Abstract
Contrary to early conceptualizations of emotional experience in schizophrenia (SZ), recent research indicates that patients do not self-report less in-the-moment pleasure than controls (CN). Rather, patients report experiencing elevated levels of negative emotionality in response to a range of evocative stimuli. In this study, we examined the possibility that elevations in negative emotionality in SZ may reflect an underlying emotion regulation abnormality. Event-related potentials (ERPs) were recorded from outpatients with SZ (n = 25) and demographically matched healthy controls (n = 21) during passive viewing of unpleasant and neutral photographs. Unpleasant images were preceded by an audio description that described the image as being either negative or neutral. Neutral images were preceded by neutral audio descriptions. The late positive potential (LPP), an ERP component sensitive to cognitive change strategies, was examined as an index of emotion regulation. Both CN and SZ showed an increased LPP to negatively described unpleasant images compared with neutral images. In addition, CN showed evidence of emotion regulation, as reflected by a smaller LPP for unpleasant images preceded by a neutral descriptor, relative to a negative descriptor. In contrast, SZ patients showed an inability to downregulate emotional response, as evidenced by no difference in the amplitude of the LPP for unpleasant images preceded by negative or neutral descriptors. Findings provide neurophysiological evidence for an emotion regulation abnormality in SZ and suggest that failures in cognitive change may underlie increased negative emotionality in SZ.
Key words: emotion regulation, affect, anhedonia, negative symptoms, psychosis
Introduction
Contrary to early conceptualizations of anhedonia,1–3 recent research indicates that individuals with schizophrenia (SZ) do not self-report less in-the-moment pleasure than controls (CN; see Kring and Moran4 for review). Specifically, individuals with SZ have been shown to report similar levels of current positive emotion to CN in response to evocative laboratory stimuli,5–10 report real-world experiences when engaged in activities,11,12 and show a similar neural response to pleasant stimuli when reporting current positive feelings (see Taylor et al.13 for meta-analysis). These findings have led some to conclude that anhedonia should be reconceptualized in SZ and no longer viewed as a diminished capacity for pleasure (see Strauss and Gold14 for review).
However, it is clear that not all aspects of emotional experience are normal in SZ. Recent meta-analyses have indicated that while patients display no reduction in self-reported positive emotion15 or arousal16 to pleasant stimuli compared with CN, they do report experiencing greater negative emotion in response to both neutral and pleasant stimuli. These elevations in state negative emotionality were seen in response to a range of stimulus types and showed a large effect size. One potential explanation for the seemingly contradictory finding of increased negative emotion to pleasant and neutral stimuli is that patients experience a coactivation of positive and negative emotions to these stimuli,15 which may reflect affective ambivalence17 or simply heightened baseline negative emotionality.18 Studies using real-world experience sampling methodology12,19,20 and trait self-report questionnaires (for review see Horan et al.21) also indicate that patients report higher negative emotionality than CN and that these elevations predict poor occupational functioning and quality of life. Collectively, these converging lines of evidence suggest that abnormalities in negative, but not positive emotional experience may be core to affective disturbance in SZ.
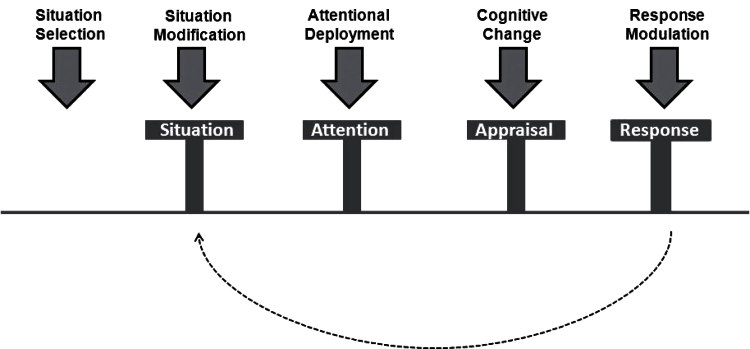
One possible explanation for these increases in state and trait negative emotionality is that individuals with SZ display impairments in “emotion regulation.”14,15,22–24 Emotion regulation refers to the processes by which we modify our negative and positive emotions with regard to their intensity, when they occur, how long they last, and how they are expressed.25 To examine how emotion regulation goes awry in SZ, we have adopted the conceptual framework of James Gross25 who proposed that emotions unfold as a multicomponential process, which can be regulated via use of strategies at different stages of emotion generation (see figure 1 and note). Emotion regulation strategies can be broadly separated into those that are antecedent focused or response focused.26 Antecedent-focused strategies are those that are applied before an emotional response has become fully activated to the point of producing behavioral and physiological changes. Examples include situation selection, situation modification, attentional deployment, and cognitive change. In contrast, response-focused strategies are those that occur late in the emotion-generation process, which are applied after an emotion and its corresponding response properties have occurred. An example is affective suppression, which involves inhibiting the outward expression of emotion. These strategies are differentially effective, with greater support for downregulation of negative emotion using antecedent-focused strategies compared with response-focused strategies.26
Fig. 1.
James Gross’ process model of emotion regulation. The “Process Model” by Gross (1998, 2002) proposes that emotions unfold as a multicomponential process, whereby a situation occurs (either external or internal) that is then attended to, giving rise to an appraisal of the situation’s valence and motivational relevance, which results in a series of experiential, behavioral, and neurophysiological response changes (see bottom row). Sometimes responses interact with the environment and lead to changes in the situation that produced the initial response, resulting in a recursive loop that engenders new emotional responses (see arrow). Importantly, a number of strategies can be applied to regulate negative and positive emotions at these different stages of the emotion generative process (see top row). These emotion regulation strategies can be divided into those that are antecedent-focused (situation selection, situation modification, attentional deployment, cognitive change) and response-focused (response modulation/affective suppression).
A limited number of studies have examined emotion regulation in SZ. In 2 studies using self-report questionnaires, individuals with SZ were found to report greater use of affective suppression and less use of cognitive change strategies than CN.27,28 However, 2 other studies have found no differences between SZ and CN in self-reported emotion regulation strategy use.29,30 Despite these inconsistencies across studies, lower use of cognitive change strategies has repeatedly been found to predict poor functional outcome.27–29 These prior studies provide valuable information regarding self-reported emotion regulation strategy use in SZ; however, self-report is only one means of studying emotion regulation. A number of paradigms have been validated in the field of affective neuroscience using event-related potentials (ERPs) and functional magnetic resonance imaging (fMRI), which allow emotion regulation to be assessed at the neural level independent of self-report.
The goal of this study was to extend the literature on emotion regulation in SZ by examining whether patients show neural evidence of impaired downregulation of negative emotion using an antecedent-focused strategy, cognitive change (ie, altering one’s appraisal of the meaning of an emotional stimulus). We used an emotion regulation ERP paradigm developed by Foti and Hajcak,31 in which individuals passively view neutral and unpleasant photographs that are preceded by an audio presentation that describes the upcoming image. Neutral images are always preceded by a neutral description of the upcoming image, whereas unpleasant images are preceded by a description that either describes the upcoming image as negative (ie, unregulated) or describes the upcoming image as being more neutral (ie, downregulated via cognitive change). Analyses focused on the late positive potential (LPP), which is a midline centroparietal ERP component that becomes evident starting around 500ms following stimulus onset that persists as a sustained positive deflection that is larger for both pleasant and unpleasant than neutral stimuli.32–36 In passive viewing paradigms with stimulus durations lasting for several seconds, the LPP is typically evaluated across early (eg, 500–1000ms), middle (eg, 1000–2000ms), and late (eg, 2000–3000ms) windows. The early window may reflect increased attention to motivationally relevant stimuli, whereas the middle and late windows reflect deeper processing and the appraisal of stimulus meaning.32,33 The magnitude of the LPP across these windows has been shown to relate to subjective arousal ratings of emotional stimuli in healthy subjects.34–36 In the Foti and Hajcak31 paradigm described above, the typical effect of a larger LPP to unpleasant images was found when unpleasant images were preceded by negative audio descriptions. In contrast, unpleasant stimuli with preceding neutral descriptions had significantly lower LPP amplitude than unpleasant images with preceding negative descriptions, suggesting that the LPP is sensitive to the cognitive change manipulation provided by the context of the preceding audio description. Using this same type of paradigm in conjunction with fMRI, it was found that unpleasant images preceded by neutral descriptors resulted in increased activation of prefrontal regions and decreased activation of the amygdala.37 Similar results have been found in emotion regulation paradigms in which participants generate their own alternate explanations of stimuli using ERP measures38 and fMRI.39–41 Collectively, these findings provide strong evidence that cognitive change strategies are successful at downregulating the neural response to unpleasant stimuli.
We hypothesized that both SZ and CN would be sensitive to the emotional content of the stimuli, as indicated by a larger LPP amplitude to negatively described unpleasant images compared with neutral images. In line with the findings of Foti and Hajcak31 on healthy individuals, we predicted that CN would display strong neurophyiological evidence for emotion regulation, as indicated by lower LPP amplitude for unpleasant stimuli with neutral descriptions relative to those with negative descriptions. In contrast, individuals with SZ were expected to show impaired emotion regulation, as indicated by no difference in LPP amplitude for unpleasant stimuli with preceding negative or neutral descriptions. This pattern of results was predicted to occur at early, middle, and late time windows. Furthermore, we predicted that the inability to downregulate the neural response to unpleasant stimuli would be associated with poorer functional outcome27–29 and elevated self-reported state and trait negative emotionality.38
Methods and Materials
Participants
Twenty-seven individuals with SZ and 23 CN completed study procedures. Two SZ and 2 CN participants were eliminated due to excessively noisy EEG data following artifact correction (see below), yielding a final sample of SZ n =25 and CN n = 21. All results presented for the SZ and CN groups reflect this final sample.
Individuals with SZ were recruited through the Outpatient Research Program at the Maryland Psychiatric Research Center and evaluated during a period of clinical stability as evidenced by no changes in medication type or dosage for a period greater than or equal to four weeks. Consensus diagnosis was established via a best-estimate approach based upon multiple interviews and a detailed psychiatric history. This diagnosis was subsequently confirmed using the Structured Clinical Interview for DSM-IV (SCID).42
CN subjects were recruited by means of random digit dialing, by word-of-mouth among recruited participants, and through the use of newspaper advertisements. CN had no current Axis I or II diagnoses as established by the SCID42 and SID-P,43 had no family history of psychosis, and were not taking psychotropic medications. All participants denied a history of significant neurological injury or disease and significant medical or substance use disorders within the last six months. All participants provided informed consent for a protocol approved by the University of Maryland Institutional Review Board.
The CN and SZ groups did not significantly differ in age, parental education, gender, or ethnicity. SZ had lower personal education, and Wechsler Test of Adult Reading estimated premorbid intelligence quotients than CN. On average, patients displayed moderately severe positive and negative symptoms at the time of testing (see table 1).
Table 1.
Participant Demographic and Clinical Characteristics
| SZ (n = 25) | CN (n = 21) | Test Statistic | P-value | |
|---|---|---|---|---|
| Age | 45.3 (12.2) | 45.7 (7.2) | F = 0.02 | .89 |
| Parental education | 13.1 (2.2) | 14.1 (2.2) | F = 2.48 | .12 |
| Participant education | 12.6 (1.8) | 15.9 (1.9) | F = 34.9 | < .001 |
| % Male | 72 % | 62% | χ2 = 0.53 | .47 |
| Race | χ2 = 2.40 | .50 | ||
| Caucasian (%) | 60 | 76 | — | — |
| African-American (%) | 32 | 24 | — | — |
| Asian-American (%) | 4 | 0 | — | — |
| American-Indian (%) | 4 | 0 | — | — |
| Neuropsychological tests | ||||
| WTAR SS | 96.6 (15.3) | 114.8 (10.1) | F = 20.96 | < .001 |
| DPX (AY-BX trials) | −7.0% | 3.0% | F = 2.15 | .15 |
| PANAS trait self-report | ||||
| NA | 19.8 (8.6) | 14.6 (3.4) | F = 6.48 | < .02 |
| PA | 26.3 (7.5) | 32.3 (5.7) | F = 7.93 | < .01 |
| TEPS self-report | ||||
| TEPS-ANT | 4.22 (0.99) | 4.63 (0.57) | F = 2.79 | .10 |
| TEPS-CON | 3.98 (0.91) | 4.93 (0.57) | F = 16.73 | < .001 |
| Symptom ratings | ||||
| BNSS total | 33.0 (19.7) | — | — | — |
| LOF total | 14.1 (8.0) | — | — | — |
| BPRS total | 44.6 (12.5) | — | — | — |
| BPRS positive | 2.6 (1.5) | — | — | — |
| BPRS negative | 2.8 (1.4) | — | — | — |
| BPRS disorganized | 1.8 (0.7) | — | — | — |
Note: SZ, schizophrenia group; CN, healthy control group; WTAR SS, Wechsler Test of Adult Reading Scale Score; DPX, Dot Pattern Expectancy Task % error difference score on AY-BX trials; PANAS NA, Positive and Negative Affect Scale Trait Negative Affect Subscale; PANAS PA, Positive and Negative Affect Scale Trait Positive Affect Subscale; TEPS-ANT, Temporal Experience of Pleasure Scale-Anticipatory Subscale; TEPS-CON, Temporal Experience of Pleasure Subscale Consummatory Pleasure Subscale; BNSS, Brief Negative Symptom Scale total score; LOF, Level of Function Scale total score; BPRS, Brief Psychiatric Rating Scale. Subjects were prescribed the following antipsychotic medications: clozapine (10), risperidone (7), quetiapine (5), olanzapine (4), aripiprazole (2), haloperidol (2), fluphenazine (1), chlorpromazine (1). Of subjects prescribed more than one antipsychotic medication: risperidone-clozapine (5), clozapine-quetiapine (1), olanzapine-quetiapine (1), haloperidol-aripiprazole (1). One subject was clinically stable and unmedicated at the time of testing.
Procedures
In addition to the ERP task, patients also completed a clinical interview after which the Brief Psychiatric Rating Scale (BPRS),44 Brief Negative Symptom Scale (BNSS),45–47 and Level of Function Scale (LOF)48 were rated. Participants also completed the Positive and Negative Affect Scale (PANAS)49 using the “in general” reporting timeframe, and the Temporal Experience of Pleasure Scale (TEPS).50 The Dot Pattern Expectancy (DPX)51 task was administered to index general cognitive control and goal maintenance, and the standard AY-BX contrast score was used to index a participant’s ability to represent and maintain contextual information relevant to task goals.
ERP Emotion Regulation Task
Participants completed the emotion regulation paradigm developed by Foti and Hajcak31 while the electroencephalogram (EEG) was recorded. Trial sequence, stimuli, and audio descriptions were identical to that prior study.31
Instructions indicated that participants would be passively viewing a series of unpleasant and neutral stimuli and that these images would be preceded by an audio file that described what would be depicted in the upcoming image. Following each image, participants then rated how negative the picture made them feel.
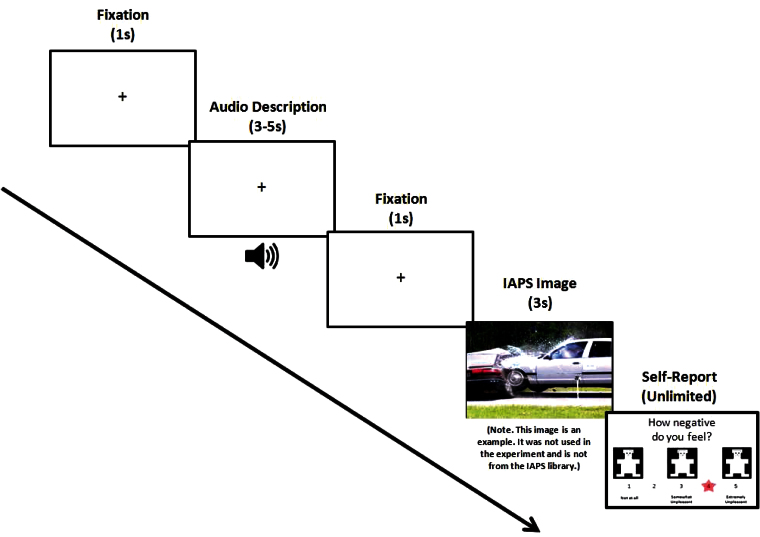
A sample trial sequence is presented in figure 2. Each trial started with a white fixation cross that was presented against a black screen for 1 s. The fixation cross remained on screen while a brief audio file (~3 s) that described the upcoming stimulus was played aloud in a man’s voice. The fixation then remained on screen for 1 s after the auditory description had finished playing. A color photograph was then displayed for 3 s across the entirety of the screen (17″ monitor, 1280 × 1024 resolution, 60 Hz refresh rate) at a viewing distance of approximately 70cm. After viewing each stimulus, a screen prompting participants to indicate their subjective emotional response (“How negative do you feel?”) was presented with unlimited time and subjects were asked to respond on a 1 (not at all) to 5 (extremely) scale using a gamepad. The next trial sequence began 1 s after behavioral response was made.
Fig. 2.
Sample trial sequence. The paradigm used in this study developed by Foti and Hajcak (2008) involves an antecedent-focused (ie, strategy employed before the emotion has been triggered) and incidental (ie, descriptions alter affective response without intentional effort) emotion regulation manipulation. It was selected to provide a “purer” test of emotion regulation ability, where all subjects received the same quality of cognitive change strategies that were not dependent upon their own ability to voluntarily generate alternative descriptions. The sequence of emotional processes involved is likely to be that suggested in Gross’ model, such that after subjects have heard the appraisal provided by the audio file, they then attend to the image, appraise the image as either neutral or unpleasant, and then apply the context provided to them in the auditory description to reappraise the image as more negative or more neutral. Downregulation therefore occurs while the image is on screen and after the context has been applied to reinterpret the meaning of the image.
Six practice trials were first presented to familiarize participants with the procedures and ensure task comprehension: 4 were neutral images with preceding neutral descriptions and 2 were unpleasant images with negative descriptions. There were a total of 75 experimental trials, 25 of which were neutral images and 50 of which were unpleasant images selected from the International Affective Picture System (IAPS).52 Of the 50 unpleasant images, 25 were preceded by a less negative audio description, and 25 were preceded by a more negative audio description. The 25 neutral images were preceded by neutral descriptions. All participants heard the same 25 audio descriptions of the neutral images, which simply described the neutral content of the image (NEU-PIC/NEU-DESC condition). Each unpleasant image had 2 possible corresponding descriptions: one that focused on the negative aspects of the image (negatively described unpleasant image condition: UNP-PIC/NEG-DESC), and another that described the upcoming stimulus in more neutral terms (neutrally described unpleasant image condition: UNP-PIC/NEU-DESC). Each image therefore had 2 possible corresponding audio descriptions, and participants randomly received one of these. For example, the audio description of an unpleasant image depicting a tarantula on a man’s shoulder either said “A poisonous tarantula is about to bite this man” (UNP-PIC/NEG-DESC) or said “This is a harmless pet tarantula sitting on his owner’s shoulder” (UNP-PIC/NEU-DESC). The goal of these audio descriptions is to provide subject’s with the type of cognitive change strategy that has typically been self-generated in studies of emotion regulation and to reduce inter and intraparticipant variability in the way in which cognitive change is used. The order in which trials and the preceding audio descriptions were presented was randomized across participants (see online supplementary material for images and their preceding descriptions).
EEG Recording and Data Processing Procedures
The EEG was recorded during the task from Ag/AgCl electrodes mounted in an elastic cap using a subset of the International 10/20 System (Fz, C3, Cz, C4, CPz, P3, Pz, P4, Oz, Fp1, Fp2, and left mastoid). The signals were recorded online using a right mastoid reference electrode, and the signals were re-referenced offline to the average of the left and right mastoid.53,54 The horizontal electrooculogram (HEOG) was used to measure horizontal eye movements and was recorded as the voltage between electrodes placed lateral to the external canthi. The vertical EOG was used to detect eyeblinks and vertical eye movements and was recorded from an electrode beneath the left eye. All electrode impedances were kept below 15 KΩ. The EEG and EOG were amplified by a Neuroscan Synamps amplifier with a gain of 5000, a bandpass filter of 0.05–100 Hz, and a 60-Hz notch filter. The amplified signals were digitized at 500 Hz and averaged offline.
All signal processing and analysis procedures were performed in Matlab using EEGLAB toolbox55 and ERPLAB toolbox (http://www.erpinfo.org/erplab). Data preprocessing included the removal of large muscle artifacts or extreme offsets (identified by visual inspection). Independent component analysis (ICA) was performed on the continuous data to identify and remove eyeblink activity.56 The ICA-corrected EEG data were segmented into epochs that began 200ms prior to the onset of the stimulus and continued for 3000ms and baseline corrected using a 200ms prestimulus period. The baseline selected is identical to Foti and Hajcak31 and should not include overlap with the preceding audio presentation given the 1000ms gap between the audio description and image. ERPs were constructed by separately averaging trials from the 3 conditions of interest: UNP-PIC/NEG-DESC, UNP-PIC/NEU-DESC, and NEU-PIC/NEU-DESC.
ERP Measurement Procedures.
The mean amplitude of the LPP was measured separately from the waveforms for the 3 conditions across electrode sites Cz, CPz, and Pz at 3 time windows following stimulus onset: an early window (500–1000ms), middle window (1000–2000ms), and late window (2000–3000ms). The N1 and P2 components were used to examine the effects of emotional content on early attention. The ERP literature on emotion indicates that the N1 typically develops between 50–150ms following stimulus offset, which is earlier than what is observed for standard spatial attention paradigms (150–200ms). The P2 for emotional stimuli typically occurs at anterior sites between 150–250ms. Thus, the N1 was defined as the peak amplitude between 50–150ms at posterior sites (Pz, Oz), and the P2 was defined as the peak amplitude between 150–250ms at an anterior site, Fz. Measurement procedures are consistent with prior work in this area and this task.29
Results
Behavioral Data
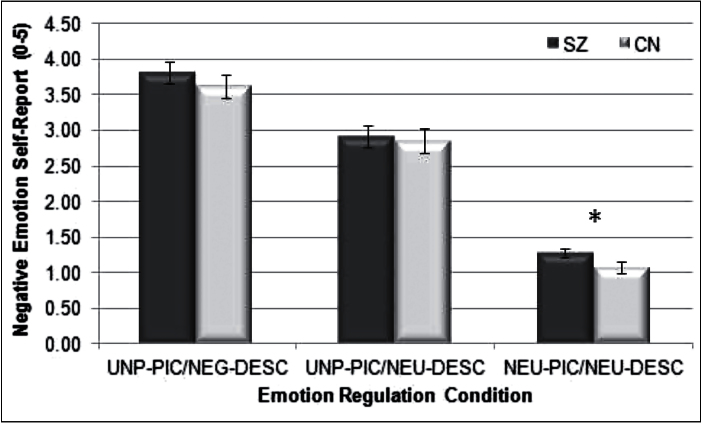
Repeated measures ANOVA with subjective negative emotional experience ratings as the dependent variable indicated a significant within-subjects effect of Condition, F(2, 88) = 354.9, P < .001, eta squared = 0.89; however, the between-subjects effect of Group, F(1,44) = 1.09, P = .30, eta squared = 0.02, and Group × Condition interaction, F(2, 88) = 0.30, P = .74, eta squared = 0.01, were nonsignificant. As can be seen in figure 3, both SZ and CN demonstrated a similar pattern of self-reported negative emotionality, where UNP-PIC/NEG-DESC stimuli were rated as more negative than UNP-PIC/NEU-DESC, which were in turn more negative than NEU-PIC/NEU-DESC. One-way ANOVAs indicated that SZ reported more negative emotion to NEU-PIC/NEU-DESC stimuli than CN (P < .05); however, there were no differences in the UNP-PIC/NEG-DESC and UNP-PIC/NEU-DESC conditions. Thus, SZ and CN self-reported similar magnitude of decrease in negative affect in response to the emotion regulation manipulation, but patients reported greater negative emotion to neutral stimuli.
Fig. 3.
Mean self-reported state negative emotional experience in response to stimuli. UNP-PIC/NEG-DESC, unpleasant image with preceding negative audio description; UNP-PIC/NEU-DESC, unpleasant image with preceding neutral description; NEU-PIC/ NEU-DESC, neutral image with preceding neutral audio description. * P < .05.
Omnibus ANOVA
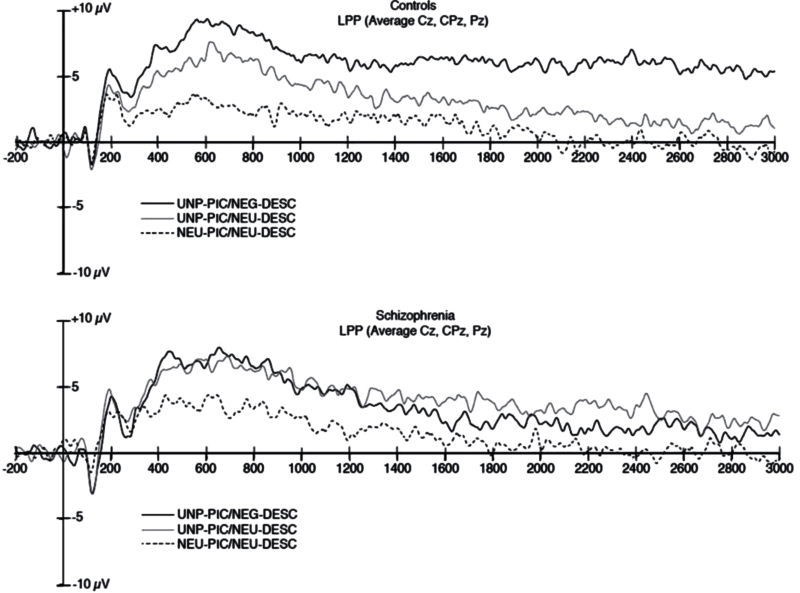
Grand average waveforms for the LPP in each of the 3 conditions are presented for CN and SZ in figure 4 panel A. An omnibus 2 Group (SZ, CN) × 3 LPP Window (Early, Middle, Late) × 3 Condition (UNP-PIC/ NEG-DESC, UNP-PIC/NEU-DESC, NEU-PIC/NEU- DESC) repeated measures ANOVA indicated a significant 3-way Group × Window × Condition interaction, F(4, 176) = 2.38, P < .05, eta squared = 0.05, as well as a significant Group × Condition interaction, F(2, 88) = 7.30, P < .001, eta squared = 0.14; significant main effects of Condition, F(2, 88) = 33.02, P < .001, eta squared = 0.43; and Window, F(2, 88) = 40.18, P < .001, eta squared = 0.48. The Window × Group, F(2, 88) = 0.58, P = .56, eta squared = 0.01, and Window × Condition, F(4, 88) = 1.2, P = .30, eta squared = 0.03, interactions were nonsignificant, as was the between-subjects effect of Group, F(1,44) = 0.39, P = .54, eta squared = 0.01.
Fig. 4.
Late positive potential (LPP) grand average waveforms. Top panel presents grand average LPP waveforms for controls; bottom panel presents grand average LPP waveforms for individuals with schizophrenia.
LPP Early Window
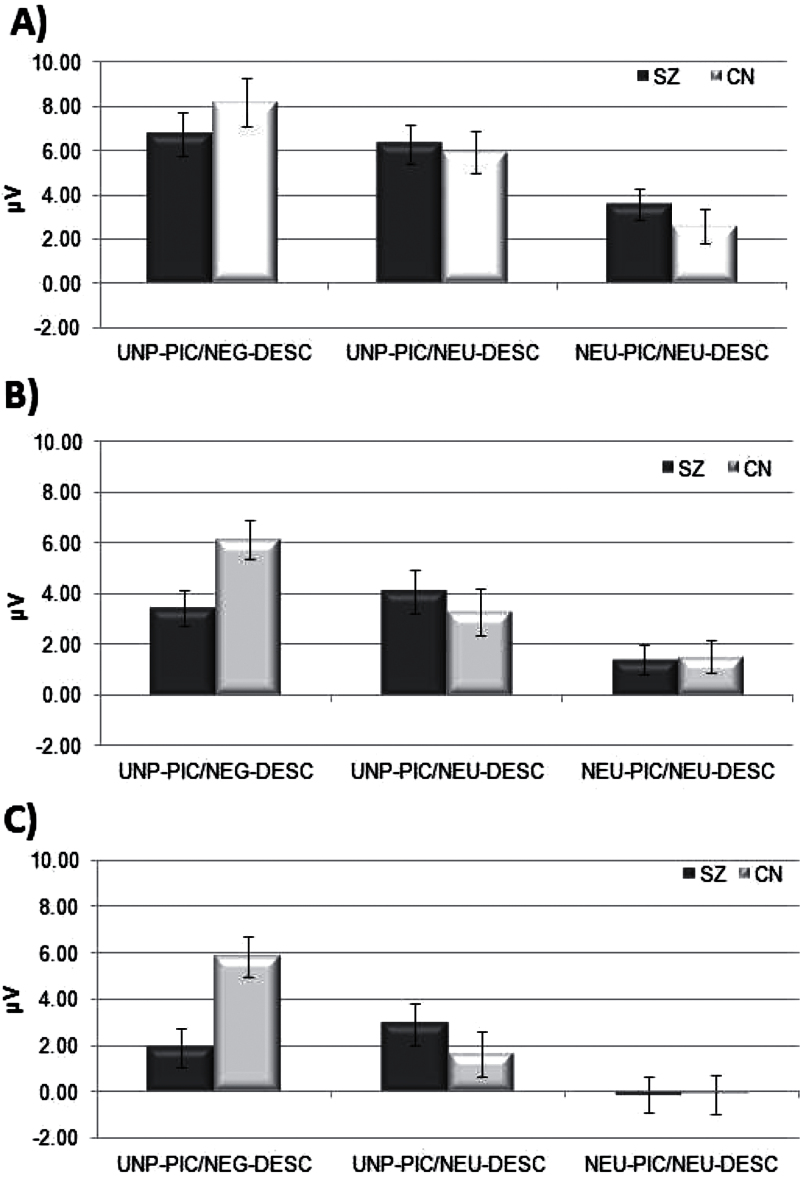
To follow up the significant interactions and directly test the hypothesized emotion regulation effects in each group, paired-samples t tests were conducted. In CN, the amplitude of the LPP was higher for UNP-PIC/NEG-DESC (t = 8.7, P < .001) and UNP-PIC/NEU-DESC (t = 4.67, P < .001) than NEU-PIC/NEU-DESC, and UNP-PIC/NEG-DESC was higher than UNP-PIC/NEU-DESC (t = 3.3, P < .01). These findings indicate that cognitive change successfully downregulated the early neural response to unpleasant stimuli in CN. Individuals with SZ also demonstrated higher amplitude of the LPP to UNP-PIC/NEG-DESC (t = 3.3, P < .01) and UNP-PIC/NEU-DESC (t = 3.7, P < .01) than NEU-PIC/NEU-DESC, suggesting a robust neural response to unpleasant stimuli; however, there was no difference between UNP-PIC/NEG-DESC and UNP-PIC/NEU-DESC (t = 0.57, P = .57), suggesting that cognitive change did not downregulate the neural response to unpleasant stimuli (see figure 5, panel A).
Fig. 5.
Mean LPP amplitudes in early, middle, and late windows per condition. Panel A presents mean LPP amplitude in the early window (500–1000ms); panel B presents mean LPP amplitude in the middle window (1000–2000ms); panel C presents mean LPP amplitude in the late window (2000–3000ms).
LPP Middle Window
In the middle window (1000–2000ms), CN had higher LPP amplitude for UNP-PIC/NEG-DESC (t = 7.2, P < .001) and UNP-PIC/NEU-DESC (t = 3.6, P = .002) than NEU-PIC/NEU-DESC, and UNP-PIC/NEU-DESC was lower than UNP-PIC/NEG-DESC (t = 4.98, P < .001). Patients with SZ demonstrated higher amplitude of the LPP to UNP-PIC/NEG-DESC (t = 2.4, P < .03) and UNP-PIC/NEU-DESC (t = 2.8, P < .01) than NEU-PIC/NEU-DESC; however, the UNP-PIC/NEG-DESC and UNP-PIC/NEU-DESC contrast was nonsignificant (t = −0.86, P = .40). Thus, cognitive change reduced the neural response to unpleasant stimuli in CN, but not in SZ (see figure 5, panel B).
LPP Late Window
In the late window (2000–3000ms), CN had higher LPP amplitude for UNP-PIC/NEG-DESC (t = 6.03, P < .001) and UNP-PIC/NEU-DESC (t = 2.46, P < .03) than NEU-PIC/NEU-DESC, and UNP-PIC/NEU-DESC was lower than UNP-PIC/NEG-DESC (t = 5.08, P < .001). In contrast, while SZ demonstrated higher amplitude of the LPP to UNP-PIC/NEG-DESC (t = 2.1, P < .05) and UNP-PIC/NEU-DESC (t = 3.04, P < .01) than NEU-PIC/NEU-DESC, the UNP-PIC/NEG-DESC and UNP-PIC/NEU-DESC contrast was nonsignificant (t = −1.1, P = .32). These results indicate intact emotion regulation in CN, but not in SZ participants (see figure 5, panel C).
Early Components: N1 and P2
Analyses of the peak amplitude for the N1 and P2 components indicated that SZ and CN showed a similar pattern of neural response across conditions (see online supplementary material).
LPP Associations With State and Trait Emotional Experience, Cognitive Control, and Symptoms
To directly index emotion regulation, a difference score of mean LPP amplitude was calculated separately for early, middle, and late windows as (UNP-PIC/NEG-DESC) – (UNP-PIC/NEU-DESC). Higher difference scores reflect better emotion regulation, as reflected in greater reduction of the LPP in the UNP-PIC/NEU-DESC condition relative to the UNP-PIC/NEG-DESC condition. In SZ, poorer neurophysiological emotion regulation was generally associated with higher self-reported state negative emotion for the UNP-PIC/NEU-DESC condition at each time window and higher PANAS trait negative affect in the early and late time windows. However, there were no significant correlations between state/trait self-reports and the LPP difference score in CN (see table 2). There were no significant correlations between LPP difference score and the DPX or TEPS in SZ or CN. In SZ, associations with BNSS total score and subscales, LOF total score, BPRS positive, BPRS negative, BPRS disorganized, and BPRS total scores were nonsignificant.
Table 2.
Correlations Between Self-Reported State and Trait Emotional Experience and LPP Difference Score (UNP-PIC/NEG-DESC – UNP-PIC/NEU-DESC)
| Early Window | Middle Window | Late Window | ||||
|---|---|---|---|---|---|---|
| SZ | CN | SZ | CN | SZ | CN | |
| State self-report | ||||||
| UNP-PIC/NEG-DESC | −0.24 | −0.28 | −0.36 | −0.14 | −0.47* | −0.15 |
| UNP-PIC/NEU-DESC | −0.44* | −0.16 | −0.40* | −0.28 | −0.52** | 0.09 |
| NEU-PIC/NEU-DESC | −0.11 | 0.01 | −0.06 | −0.21 | −0.41* | −0.09 |
| Trait self-report | ||||||
| PANAS NA | −0.49* | 0.04 | −0.09 | −0.12 | −0.41* | 0.20 |
| PANAS PA | −0.13 | 0.03 | 0.01 | 0.24 | 0.25 | 0.13 |
Note: UNP-PIC/NEG-DESC, unpleasant image with preceding negative audio description; UNP-PIC/NEU-DESC, unpleasant image with preceding neutral description; NEU-PIC/NEU-DESC, neutral image with preceding neutral audio description; LPP, late positive potential. Higher LPP difference score values reflect better emotion regulation; higher behavioral ratings reflect greater self-reported negative affect.
* = p < .05; ** = p < .01
Discussion
Results supported the hypothesis that both CN and SZ would be sensitive to the emotional content of IAPS stimuli, as indicated by larger LPP amplitude to negatively described unpleasant images compared with neutral images. This pattern of differences was most prominent in the early window but present for both groups at the middle and late windows as well. There was also support for the hypothesis that individuals with SZ would show neurophysiological evidence for an emotion regulation abnormality. Specifically, CN had higher LPP amplitude for unpleasant stimuli with preceding negative descriptions relative to unpleasant stimuli with preceding neutral descriptions. These findings are consistent with those of Foti and Hajcak31 using this same ERP paradigm, as well as numerous other ERP and fMRI findings using other tasks, which collectively indicate that cognitive change successfully downregulates the neural response to unpleasant stimuli. In contrast, individuals with SZ failed to show differences in the amplitude of the LPP between unpleasant stimuli with preceding negative descriptions and those with neutral descriptions, suggesting that cognitive change was ineffective at downregulating the neural response to unpleasant stimuli in patients.
Interestingly, the neurophysiological evidence for an emotion regulation abnormality in SZ occurred in the context of intact self-reported emotion regulation to the UNP-PIC/NEU-DESC condition. These behavioral findings are consistent with several other studies examining self-reported emotion regulation strategy use in SZ using questionnaires, which found that patients indicated using cognitive change strategies to decrease negative emotion as frequently and effectively as CN.27–30 However, SZ did report higher negative emotion to the neutral stimuli than CN, which may indicate that SZ have elevated baseline negative emotions that bleed into these reports and reflect underlying emotion regulation impairments.
Although the group differences in self-report to the unpleasant stimuli were nonsignificant, there was a different pattern of correlations between neural response and subjective experience in SZ and CN. Individuals with SZ showed robust correlations between the LPP difference score and state negative emotion across the LPP time windows, such that poor neurophysiological emotion regulation was associated with higher state negative emotion to stimuli. Furthermore, patients also showed a significant correlation between self-reported trait negative emotion on the PANAS and the LPP emotion regulation difference score. In CN, the LPP difference score was not significantly correlated with state or trait self-reported negative emotion. It is unclear why these correlations were nonsignificant in our CN group because some prior studies have found significant associations between the LPP and self-reported arousal34–36; however, one possibility is that these associations are most robust when negative emotionality exceeds a certain threshold and becomes pathological. Alternatively, the LPP may be more strongly related to arousal than valence, and arousal was not measured in this study. Altogether, the correlational findings evidenced by the patient group support our novel hypothesis that elevated self-reported state and trait negative emotion is related to an emotion regulation abnormality in SZ. It is plausible that emotion regulation dysfunction results in increased state and trait negative emotions, and/or that chronically elevated negative emotions make it harder for patients to learn and successfully apply cognitive change strategies to decrease their negative emotions.
The neural substrates responsible for these emotion regulation abnormalities cannot be definitively determined using ERP alone. Although the excellent temporal resolution of ERP enables a precise evaluation of the timecourse of neural response in relation to the emotion regulation manipulation, its low spatial resolution makes it difficult to draw accurate conclusions regarding the neural circuitry involved. Based upon prior fMRI findings with this and similar tasks indicating that successful cognitive change is associated with increased prefrontal cortex activity and decreased amygdala activity,37,39–41 it is possible that the present ERP findings reflect that SZ patients either have ineffective cortical control over the amygdala and limbic regions or a failure to adequately engage prefrontal and limbic regions when applying cognitive change strategies. Another possibility is that abnormal activation of dorsal regions of the anterior cingulate cortex cause failures in monitoring the extent to which cognitive change strategies effectively decrease negative emotions. Future studies should examine these possibilities directly in SZ using fMRI.
Several alternative explanations for these findings should be ruled out. One possibility is that the LPP abnormalities noted in SZ occur due to some process other than cognitive change, such as a failure to attend to the auditory stimuli, impaired priming, or a generalized context processing deficit. Given that SZ and CN had similar self-reported negative emotionality to the UNP-PIC/NEG-DESC and UNP-PIC/NEU-DESC conditions, it is unlikely that SZ simply did not attend to the auditory stimuli. We also doubt that the findings reflect abnormal priming for 3 reasons: (1) the temporal characteristics of the task are not amenable to a priming explanation; the duration between the auditory description and the image is much longer than the very brief durations needed for priming to reliably take place; (2) there were no group × condition interaction effects at the N1 and P2 early ERP components, which would be expected if priming were a potential explanation; and (3) the results obtained on healthy individuals in our sample and 2 prior studies using this implicit cognitive change paradigm31,57 are very similar to those obtained via explicit ERP regulation paradigms when healthy subjects generate their own reappraisals.38 Given that the timecourse of the LPP response to reappraisals is similar in this task and those where subjects generate their own reappraisals, we think it is unlikely that this paradigm reflects priming or some process other than cognitive change and reappraisal. Finally, it is unlikely that the LPP findings in patients are best explained by a generalized context processing deficit because the correlation between the LPP difference score and the DPX task was nonsignificant.
These findings have important treatment implications. A number of psychosocial treatment programs have been developed and shown to be efficacious in other disorders (eg, mood and anxiety), which use cognitive-behavioral therapy techniques to improve emotion regulation by teaching cognitive change techniques, facilitating adaptive action tendencies, and preventing emotional avoidance.58 These methods could be adapted for use in a SZ population and may have utility for improving emotion regulation, symptoms, and functional outcome.
Supplementary Material
Supplementary material is available at http://schizophre niabulletin.oxfordjournals.org.
Funding
US National Institutes of Mental Health (K23MH092530 to Dr Strauss).
Supplementary Material
Acknowledgments
We are indebted to the subjects who participated in the study and staff at the Maryland Psychiatric Research Center who made the completion of the study possible. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Kraepelin E. Dementia Praecox and Paraphrenia. Translated by Barclay RM; edited by Robertson GM. New York: Robert E Krieger; 1971. [Google Scholar]
- 2. Bleuler E. Dementia Praecox or the Group of Schizophrenias. Translated by Zinkin J New York: International Universities Press; 1950. [Google Scholar]
- 3. Rado S. Dynamics and classification of disordered behavior. Am J Psychiatry. 1953;110:406–416 [DOI] [PubMed] [Google Scholar]
- 4. Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophr Bull. 2008;34:819–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berenbaum H, Oltmanns TF. Emotional experience and expression in schizophrenia and depression. J Abnorm Psychol. 1992;101:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Earnst KS, Kring AM. Emotional responding in deficit and non-deficit schizophrenia. Psychiatry Res. 1999;88:191–207 [DOI] [PubMed] [Google Scholar]
- 7. Horan WP, Green MF, Kring AM, Nuechterlein KH. Does anhedonia in schizophrenia reflect faulty memory for subjectively experienced emotions? J Abnorm Psychol. 2006;115:496–508 [DOI] [PubMed] [Google Scholar]
- 8. Kring AM, Kerr SL, Smith DA, Neale JM. Flat affect in schizophrenia does not reflect diminished subjective experience of emotion. J Abnorm Psychol. 1993;102:507–517 [DOI] [PubMed] [Google Scholar]
- 9. Herbener ES, Song W, Khine TT, Sweeney JA. What aspects of emotional functioning are impaired in schizophrenia? Schizophr Res. 2008;98:239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reske M, Kellermann T, Habel U, et al. Stability of emotional dysfunctions? A long-term fMRI study in first-episode schizophrenia. J Psychiatr Res. 2007;41:918–927 [DOI] [PubMed] [Google Scholar]
- 11. Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93:253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oorschot M, Lataster T, Thewissen V, et al. Emotional experience in negative symptoms of schizophrenia: no evidence for a generalized hedonic deficit Schizophr Bull. 2013;39: 217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor SF, Kang J, Brege IS, Tso IF, Hosanagar A, Johnson TD. Meta-analysis of functional neuroimaging studies of emotion perception and experience in schizophrenia. Biol Psychiatry. 2012;71:136–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. Am J Psychiatry. 2012;169:364–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr Bull. 2010;36:143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Llerena K, Strauss GP, Cohen AS. Looking at the other side of the coin: a meta-analysis of self-reported emotional arousal in people with schizophrenia. Schizophr Res. 2012;142:65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trémeau F, Antonius D, Cacioppo JT, et al. Anticipated, on-line and remembered positive experience in schizophrenia. Schizophr Res. 2010;122:199–205 [DOI] [PubMed] [Google Scholar]
- 18. Strauss GP, Herbener ES. Patterns of emotional experience in schizophrenia: differences in emotional response to visual stimuli are associated with clinical presentation and functional outcome. Schizophr Res. 2011;128:117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Myin-Germeys I, Delespaul PA, deVries MW. Schizophrenia patients are more emotionally active than is assumed based on their behavior. Schizophr Bull. 2000;26:847–854 [DOI] [PubMed] [Google Scholar]
- 20. Myin-Germeys I, Krabbendam L, Delespaul PA, van Os J. Sex differences in emotional reactivity to daily life stress in psychosis. J Clin Psychiatry. 2004;65:805–809 [DOI] [PubMed] [Google Scholar]
- 21. Horan WP, Blanchard JJ, Clark LA, Green MF. Affective traits in schizophrenia and schizotypy. Schizophr Bull. 2008;34:856–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Horan WP, Green MF, Kring AM, Nuechterlein KH. Does anhedonia in schizophrenia reflect faulty memory for subjectively experienced emotions? J Abnorm Psychol. 2006;115:496–508 [DOI] [PubMed] [Google Scholar]
- 23. Cohen AS, Najolia GM, Brown LA, Minor KS. The state-trait disjunction of anhedonia in schizophrenia: potential affective, cognitive and social-based mechanisms. Clin Psychol Rev. 2011;31:440–448 [DOI] [PubMed] [Google Scholar]
- 24. Strauss GP, Llerena K, Gold JM. Attentional disengagement from emotional stimuli in schizophrenia. Schizophr Res. 2011;131:219–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gross JJ. The emerging field of emotion regulation: an integrative review Review of General Psychology. 1998;2:271–299 [Google Scholar]
- 26. Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–291 [DOI] [PubMed] [Google Scholar]
- 27. van der Meer L, van’t Wout M, Aleman A. Emotion regulation strategies in patients with schizophrenia Psychiatry Res. 2009;170:108–113 [DOI] [PubMed] [Google Scholar]
- 28. Kimhy D, Vakhrusheva J, Jobson-Ahmed L, Tarrier N, Malaspina D, Gross JJ. Emotion awareness and regulation in individuals with schizophrenia: implications for social functioning. Psychiatry Res. 2012;200:193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Henry JD, Rendell PG, Green MJ, McDonald S, O’Donnell M. Emotion regulation in schizophrenia: affective, social, and clinical correlates of suppression and reappraisal. J Abnorm Psychol. 2008;117:473–478 [DOI] [PubMed] [Google Scholar]
- 30. Perry Y, Henry JD, Grisham JR. The habitual use of emotion regulation strategies in schizophrenia Brit J Clin Psychol. 2011;50:217–222 [DOI] [PubMed] [Google Scholar]
- 31. Foti D, Hajcak G. Deconstructing reappraisal: descriptions preceding arousing pictures modulate the subsequent neural response. J Cogn Neurosci. 2008;20:977–988 [DOI] [PubMed] [Google Scholar]
- 32. Hajcak G, Weinberg A, MacNamara A, Foti D. ERPs and the study of emotion. In Luck SJ, Kappenman ES. (Eds.) Oxford Handbook of Event-Related Potential Components. New York: Oxford University Press; [Google Scholar]
- 33. Hajcak G, MacNamara A, Olvet DM. Event-related potentials, emotion, and emotion regulation: an integrative review. Dev Neuropsychol. 2010;35:129–155 [DOI] [PubMed] [Google Scholar]
- 34. Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol Psychol. 2000;52:95–111 [DOI] [PubMed] [Google Scholar]
- 35. Schupp H, Cuthbert B, Bradley M, Hillman C, Hamm A, Lang P. Brain processes in emotional perception: motivated attention Cognition & Emotion. 2004;18:593–561 [Google Scholar]
- 36. Weinberg A, Hajcak G. Beyond good and evil: the time-course of neural activity elicited by specific picture content. Emotion. 2010;10:767–782 [DOI] [PubMed] [Google Scholar]
- 37. Mocaiber I, Sanchez TA, Pereira MG, et al. Antecedent descriptions change brain reactivity to emotional stimuli: a functional magnetic resonance imaging study of an extrinsic and incidental reappraisal strategy. Neuroscience. 2011;193:241–248 [DOI] [PubMed] [Google Scholar]
- 38. Hajcak G, Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cogn Affect Behav Neurosci. 2006;6:291–297 [DOI] [PubMed] [Google Scholar]
- 39. Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229 [DOI] [PubMed] [Google Scholar]
- 40. Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249 [DOI] [PubMed] [Google Scholar]
- 41. Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499 [DOI] [PubMed] [Google Scholar]
- 42. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders — Patient Edition (SCID-I/P 2/2001 Revision). New York: Biometrics Research Department, New York State Psychiatric Institute; 2001. [Google Scholar]
- 43. Pfohl BM, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality. 1st ed. American Psychiatric Publishing, Inc; 1997. [Google Scholar]
- 44. Overall JE, Gorham DR. The brief psychiatric rating scale Psychol Rep. 1962;10:799–812 [Google Scholar]
- 45. Kirkpatrick B, Strauss GP, Nguyen L, et al. The Brief Negative Symptom Scale: psychometric properties. Schizophr Bull. 2011;37:300–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Strauss GP, Keller WR, Buchanan RW, et al. Next-generation negative symptom assessment for clinical trials: validation of the Brief Negative Symptom Scale. Schizophr Res. 2012;142:88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Strauss GP, Hong LE, Keller WR, et al. Factor structure of the Brief Negative Symptom Scale. Schizophr Res. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hawk AB, Carpenter WT, Jr, Strauss JS. Diagnostic criteria and five-year outcome in schizophrenia. A report from the International Pilot Study of schizophrenia. Arch Gen Psychiatry. 1975;32:343–347 [DOI] [PubMed] [Google Scholar]
- 49. Watson D, Clark LA. On traits and temperament: general and specific factors of emotional experience and their relation to the five-factor model. J Pers. 1992;60:441–476 [DOI] [PubMed] [Google Scholar]
- 50. Gard DE, Gard-Germans M, Kring AM, Oliver JP. Anticipatory and consummatory components of the experience of pleasure: a scale development study J Res Personality. 2006;40:1086–1102 [Google Scholar]
- 51. Henderson D, Poppe AB, Barch DM, et al. Optimization of a goal maintenance task for use in clinical applications. Schizophr Bull. 2012;38:104–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Digitized Photographs, Instruction Manual and Affective Ratings, Technical Report A-6. Gainesville, FL: University of Florida; 2001. [Google Scholar]
- 53. Luck SJ. An Introduction to the Event-Related Potential Technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- 54. Nunez PL. Electric Fields of the Brain. New York: Oxford University Press; 1981. [Google Scholar]
- 55. Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21 [DOI] [PubMed] [Google Scholar]
- 56. Jung TP, Makeig S, Humphries C, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37:163–178 [PubMed] [Google Scholar]
- 57. Macnamara A, Foti D, Hajcak G. Tell me about it: neural activity elicited by emotional pictures and preceding descriptions. Emotion. 2009;9:531–543 [DOI] [PubMed] [Google Scholar]
- 58. Barlow DH, Allen LB, Choate ML. Towards a unified treatment of emotional disorders Behavior Therapy. 2004;35:205–230 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.