Abstract
A recombinant antigen vaccine against Schistosoma mansoni remains elusive, in part because the parasite deploys complex defensive and offensive strategies to combat immune attack. Nevertheless, research on rodent and primate models has shown that schistosomes can be defeated when appropriate responses are elicited. Acquired protection appears to involve protracted inhibition of larval migration or key molecular processes at the adult surfaces, not rapid cytolytic killing mechanisms. A successful vaccine will likely require a cocktail of antigens rather than a single recombinant protein. In addition, ways need to be found of keeping the immune system on permanent alert, either to achieve adequate inhibition of protein function in adults, or because a trickle of incoming parasites does not amplify the secondary response.
Schistosomes are survivors
That schistosomes persist for years in the potentially hostile environment of the human bloodstream means they must protect themselves from immune attack and/or actively intervene to render host responses ineffective. This is akin to medieval warfare, with the vaccinologist looking for a chink in the parasite’s armour. During childhood, both the rising intensity of infection and re-infection after chemotherapy [1] indicate that the parasites have the upper hand. They can successfully establish and mature, with the deposition of eggs in the tissues initiating pathogenesis and morbidity. After puberty, infection intensity and re-infection rate are muted, suggesting that a more successful counter-attack is mounted against incoming larvae. However, a clearly defined mechanism of immunity in humans has proved difficult to pinpoint [2]. IgE responses to a group of calcium-binding proteins [3] in the tegument cytosol [4] provide the best correlate of reduced susceptibility in adults, but these appear to result from the death of worms over an extended period [5]. The dominance of immune responses to the egg, which shares highly immunogenic and abundant house-keeping proteins with larvae and adult worms [6], makes more subtle protective responses hard to identify. Given the paucity of feasible leads from studies on human immunity that could inform vaccine development, we here consider the passive and active defences deployed by schistosomes and review the alternative evidence from animal models that schistosomes can be defeated by immunological attack.
The shield and the sword
The infective schistosome undergoes a programmed sequence of adaptive developmental changes during its migration from the skin to the portal system (Box 1), which provide distinct opportunities for interaction with the immune system. Current research is illuminating the complexity of both the defensive (i.e. ‘shield’) and offensive (i.e. ‘sword’) strategies that the parasite deploys.
Box 1. The potential intra-mammalian schistosome targets for immune attack.
Human infection is initiated by entry into water containing free swimming cercariae, which locate and attach to the skin surface. In the absence of human data, the principal features of the individual life cycle stages in the mammalian host that might serve as targets for immune attack are summarised, based on experimental data from hamsters and mice.
Skin
Initial penetration of cercariae is facilitated by release of acetabular gland secretions containing enzymes and immunomodulators [19, 60] into the epidermis. After entry, transformation to the schistosomulum involves shedding and replacement of the tegument membranes; whether this acts as a diversionary tactic is unclear. Head gland secretions are used for the later phase of migration [61].
Apparent obstacles include: basement of epidermis; dermal extracellular matrix; and blood vessel wall.
Kinetics of migration: Mean duration of stay in epidermis, 53 hours; in dermis, 18 hours, of which time taken to penetrate a venule, 8+/− 1 hours (Figure Ia, Ib; [62]).
The parasite should represent a stationary or slow-moving target for the immune system, but dermal migration is possible in three dimensions. Replacement of the parasite surface during transformation in vitro provides a brief window for antibody binding, but vulnerability to cytotoxic mechanisms is lost within 24 hours.
No convincing parallel in vivo studies of mass parasite death in the skin.
Lung and systemic organs
Schistosomula are delivered to the pulmonary vasculature by venous blood flow, in a semi-quiescent metabolic state with no cell division, or consumption of erythrocytes apparent [63].
Apparent obstacles include: narrow capillaries – to cope with these, the schistosomulum becomes very elongate and thin and loses its mid-body spines (Figure Ic) to facilitate passage along the lumen. Fragile capillary walls - as a result some larvae blunder into an alveolus from which return to the bloodstream is difficult (this may be the principal mechanism by which migrating parasites are ‘lost’).
Kinetics of migration: arrival in the lungs between Days 2 and 7. Mean time for first passage through pulmonary capillary bed estimated as 3–6 days and for subsequent passes ~ 30–35 hours; the schistosomulum provides a stationary or slow-moving target for immune attack. Transit times through systemic organs and splanchnic (visceral) capillary beds of 11–16 hours and 7–9 hours, respectively [64–66].
Portal system
Parasites traverse the splanchnic capillary beds to reach the portal system and transform in the intra-hepatic distributaries into blood-feeding, growing juveniles (Figure Id).
Apparent obstacles include: the hepatic ‘filter’ that traps schistosomula in the liver but is not completely efficient. It is qualitatively different from other vascular beds and may reflect partly the architecture of portal sinusoids and partly the unique composition of portal blood.
Kinetics of migration: Arrival between Days 7 and 21, followed by a three-week stay in the portal vessels, before mating and migration up the mesenteric veins to the intestine walls. Adult pairs resident but peregrinating in the distal portal system thereafter; this may explain why they are not a target for immune attack.
In a naïve permissive host, the proportion of penetrant cercariae that reaches maturity ranges from 30% in mice to 65% in rhesus macaques [67] and 80% in baboons; once in the portal system survival seems assured.
The external shield
By any criteria, both larval and adult worms in the bloodstream, covered by a syncytial layer of cytoplasm, bathed in antibodies and plasma proteins, and exposed to passing leukocytes, ought to be vulnerable to immune attack. The properties of the parasite’s ‘external’ surfaces that endow it with the ability to evade host responses are summarized in Box 2. In the case of the tegument, the composition and properties of the membranocalyx are crucial in shielding the underlying plasma membrane, so it follows that this layer should possess few exposed proteins to be recognised by the immune system [7]. The coating of host erythrocyte blood group antigens, most likely held in the membranocalyx by a glycolipid anchor, could have a protective role in masking parasite proteins, a conjecture that has proved difficult to confirm by experimentation [8]. By inference, hydrophilic pores should exist in the membranocalyx to permit access of solutes and substrates to the transporters and enzymes in the underlying plasma membrane [4, 9, 10]. Whether the shield is a perfect defence or can be penetrated by antibodies and complement may depend upon the precise disposition of proteins in the different levels of the surface complex.
Box 2. Life in the blood stream: the schistosome structures that comprise the parasite–host interface.
The major epithelial surfaces that constitute the adult schistosome’s interface with its mammalian host are the tegument (outer surface) and the gastrodermis (gut lining), together with their secretions. The tegument, in continuous contact with the bloodstream, is actually a syncytial layer (Figure Ia), connected to numerous cell bodies that lie beneath the peripheral muscle layer [7, 68]. Its surface has a multilaminate appearance that, according to our working model, comprises a normal plasma membrane overlain by an external secreted membranocalyx. It has been demonstrated by proteomic studies [4, 10] that transporter proteins and enzymes are located in the plasma membrane, whereas the membranocalyx, of predominantly lipid composition with few identified macromolecular constituents, acts as a shield against immune attack. The membranocalyx is derived from multilaminate vesicles, produced in the subtegumental cell bodies and exported to the syncytium where they fuse with the bounding plasma membrane at the base of the pits to release their contents [7].
The gastrodermis [69] of the blind-ending, tidal gut (Figure Ib) comprises a single layer of epithelial cells with lamellate extensions (Figure Ic) that display both secretory and endocytic activity at the luminal surface. This surface is covered by a glycocalyx [70] that might act as a barrier to antibody binding. Proteomic analysis of gut contents vomited by worms has revealed the presence of hydrolases and lipid-transport proteins of lysosomal origin, in accord with the acidic pH at which digestion operates. In addition, circum-esophageal glands (Figure Id) lying at the extreme anterior of the gut release secretions that may lyse erythrocytes as blood is ingested. An unusual protein known as Antigen 10.3 [71] expressed in the oesophageal glands [28] is a candidate for this process.
The gut epithelium of the parasite (Box 2) is equally exposed to plasma constituents and leukocytes, so, theoretically, represents a target for immune attack. It has tacitly been assumed that host proteins are rapidly digested and leukocytes lysed in the acidic hydrolytic environment of the gut lumen. However, this does not appear to have been tested by reacting blood constituents with worm gut contents. Owing to the location of the oesophageal glands (Box 2), their secretions may also serve a protective role by denaturing antibodies and complement factors as they are ingested.
Box 3. Characteristics of the radiation-attenuated vaccine in mice.
Detailed information on the mechanisms of immunity elicited by the radiation-attenuated (RA) vaccine has been derived from studies primarily in the high-responder C57/Bl6 strain mouse (for a 1997 review, see Ref. [37]; only later papers are cited here).
Priming
Maximum protection after a single vaccination is achieved when cercariae receive an optimum dose of radiation that restricts parasites to the skin, its draining lymph nodes, and the lungs. A comparison of the gene expression patterns of irradiated with normal parasites has revealed deficiencies in receptor signaling pathways and cytoskeletal gene expression that accord with the altered migratory capacity [72].
Prolonged residence of attenuated larvae in the skin-draining lymph nodes provokes intense lympho-proliferation. Direct contact is made between resident dendritic cells and the tegument, implicating surface antigens as vaccine candidates (Figure Ia).
CD4+ T cells with T helper cell type 1 (Th1) characteristics enter the circulation and are recruited to the pulmonary parenchyma, where they arm the lungs against a challenge with normal larvae. Administration of recombinant IL-12 with the RA vaccine enhances Th1 reactivity and drives protection to very high levels (>90%).
Multiple vaccinations enhance Th2 responses and the antibody-mediated component of protection, although passive transfer of protection is never high, except with serum from mice lacking the interferon gamma receptor (IFNγR−/−), where antibody production is not constrained by Th1 feedback [73].
Effector response
In the singly vaccinated mouse, challenge parasites travelling through the lungs, in intimate contact with the vascular endothelium (Figure Ib), attract an inflammatory focus of T cells and monocytes that builds up over several days (Figure Ic) and might terminate their migration. The production of IFNγ and tumor necrosis factor alpha (TNFα) by cells of the inflammatory focus [74] in response to parasite antigen is essential to the functioning of this effector response.
Parasites trapped within a focus show an absence of surface damage and some are deflected into alveoli. The ability of sequestered parasites to mature, when transferred experimentally to the portal vein of a naïve animal, confirms their viability, revealing that the effector mechanism acts to block migration and not through eliciting an acute lethal hit.
Less is known about antibody-mediated mechanisms in multiply vaccinated animals. However, successful induction of protection in FcRγ-chain-deficient [75] mice and in complement-C3-depleted mice again implies that the effector mechanism does not involve an acute lethal cytolytic hit.
The internal shield
Host phagocytes are capable of generating toxic oxygen- and nitrogen-based compounds with the potential to damage and kill schistosome larvae and adults. It was shown 20 years ago that, whereas newly transformed schistosomula were vulnerable, later stages were much less susceptible to in vitro killing by products of the oxidative burst [11]. This was attributed to the presence of antioxidant proteins, levels of which increased progressively as the intravascular parasites developed [12] [13]. The sequencing of the schistosome transcriptome and genome affords us a clearer idea of the range of antioxidant activities that schistosomes possess. The high-level expression of such proteins within the tegument and gut epithelium [13] implicates them as a first line of defence, providing an internal interface against immune attack.
Superoxide, the main cause of oxidative stress, reacts with parasite tissues to produce hydroxyl radicals, and initiates the peroxidation of polyunsaturated fatty acids. Schistosomes possess four superoxide dismutases [14] to disarm this threat, and three peroxiredoxins to reduce hydrogen peroxide [15]. Indeed, the parasite redox balance appears skewed towards the maintenance of a reducing environment, primed against oxidative attack. This is achieved by a unique multifunctional protein, thioredoxin glutathione reductase, which keeps the electron donors glutathione and the protein thioredoxin in the reduced state [16]. To repair oxidative damage, schistosomes also possess several glutathione-S-transferases and glutathione peroxidases that, among other functions, can salvage lipids by conjugating their hydroperoxides to glutathione [17]. The effectiveness of the internal interface in protecting the parasite is apparent from the lack of reports of dead schistosomes either in situ or ex vivo.
The sword
Recent research indicates that schistosomes do not just adopt a defensive posture but can also fight back. For example, cercarial penetration into host skin appears to elicit inflammation, which is regulated to the benefit the parasite [18]. Proteomic analysis is turning up a variety of novel proteins, both in larval secretions and expressed on the tegument surface, that may constitute the parasites’ arsenal.
Counterattack by immunomodulation?
Sm16, a cercaria-specific protein [19] with similarities to the human microtubule regulator stathmin [20], has been ascribed anti-inflammatory properties [21], implying that invading parasites use it to modify host responses in the skin. However, doubt has been cast on this function [22] and, instead, a pro-apoptotic action after endocytosis by host cells suggested; it has recently been shown that Sm16 can inhibit Toll-like receptor signalling by human leukocytes in vitro [23]. Other intriguing proteins are released by the cercaria as it transforms into the schistosomulum, including SmKK7 [19] that has homology to potassium-channel blockers in scorpion venom. Such proteins can inhibit human T-cell activation by interfering with the calcium influx, and the schistosome protein shows similar properties in preliminary experiments (G.P. Dillon, pers. commun.). Cercariae also secrete three sperm coat-domain proteins (SCPa, b and c) [19] that are members of a large family of wasp venom homologues [24]. Diverse functions have been ascribed to this class of proteins, most notably from a study of a homologue in the hookworm Necator [25], where the three-dimensional structure points to similarities with host chemokines, suggesting a propensity to modify dermal immunity. Finally, a microarray analysis of transcripts highly expressed in the lung-stage schistosomulum has identified another SCP protein (SPCd) and Antigen 5 [26]; Antigen 5 has immunomodulatory properties in infections by the tapeworm Echinococcus [27]; whole-mount in situ hybridisation shows expression of this gene in the rudimentary gut [28], possibly indicating that the migrating larva is also able to manipulate host responses. Indeed, recent phylogenetic research has revealed structural similarities between Antigen 5 and the venoms of various animal species [29].
Subversion of immune attack on the parasite surface?
Recent proteomic analyses have confirmed that antibodies of several isotypes are associated with the adult parasite surface [4, 9, 10]. Complement factors C3 and C4 have also been identified, but failure to find C5 to C9 (the elements of the culminating membrane attack complex [MAC]), suggests that the cascade is inhibited at an intermediate step by parasite or acquired host factors. It is also surprising that the antibodies and complement factors do not trigger leukocyte adherence to parasites in blood vessels, although the margination of leukocytes to the endothelium in proximity to the intravascular parasite confirms that the host does recognise its presence [30].
There is some evidence that the host’s complement defence mechanisms may be turned against it to protect the parasite. The regulatory protein known as decay accelerating factor (DAF) that protects host cells by blocking formation of MAC, has been detected on the tegument by immunocytochemistry [31]. Furthermore, proteomic analysis of the tegument membranes [9] has revealed the analogous murine complement-receptor-related protein y (Crry) [32]. The complement alpha chain C3c/C3dg fragment has also been identified, implying covalent linkage of C3 to tegument proteins but subsequent inactivation by proteolysis [10]. Perhaps the most exciting observation in the complement context is the discovery in the schistosome genome of six homologues of human CD59 (which is a potent inhibitor of the MAC), and the identification of four of them at the tegument surface by proteomics (S. Braschi and W. Borges, pers. commun.). This group of proteins shows 20–30% identity with the human counterpart, rising to >40% if conservative amino acid substitutions are included, and displays the consensus CCXXXCN sequence at the C terminus. These CD59 homologues appear to be better candidates for complement inhibition at the tegument surface than schistosome complement inhibitory protein 1 (SCIP-1) [33], later identified as paramyosin [34], which has not been found in the tegument membrane proteome [7–9].
A schistosome integrin has been identified in the tegument surface, with homology to human T-cell immunomodulatory protein (TIP) that affects cytokine secretion by T cells and has a protective effect in a murine model of acute graft-versus-host disease [35]. Finally, several unique schistosome proteins (Sm200, Sm29, Sm25, Sm14) of unknown function but possible external location [10] should not be neglected as potential subverting agents. We emphasise that the above proteins were all annotated on the basis of their homology with known or conjectured inhibitory molecules, but functional evidence is required to confirm a role in immune subversion. The obvious way to tackle questions about protein function would be targeted gene disruption. Unfortunately, in spite of initial optimism, RNA interference in schistosomes has not become a routine and reproducible technique, and germ-line disruption remains elusive [36].
Are there chinks in the parasite’s armour?
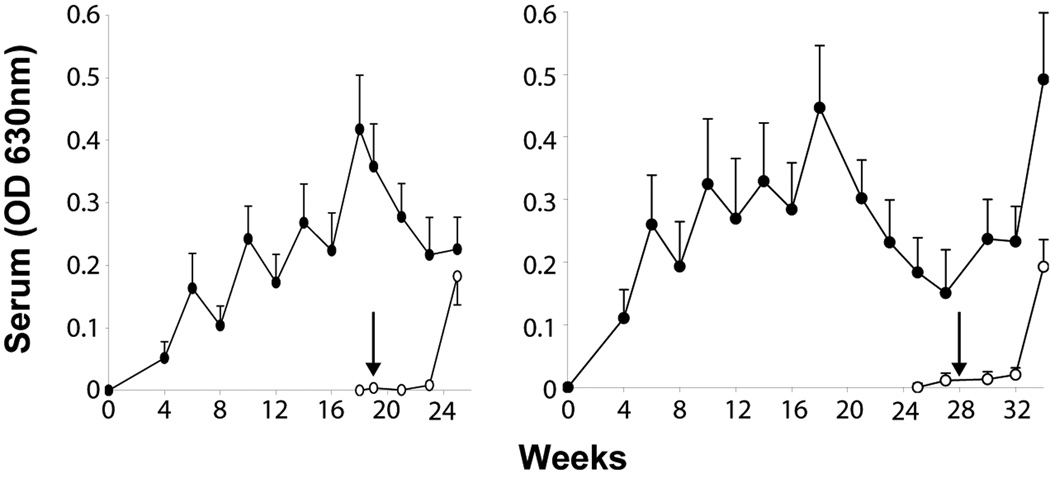
We believe there are three animal models where chinks in the schistosomes’ armour are successfully exploited by an acquired immune response to eliminate the parasite. There could be other points of vulnerability, but no model has so far identified them. Most is known about the radiation-attenuated (RA) cercaria vaccine in mice (Box 3) and primates (reviewed in Ref. [37]). After the murine immune system has been primed by a single exposure to the vaccine, a large fraction of incoming challenge larvae is eliminated relative to non-vaccinated controls. Multiple exposures are essential to elicit protection in the baboon (up to 86% reduction in worm burden relative to controls), the level achieved being proportional to the number of vaccinations [38]. Protection in the baboon diminished from 72% to 53%, in parallel with antibody titre (Figure 1), when the interval between the last vaccination and challenge was extended from three to 12 weeks. By contrast, it did not diminish in mice for at least 15 weeks [39]. However, the level of protection elicited in baboons is not affected when the vaccine is superimposed on, or given after, drug treatment of a chronic infection [40]. This means that the dominant response to egg antigens does not prevent immune priming for protection, in spite of the extent of shared glycan structures between egg and cercarial secretions [41]. The relevance of the RA vaccine model to humans has been demonstrated by the successful induction of protection in chimpanzees [42], but the experiment also suggested that anti-glycan responses were a diversionary tactic [43], and in no way protective. The baboon and mouse studies have reinforced this conclusion [44].
Figure 1.
Antibody responses to cercarial secretions in baboons receiving five exposures to irradiated cercariae at 4 week intervals before challenge with normal cercariae at 3 or 12 weeks after the last vaccination [38]. The saw-tooth response reveals that persistent antigenic pressure is needed to increase IgG titre. When that is removed, it rapidly falls, and with it the level of protection. This decline is one reason why we have not sought to emulate malaria researchers [77] by advocating the development of the irradiated cercaria vaccine for human use.
There is evidence from one laboratory host, the brown rat, that the pre-adult liver worm is also vulnerable to attack. Here, the initial infection primes the immune system in a highly idiosyncratic way so that the worms are rapidly eliminated around 28 days post infection, while resident in the hepatic portal distributaries of the liver. Finally, recent work in the rhesus macaque has revealed that even adult worms can be targeted for immune-mediated elimination [45]. An infection proceeds normally, eggs are produced and egg excretion commences, but subsequently, over a protracted period, egg excretion falls to zero and worms are eliminated in a self-cure process by a mechanism quite distinct from that in the brown rat. The timing and extent of worm deterioration varies with the individual rhesus macaque, being first detectable in rapid responders around 12 weeks and it may take 4–6 weeks for worms to succumb.
What are the secrets of success?
The RA vaccine in mice and primates
In the wild-type mouse given a single vaccination with irradiated cercariae, the lung-stage parasite is the pre-eminent target of the effector response, not via an acute lethal cytotoxic attack but, rather, by slow strangulation (Box 3). Why should this life cycle stage be vulnerable? On first pass through the lungs, it represents a stationary or slow-moving target (Box 1) that gives time for a cell-mediated response to develop (Box 3). From the dependence of the effector response on CD4+ T cells, it can be inferred that the relevant antigens are surface-exposed or secreted. Furthermore, the intimate contact between the parasite tegument and capillary endothelium would permit adhesion interactions, as well as transcytosis of antigen to interstitial spaces. The fact that the parasite is in a confined space should limit diffusion of secretions, so contributing to the focal nature of the response. Where antibody is the major component of the protective mechanism, the timing of successful passive transfer of serum again suggests that the lung-stage parasite is the target [46]. If the action of antibodies to opsonise or fix complement is ruled out as the likely effector mechanism by earlier experiments (Box 3), this leaves their neutralising activity against proteins essential for physiological maintenance or onward migration. Sm29 is one such candidate, recently demonstrated on the lung schistosomulum surface, and giving good protection in vaccination experiments [47].
Compared with mice, primates are a ‘black box’ in terms of effector responses. We infer that the target is a pre-adult stage because challenge parasites are eliminated before they begin egg laying [38] – cf. the rhesus macaque [45]. As for mechanism, in both chimpanzees and baboons, the progressively rising antibody titre with each vaccination [38, 42] implicates IgG. This inference is reinforced by the inverse correlation between a baboon’s titre at challenge and its worm burden at perfusion [38, 48]. In addition to lung-stage antigens, secretions from acetabular and head glands during skin invasion might serve as targets for antibody-mediated protection. However, their diffusion away from the parasite through the skin matrix could reduce their potential because neither antibodies nor cells would be focused on the larva, except when it is penetrating a blood vessel wall.
Self cure in the rat
Although it has been suggested that worm elimination in the rat results from a nutritional deficiency, its delayed occurrence in immunologically compromised animals implicates an immune effector response [49]. Worm death is preceded by two events: rising levels of schistosome-specific IgE in the circulation [49] and the recruitment of mucosal-type mast cells to the peri-portal hepatic tissues [50]. The appearance of specific proteases in the blood as early as Day 14 is evidence for mast cell degranulation. These sequelae appear to be unique to the brown rat (Rattus norvegicus), not occurring in the black rat (R. rattus), where an infection becomes patent [51], nor the laboratory mouse where hepatic mastocytosis is absent [50]. (The explanation may lie in the brown rat’s predisposition to mount allergic responses [52].)
Worms located in the portal vessel lumen will encounter both pre-synthesised and de-novo products of antigen-stimulated mast cells. These could inflict direct damage (e.g. the mast cell proteases), but a potential effect of the biogenic amines (histamine and serotonin) is more intriguing. Schistosomes possess a histamine receptor [53] and at least three serotonin receptor homologues (www.GeneDB.org) that could mediate hyperstimulation, potentially via the nervous system, leading to exhaustion and death. An effect of the products synthesised de-novo, such as prostaglandins and leucotrienes should also not be ignored. Western blotting has revealed the principal targets of the rat IgE as proteins of 67 and 36–38 kDa in adult worm homogenates, the 67 kDa protein is possibly gut-derived, whereas glycan epitopes could be responsible for the activity of the 36–38 kDa protein [49]. Once animals have been sensitised by the primary infection they are strongly resistant to a challenge [54].
Self cure in the rhesus macaque
Rhesus macaques show a wide variation in responsiveness to a primary schistosome infection, perhaps a reflection of their outbred status. A notable feature of the only recent study [45] was the strong association between the timing and intensity of IgG production and adult worm deterioration. There was no evidence for an acute antibody-mediated lethal hit; instead the mechanism appeared to involve sustained immunological pressure over a period of weeks to months. This was manifested as a sequence of degenerative changes in the worms, involving cessation of feeding, starvation and ultimately organ failure. These conclusions were reinforced by the retarded growth of blood feeding worms during in vitro culture with rapid-responder serum versus slow-responder or naïve rhesus serum. Earlier studies demonstrated that, several months after infection, rhesus macaques were able to resist a challenge almost completely [55]; however, as with the brown rat, nothing is known about the target stages of this induced protection. As 75Se methionine is no longer available for parasite tracking experiments, resolution of this problem may depend on the application of modern in vivo imaging tachniques [56].
Using immunoproteomics, gut digestive enzymes, tegument surface hydrolases and antioxidant enzymes were identified as targets of IgG in the high-responder animals [45]. In view of the long time taken for worms to expire, it was speculated that the mechanism did not involve opsonisation, complement fixation and cytolysis. More plausibly, the role of IgG appears to depend on its potential blocking or stimulatory properties. Thus, antibodies that neutralise the function of tegument and/or gut proteins involved in nutrient uptake could lead directly to starvation. Conversely, antibodies stimulating signalling receptors could trigger hyperactivity, leading to exhaustion of reserves. A third possibility is that targeting of antioxidant proteins culminates in progressive worm damage that is eventually fatal.
Can we replicate any of these mechanisms in a vaccine?
It is clear from the foregoing account that schistosomes have evolved complex strategies that enable them to survive in the mammalian host. In order to defeat them, the vaccinologist will have to be equally cunning.
How do we identify the antigenic targets?
Secreted and/or surface-exposed proteins appear to be the key immunogens mediating protection, and post-genomic technologies now permit us to identify their full range in larvae and adults [36]. The adult inventory is more advanced [4, 9, 10] not least because mature worms provide abundant material, compared with schistosomula where additional identities are needed especially from structures such as the head gland. There is no simple in vitro screen to evaluate vaccine potential, so protein expression for direct testing of protection in an animal model is essential – i.e. ‘reverse vaccinology’ [57].
Vaccine testing raises another fundamental question – is there a single ‘magic bullet’ or must we hit a number of targets to disable the worm? The ‘magic bullet’ is preferable from a commercial vaccine viewpoint because of the expense of generating multiple recombinants. However, the animal models suggest that targeting multiple functions is more likely to achieve worm elimination, thereby requiring administration of a cocktail of antigens. For example, if the three phosphohydrolases located at the external surface of the adult tegument [10] have overlapping substrate specificities, is it necessary to inhibit all of them to a high degree to achieve an effect on the worm’s fitness?
How to prime the immune system?
The dependence on IgE and mast cells for worm elimination in the rat presents a significant obstacle to replication in a human vaccine. It is difficult to envisage how anaphylactic antibodies might be elicited to specific schistosome antigens, but not to bystander environmental immunogens, with immunopathological consequences [49]. Moreover, the selective recruitment of mast cells to the human liver poses another challenge. Ironically the small number of IgE targets detected by immune rat serum [49] might be the nearest approximation to a ‘magic bullet’ yet described!
The mechanisms operating in two other animal models offer more promise. The most effective anti-larval mechanisms elicited by the RA vaccine (in mice) involve cell-mediated immunity. They rely on the generation of CD4+ effector/memory cells with a T helper cell type 1 (Th1) phenotype, and their recruitment to the lungs [37]. In seeking to emulate such cell-mediated mechanisms, we need to bear in mind that, even with the RA vaccine under the most favourable conditions, there are limits to protection. Taking each parasite as an independent event, the important stochastic element setting the limit may be the frequency of specific responder cells recruited to the pulmonary parenchyma. It is likely that a parasite needs to interact with more than one Th cell to generate an effector focus. However, it is also likely that a ceiling to Th cell expansion and recruitment will result from the co-induction of T regulatory cells (Tregs) that produce inhibitory cytokines.
If we accept that the alternative anti-larval mechanism elicited by the RA vaccine relies upon the neutralising activity of antibodies, not cytolysis, then the titre achieved by vaccination becomes paramount. It is not known what percentage of the activity of crucial parasite molecules would need to be inhibited to prevent migration. The key here may be to develop strategies that elicit long-lived plasma cells to maintain titre [58]. Correctly folded proteins are a likely requirement to elicit neutralising antibodies against conformational epitopes, which may be essential for inhibition of function. As secreted and membrane proteins often possess several disulphide bridges, they may need to be produced in the more demanding eukaryotic systems to ensure this.
Emulating the anti-adult mechanisms employed by the rhesus macaque could have therapeutic as well as prophylactic properties. As the mechanism appears to rely on antibody-mediated protection, the same considerations about persistent high antibody titre apply, irrespective of the specific target(s). In this case, key questions include: what level of neutralising activity needs to be achieved to disable an adult worm over an extended period? And, how many targets need to be hit?
All the above considerations point to a task of some magnitude before a schistosome vaccine can become a reality. Our thoughts on the problems that need to be solved are summarised in Box 4. On a personal note, after nearly 30 years research on schistosome vaccines, we still hope that a magic bullet akin to the Taenia onchosphere antigens [59] will be found by serendipity. Experience tells us that more likely, the laudable goal will require a ‘long march’.
Box 4. The long march to a schistosome vaccine.
Research on in vivo models has revealed that schistosomes are undoubtedly vulnerable to immune attack, provided that an appropriate response is mounted by the host. The challenge is to replicate such a response in humans, who seem poorly equipped to eliminate schistosomes under conditions of natural infection, with a vaccine strategy. However, significant obstacles remain that require systematic and intensive attention if we are to succeed.
Pinpointing proteins with strong protective capacity is a key task and reverse vaccinology provides the route. The advent of genome [76], transcriptome and proteome information now permits characterisation of the full complement of proteins expressed at the interface between parasite and mammalian host and thus accessible to the immune system in the live parasite. This is very much ‘work in progress’.
There appears to be no substitute for expression of antigenic targets as recombinants (to date the short-cut represented by DNA vaccines has not lived up to its promises). The same goes for the trialling of antigens in the mouse and ultimately primate models, primed, boosted and given a percutaneous cercarial challenge. No in vitro correlate of protection has emerged, or indeed appears feasible, given the nature of the protective mechanisms that we espouse. In this context, a potential pitfall for antigen trials is that if protection requires responses to a cocktail of antigens, then the normal practice of testing one at a time is unlikely to bear fruit. .
Different rules are likely to apply for vaccines designed to elicit anti-larval versus anti-adult immunity. We contend that the absence of parasite replication after invasion to amplify secondary responses (cf. microbes or viruses), plus the trickle nature of schistosome infection delivering only minute quantities of larval antigen, mean that memory recall of vaccine-induced protection is likely to be weak or non-existent. We conclude, therefore, that vaccination for anti-larval immunity will need not just to prime the requisite responses but also to maintain them in a ‘ready to go’ state for months to years. For anti-adult immunity targeting abundant gut or tegument antigens, boosting should occur naturally as worms mature (they get bigger and produce antigen continuously), hopefully before egg production gets under way. Vaccination would still need to generate high antibody titres, but there would be time for memory to be activated.
This raises a final question about the adjuvant needed to elicit the strong responses that will eliminate the tenacious schistosomes. It appears unlikely that conventional adjuvants such as aluminium hydroxide will do the trick, so more experimental products or live vectors will need to be employed, thus raising drug regulation issues that might impede field trials or implementation.
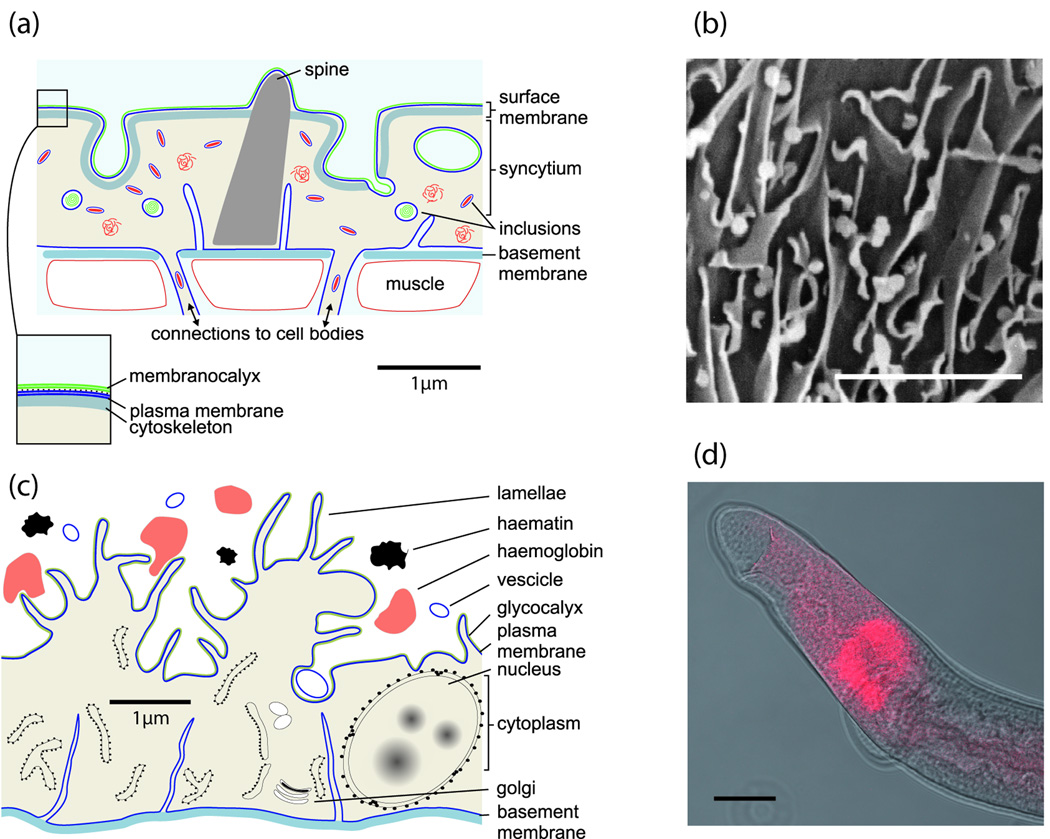
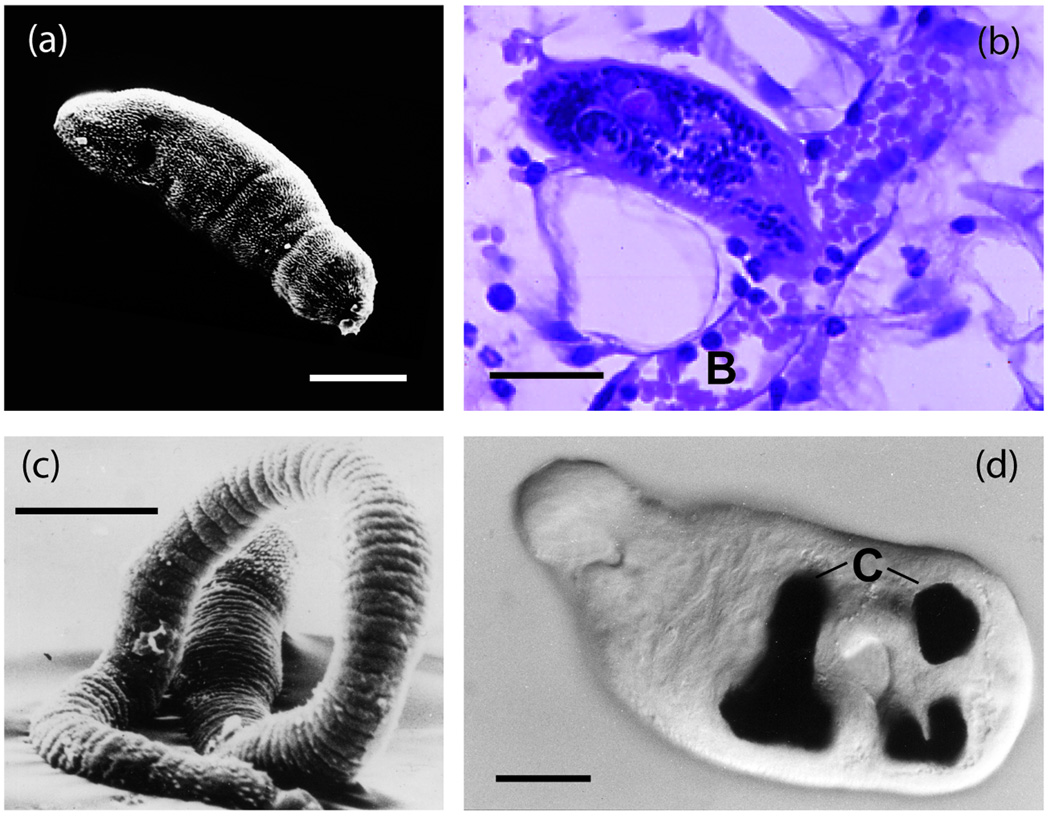
Figure I.
Micrographs of key larval stages. (a) Skin schistosomulum ex vivo after transformation from the cercaria, illustrating the dense spination; (b) skin schistosomulum exiting the dermis by penetrating a blood vessel (V) using head gland secretions; (c) lung schistosomulum (cf. part a) showing extension of the body and loss of spination to faciliatate transit through capillary beds; (d) juvenile liver worm with black haematin pigment in the caecum (C) revealing the start of feeding on erythrocytes and increase in body mass. Scale bar = 20 µm (a,c,d), and 50µm (b).
Figure I.
(a) Diagram showing the main features of the syncytial tegument. The plasma membrane is overlain by a secreted membranocalyx that acts as a physical barrier to antibody binding and complement fixation, and is formed from the contents of multilaminate inclusions in the tegument cytosol. (b) Diagram showing the main features of the gut epithelial monolayer that is active both as secretory layer, and in the absorption of nutrients. (c) Stereoscan electron micrograph of a starved worm reveals the lamellate nature of the gut epithelial surface extensions; scale bar = 2 µm. (d) The location of the oesophageal glands astride the posterior oesophagus revealed by whole mount in situ hybridisation of Antigen 10.3 mRNA that encodes the only protein currently known to be synthesised there [28]; scale bar = 50 µm.
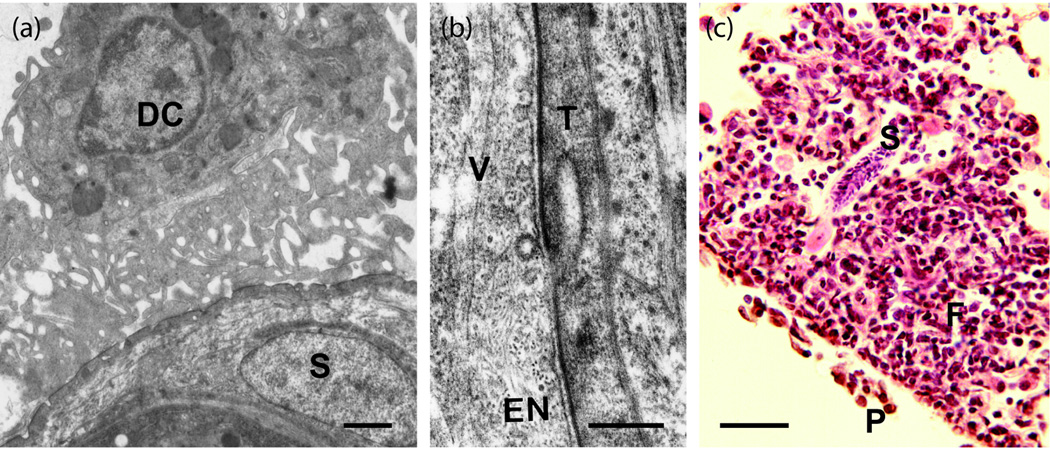
Figure I.
(a) Dendritic cell (DC) grazing on the surface of an attenuated schistosomulum (S) in a skin-draining lymph node primes the immune system for a Th1 response. (The tissue was not post-fixed in uranyl acetate to stabilise the membranocalyx.) Scale bar = 1 µm. (b) Intimate contact between the schistosomulum tegument (T) and pulmonary capillary endothelium (EN) may facilitate transfer of surface proteins to the interstitial spaces as vesicles (V) mediating transcytosis are evident at the luminal surface. Bar = 0.25 µm. (c) Challenge schistosomulum (S) in the lungs of a vaccinated mouse surrounded by an inflammatory focus largely composed of lymphocytes and monocytes. A pleural infiltrate (P) is also evident on the lung surface. The focus impedes migration and deflects some larvae into the alveoli, rather than killing them by cytolytic mechanisms. Scale bar = 50 µm.
Acknowledgements
Our work is supported by The Biotechnology and Biological Sciences Research Council, and NIH grant AI54711-02, PI Dr Chris King, Center for Global Health & Diseases, Case Western Reserve University, Cleveland, OH, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fulford AJ, et al. On the use of age-intensity data to detect immunity to parasitic infections, with special reference to Schistosoma mansoni in Kenya. Parasitology. 1992;105:219–227. doi: 10.1017/s003118200007414x. [DOI] [PubMed] [Google Scholar]
- 2.Dunne DW, Mountford AP. Resistance to infection in humans and animal models. In: Mahmoud AFF, editor. Schistosomiasis. Imperial College Press; 2001. pp. 133–212. [Google Scholar]
- 3.Fitzsimmons CM, et al. Factors affecting human IgE and IgG responses to allergen-like Schistosoma mansoni antigens: Molecular structure and patterns of in vivo exposure. Int Arch Allergy Immunol. 2007;142:40–50. doi: 10.1159/000095997. [DOI] [PubMed] [Google Scholar]
- 4.Braschi S, et al. The tegument surface membranes of the human blood parasite Schistosoma mansoni: a proteomic analysis after differential extraction. Proteomics. 2006;6:1471–1482. doi: 10.1002/pmic.200500368. [DOI] [PubMed] [Google Scholar]
- 5.Walter K, et al. Increased human IgE induced by killing Schistosoma mansoni in vivo is associated with pretreatment Th2 cytokine responsiveness to worm antigens. J Immunol. 2006;177:5490–5498. doi: 10.4049/jimmunol.177.8.5490. [DOI] [PubMed] [Google Scholar]
- 6.Curwen RS, et al. The Schistosoma mansoni soluble proteome: a comparison across four life-cycle stages. Mol Biochem Parasitol. 2004;138:57–66. doi: 10.1016/j.molbiopara.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Skelly PJ, Wilson RA. Making sense of the schistosome surface. Adv Parasitol. 2006;63:185–284. doi: 10.1016/S0065-308X(06)63003-0. [DOI] [PubMed] [Google Scholar]
- 8.McLaren DJ, Terry RJ. The protective role of acquired host antigens during schistosome maturation. Parasite Immunol. 1982;4:129–148. doi: 10.1111/j.1365-3024.1982.tb00426.x. [DOI] [PubMed] [Google Scholar]
- 9.Braschi S, et al. Proteomic analysis of the schistosome tegument and its surface membranes. Mem Inst Oswaldo Cruz. 2006;101(Suppl 1):205–212. doi: 10.1590/s0074-02762006000900032. [DOI] [PubMed] [Google Scholar]
- 10.Braschi S, Wilson RA. Proteins exposed at the adult schistosome surface revealed by biotinylation. Mol Cell Proteomics. 2006;5:347–356. doi: 10.1074/mcp.M500287-MCP200. [DOI] [PubMed] [Google Scholar]
- 11.Mkoji GM, et al. Antioxidant systems in Schistosoma mansoni: evidence for their role in protection of the adult worms against oxidant killing. Int J Parasitol. 1988;18:667–673. doi: 10.1016/0020-7519(88)90102-6. [DOI] [PubMed] [Google Scholar]
- 12.Nare B, et al. Schistosoma mansoni: levels of antioxidants and resistance to oxidants increase during development. Exp Parasitol. 1990;70:389–397. doi: 10.1016/0014-4894(90)90122-s. [DOI] [PubMed] [Google Scholar]
- 13.Mei H, LoVerde PT. Schistosoma mansoni: the developmental regulation and immunolocalization of antioxidant enzymes. Exp Parasitol. 1997;86:69–78. doi: 10.1006/expr.1997.4150. [DOI] [PubMed] [Google Scholar]
- 14.LoVerde PT. Do antioxidants play a role in schistosome host-parasite interactions? Parasitol Today. 1998;14:284–289. doi: 10.1016/s0169-4758(98)01261-7. [DOI] [PubMed] [Google Scholar]
- 15.Sayed AA, Williams DL. Biochemical characterization of 2-Cys peroxiredoxins from Schistosoma mansoni. J Biol Chem. 2004;279:26159–26166. doi: 10.1074/jbc.M401748200. [DOI] [PubMed] [Google Scholar]
- 16.Kuntz AN, et al. Thioredoxin glutathione reductase from Schistosoma mansoni: an essential parasite enzyme and a key drug target. PLoS Med. 2007;4:e206. doi: 10.1371/journal.pmed.0040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai H, Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med. 2003;34:145–169. doi: 10.1016/s0891-5849(02)01197-8. [DOI] [PubMed] [Google Scholar]
- 18.Mountford AP, Trottein F. Schistosomes in the skin: a balance between immune priming and regulation. Trends Parasitol. 2004;20:221–226. doi: 10.1016/j.pt.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Curwen RS, et al. Identification of novel proteases and immunomodulators in the secretions of schistosome cercariae that facilitate host entry. Mol Cell Proteomics. 2006;5:835–844. doi: 10.1074/mcp.M500313-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Valle C, et al. Stage-specific expression of a Schistosoma mansoni polypeptide similar to the vertebrate regulatory protein stathmin. J Biol Chem. 1999;274:33869–33874. doi: 10.1074/jbc.274.48.33869. [DOI] [PubMed] [Google Scholar]
- 21.Rao KV, Ramaswamy K. Cloning and expression of a gene encoding Sm16, an anti-inflammatory protein from Schistosoma mansoni. Mol Biochem Parasitol. 2000;108:101–108. doi: 10.1016/s0166-6851(00)00209-7. [DOI] [PubMed] [Google Scholar]
- 22.Holmfeldt P, et al. The Schistosoma mansoni protein Sm16/SmSLP/SmSPO-1 is a membrane-binding protein that lacks the proposed microtubule-regulatory activity. Mol Biochem Parasitol. 2007;156:225–234. doi: 10.1016/j.molbiopara.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Brannstrom K, et al. The Schistosoma mansoni protein Sm16/SmSLP/SmSPO-1 assembles into a nine-subunit oligomer with potential To inhibit Toll-like receptor signaling. Infect Immun. 2009;77:1144–1154. doi: 10.1128/IAI.01126-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chalmers IW, et al. Developmentally regulated expression, alternative splicing and distinct sub-groupings in members of the Schistosoma mansoni venom allergen-like (SmVAL) gene family. BMC Genomics. 2008;9:89. doi: 10.1186/1471-2164-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asojo OA, et al. X-ray structure of Na-ASP-2, a pathogenesis-related-1 protein from the nematode parasite, Necator americanus, and a vaccine antigen for human hookworm infection. J Mol Biol. 2005;346:801–814. doi: 10.1016/j.jmb.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 26.Dillon GP, et al. Microarray analysis identifies genes preferentially expressed in the lung schistosomulum of Schistosoma mansoni. Int J Parasitol. 2006;36:1–8. doi: 10.1016/j.ijpara.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Lorenzo C, et al. Echinococcus granulosus antigen 5 is closely related to proteases of the trypsin family. Biochem J. 2003;369:191–198. doi: 10.1042/BJ20021402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dillon GP, et al. Patterns of gene expression in schistosomes: localization by whole mount in situ hybridization. Parasitology. 2007;134:1589–1597. doi: 10.1017/S0031182007002995. [DOI] [PubMed] [Google Scholar]
- 29.Fry BG, et al. Tentacles of venom: toxic protein convergence in the Kingdom Animalia. J Mol Evol. 2009;68:311–321. doi: 10.1007/s00239-009-9223-8. [DOI] [PubMed] [Google Scholar]
- 30.Keating JH, et al. No overt cellular inflammation around intravascular schistosomes in vivo. J Parasitol. 2006;92:1365–1369. doi: 10.1645/GE-864R.1. [DOI] [PubMed] [Google Scholar]
- 31.Pearce EJ, et al. Host-specific evasion of the alternative complement pathway by schistosomes correlates with the presence of a phospholipase C-sensitive surface molecule resembling human decay accelerating factor. J Immunol. 1990;144:2751–2756. [PubMed] [Google Scholar]
- 32.Wu X, et al. Membrane protein Crry maintains homeostasis of the complement system. J Immunol. 2008;181:2732–2740. doi: 10.4049/jimmunol.181.4.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parizade M, et al. Functional and antigenic similarities between a 94-kD protein of Schistosoma mansoni (SCIP-1) and human CD59. J Exp Med. 1994;179:1625–1636. doi: 10.1084/jem.179.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng J, et al. Inhibition of the complement membrane attack complex by Schistosoma mansoni paramyosin. Infect Immun. 2003;71:6402–6410. doi: 10.1128/IAI.71.11.6402-6410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiscella M, et al. TIP, a T-cell factor identified using high-throughput screening increases survival in a graft-versus-host disease model. Nat Biotechnol. 2003;21:302–307. doi: 10.1038/nbt797. [DOI] [PubMed] [Google Scholar]
- 36.Wilson RA, et al. 'Oming in on schistosomes: prospects and limitations for post-genomics. Trends Parasitol. 2007;23:14–20. doi: 10.1016/j.pt.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Coulson PS. The radiation-attenuated vaccine against schistosomes in animal models: paradigm for a human vaccine? Adv Parasitol. 1997;39:271–336. doi: 10.1016/s0065-308x(08)60048-2. [DOI] [PubMed] [Google Scholar]
- 38.Kariuki TM, et al. Parameters of the attenuated schistosome vaccine evaluated in the olive baboon. Infect Immun. 2004;72:5526–5529. doi: 10.1128/IAI.72.9.5526-5529.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minard P, et al. Immunization of mice with cobalt-60 irradiated Schistosoma mansoni cercariae. Am J Trop Med Hyg. 1978;27:76–86. doi: 10.4269/ajtmh.1978.27.76. [DOI] [PubMed] [Google Scholar]
- 40.Kariuki TM, et al. Previous or ongoing schistosome infections do not compromise the efficacy of the attenuated cercaria vaccine. Infect Immun. 2006;74:3979–3986. doi: 10.1128/IAI.01657-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jang-Lee J, et al. Glycomics analysis of Schistosoma mansoni egg and cercarial secretions. Mol Cell Proteomics. 2007;6:1485–1499. doi: 10.1074/mcp.M700004-MCP200. [DOI] [PubMed] [Google Scholar]
- 42.Eberl M, et al. Cellular and humoral immune responses and protection against schistosomes induced by a radiation-attenuated vaccine in chimpanzees. Infect Immun. 2001;69:5352–5362. doi: 10.1128/IAI.69.9.5352-5362.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eberl M, et al. Antibodies to glycans dominate the host response to schistosome larvae and eggs: is their role protective or subversive? J Infect Dis. 2001;183:1238–1247. doi: 10.1086/319691. [DOI] [PubMed] [Google Scholar]
- 44.Kariuki TM, et al. Antibodies elicited by the secretions from schistosome cercariae and eggs are predominantly against glycan epitopes. Parasite Immunol. 2008;30:554–562. doi: 10.1111/j.1365-3024.2008.01054.x. [DOI] [PubMed] [Google Scholar]
- 45.Wilson RA, et al. Elimination of Schistosoma mansoni Adult Worms by Rhesus Macaques: Basis for a Therapeutic Vaccine? PLoS Negl Trop Dis. 2008;2:e290. doi: 10.1371/journal.pntd.0000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mangold BL, Dean DA. Passive transfer with serum and IgG antibodies of irradiated cercaria-induced resistance against Schistosoma mansoni in mice. J Immunol. 1986;136:2644–2648. [PubMed] [Google Scholar]
- 47.Cardoso FC, et al. Schistosoma mansoni Tegument Protein Sm29 Is Able to Induce a Th1-Type of Immune Response and Protection against Parasite Infection. PLoS Negl Trop Dis. 2008;2:e308. doi: 10.1371/journal.pntd.0000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yole DS, et al. Protective immunity to Schistosoma mansoni induced in the olive baboon Papio anubis by the irradiated cercaria vaccine. Parasitology. 1996;112(Pt 1):37–46. doi: 10.1017/s0031182000065057. [DOI] [PubMed] [Google Scholar]
- 49.Cutts L, Wilson RA. Elimination of a primary schistosome infection from rats coincides with elevated IgE titres and mast cell degranulation. Parasite Immunol. 1997;19:91–102. doi: 10.1046/j.1365-3024.1997.d01-184.x. [DOI] [PubMed] [Google Scholar]
- 50.Miller HR, et al. Hepatic recruitment of mast cells occurs in rats but not mice infected with Schistosoma mansoni. Parasite Immunol. 1994;16:145–155. doi: 10.1111/j.1365-3024.1994.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 51.Imbert-Establet D, et al. Schistosome-induced portacaval haemodynamic changes in Rattus rattus are associated with translocation of adult worms to the lungs. Parasitology. 1998;116:237–241. doi: 10.1017/s0031182097002199. [DOI] [PubMed] [Google Scholar]
- 52.Tryphonas H, et al. Animal models to detect allergenicity to foods and genetically modified products: workshop summary. Environ Health Perspect. 2003;111:221–222. doi: 10.1289/ehp.5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamdan FF, et al. A novel Schistosoma mansoni G protein-coupled receptor is responsive to histamine. Mol Biochem Parasitol. 2002;119:75–86. doi: 10.1016/s0166-6851(01)00400-5. [DOI] [PubMed] [Google Scholar]
- 54.Phillips SM, et al. The cellular and humoral immune response to Schistosoma mansoni infections in inbred rats. II. Mechanisms during reexposure. Cell Immunol. 1977;28:75–89. doi: 10.1016/s0008-8749(77)80008-7. [DOI] [PubMed] [Google Scholar]
- 55.Smithers SR, Terry RJ. Naturally acquired resistance to experimental infections of Schistosoma mansoni in the rhesus monkey (Macaca mulatta) Parasitology. 1965;55:701–710. [PubMed] [Google Scholar]
- 56.Krautz-Peterson G, et al. Imaging schistosomes in vivo. Faseb J. 2009 doi: 10.1096/fj.08-127738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giuliani MM, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci U S A. 2006;103:10834–10839. doi: 10.1073/pnas.0603940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moser K, et al. Long-lived plasma cells in immunity and immunopathology. Immunol Lett. 2006;103:83–85. doi: 10.1016/j.imlet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 59.Lightowlers MW. Cestode vaccines: origins, current status and future prospects. Parasitology. 2006;133(Suppl):S27–S42. doi: 10.1017/S003118200600179X. [DOI] [PubMed] [Google Scholar]
- 60.Knudsen GM, et al. Proteomic analysis of Schistosoma mansoni cercarial secretions. Mol Cell Proteomics. 2005;4:1862–1875. doi: 10.1074/mcp.M500097-MCP200. [DOI] [PubMed] [Google Scholar]
- 61.Crabtree JE, Wilson RA. Schistosoma mansoni: an ultrastructural examination of skin migration in the hamster cheek pouch. Parasitology. 1985;91(Pt 1):111–120. doi: 10.1017/s0031182000056559. [DOI] [PubMed] [Google Scholar]
- 62.Wilson RA, Lawson JR. An examination of the skin phase of schistosome migration using a hamster cheek pouch preparation. Parasitology. 1980;80:257–266. doi: 10.1017/s0031182000000731. [DOI] [PubMed] [Google Scholar]
- 63.Lawson JR, Wilson RA. Metabolic changes associated with the migration of the schistosomulum of Schistosoma mansoni in the mammal host. Parasitology. 1980;81:325–336. doi: 10.1017/s0031182000056067. [DOI] [PubMed] [Google Scholar]
- 64.Mangold BL, et al. Site requirements and kinetics of immune-dependent elimination of intravascularly administered lung stage schistosomula in mice immunized with highly irradiated cercariae of Schistosoma mansoni. Am J Trop Med Hyg. 1986;35:332–344. doi: 10.4269/ajtmh.1986.35.332. [DOI] [PubMed] [Google Scholar]
- 65.Wilson RA, Coulson PS. Schistosoma mansoni: dynamics of migration through the vascular system of the mouse. Parasitology. 1986;92(Pt 1):83–100. doi: 10.1017/s0031182000063472. [DOI] [PubMed] [Google Scholar]
- 66.Wilson RA. The saga of schistosome migration and attrition. Parasitology. 2009 doi: 10.1017/S0031182009005708. in press. [DOI] [PubMed] [Google Scholar]
- 67.Fallon PG, et al. Juvenile rhesus monkeys have lower type 2 cytokine responses than adults after primary infection with Schistosoma mansoni. J Infect Dis. 2003;187:939–945. doi: 10.1086/368130. [DOI] [PubMed] [Google Scholar]
- 68.Kusel JR, et al. The schistosome in the mammalian host: understanding the mechanisms of adaptation. Parasitology. 2007;134:1477–1526. doi: 10.1017/S0031182007002971. [DOI] [PubMed] [Google Scholar]
- 69.Morris GP. Fine structure of the gut epithelium of Schistosoma mansoni. Experientia. 1968;24:480–482. doi: 10.1007/BF02144405. [DOI] [PubMed] [Google Scholar]
- 70.Wilson RA, Barnes PE. The tegument of Schistosoma mansoni: observations on the formation, structure and composition of cytoplasmic inclusions in relation to tegument function. Parasitology. 1974;68:239–258. [PubMed] [Google Scholar]
- 71.Davis RE, et al. Tandemly repeated exons encode 81-base repeats in multiple, developmentally regulated Schistosoma mansoni transcripts. Mol Cell Biol. 1988;8:4745–4755. doi: 10.1128/mcb.8.11.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dillon GP, et al. Altered patterns of gene expression underlying the enhanced immunogenicity of radiation-attenuated schistosomes. PLoS Negl Trop Dis. 2008;2:e240. doi: 10.1371/journal.pntd.0000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilson RA, et al. Immune responses to the radiation-attenuated schistosome vaccine: what can we learn from knock-out mice? Immunol Lett. 1999;65:117–123. doi: 10.1016/s0165-2478(98)00134-5. [DOI] [PubMed] [Google Scholar]
- 74.Street M, et al. TNF is essential for the cell-mediated protective immunity induced by the radiation-attenuated schistosome vaccine. J Immunol. 1999;163:4489–4494. [PubMed] [Google Scholar]
- 75.Jankovic D, et al. Optimal vaccination against Schistosoma mansoni requires the induction of both B cell- and IFN-gamma-dependent effector mechanisms. J Immunol. 1999;162:345–351. [PubMed] [Google Scholar]
- 76.Berriman M. The Genome of the blood fluke Schistosoma mansoni. Nature. 2009 doi: 10.1038/nature08160. 56 others. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luke TC, Hoffman SL. Rationale and plans for developing a non-replicating, metabolically active, radiation-attenuated Plasmodium falciparum sporozoite vaccine. J Exp Biol. 2003;206:3803–3808. doi: 10.1242/jeb.00644. [DOI] [PubMed] [Google Scholar]