cluster significant for AA comprising parietal, occipital and frontal WM (r s = −0.390, P = .040) and of the clusters significant for AA comprising right temporal WM (r s = −0.379, P = .037), left temporal WM (r s = −0.423, P = .025), and right temporal WM in the inferior longitudinal fasciculus (r s = −0.480, P = .010). There were no significant correlations between positive symptom rating on the PANSS and any of the (P)UFA concentrations or mean FA values of the significant clusters.
Discussion
In patients with a recent-onset psychotic disorder, we found significant positive correlations between total erythrocyte membrane (P)UFA concentration and FA in the CC and bilateral parietal, occipital, temporal and frontal WM. These correlations were mainly driven by AA concentration, and to a lesser extent NA, LA, and DPA concentrations; correlations between DHA concentration and FA were not significant, yet of similar direction. No significant or trend-level negative correlations were observed.
These results are consistent with the hypothesis that (P)UFA concentrations, as measured in peripheral membranes, are related to microstructural WM integrity in the early phase of psychosis. Our findings suggest that a generalized disturbance of membrane (P)UFA metabolism is involved in WM abnormalities in psychotic disorders, more than a specific (P)UFA being primary. However, because of intercorrelations between the individual (P)UFAs, basic laboratory studies will be necessary to examine the specific effects of individual (P)UFAs, beyond these statistical associations. The fact that RD was found to drive these correlations with FA supports the hypothesis that a disturbance in myelin may play a role in this process.28 RD indexes water diffusion perpendicular to the axon bundles and has been found to indicate demyelination in mouse models of WM disease, while AD indexes diffusion parallel to the axons and has been found to indicate axonal damage.28 However, we note that there are no direct human comparisons to confirm this for humans.
The mechanism of how (P)UFAs may affect myelination is not known. Myelin is not a static structure, and turnover studies showed that a considerable portion of myelin lipids in the young and adult animal undergoes rapid turnover.32 O’Brien proposed that, because carbon-carbon interactions are important in membrane cohesion and because very long-chain fatty acids (ie, 22–26 carbons) have a large surface of carbon atoms and are long enough to cross-link to the opposite membrane layer, they may be important in stabilizing the myelin membrane.33 Alternatively, PUFAs may influence myelination by stimulating the expression of myelin proteins.9 Furthermore, reduced (P)UFA concentrations may be related to WM integrity through inflammation or oxidative stress, both of which have been implicated in the pathophysiology of schizophrenia.34 AA, after being liberated from the membrane, transforms to eicanosoids, which include proinflammatory prostaglandins and leukotrienes; omega-3 fatty acids on the other hand can be transformed to several anti-inflammatory factors.34 Thus, increased metabolism of omega-3 and omega-6 fatty acids, perhaps through altered immune function and increased phospholipase A2 activity in psychosis,34,35 could lead to synergistic effects that promote WM inflammation and thereby disturbed myelination. Oxidative stress may be another pathway to affect PUFA concentrations and cause myelin disruptions.36 In first episode and never-medicated patients, reduced levels of antioxidant enzymes and increased plasma lipid peroxides have been associated with reduced membrane PUFA concentrations.13,15,16
Our results confirm that NA is important to WM integrity.37 NA is a major constituent of the myelin membranes,37 and during the first years of brain development NA content of sphingomyelin increases dramatically.38 Thus, decreases in NA concentration could directly affect myelination. Interestingly, recent data indicate that decreased NA levels predict transition to psychosis in individuals at clinical high risk for psychosis.39
The observed associations between the (P)UFA concentrations and FA remained largely present when the analyses were restricted to patients without recent cannabis use. Yet some differences were observed, which suggests that the observed (P)UFA-FA relationships were partially related to recent cannabis use by some patients. The main psychoactive ingredient of cannabis, ∆9-THC, displays a high affinity to lipid membranes, and recent cannabis use has been associated with dysfunction of the lipid-arachidonic pathways, possibly through systemic exhaustion of AA by ∆9-THC.29 However, excluding the patients with recent cannabis use in our sample attenuated the findings for total (P)UFA concentration but not for AA concentration, which implies that the effects of cannabis on lipid membranes may affect other (P)UFAs besides AA. In addition, in our sample, illness-related decreases may have reached a ceiling effect in depleting AA concentrations.
Greater severity of negative symptoms was associated with lower FA for several of the WM clusters associated with total (P)UFA or AA concentration, while no significant associations with positive symptoms were observed. In addition, lower NA levels were significantly associated with greater severity of negative symptoms, which is consistent with findings in patients at clinical high risk for psychosis.39 FA may mediate the association between NA levels and negative symptoms. Previous DTI studies found that reduced frontal FA was related to severity of negative symptoms,40 and omega-3 fatty acid supplementation was found to improve negative symptoms in patients with first-episode psychosis.41
Antipsychotic treatment in the majority of our patients may be a limitation of our study. However, partial correlations adjusting for antipsychotic dose indicated little effect of antipsychotics on the relationships between (P)UFAs and FA. Consistent with this, several studies have observed PUFA abnormalities in never-medicated patients,14–16 and DTI studies comparing chronically medicated with briefly or never-medicated patients suggest no significant effect of antipsychotics on FA values.5,40
The time between MRI scanning and collection of the blood samples (median 68.5 days) is unlikely to have influenced the results. Fatty acid composition of erythrocytes is relatively stable; it reflects fatty acid incorporation and metabolism in the membrane phospholipids and dietary intake of the previous couple of months.42 Bentsen et al.43 recently showed that erythrocyte PUFA concentrations in schizophrenia patients and healthy controls did not significantly change over a 4-month period. In addition, partial correlations between total (P)UFA or AA concentration and FA values, adjusting for time between MRI and blood collection, did not alter our results.
Conclusions and Future Directions
In young-adult males in the early phase of a psychotic disorder, erythrocyte membrane (P)UFA concentrations appear to be robustly related to WM integrity. The observed associations were mostly related to AA concentration, and to a lesser extent NA, LA, and DPA concentrations. Because these effects were mainly driven by a decrease in RD, decreased (P)UFA concentrations most likely contribute to WM abnormalities through a disturbance in myelination. Furthermore, lower NA concentration and lower FA values both correlated with greater severity of negative symptoms. Further studies should test these findings, in first-episode psychosis patients and patients at clinical high risk for psychosis, as well as in healthy subjects, including female subjects. These results may also provide a basis for future studies to investigate the effects of fatty acid supplementation on WM integrity in the early phase of schizophrenia and other psychotic disorders.
Funding
National Institute of Mental Health (5P50MH080173 to Dr. Malhotra). This work used resources of the BiGGrid project, the Dutch e-Science Grid, which is financially supported by the Netherlands Organization for Scientific Research (NWO).
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Footnotes
The original version contained minor abbreviation and table errors.
References
- 1. Li D, Collier DA, He L. Meta-analysis shows strong positive association of the neuregulin 1 (NRG1) gene with schizophrenia. Hum Mol Genet. 2006;15:1995–2002 [DOI] [PubMed] [Google Scholar]
- 2. Hodgkinson CA, Goldman D, Jaeger J, et al. Disrupted in schizophrenia 1 (DISC1): association with schizophrenia, schizoaffective disorder, and bipolar disorder. Am J Hum Genet. 2004;75:862–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tkachev D, Mimmack ML, Ryan MM, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805 [DOI] [PubMed] [Google Scholar]
- 4. Hof PR, Haroutunian V, Copland C, Davis KL, Buxbaum JD. Molecular and cellular evidence for an oligodendrocyte abnormality in schizophrenia. Neurochem Res. 2002;27:1193–1200 [DOI] [PubMed] [Google Scholar]
- 5. Peters BD, Blaas J, de Haan L. Diffusion tensor imaging in the early phase of schizophrenia: what have we learned? J Psychiatr Res. 2010;44:993–1004 [DOI] [PubMed] [Google Scholar]
- 6. Bourre JM, Pascal G, Durand G, Masson M, Dumont O, Piciotti M. Alterations in the fatty acid composition of rat brain cells (neurons, astrocytes, and oligodendrocytes) and of subcellular fractions (myelin and synaptosomes) induced by a diet devoid of n-3 fatty acids. J Neurochem. 1984;43:342–348 [DOI] [PubMed] [Google Scholar]
- 7. Trapp BD, Bernsohn J. Essential fatty acid deficiency and CNS myelin. Biochemical and morphological observations. J Neurol Sci. 1978;37:249–266 [DOI] [PubMed] [Google Scholar]
- 8. Selivonchick DP, Johnston PV. Fat deficiency in rats during development of the central nervous system and susceptibility to experimental allergic encephalomyelitis. J Nutr. 1975;105:288–300 [DOI] [PubMed] [Google Scholar]
- 9. Salvati S, Natali F, Attorri L, et al. Eicosapentaenoic acid stimulates the expression of myelin proteins in rat brain. J Neurosci Res. 2008;86:776–784 [DOI] [PubMed] [Google Scholar]
- 10. Virtanen JK, Siscovick DS, Longstreth WT, Jr, Kuller LH, Mozaffarian D. Fish consumption and risk of subclinical brain abnormalities on MRI in older adults. Neurology. 2008;71:439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nordvik I, Myhr KM, Nyland H, Bjerve KS. Effect of dietary advice and n-3 supplementation in newly diagnosed MS patients. Acta Neurol Scand. 2000;102:143–149 [DOI] [PubMed] [Google Scholar]
- 12. Assies J, Lieverse R, Vreken P, Wanders RJ, Dingemans PM, Linszen DH. Significantly reduced docosahexaenoic and docosapentaenoic acid concentrations in erythrocyte membranes from schizophrenic patients compared with a carefully matched control group. Biol Psychiatry. 2001;49:510–522 [DOI] [PubMed] [Google Scholar]
- 13. Evans DR, Parikh VV, Khan MM, Coussons C, Buckley PF, Mahadik SP. Red blood cell membrane essential fatty acid metabolism in early psychotic patients following antipsychotic drug treatment. Prostaglandins Leukot Essent Fatty Acids. 2003;69:393–399 [DOI] [PubMed] [Google Scholar]
- 14. Reddy RD, Keshavan MS, Yao JK. Reduced red blood cell membrane essential polyunsaturated fatty acids in first episode schizophrenia at neuroleptic-naive baseline. Schizophr Bull. 2004;30:901–911 [DOI] [PubMed] [Google Scholar]
- 15. Khan MM, Evans DR, Gunna V, Scheffer RE, Parikh VV, Mahadik SP. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophr Res. 2002;58:1–10 [DOI] [PubMed] [Google Scholar]
- 16. Arvindakshan M, Sitasawad S, Debsikdar V, et al. Essential polyunsaturated fatty acid and lipid peroxide levels in never-medicated and medicated schizophrenia patients. Biol Psychiatry. 2003;53:56–64 [DOI] [PubMed] [Google Scholar]
- 17. Yao JK, Leonard S, Reddy RD. Membrane phospholipid abnormalities in postmortem brains from schizophrenic patients. Schizophr Res. 2000;42:7–17 [DOI] [PubMed] [Google Scholar]
- 18. Amminger GP, Schäfer MR, Papageorgiou K, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–154 [DOI] [PubMed] [Google Scholar]
- 19. Peters BD, Duran M, Vlieger EJ, et al. Polyunsaturated fatty acids and brain white matter anisotropy in recent-onset schizophrenia: a preliminary study. Prostaglandins Leukot Essent Fatty Acids. 2009;81:61–63 [DOI] [PubMed] [Google Scholar]
- 20. Richardson AJ, Allen SJ, Hajnal JV, Cox IJ, Easton T, Puri BK. Associations between central and peripheral measures of phospholipid breakdown revealed by cerebral 31-phosphorus magnetic resonance spectroscopy and fatty acid composition of erythrocyte membranes. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1513–1521 [DOI] [PubMed] [Google Scholar]
- 21. Yao J, Stanley JA, Reddy RD, Keshavan MS, Pettegrew JW. Correlations between peripheral polyunsaturated fatty acid content and in vivo membrane phospholipid metabolites. Biol Psychiatry. 2002;52:823–830 [DOI] [PubMed] [Google Scholar]
- 22. Highley JR, Esiri MM, McDonald B, Cortina-Borja M, Herron BM, Crow TJ. The size and fibre composition of the corpus callosum with respect to gender and schizophrenia: a post-mortem study. Brain. 1999;122(Pt 1):99–110 [DOI] [PubMed] [Google Scholar]
- 23. Olabarriaga SD, Glatard T, de Boer PT. A virtual laboratory for medical image analysis. IEEE Trans Inf Technol Biomed. 2010;14:979–985 [DOI] [PubMed] [Google Scholar]
- 24. Caan MW, Khedoe G, Poot D, et al. Adaptive noise filtering for accurate and precise diffusion estimation in fiber crossings. Med Image Comput Comput Assist Interv. 2010;13:167–174 [DOI] [PubMed] [Google Scholar]
- 25. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505 [DOI] [PubMed] [Google Scholar]
- 26. Assies J, Lok A, Bockting CL, et al. Fatty acids and homocysteine levels in patients with recurrent depression: an explorative pilot study. Prostaglandins Leukot Essent Fatty Acids. 2004;70:349–356 [DOI] [PubMed] [Google Scholar]
- 27. Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455 [DOI] [PubMed] [Google Scholar]
- 28. Hofling AA, Kim JH, Fantz CR, Sands MS, Song SK. Diffusion tensor imaging detects axonal injury and demyelination in the spinal cord and cranial nerves of a murine model of globoid cell leukodystrophy. NMR Biomed. 2009;22:1100–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smesny S, Rosburg T, Baur K, Rudolph N, Sauer H. Cannabinoids influence lipid-arachidonic acid pathways in schizophrenia. Neuropsychopharmacology. 2007;32:2067–2073 [DOI] [PubMed] [Google Scholar]
- 30. Hibbeln JR, Makino KK, Martin CE, Dickerson F, Boronow J, Fenton WS. Smoking, gender, and dietary influences on erythrocyte essential fatty acid composition among patients with schizophrenia or schizoaffective disorder. Biol Psychiatry. 2003;53:431–441 [DOI] [PubMed] [Google Scholar]
- 31. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276 [DOI] [PubMed] [Google Scholar]
- 32. Sastry PS. Lipids of nervous tissue: composition and metabolism. Prog Lipid Res. 1985;24:69–176 [DOI] [PubMed] [Google Scholar]
- 33. O’Brien JS. A molecular defect of myelination. Biochem Biophys Res Commun. 1964;15:484–490 [DOI] [PubMed] [Google Scholar]
- 34. Yao JK, van Kammen DP. Membrane phospholipids and cytokine interaction in schizophrenia. Int Rev Neurobiol. 2004;59:297–326 [DOI] [PubMed] [Google Scholar]
- 35. Smesny S, Kinder D, Willhardt I, et al. Increased calcium-independent phospholipase A2 activity in first but not in multiepisode chronic schizophrenia. Biol Psychiatry. 2005;57:399–405 [DOI] [PubMed] [Google Scholar]
- 36. Bongarzone ER, Pasquini JM, Soto EF. Oxidative damage to proteins and lipids of CNS myelin produced by in vitro generated reactive oxygen species. J Neurosci Res. 1995;41:213–221 [DOI] [PubMed] [Google Scholar]
- 37. Babin F, Sarda P, Limasset B, et al. Nervonic acid in red blood cell sphingomyelin in premature infants: an index of myelin maturation? Lipids. 1993;28:627–630 [DOI] [PubMed] [Google Scholar]
- 38. Martínez M, Mougan I. Fatty acid composition of human brain phospholipids during normal development. J Neurochem. 1998;71:2528–2533 [DOI] [PubMed] [Google Scholar]
- 39. Amminger GP, Schäfer MR, Klier CM, et al. Decreased nervonic acid levels in erythrocyte membranes predict psychosis in help-seeking ultra-high-risk individuals [published online ahead of print December 20, 2011]. Mol Psychiatry. 10.1038/mp.2011.167 [DOI] [PubMed] [Google Scholar]
- 40. Szeszko PR, Robinson DG, Ashtari M, et al. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology. 2008;33:976–984 [DOI] [PubMed] [Google Scholar]
- 41. Berger GE, Wood SJ, Wellard RM, et al. Ethyl- eicosapentaenoic acid in first-episode psychosis. A 1H-MRS study. Neuropsychopharmacology. 2008;33:2467–2473 [DOI] [PubMed] [Google Scholar]
- 42. Arab L. Biomarkers of fat and fatty acid intake. J Nutr. 2003;133(Suppl 3):925–932 [DOI] [PubMed] [Google Scholar]
- 43. Bentsen H, Solberg DK, Refsum H, et al. Bimodal distribution of polyunsaturated fatty acids in schizophrenia suggests two endophenotypes of the disorder. Biol Psychiatry. 2011;70:97–105 [DOI] [PubMed] [Google Scholar]