Abstract
Prenatal cocaine exposure (PCE) is associated with blunted stress responsivity within the extrauterine environment. This study investigated the association between PCE and diurnal salivary cortisol levels in preadolescent children characterized by high biological and/or social risk (N = 725). Saliva samples were collected at their home. Analyses revealed no group differences in basal evening or morning cortisol levels; however, children with higher degrees of PCE exhibited blunted overnight increases in cortisol, controlling for additional risk factors. Race and caregiver depression were also associated with diurnal cortisol patterns. While repeated PCE may contribute to alterations in the normal or expected stress response later in life, sociodemographic and environmental factors are likewise important in understanding hormone physiology, especially as more time elapses from the PCE. Anticipating the potential long-term medical, developmental, or behavioral effects of an altered ability to mount a normal protective cortisol stress response is essential in optimizing the outcomes of children with PCE.
Introduction
The hypothalamic-pituitary-adrenal (HPA) axis of the neuroendocrine system maintains physiologic homeostasis by regulating the body's response to internal and external stressors. Following a threatening exposure, cortisol, a stress hormone secreted by the adrenal gland, is released, resulting in immediate elevations of heart rate and blood pressure (i.e., the “fight or flight” response). As the perceived threat dissipates, elevated hormone levels and physiological parameters gradually return to baseline. Under normal, nonstressful conditions, cortisol is released in a diurnal pattern with the highest concentrations present in the morning in preparation for daily activities (1). Levels rapidly decline toward midday, dropping more gradually into the evening. Repetitive stress exposures over time may permanently disrupt this typical pattern, leading to prolonged elevations in cortisol levels and eventual system desensitization and down-regulation (2). The individual may then display decreased morning cortisol levels that remain low throughout the day. This lowered responsivity has been associated with alterations in attention regulation, motivation, immune system functioning, sleep cycle, and onset of stress-related diseases (3).
Atypical cortisol patterns have been reported in children living in high-stress environments (1, 4-9). Acute (or periodic) stress exposures may result in increased cortisol levels while chronic (or pervasive) stress exposures may result in decreased levels (10). For instance, children in foster care who experienced severe, chronic physical neglect have exhibited lowered morning cortisol levels as opposed to children experiencing severe, intermittent emotional maltreatment who had the highest levels (5). These findings support evidence that chronic stress may affect system down-regulation and blunted cortisol production (2, 10, 11). Flattened diurnal cortisol patterns have also been observed in children who continued to live with birth parents following Child Protective Services involvement. However, children who were removed from similar chronic threatening situations and placed in foster care exhibited more normal cortisol patterns (8). Other variables associated with alterations in child cortisol patterns include premature birth, secondhand smoke, low socioeconomic status (SES), caregiver depression, and domestic violence (1, 4, 7, 9, 12, 13). Documenting the details of a child's environment is essential to better understand factors that may alter the integrity of their stress physiology.
Prolonged stress exposure in utero may also disrupt the usual pattern of cortisol secretion later in life. Evidence suggests that maternal cocaine use during pregnancy may permanently affect developing fetal systems that control arousal regulation and stress response, altering the threshold response of the HPA axis to extrauterine stressors (14). Specific human brain regions, such as the hippocampus and amygdala, which primarily regulate other systems (e.g., emotional functioning) are also affected by stress hormones (15). Understanding mechanisms that alter stress reactions in children may provide insights into potential dysfunctions in other developmental realms.
Limited and somewhat contradictory data have been published regarding the association between prenatal cocaine exposure (PCE) and cortisol physiology. One study reported elevated cortisol levels in preterm neonates with PCE (12), while another reported lowered basal cortisol levels in exposed infants (gestational age ≥ 32 weeks) (16). Another study reported no group differences in basal cortisol levels; however, exposed infants demonstrated suppressed cortisol reactivity (the difference between basal cortisol and peak levels) following an induced stress event (17). Blunted reactivity has also been observed in preadolescent children with PCE with the greatest effects seen in exposed children whose caregivers had experienced domestic violence (13). In contrast, an infant study reported greater cortisol reactivity, especially among exposed boys (18). Differences in child age or level of PCE may contribute somewhat to inconsistencies in study findings. In addition, these studies reflect the cortisol levels of children in a clinic setting (an atypical environment that may induce additional stress). To our knowledge, no published reports have examined the diurnal cortisol variation in older children with PCE or have examined cortisol levels of exposed children within the more naturalistic home setting.
Multiple comorbid risk factors besides PCE may alter the stress response system of infants and older children. Women who use cocaine during pregnancy typically use greater amounts of legal and illegal substances and are more likely to raise their children in compromised environments (19). The objective of the current study was to examine the basal cortisol levels of 11-year-old children at high social and/or biological risk within the home setting. This report examines both the association between levels of PCE and basal cortisol levels and the change in these levels between evening and morning, controlling for various biological, social, and environmental characteristics of this high-risk sample.
Methods
Participants
Children were enrolled at birth in the Maternal Lifestyle Study (MLS), a prospective, longitudinal investigation of the effects of prenatal cocaine and/or opiate exposure on acute and long-term child outcomes. Four institutions participated: Brown University, the University of Miami, the University of Tennessee-Memphis, and Wayne State University. Institutional review board approval occurred at each site and informed maternal consent was obtained. Exposure status was determined by maternal admission of drug use and/or a positive enzyme multiplied immunoassay technique (EMIT) screening for drug metabolites in meconium confirmed with gas chromatography/mass spectroscopy (GC/MS) (20). Nonexposure was determined by denial of cocaine/opiate use during pregnancy and a negative meconium toxicology screen. Detailed information regarding screening and participant enrollment has been previously reported (21). A total of 1388 dyads (658 cocaine/opiate exposed and 730 nonexposed) attended the first clinic visit at 1 month of age, which defined enrollment into the follow-up component. There were 18 visits scheduled between the 1-month and 11-year visits.
Of these 1388 children, 1023 attended the 11-year clinic visit. Children who attended the visit were less likely to have prenatal opiate exposure or to be white compared to children who did not attend the visit (p's < .001). Groups were comparable on PCE level, prenatal tobacco, alcohol, or marijuana exposure, maternal education, race, small for gestational age (SGA), low birth weight (LBW), and preterm birth. Of the 1023 children who attended the 11-year clinic visit, 725 were included in the current sample (see Figure 1). Similar contrasts revealed that children included in the current sample were less likely to be white and to be born preterm compared to excluded children (p's < .05). Groups were otherwise comparable.
Figure 1.
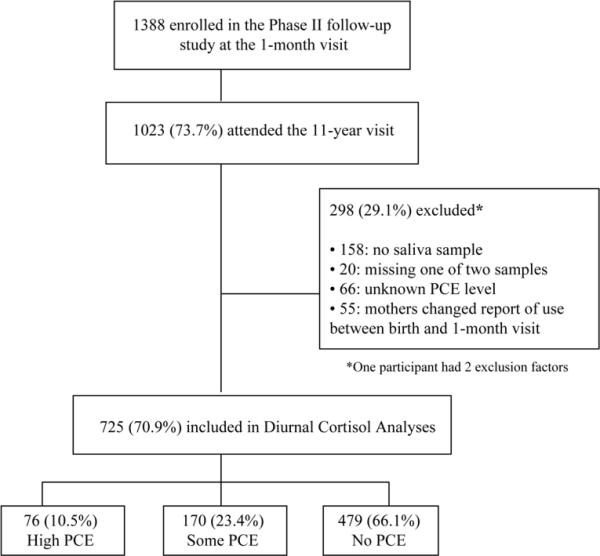
Delineation of Study Sample
Measures
Child birth outcomes, gender, and maternal demographics
Preterm birth (<37 weeks gestation), SGA (<10th percentile), LBW (<2500 grams), child gender, maternal education (<high school (HS), HS diploma, >HS), and maternal race (black, white, other) were included as control variables.
Prenatal drug exposure
At the 1-month visit, detailed information regarding drug use was collected through maternal interview. Level of PCE was categorized as high (≥3 times/week during the first trimester), some (any other use during pregnancy), or none. Other drug exposures were entered as control variables. Prenatal alcohol (average ounces of absolute alcohol consumed p/day), tobacco (average number of cigarettes smoked p/day), marijuana (average number of joints smoked p/day) were calculated for the entire pregnancy consistent with our previous work (13, 22). Binge drinking (≥5 drinks at one time) and opiate use during pregnancy were recorded dichotomously (yes/no).
Salivary Cortisol
Saliva is a reliable and accessible source of cortisol levels in both children and adults (23). Families were provided with instructions and materials to collect children's saliva at home. One evening and one morning sample were collected in individual test tubes using a small straw until the amount met or exceeded the line indicated on the tube. On the night prior to their clinic appointment, evening samples were collected around bedtime at least one hour after eating or drinking and before brushing their teeth. Morning samples were collected the following day right after waking and before eating or brushing their teeth. Drinking water was acceptable at least 15 minutes prior to saliva collection. Documentation of collection times was required and both saliva samples were expected to be capped and kept in their freezer at home until their clinic appointment. Returned samples were placed in a -20°C freezer until they were shipped in dry ice to Salimetrics Laboratory (Salimetrics, LLC) for analysis.
Caregiving Environment
Potential risk factors related to the child's caregiving environment were investigated. SES was based on the Hollingshead Index of Social Position score (Hollingshead, 1975; unpublished report) at the 11-year visit. Higher scores indicated higher SES. The total number of caretaker changes and foster care placement (yes/no) was based on information gathered at any visit. As reported at the 11-year visit, caretaker abuse (yes/no) was determined by caregiver-report of experiencing physical or sexual violence and child abuse (yes/no) was based on any report of abuse or neglect since their last clinic visit (usually 12 months prior). Community violence exposure was based on caregiver-report on the 12-item Survey of Exposure to Community Violence interview (24) at the 9-year visit. Higher scores indicated more exposure. Caregiver depression was assessed using the Beck Depression Inventory (BDI) (25) at the 11-year visit. Caregivers with scores ≥16 were classified as being depressed within the past 7 days. Caregiver postnatal alcohol, tobacco, and marijuana intake and binge drinking were assessed using the same quantification definitions as for prenatal exposures and refer to occurrence since their last clinic visit as reported during the 11-year visit.
The aforementioned environmental variables were used to assess recent environmental stress. To assess the potential differential effects of cumulative stress exposure, a modified set of environmental covariates were created for use in a logistic regression model. SES was redefined as the mean SES score recorded across the 1-month to 11-year visits. Caregiver abuse and child abuse were determined by caregiver-report (yes/no) at any visit. Chronic caregiver depression referred to the total number of times the caregiver scored ≥16 on the BDI at the 4-month, 30-month, and 4-, 7-, 9-, and 11-year visits. Caregiver postnatal substance use variables were redefined based upon self-report data at any visit. The remaining covariate definitions were unchanged for this cumulative stress model.
Analyses
Differences between PCE groups (high, some, and none) were assessed on all covariates using chi-square tests for categorical variables and analyses of variance (ANOVAs) for continuous variables. Evening and morning cortisol values were compared by PCE level using linear regression. Distributions of raw cortisol values were highly positively skewed; therefore, square root transformations were applied to achieve more normal distributions. We also tested whether change in diurnal cortisol values between evening and morning measurements varied by PCE (i.e., time x exposure interactions) using generalized estimating equations (GEE) models to account for repeated measurements. Follow-up analyses were conducted for linear regression and GEE models controlling for site, other prenatal drug exposures, birth outcomes, gender, maternal demographics, and caretaking environment.
Finally, we assessed whether children had typical versus atypical patterns of change in diurnal cortisol levels. Patterns were defined as atypical if their increase in cortisol levels between evening and morning measurements fell at or below the 25th percentile (i.e., increased 0.091 μg/dL or less). A logistic regression model was conducted to examine the relationship between PCE and atypical cortisol patterns, controlling for site, other prenatal drug exposures, birth outcomes, gender, maternal demographics, and caretaking environment. An additional logistic regression was then conducted for the cumulative environmental stress model which examined the association between PCE and atypical cortisol patterns, controlling for site, other prenatal drug exposures, birth outcomes, gender, maternal demographics, and the modified set of environmental covariates (i.e., variables based on longitudinal data).
A majority of the sample (84%) completed saliva sampling as instructed collecting the evening sample first and the morning sample second. The remaining children collected their samples in the opposite order. Overnight change in cortisol levels was calculated the same (morning-evening) regardless of the order of sample collection. Excluding these children did not affect results; therefore, all 725 children were included in analyses.
Results
Anthropometric, sociodemographic, and environmental characteristics of the analytic sample are presented in Table 1. PCE groups (high, some, and none) were comparable on gender, LBW, preterm birth, prenatal opiate exposure, caregiver abuse, child abuse, caregiver depression, and caregiver postnatal marijuana intake. Group differences occurred on the remaining variables. PCE was associated with an increased likelihood of prenatal exposure to alcohol, binge drinking, tobacco, or marijuana. Children with any cocaine exposure had mothers with less education, had caregivers with higher SES, experienced more caretaker changes, were more likely to have been placed in foster care, and were exposed to more community violence in contrast to children with no PCE. Children with high PCE (vs. nonexposed) were more likely to be born SGA. Lastly, children with some PCE (vs. nonexposed) were more likely to be black and to have caregivers who reported recent alcohol consumption, binge drinking, and greater tobacco use. Lastly, supplemental, exploratory analyses revealed that exposed children were less likely to live with a biological parent(s) than nonexposed children (65% vs. 94%) at the 11-year visit. Similarly, exposed children were more likely to have an adoptive parent as their primary caregiver than nonexposed children (11% vs. 1%; p's < .001).
Table 1.
Sociodemographic, Anthropometric, and Environmental Characteristics of Study Sample
| Characteristic | All (N = 725) | High PCE (n = 76) | Some PCE (n = 170) | No PCE (n = 479) | H vs. N p | S vs. N p | H vs. S p |
|---|---|---|---|---|---|---|---|
| Male, n (%) | 373 (51) | 41 (54) | 87 (51) | 245 (51) | .65 | .995 | .688 |
| Maternal education, n (%) | |||||||
| > HS | 163 (22) | 17 (22) | 29 (17) | 117 (24) | .002 | .001 | .309 |
| HS | 299 (41) | 21 (28) | 63 (37) | 215 (45) | |||
| < HS | 262 (36) | 38 (50) | 78 (46) | 146 (30) | |||
| Race, n (%) | |||||||
| Black | 599 (83) | 63 (83) | 152 (89) | 384 (80) | .813 | .024 | .346 |
| White | 83 (11) | 8 (11) | 12 (7) | 63 (13) | |||
| Other | 43 (6) | 5 (7) | 6 (4) | 32 (7) | |||
| SES, mean (SD) | 3.48 (0.99) | 3.65 (0.86) | 3.70 (0.97) | 3.38 (1.00) | .025 | < .001 | .741 |
| SGA, n (%) | 166 (23) | 24 (32) | 42 (25) | 100 (21) | .037 | .283 | .272 |
| LBW, n (%) | 293 (40) | 33 (43) | 71 (42) | 189 (39) | .512 | .598 | .808 |
| Preterm birth, n (%) | 288 (40) | 26 (34) | 71 (42) | 191 (40) | .34 | .601 | .234 |
| Prenatal alcohol use, mean (SD) | 0.33 (0.98) | 0.89 (1.81) | 0.69 (1.27) | 0.11 (0.48) | < .001 | < .001 | .117 |
| Prenatal binge drinking, n (%) | 129 (18) | 24 (32) | 66 (39) | 39 (8) | < .001 | < .001 | .276 |
| Prenatal tobacco use, mean (SD) | 6.12 (9.65) | 11.42 (11.01) | 10.86 (10.78) | 3.60 (7.88) | < .001 | < .001 | .655 |
| Prenatal marijuana use, mean (SD) | 0.08 (0.30) | 0.19 (0.45) | 0.12 (0.40) | 0.04 (0.21) | < .001 | .002 | .112 |
| Prenatal opiate exposure, n (%) | 49 (7) | 8 (11) | 11 (6) | 30 (6) | .172 | .924 | .271 |
| Foster care, n (%) | 76 (10) | 22 (29) | 34 (20) | 20 (4) | < .001 | < .001 | .122 |
| Caretaker changes, mean (SD) | 0.75 (1.34) | 1.89 (2.08) | 1.25 (1.41) | 0.38 (0.96) | <.001 | < .001 | < .001 |
| Caretaker abuse, n (%) | 17 (2) | 2 (3) | 6 (4) | 9 (2) | .622 | .206 | .738 |
| Child abuse, n (%) | 9 (1) | 2 (3) | 3 (2) | 4 (1) | .155 | .311 | .650 |
| Community violence, mean (SD) | 3.98 (3.97) | 4.74 (4.12) | 5.20 (4.16) | 3.44 (3.77) | .009 | < .001 | .417 |
| Caregiver depression, n (%) | 64 (9) | 6 (8) | 15 (9) | 43 (9) | .886 | .99 | .892 |
| Caregiver alcohol use, mean (SD) | 0.24 (0.72) | 0.25 (0.69) | 0.43 (1.04) | 0.17 (0.56) | .401 | < .001 | .081 |
| Caregiver binge drinking, n (%) | 100 (14) | 7 (9) | 38 (22) | 55 (11) | .629 | < .001 | .016 |
| Caregiver tobacco use, mean (SD) | 5.59 (9.08) | 6.39 (8.66) | 7.96 (9.98) | 4.64 (8.65) | .125 | < .001 | .22 |
| Caregiver marijuana use, mean (SD) | 0.05 (0.28) | 0.05 (0.25) | 0.05 (0.17) | 0.05 (0.31) | .932 | .986 | .948 |
Note. H = high, S = some, and N = none. Statistical comparisons are based on chi-square tests for categorical variables and ANOVAs for continuous variables.
Mean time for evening samples was 8:46 p.m. (SD = 3:42) and 7:57 a.m. (SD = 1:27) for morning samples. No differences were found among exposure groups for basal evening or morning cortisol levels (see Table 2). Results generally remained the same when controlling for site, birth outcomes, gender, maternal demographics, other prenatal drug exposures, and caretaking environment. However, children of black mothers had lower morning cortisol levels compared to children of white mothers (b = -6.50, p = .026). Results of unadjusted GEE analyses are reported in Table 2 and Figure 2. These analyses revealed that children with none and some PCE had similar patterns of overnight increase; however, children with high PCE had smaller increases in cortisol from the evening to morning measurements than those with some or no PCE. In adjusted analyses controlling for similar covariates, children with high PCE continued to demonstrate smaller increases in cortisol compared to children with no PCE (p = .046). There was no longer a difference between the high and some PCE groups. =
Table 2.
Diurnal Cortisol Values by Level of PCE
| Measurement | All | PCE | H vs. N | S vs. N | H vs. S | ||
|---|---|---|---|---|---|---|---|
| High | Some | None | p | p | p | ||
| Diurnal Cortisol values (μg/dL), mean (SD) | |||||||
| Evening | 0.20 (0.52) | 0.26 (0.59) | 0.19 (0.56) | 0.19 (0.49) | .180 | .791 | .170 |
| Morning | 0.38 (0.33) | 0.33 (0.21) | 0.40 (0.45) | 0.38 (0.29) | .093 | .969 | .127 |
| Morning-Evening | 0.18 (0.49) | 0.07 (0.59) | 0.20 (0.45) | 0.20 (0.48) | .036 | .816 | .043 |
| Atypical pattern of change, n (%) | 181 (25) | 26 (34) | 46 (27) | 109 (23) | .032 | .259 | .255 |
Note. H = high, S = some, and N = none. Statistical comparisons are based on unadjusted linear regression models for diurnal cortisol values, an unadjusted GEE regression model for change in cortisol values over time, and an unadjusted logistic regression model for atypical pattern of change (i.e., increase in diurnal cortisol levels at or below the 25th percentile). Although data transformations were applied in analyses, original units are presented here for easier interpretability.
Figure 2.
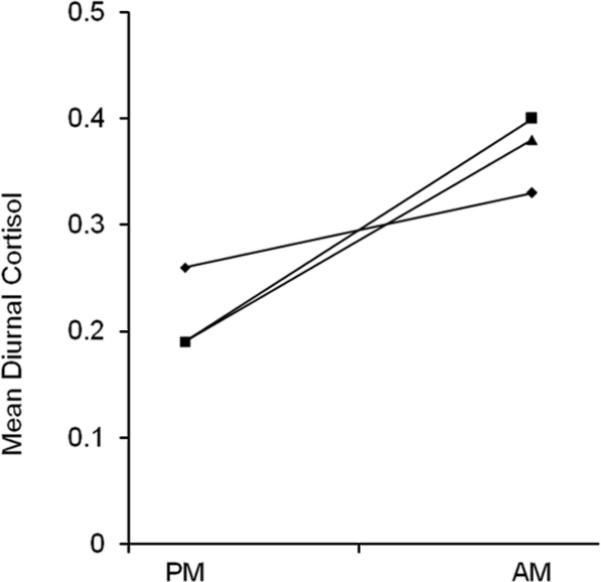
Mean Evening and Morning Diurnal Cortisol Values by Level of PCE. ◆: high PCE; ■: some PCE; ▲: no PCE.
When examining typical versus atypical patterns of change, children with high PCE (vs. nonexposed children) were more likely to demonstrate an atypical change (increase ≤ the 25th percentile) in cortisol levels from evening to morning (Table 2). This difference remained after controlling for site, birth outcomes, gender, maternal demographics, other prenatal drug exposures, and caretaking environment (see Table 3). Children with high PCE had more than twice the odds of demonstrating an atypical pattern of change in diurnal cortisol. Race and caregiver depression were also predictive of cortisol patterns. Children with black mothers were more than twice as likely to demonstrate a blunted increase in cortisol levels compared to children with white mothers. Children with caregivers reporting recent depression (vs. children with non-depressed caregivers) were approximately one-third as likely to demonstrate this atypical pattern of change.
Table 3.
Logistic Regression Model of Atypical Pattern of Change in Diurnal Cortisol from Evening to Morning
| Variable | Adjusted OR | 95% CI | p |
|---|---|---|---|
| Site | |||
| A | 0.47 | 0.22, 1.00 | 0.051 |
| B | 0.66 | 0.30, 1.44 | 0.295 |
| C | 0.42 | 0.18, 1.00 | 0.050 |
| Male | 1.14 | 0.77, 1.67 | 0.511 |
| Maternal education level | |||
| HS | 1.03 | 0.59, 1.79 | 0.925 |
| < HS | 1.32 | 0.80, 2.20 | 0.279 |
| Race | |||
| Black | 2.91 | 1.32, 6.44 | 0.008 |
| Other | 1.10 | 0.36, 3.36 | 0.873 |
| SES | 1.04 | 0.84, 1.28 | 0.745 |
| SGA | 1.18 | 0.71, 1.96 | 0.524 |
| LBW | 0.57 | 0.32, 1.02 | 0.057 |
| Preterm birth | 1.62 | 0.93, 2.82 | 0.091 |
| Prenatal cocaine exposure | |||
| High | 2.23 | 1.11, 4.47 | 0.025 |
| Some | 1.51 | 0.89, 2.56 | 0.129 |
| Prenatal alcohol use | 0.77 | 0.55, 1.06 | 0.112 |
| Prenatal binge drinking | 1.44 | 0.77, 2.69 | 0.249 |
| Prenatal tobacco use | 0.99 | 0.97, 1.02 | 0.648 |
| Prenatal marijuana use | 0.90 | 0.45, 1.82 | 0.779 |
| Prenatal opiate exposure | 1.95 | 0.95, 3.98 | 0.068 |
| Caretaker changes | 1.03 | 0.88, 1.20 | 0.749 |
| Foster care placement | 0.79 | 0.37, 1.67 | 0.538 |
| Caregiver depression | 0.38 | 0.17, 0.89 | 0.025 |
| Caretaker abuse | 2.06 | 0.68, 6.21 | 0.200 |
| Child abuse | 0.98 | 0.17, 5.81 | 0.982 |
| Community violence exposure | 0.97 | 0.92, 1.03 | 0.321 |
| Caregiver alcohol use | 1.03 | 0.72, 1.47 | 0.865 |
| Caregiver binge drinking | 0.84 | 0.42, 1.71 | 0.639 |
| Caregiver tobacco use | 1.00 | 0.97, 1.02 | 0.782 |
| Caregiver marijuana use | 0.62 | 0.20, 1.94 | 0.409 |
Note. Atypical change is defined as those in the 25th percentile for least increase in diurnal cortisol levels (μg/dL) from evening to morning measurements. Reference categories are site D, female, more than high school, white, average for gestational age, normal birth weight, term, no PCE, no binge drinking, no foster care, and no abuse.
For the cumulative environmental stress model (controlling for site, birth outcomes, gender, maternal demographics, other prenatal drug exposures, and longitudinal environmental variables), results of a logistic regression generally remained the same. High PCE (Odds ratio (OR), 2.06; 95% confidence interval (CI), 1.07-4.00) and race (OR, 2.45; 95% CI, 1.13-5.30) were associated with atypical diurnal cortisol patterns (p's < .05). Caregiver depression over time, however, was not associated with cortisol patterns (p = .614). As in the previous model, no other covariates predicted atypical cortisol patterns.
Supplemental analyses were conducted to examine issues related to collinearity and the potential effect of differences in sample collection time across children on diurnal cortisol patterns. With regard to collinearity, associations among the original predictor variables were examined. As expected, prenatal alcohol, binge drinking, tobacco, marijuana exposure were strongly associated with PCE. There was also a strong association between PCE and number of caretaker changes. It is essential to control for other prenatal drug exposures to isolate the effect of PCE on diurnal cortisol patterns from the potential effect of other substances; thus, these variables remained in our model. However, a logistic regression was re-run excluding caretaker changes to avoid potential overlap with PCE. Results were consistent with a slightly larger effect for high PCE (OR, 2.29; 95% CI, 1.17-4.50; p = .016). A median split was then used to classify children as having early or late collection times for the evening and morning measurements. Results of a logistic regression model (controlling for timing of sample collections) were consistent. Children with high PCE (vs. nonexposed) were more likely to have atypical diurnal cortisol patterns (OR, 2.28; 95% CI, 1.12-4.66; p = .023). There were no differences for children with some versus no PCE.
Discussion
Early stressful experiences have been associated with altered development and subsequent dysfunction of the HPA system, specifically in regard to cortisol secretion. In this study, preadolescent children with higher levels of PCE showed a blunted increase in cortisol levels between evening and morning measurements, especially compared to nonexposed children. Extensive maternal use of cocaine during pregnancy may constitute a pervasive stress resulting in increased maternal and fetal cortisol secretion and prolonged exposure to elevated cortisol levels (14). Chronic over-stimulation of the HPA axis during this critical developmental period may lead to later dysfunction of the normal stress response, resulting in physiologic down-regulation of systems that are normally responsive to cortisol. Blunted periodicity could place the body at chronic disadvantage in mounting the normal protective response to day-to-day stresses or to an acute, perhaps, life-threatening event.
Physiologic down-regulation of the neuroendocrine system during early development may have important health implications later in life. Abnormalities in arousal regulation and stress responsivity have been associated with cognitive, behavioral, and socioemotional dysfunction, as well as increased risk for stress-related diseases (e.g., chronic fatigue syndrome, and fibromyalgia) (1, 2, 4, 11, 26, 27). Recent evidence also suggests that several adult diseases, such as cardiovascular and psychiatric diseases, may be etiologically linked to developmental and biological disturbances during early childhood (28). Chronic adverse exposures during sensitive developmental periods may result in significant biologic disruptions that contribute to permanent damage over time. Blunted cortisol reactivity was recently observed in preadolescent children with PCE within a clinic setting (13). Similar to current study findings, this blunted response was associated with higher levels of PCE as well as extrauterine stressors supporting evidence that intrauterine and extrauterine stressors may disrupt the neuroendocrine system. Lester et al. also found that exposure to domestic violence mediated the effect of PCE on cortisol reactivity. Domestic violence was used as a control variable in the current study but mediating analyses were not conducted.
In addition to PCE, race and recent caregiver depression were associated with patterns of overnight change in diurnal cortisol levels. Specifically, children with black mothers were more likely to have low cortisol levels in the evening that remained low in the morning, rather than following the more typical pattern of increase. Few studies have reported racial/ethnic disparities in diurnal cortisol values. Flattened diurnal cortisol patterns have been observed in black and Hispanic adolescents in contrast to white peers, regardless of socioenvironmental factors (29). Similar patterns have also been observed in adults (30). Additional research is necessary to determine what specific characteristics of minority populations may help explain this racial disparity in hormonal response. For instance, race-based discrimination has been associated with increased stress in blacks, subsequently increasing risk for involvement in socially deviant behaviors (e.g., substance use) (31). Other factors such as a higher incidence of adverse health conditions (e.g., hypertension, heart disease) among blacks may also help explain variance in stress hormone levels (32).
In contrast, children with caregivers experiencing recent depression were less likely to demonstrate this atypical cortisol pattern compared to children of non-depressed caregivers. Timing and duration of a child's exposure to caregiver depression may differentially affect cortisol levels. Elevated cortisol levels have been reported in school-aged children exposed to maternal depression during early childhood and in children of recently depressed mothers (9). However, chronic maternal depression has been associated with lower basal cortisol levels in children (33). In this study, recent caregiver depression may have had less of a negative impact on child cortisol patterns because the definition of the exposure was limited to the past week. When assessing reports of caregiver depression occurring over the 11-year period, no group differences were found. Recent caregiver depression in this study may represent a transient rather than a chronic stressor. Given that a hypocortisolism is typically associated with chronic stress exposure, it is not surprising that this specific aspect of the child's environment did not have the same blunting effect on their cortisol secretion as did more chronic variables (PCE and race).
While other anthropometric and environmental variables (e.g., prematurity, foster care, secondhand smoke, SES, and domestic violence) have been associated with variations in child cortisol levels, no such associations were found in the current study between similar variables and diurnal cortisol patterns. This may be partly due to the fact that our sample as a whole was characterized by increased sociodemographic, environmental, and/or biological risk, regardless of PCE. Nonetheless, controlling for these various risk factors is essential in isolating factors that have a stronger impact on the integrity of at-risk children's stress response physiology.
Limitations to the current study must be acknowledged. First, the elevated risk for this cohort (e.g., low SES, predominantly ethnic minority participants, licit prenatal drug exposure) limits the generalizability of results to other high-risk populations. Second, we attempted to statistically control for potential confounders that may have coexisted (e.g., prenatal licit and illicit drug exposure) or arisen over time (e.g., exposure to violence, caregiver depression); however, it was not possible to identify all potential stressors within this sample. The presence of multiple risk factors also limits our ability to determine causality between PCE and later cortisol patterns. In addition, factors such as child caffeine intake may influence cortisol levels. Such data was not available in this study. Lastly, cortisol levels are known to vary between individuals as well as within individuals (e.g., across different days or times of day). Availability of cortisol samples for more than two time-points and across multiple days would have likely enhanced our assessment of diurnal cortisol rhythm. Unfortunately, such methodology was not feasible in this study.
There are also important strengths of this study. To our knowledge, this is the first report of diurnal cortisol patterns in older children with PCE. In addition, reliability and generalizability of study findings were enhanced given that PCE was confirmed through positive EMIT screening and GC/MS confirmation of drug metabolites in meconium, as opposed to a classification based purely on self-report or urine determination of only recent drug metabolites, in a large, multi-site sample. Finally, this study contributes to the growing body of evidence supporting the potential long-term impact of PCE on child health outcomes.
Limited information is available regarding the potential long-term impact of PCE on alterations in cortisol physiology. The current findings suggest that higher levels of PCE may have a long-term effect on a child's neuroendocrine physiology, even after controlling for other acute or pervasive stressors. Being able to anticipate the potential long-term medical, developmental, or behavioral dysfunctions related to altered cortisol physiology is important in optimizing outcomes for children with PCE. Given the potential impact specific stressors may have on children's stress regulation abilities, health professionals (e.g., pediatricians, social workers, counselors) may need to intervene to help reduce the stress load of children with PCE (e.g., referrals for appropriate social services, avoid multiple caretaker changes, address caregiver psychopathology). Ongoing research is needed to understand the long-term consequences that prenatal substance exposure may have on physiology and health into adolescence and adulthood.
Acknowledgements
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
The following individuals, in addition to those listed as authors, and federal funding grants contributed to this study:
Brown University Warren Alpert Medical School Women & Infants Hospital of Rhode Island (U10 DA24119, U10 HD27904, N01 HD23159) – Margarita Andrade; Laura Dietz; Katherine Halloran; Seamus Hearne; Matthew Hinkley; Melissa Hooks; Melissa Kupchak; Richard Lin; Jing Liu; Cynthia Miller-Loncar; Sandra Muldowney; Geidy Nolasco; Lia O'Brien; Matt Pescosolido; Sonia Tobon; Jean Twomey.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Rosemary D. Higgins; Linda L. Wright.
National Institute on Drug Abuse – Nicolette Borek.
RTI International (U10 HD36790) – W. Kenneth Poole; Abhik Das; Debra Fleischmann.
University of Miami Holtz Children's Hospital (U10 DA24118, U10 HD21397) – Carmel Azemar; Tonya Barriere-Perez; Miriam Borges; Janine Closius; Khania Contreras; Diedre Gallop; Edgar Garcia; Susan Gauthier; Ann L. Graziotti; Wendy Griffin; Rafael Guzman; Elizabeth Jacque; Brittany Lambert; Jennifer Lewis; Michelle Lugo; Soraya Melegi-Diaz; Daniel S. Messinger; Amy Mur Worth; Mary Triolo; Yamille Valdez.
University of Tennessee (U10 DA24128, U10 HD21415, U10 HD42638) – Regina Barnes; Ashley Bayne; Teresa Beck; Beth Brewer; Vickie Brewer; Charlotte C. Bursi; Gail Campbell; Kelly Chapman; Vivian Crawford; Sheila Dempsey; Daneen Deptula; Claudia Duncan; Betty Eady; Levy A. Eymard; Mary Georgeson; Sandra Grimes; Wendy Hadley; Denise Head; Tracy Hopkins Golightly; Tina Hudson; Lillie Hughey; Lisa Jackson; Vickie Jones; Sheldon B. Korones; Deloris Lee; Pamela LeNoue; Laura Manejwala; Sue Meewes; AlanE. Miller; Laura Murphy; Sidney Ornduff; Beth Owens; Mario C. Petersen; Leanne Plumlee Pollard; Jonathan Rowland; Angelyn Sherrod; Michelle Silcox Miller; Andrea Simmons; Nanise Tomlinson; Chandra Ward; Toni Whitaker; Lucia White; Marilyn Williams; Kimberly A. Yolton.
Wayne State University Hutzel Women's Hospital and Children's Hospital of Michigan (U10 DA24117, U10 HD21385) – Catherine Bartholomay; Jay Ann Nelson; Suzanne Deprez Piziali; Lisa Sulkowski; Nicole Walker; Eunice Woldt.
Financial Suppoert
Support for the Maternal Lifestyle Study was provided by the National Institutes of Health through the National Institute on Drug Abuse and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with supplemental funding from the National Institute of Mental Health, the Administration on Children, Youth, and Families and the Center for Substance Abuse and Treatment, U.S. Department of Health and Human Services.
Abbreviations
- HPA
Hypothalamic-pituitary-adrenal
- HS
high school
- PCE
prenatal cocaine exposure
- SES
socioeconomic status
- SGA
small for gestational age
Footnotes
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Dev Psychopathol. 2001;13:695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- 2.Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Dev Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- 3.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Dat Fisher D, Serbin LA, Stack DM, Ruttle PL, Ledingham JE, Schwartzman AE. Intergenerational predictors of diurnal cortisol secretion in early childhood. Infant Child Dev. 2007;16:151–170. [Google Scholar]
- 5.Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool-aged foster children: differential effects of maltreatment type. Dev Psychobiol. 2009;51:14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall-McClough K, Eldreth D, Levine S. Foster children's diurnal production of cortisol: an exploratory study. Child Maltreat. 2006;11:189–197. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- 7.Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- 8.Bernard K, Butzin-Dozier Z, Rittenhouse J, Dozier M. Cortisol production patterns in young children living with birth parents vs. children placed in foster care following involvement of Child Protective Services. Arch Pediatr Adolesc Med. 2010;164:438–443. doi: 10.1001/archpediatrics.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lupien SJ, King S, Meaney MJ, McEwen BS. Child's stress hormone levels correlate with mother's socioeconomic status and depressive state. Biol Psychiatry. 2000;48:976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- 10.Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 12.Scafidi FA, Field TM. Cocaine-exposed preterm neonates show behavioral and hormonal differences. Pediatrics. 1996;97:851–855. [PubMed] [Google Scholar]
- 13.Lester BM, LaGasse LL, Shankaran S, Bada HS, Bauer CR, Lin R, Das A, Higgins R. Prenatal cocaine exposure related to cortisol stress reactivity in 11-year-old children. J Pediatr. 2010;157:288–295. doi: 10.1016/j.jpeds.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lester BM, Padbury JF. Third pathophysiology of prenatal cocaine exposure. Dev Neurosci. 2009;31:23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- 15.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson SW, Bihun JT, Chiodo LM. Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Dev Psychopathol. 1999;11:195–208. doi: 10.1017/s0954579499002011. [DOI] [PubMed] [Google Scholar]
- 17.Magnano CL, Gardner JM, Karmel BZ. Differences in salivary cortisol levels in cocaine-exposed and noncocaine-exposed NICU infants. Dev Psychobiol. 1992;25:93–103. doi: 10.1002/dev.420250203. [DOI] [PubMed] [Google Scholar]
- 18.Eiden RD, Veira Y, Granger DA. Prenatal cocaine exposure and infant cortisol reactivity. Child Dev. 2009;80:528–543. doi: 10.1111/j.1467-8624.2009.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eiden RD, Peterson M, Coleman T. Maternal cocaine use and the caregiving environment during early childhood. Psychol Addict Behav. 1999;13:293–302. [Google Scholar]
- 20.Lester BM, ElSohly M, Wright LL, Smeriglio VL, Verter J, Bauer CR, Shankaran S, Bada HS, Walls HC, Huestis MA, Finnegan LP, Maza PL. The Maternal Lifestyle Study: drug use by meconium toxicology and maternal self-report. Pediatrics. 2001;107:309–317. doi: 10.1542/peds.107.2.309. [DOI] [PubMed] [Google Scholar]
- 21.Bauer CR, Langer JC, Shankaran S, Bada HS, Lester B, Wright LL, Krause-Steinrauf H, Smeriglio VL, Finnegan LP, Maza PL, Verter J. Acute neonatal effects of cocaine exposure during pregnancy. Arch Pediatr Adolesc Med. 2005;159:824–834. doi: 10.1001/archpedi.159.9.824. [DOI] [PubMed] [Google Scholar]
- 22.Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Lu J, Finnegan LP, Maza PL. The Maternal Lifestyle Study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002;110:1182–1192. doi: 10.1542/peds.110.6.1182. [DOI] [PubMed] [Google Scholar]
- 23.Kiess W, Meidert A, Dressendorfer RA, Schriever K, Kessler U, Konig A, Schwarz HP, Strasburger CJ. Salivary cortisol levels throughout childhood and adolescence: Relation with age, pubertal stage, and weight. Pediatr Res. 1995;37:502–506. doi: 10.1203/00006450-199504000-00020. [DOI] [PubMed] [Google Scholar]
- 24.Richters JE, Martinez P. The NIMH community violence project, I: Children as victims of and witnesses to violence. Psychiatry. 1993;56:7–21. doi: 10.1080/00332747.1993.11024617. [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 26.Maldonado EF, Fernandez FJ, Trianes MV, Wesnes K, Petrini O, Zangara A, Enguix A, Ambrosetti L. Cognitive performance and morning levels of salivary cortisol and α-amylase in children reporting high vs. low daily stress perception. Span J Psychol. 2008;11:3–15. doi: 10.1017/s1138741600004066. [DOI] [PubMed] [Google Scholar]
- 27.Smider NA, Essex MJ, Kalin NH, Buss KA, Klein MH, Davidson RJ, Goldsmith HH. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: a prospective study. Child Dev. 2002;73:75–92. doi: 10.1111/1467-8624.00393. [DOI] [PubMed] [Google Scholar]
- 28.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 29.DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J Adolesc Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- 31.Neblett EW, Jr, Terzian M, Harriott V. From racial discrimination to substance use: the buffering effects of racial socialization. Child Dev Perspect. 2010;4:131–137. doi: 10.1111/j.1750-8606.2010.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nesbitt S, Victor RG. Pathogenesis of hypertension in African Americans. Congest Heart Fail. 2004;10:24–29. doi: 10.1111/j.1527-5299.2004.02021.x. [DOI] [PubMed] [Google Scholar]
- 33.Gump BB, Reihman J, Stewart P, Lonky E, Darvill T, Granger DA, Matthews KA. Trajectories of maternal depressive symptoms over her child's life span: relation to adrenocortical, cardiovascular, and emotional functioning in children. Dev Psychopathol. 2009;21:207–225. doi: 10.1017/S0954579409000133. [DOI] [PMC free article] [PubMed] [Google Scholar]


