Abstract
Background
Erratic regulation of glucose metabolism including hyperglycemia is a common condition of premature infants and is associated with increased morbidity and mortality.
Objective
To examine histological and ultra-structural differences in the endocrine pancreas in fetal (throughout gestation) and neonatal baboons.
Methods
Twelve fetal baboons were delivered at 125 days (d) gestational age (GA), 140dGA, or 175dGA. Eight animals were delivered at term (185dGA); half were fed for 5d. Seventy-three non-diabetic adult baboons were used for comparison. Pancreatic tissue was studied utilizing light microscopy, confocal imaging and electron microscopy.
Results
The fetal and neonatal endocrine pancreas islet architecture became more organized as GA advanced. The percent areas of α-β-δ-cell type were similar within each fetal and newborn GA (NS), but were higher than the adults (P<0.05) regardless of GA. The ratio of β-cells within the islet (whole and core) increased with gestation (P<0.01). Neonatal baboons who survived for 5 days (feeding), had a 2.5-fold increase in pancreas weight compared to their counterparts euthanized at birth (P=0.01). Endocrine cells were found amongst exocrine ductal and acinar cells in 125,140 and 175dGA fetuses. Subpopulation of cells that co-expressed trypsin and glucagon/insulin show the presence of cells with mixed endo-exocrine lineage in fetuses.
Conclusions
The fetal endocrine pancreas has no prevalence of a of α-β-δ-cell type with larger endocrine cell percent areas than adults. Cells with mixed endocrine/exocrine phenotype occur during fetal development. Developmental differences may play a role in glucose homeostasis during the neonatal period and may have long term implications.
Keywords: Insulin, glucagon, fetus, islet cells, primates
INTRODUCTION
Type 1 and type 2 diabetes mellitus affect millions of Americans leading to long-term consequences which can be severe (Halban et al. 2010; Polak et al. 2000; Zoungas & Patel 2010). The impaired glucose homeostasis seen in both types of this disease has been linked to loss of β-cell mass, impaired β-cell function, as well as increased glucagon secretion. The potential for β-cell regeneration in humans is an area under active investigation (Karaca et al. 2009; Dhawan et al. 2007; Halban et al. 2010; Collombat et al. 2010). In order to design therapies with this goal in mind, it is imperative to elucidate the natural development of the endocrine pancreas during fetal life. Pancreas morphogenesis has been studied in rodent models, but has not been completely investigated in the fetal human because of the extremely limited availability of fetal pancreas tissue (Svenstrup et al. 2002; Piper et al. 2004; Kahan et al. 2003; Sarkar et al. 2008). In addition, there are large gaps of knowledge in the current literature, particularly during late gestation.
Hyperglycemia has been reported in up to 80% of extremely premature infants (birth weight <1,000 grams) and has been associated with intra-ventricular hemorrhage, retinopathy of prematurity and increased risk of death (Blanco et al. 2006; Garg et al. 2003; Hall et al. 2004; Liechty 2010; Pildes 1986). Extremely low birth weight infants lack normal glucose homeostasis as evidenced by nearly absent glycogen stores at birth (Mena et al. 2001). There are long term consequences of disrupted glucose metabolism in both term and preterm infants, as demonstrated by the development of adult diseases (including type 2 diabetes) earlier in life in infants born to diabetic mothers, premature infants, and infants of low birth weight (Darendeliler et al. 2008; Whincup et al. 2008; Catalano et al. 2003; Meier 2009; Pilgaard et al. 2010). Hyperglycemia is highly prevalent in the preterm baboon and develops spontaneously with a similar incidence to that reported in extremely premature human infants (Blanco et al. 2006; Blanco et al. 2010). Furthermore, baboons have close (97%) phylogenetic proximity with humans, and can develop insulin resistance and diabetes spontaneously. Conversely, the genomic similarity between humans and mice has been reported to be 80% (Waterston RH et al. 2002). These features distinguish baboons from rodents, which are frequently utilized in diabetes research (Chavez et al. 2008). Previously we have shown that the adult baboon is a pertinent non-human primate model to examine the underlying cellular/molecular mechanisms responsible for insulin resistance, beta cell failure with islet of Langerhans remodeling and expansion of the glucagon cell component (Chavez et al. 2008; Guardado et al. 2009b; Guardado et al. 2009a; Chavez et al. 2009; Kamath et al. 2011). Preterm baboons have significant gestational differences of key insulin signaling proteins in skeletal muscle, which may also contribute to neonatal hyperglycemia (Blanco et al. 2010). Moreover, the human endocrine islet architecture is similar to the rhesus macaque and baboon as opposed to the significant differences found when compared to the rodent model (Brissova et al. 2005).
The morphology of the adult pancreas has been well-defined through numerous studies in humans and several animal models (Brissova et al. 2005; Guardado et al. 2009b; Guardado et al. 2009a; Orci L 1982). However, information is relatively sparse regarding the morphogenesis of the pancreas throughout gestation, in particular late gestation (Bock et al. 2003; Svenstrup et al. 2002; Kahan et al. 2003; Lukinius et al. 1992; Polak et al. 2000; Sarkar et al. 2008; Bouwens et al. 1997; Meier et al. 2010). With the increased survival of premature infants and the high incidence of diabetes in the pregnant population, studying the fetal pancreas during this vulnerable period is of utmost importance. This could have important implications for future targeted therapies in adult diseases. One of many advantage of utilizing baboons is that they are long lived animals and therefore, it is possible to study the potential long term effects of disrupted glucose metabolism in-utero and at early stages of development.
In this study, we hypothesized that maturational differences in the endocrine cell compartment were present among different gestational ages with morphological changes approaching those of adults as gestation increased. We analyzed the pancreases of 12 fetal (4 animals per group at 3 different gestational ages) and 8 newborn baboons (4 exposed to enteral feeds, 4 non-exposed) and used adult baboon specimens (73 control animals) for comparisons.
MATERIALS AND METHODS
Animals
Fetuses
Twelve fetal baboons (7 males, 5 females) at the Texas Biomedical Research Institute (TBRI) in San Antonio, Texas were delivered prematurely via c-section under general anesthesia from healthy, non-diabetic mothers at 125d GA, 140d GA, or near term at 175d GA (full term=185d GA), and euthanized immediately after birth. 4 animals were delivered in each GA group.
Newborns
Animals were born at either TBRI or at Baboon Research Resources at the University of Oklahoma Health Sciences Center in Oklahoma City, Oklahoma (UOHSC). Four term baboons (all female) were delivered via spontaneous vaginal delivery from healthy, non-diabetic mothers and euthanized shortly after birth. Four additional animals (1 male, 3 females) were born at term, fed ad libitum by their mothers, and subsequently by veterinary staff with infant formula (Similac, Abbott, Columbus, OH, USA); these animals are referred to as neonatal throughout the paper. At day of life 4±2 days, these animals underwent general anesthesia for 6 hours and were exposed to a euglycemic-hyperinsulinemic clamp for 2 hours followed by euthanasia to collect data in regards to endogenous glucose production.
Adults
Seventy three adult, non-diabetic animals were utilized as controls. Pancreas tissues from these animals were used as normal adult pancreas (25).
Blood samples were obtained from umbilical cord in fetal baboons (shortly after birth) and prior to euthanasia by venous puncture in term animals. Plasma glucose was measured using the Analox GM9 analyzer (Analox Instruments, London, UK). Plasma insulin was measured by ELISA (Alpco Diagnostics, Salem, NH, USA).
All studies were approved by the Institutional Animal Care Committee at the TBRI, and at the University of Texas Health Science Center San Antonio. Administrative approval was obtained from UOHSC. Animal experiments were conducted in accordance with accepted standards of humane animal care.
Tissue Preparation
Fetal, term, neonatal and adult pancreatic tissues were obtained post-mortem for routine histological staining. The weight of the each animal and of the pancreatic tissue was recorded at necropsy and pancreas as a percentage of body weight was calculated. The majority of the pancreas was fixed in 10% neutral buffered formalin and stored in 70% EtOH solution. The specimens were then processed using a Tissue-Tek VIP5 (Sakura Finetek, Torrance, CA) and embedded in paraffin with the Tissue-Tek Embedding Console System (Sakura Finetek, Torrance, CA, USA). Serial sections were cut at 4 µm with the HM325 Rotary Microme (Thermo Scientific, Austin, TX, USA) and mounted on coated Plus slides (Fisher Scientific, Pittsburg, PA, USA).
Histology and Immunohistochemistry for light microscopy
Consecutive sections from the pancreatic body region were stained with hematoxylin and eosin, and immunostained for insulin, glucagon, and somatostatin for light microscopy. Slides from each paraffin block were processed for immunohistochemistry utilizing the Benchmark XT automatic slide preparation system (Ventana Medical Systems, Tucson, AZ, USA). After dewaxing, the sections underwent a heat-induced antigen retrieval step with a high pH antigen retrieval solution (Cell Conditioning 1 Solution, Ventana Medical Systems, Tucson, AZ, USA) for 30 minutes. An anti-insulin polyclonal guinea pig antibody (Cell Marque, Hot Springs, AZ, USA) was used to label β-cells, an anti-glucagon polyclonal rabbit antibody (Leica Biosystems, Bannoockburn, IL, USA) for the labeling of the α-cells, and anti-somatostatin polyclonal rabbit antibody (Cell Marque, Hot Springs, AZ, USA) for the labeling of δ-cells. This step was followed by application of a biotinylated secondary antibody (all antibodies were pre-diluted from manufacturers). Sections were subsequently incubated with a streptavidin-HRP complex. Visualization was obtained with a diaminobenzidine (DAB) detection kit (Ventana Medical Systems, Tucson, AZ, USA). All control incubation without application of the primary antibodies yielded no labeling. Finally, the specificity of the antibody staining for α-, β-, and δ-cells was also validated by competitive displacement experiments, in which the primary antibodies to insulin, glucagon and somatostatin were pre-incubated overnight at 4°C with chemical grade insulin, glucagon and somatostatin at a final concentration of 500 nmol. Immunocytochemistry experiments were then completed as described above (Supplemental Figure 1).
Immunofluorescence staining and confocal image analysis
Pancreas specimens were immunostained as previously described (Federici et al. 2001). After heat-induced antigen retrieval (2×5min microwave treatment in 10 mM citrate buffer, pH 6.0), the sections were incubated overnight with the primary antibodies. The following primary antibodies were used: anti-insulin polyclonal guinea pig (Roche, Tucson, AZ, USA; diluted 1:300), anti-glucagon polyclonal rabbit (R&D Systems, Minneapolis, MN, USA; diluted 1:150), and anti-trypsin monoclonal mouse (Chemicon, Temecula, CA, USA; diluted 1:100). Staining with primary antibody was followed by incubation for two hours with FITC (diluted 1:200), TRITC (diluted 1:150), or CY5 (diluted 1:200)-conjugated secondary antibodies (Jackson Immunoresearch, Baltimore, MD, US).
Image acquisition
Islets were imaged using a Bio-Rad MRC 1024 confocal laser scanning microscope (Bio-Rad, Philadelphia, PA, USA). To reduce the bleed through from below, confocal images were acquired sequentially, using the LaserSharp2000 software with a low iris diameter (1–2). The florophores (FITC, TRITC and Cy5) are all commonly used for triple immunostaining and the bleed through for these florophores is negligible when sequential scanning is employed. Identical parameters (laser power, iris diameter and gain) were maintained to acquire images from all sections. Background signal due to non-specific binding was subtracted from a “test” image. “Test” image is the image obtained from a pancreas section processed exactly as the experimental samples, but with secondary antibodies only (no primary antibody).
Image analysis and quantification
Quantifications of red and blue fluorescence on digital images were performed using the Image-Pro 3D Analyser 5.1 Image Software (Media Cybernetics). Briefly, single-stain confocal images were merged and enhanced, and then a macro was created in order to automatically quantify the green- and red-stained areas in defined islet regions. Areas labelled for glucagon (green = a) and for insulin (red = b) were quantified in the whole islet, in the islet mantle and in the islet core. The mantle region is defined as the region of 20-µm deep that follows the external perimeter of the islet (Bosco et al, 2010). The core region is the total islet area minus the 20-µm deep mantle area. Data are expressed as a ratio between single hormone-stained areas/(insulin + glucagon stained areas). Fifteen islets per section were analysed from three different animals for each category. Experiments were performed in duplicate. Differences between means were assessed by one way Anova, followed by Kruskal-Wallis test. A P value <0.05 was considered statistically significant.
Electron Microscopy and Immunoelectron Microscopy
For ultrastructural study, pancreatic tissues were fixed for two hours at 4°C in a mixture of 2% paraformaldehyde and 2% glutaraldehyde in 0.05 M pH7.3 cacodylate buffer, post-fixed in 1% osmium tetroxide for one hour at room temperature, dehydrated in ethanol and embedded in Epon-Araldite.
For electron microscopy immunocytochemistry, pancreatic tissues were fixed for two hours at 4°C in a mixture of 2% paraformaldehyde and 0.5% glutaraldehyde in 0.05 M pH7.3 cacodylate buffer, and embedded in London White Resin (Polysciences, Warrington, PA, USA). Thin sections, after pretreatment with ovoalbumin1% for 5 minutes, were incubated for 24 hours at 4°C with the following primary antibodies: guinea pig polyclonal anti-insulin antibody (Dako, Carpinteria, CA, USA; diluted 1:50), rabbit polyclonal anti-glucagon antibody (Milab, Malmoe, Sweden; diluted 1:1000) and mouse monoclonal anti-trypsin antibody (Chemicon, Temecula, CA, USA; diluted 1:50). Then, sections were incubated with 12nm Colloidal Gold-AffiniPure donkey anti-rabbit (Jackson Immunoresearch Laboratories, Baltimore, MD, USA) diluted 1:50, or 18nm Gold-AffiniPure (Jackson Immunoresearch Laboratories) goat anti-mouse diluted 1:20, or 12 nm Colloidal Gold-AffiniPure donkey anti-guinea pig (Jackson Immunoresearch Laboratories) diluted 1:20 for one hour at room temperature. Finally, after counterstaining with uranyl acetate and lead citrate, all thin sections were examined with a Philips (Morgagni 268 D) electron microscope. Specificity controls consisted of omission of the first layer, use of tissues with or without pertinent antigens, and substitution of the primary antibody with normal serum as previously described (Van Noorden S 1986). The pancreas from 4 fetal baboons (67% gestation) and 7 adult baboons were analyzed. For each case, we studied 2 sections evaluating 200 cells per section, for a total of 400 cells per case.
Tissue Analysis
For the morphological assessment of pancreatic development, cases were grouped into six development categories: 1) 67% gestation (125d GA); 2) 75% gestation (140d GA); 3) near term, 95% gestation (175d GA); 4) term, 100% gestation (approximately 185d GA); 5) term gestation exposed to enteral feeds (185d GA + 5 days); and 6) adults.
Morphometric Analysis
Morphometric analysis was performed using the Computer Assisted Stereology Toolbox (CAST) 2.0 system (Visiopharm, Ballerup, Denmark) coupled with an Olympus BX61 microscope (Olympus Corporation of the Americas, Center Valley, PA, USA). By utilizing the stereology fundamentals previously described on pancreatic sections randomly collected (Mandarim-de-Lacerda 2003), we calculated the percent areas of the different tissue structures through the use of lines and points. Four serial sections per animal (H&E, insulin, glucagon and somatostatin) were taken from the pancreas to quantify endocrine cells. Each field was selected randomly from the whole section using the CAST sampling. On average, we analyzed 48 fields per slide; in each field, point counting of total pancreatic tissue, islets, and individual endocrine cells was performed at the magnification of 40× to calculate the percent area of each component; 180 points were inserted on each field and an average of 8028 points were used per slide. The quantification of the cell percent areas within the total pancreas was calculated by using the following formula: (CP/TP) × 100, where CP = points that hit specified endocrine cells and TP = total pancreas points (Guardado et al. 2009a).
Data Analyses
Statistical calculations were performed with SPSS for Windows (Version 16.5, SPSS, Inc., Chicago, IL, USA). Power calculation was based on pancreas weight from term +5 day animals (0.941±0.24 grams); with an expected 33% decrease in pancreas weight in fetal animals, a one sided t-test, alpha of 0.05 and 80% power, a total of 4 animals per group were calculated for significance. Differences between groups were analyzed utilizing ANOVA after arcsin data transformation. A P<0.05 was considered to be statistically significant. Confocal data analysis was performed with GraphPad Prism, one way ANOVA, Kruskal-Wallis test, and Dunn’s multiple comparisons tests.
RESULTS
Gross findings
Demographics for the fetal and neonatal animals studied are shown in Table 1. As expected, the weights of the baboons at term or near-term are significantly higher than at lower gestational ages (P<0.05). The pancreas weights were similar between fetal baboons prior to term, regardless of GA. Surprisingly, for those baboons born at term, the pancreas weight increased by greater than 2-fold at 5 days after birth when compared to their term counterparts euthanized immediately after birth (P=0.02).
Table 1.
Demographic Characteristics
|
GA |
Weight (grams) |
Pancreas weight (grams) |
Pancreas % of body weight |
Gender |
Fasting Serum Insulin (mIU/dL) |
Fasting Serum Glucose (mg/dL) |
|---|---|---|---|---|---|---|
| 125d (n=4) | 421.5 ± 69.6 | 0.353 ± 0.26 | 0.08% | 3 Male/1 Female | 2.6 ± 2.2 | 40.6 ± 4.7 |
| 140d (n=4) | 493 ± 40.9 | 0.247 ± 0.10 | 0.05% | 2 Male/2 Female | 2.9 ± 1.1 | 37 ± 7.9 |
| 175d (n=4) | 1046.5 ± 163.1 | 0.352 ± 0.25 | 0.03% | 2 Male/2 Female | 2.4 ± 2.7 | 31.4 ± 4.7 |
| 185d (n=4) | 862 ± 74.5 | 0.394 ± 0.178 | 0.04% | 0 Male/4 Female | N/A | N/A |
| 185+5d (n=4) | 935.5 ± 94.9 | 0.941 ± 0.24** | 0.10%* | 0 Male/4 Female | 8.7 ± 6.1 | 59.2±10.6 |
| Adult (19y) | 27000 ± 9000 | 17.93 ± 1.98† | 0.07% | 29 Male/44 Female | 32.1±41.0 | 87.0±16.6 |
P<0.05 when compared to all GA’s for pancreas % of body weight.
P=0.01 when compared to 185d for pancreas weight.
Average adult pancreas weight as previously reported (Mahaney MC, Leland NM, Williams-Blangero S, Marinez YN 1993 Cross-sectional growth standards for captive baboons: Organ weight by chronological age. Journal of Medical Primatology 22 400–414)
Qualitative Analysis/Morphology
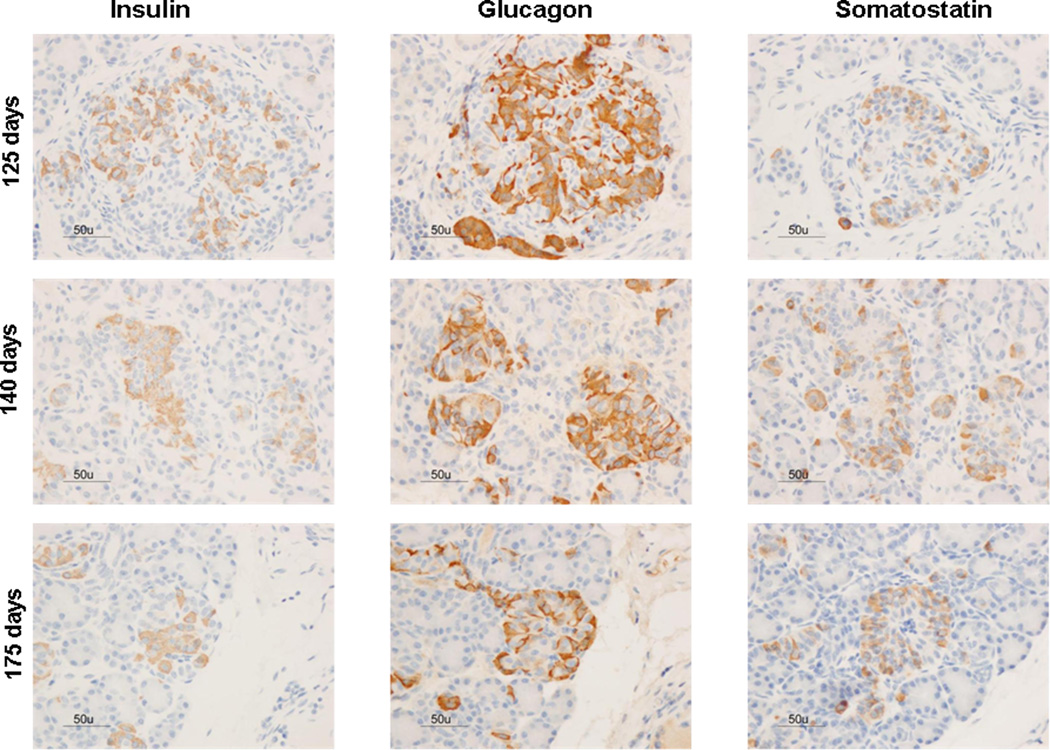
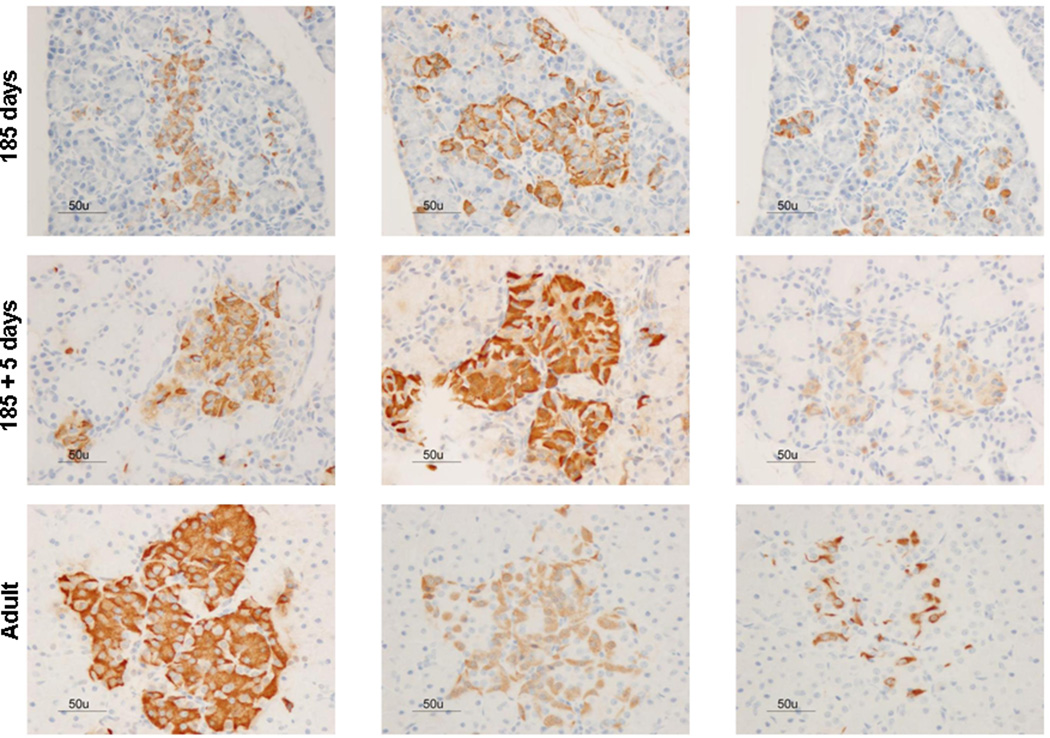
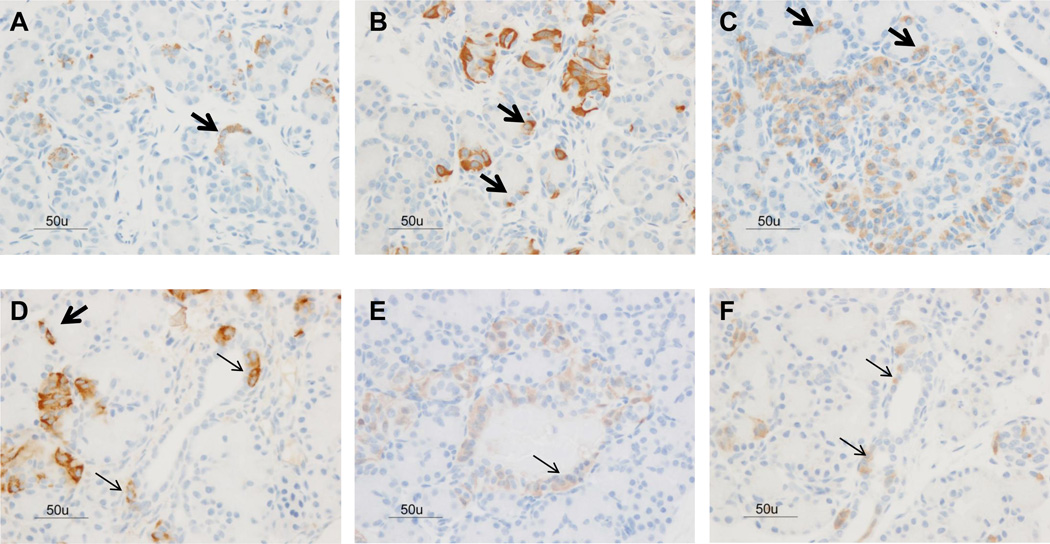
Immunoreactivity for insulin, glucagon, and somatostatin was found throughout all gestational ages (Figure 1). The percent area of endocrine cellular staining for insulin, glucagon and somatostatin were consistent throughout the fetal gestational ages, regardless of the hormone studied (Figure 2 and supplemental Figure 2). Additionally, scattered endocrine stained cells were observed among exocrine cells in fetal pancreas samples (Figure 3). Although fetal islets resembled the postnatal (term and neonatal) baboon islets, the architectural structure became more organized as gestational age advanced. Regardless of gestational age, baboon islet architecture was composed of centralized β-cells with α and δ cells mainly peripherally located. The displacement experiments confirmed the specificity of the staining techniques as antigen/hormone specific (Supplemental Figure 1).
Figure 1. Hormone expression in the developing baboon pancreas.
Gestational age in fetal baboons is shown at 125d, 140d, 175d, 185d gestation (full term = 185 days). 185+5 days represent baboons that survived 5 days after natural delivery, adult baboons fed normal diets are shown. Immunohistochemistry panels for insulin, glucagon, and somatostatin for each time point. Bar = 50µm; all images were taken at the same magnification.
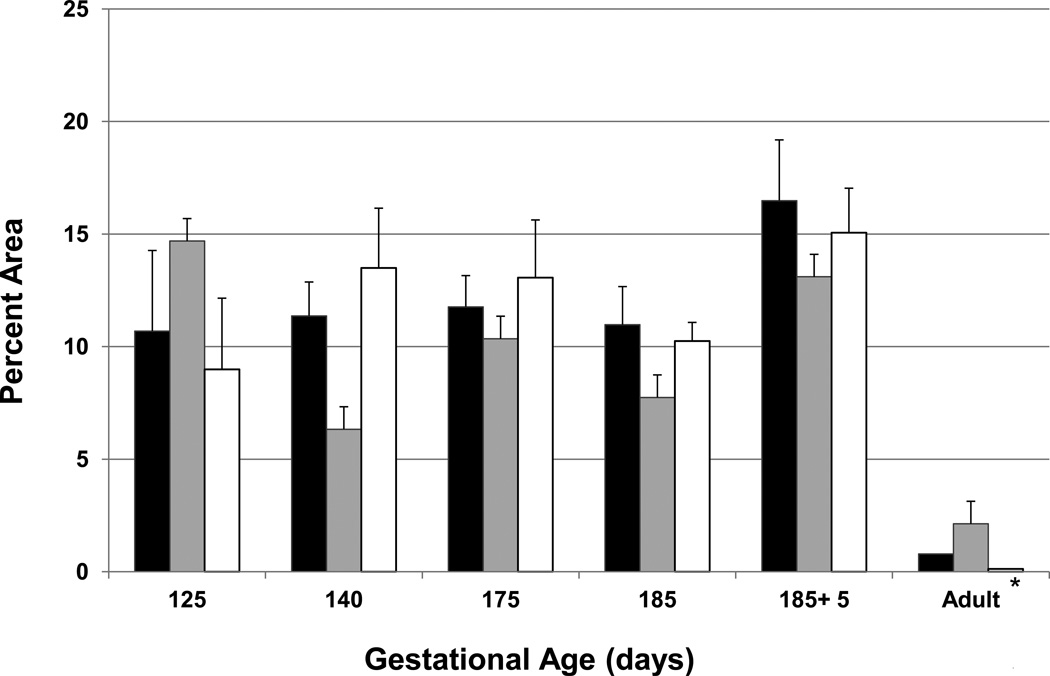
Figure 2. Percent area of islet by cell type within the entire pancreas at different gestational ages.
The volume of α-cells is represented by the black bars, β-cells by gray bars and δ-cells by white bars. *P<0.05 for each adult cell type compared to all other gestational ages.
Figure 3.
Hormonal expression in ductal (thin arrows) and acinar (thick arrow) components of the fetal exocrine pancreas (A= insulin stain, B= glucagon stain, C= somatostatin) and term newborn pancreas (D= insulin stain, E= glucagon stain, F= somatostatin). Bar = 50µm.
Quantitative Analysis/Morphometry/Immunoelectron microscopy
The percent area of α-, β- and δ-cells within the endocrine pancreas was similar between and within each fetal and neonatal gestations (N.S); however, the percent area of each cell type (α-, β- and δ) was 15, 5 and 94 fold higher than adult animals respectively, P<0.05 (Figure 2). Representative immuno-stains from each cell type from fetal and adult animals are depicted on Supplemental Figure 2.
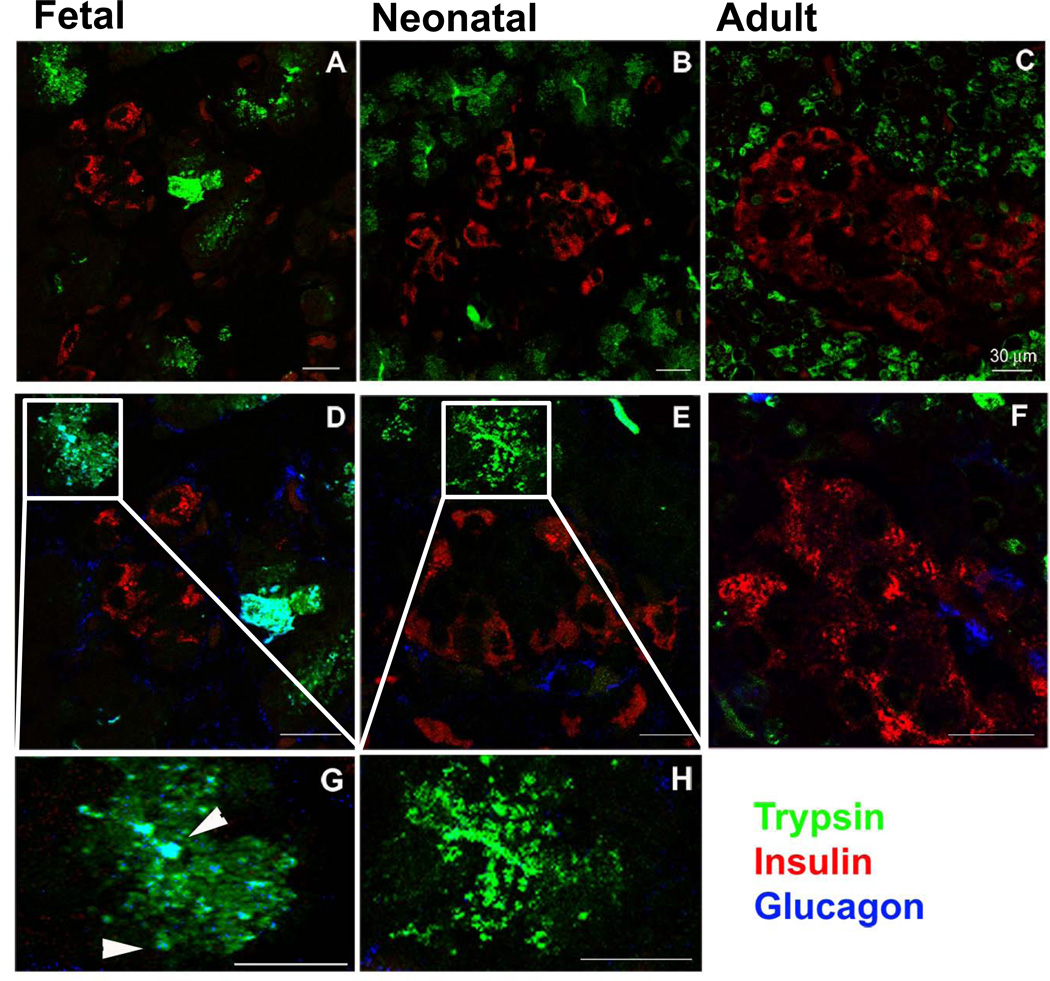
Confocal Microscopy: The organization of fetal, neonatal and adult pancreas was analysed by confocal microscopy after immunostaining with specific hormones as markers of the endocrine pancreas and trypsin as a marker of acinar cells (Figure 4A – 4C). In adult pancreases, well defined islets were surrounded by trypsin-positive acinar cells. In neonatal pancreases, islets were clearly recognizable and separated from the exocrine tissue while in fetal pancreases islet morphology was less well defined. In the adult islet, the majority of cells were insulin-positive; fetal and neonatal islets had a more even mixture of insulin-, glucagon-, and somatostatin-positive cells. The islet architecture was also analysed using triple staining with insulin, glucagon and trypsin (Figure 4D – 4H). In adult and neonatal pancreases, glucagon-positive cells had a prevalent peripheral localization, while insulin-positive cells were mainly detected in the islet core. In fetal samples, insulin-positive cells were surrounded by cells slightly positive for glucagon. At the islet periphery, the margin between the endocrine and the exocrine tissue was not always clearly defined.
Figure 4. Confocal microscopy of fetal, neonatal, and adult baboon pancreas.
Islets of Langerhans are clearly recognizable and well organized in the adult and neonatal pancreas samples. 4A–C: Double immunostaining with trypsin (green) and insulin (red). (A= fetal pancreas, B= neonatal pancreas, C= adult pancreas). Insulin positive cells are interspersed in the exocrine pancreas of the fetal sample only. 4D–4F: Triple immunostaining with trypsin (green), insulin (red), and glucagon (blue). (D= fetal pancreas, E= neonatal pancreas, F= adult pancreas). Glucagon staining was detected amongst trypsin-positive cells in the fetal pancreas only. 4G–4H: Enlarged images of the triple immunostaining (G= fetal pancreas, H= neonatal pancreas). Bar = 30µm.
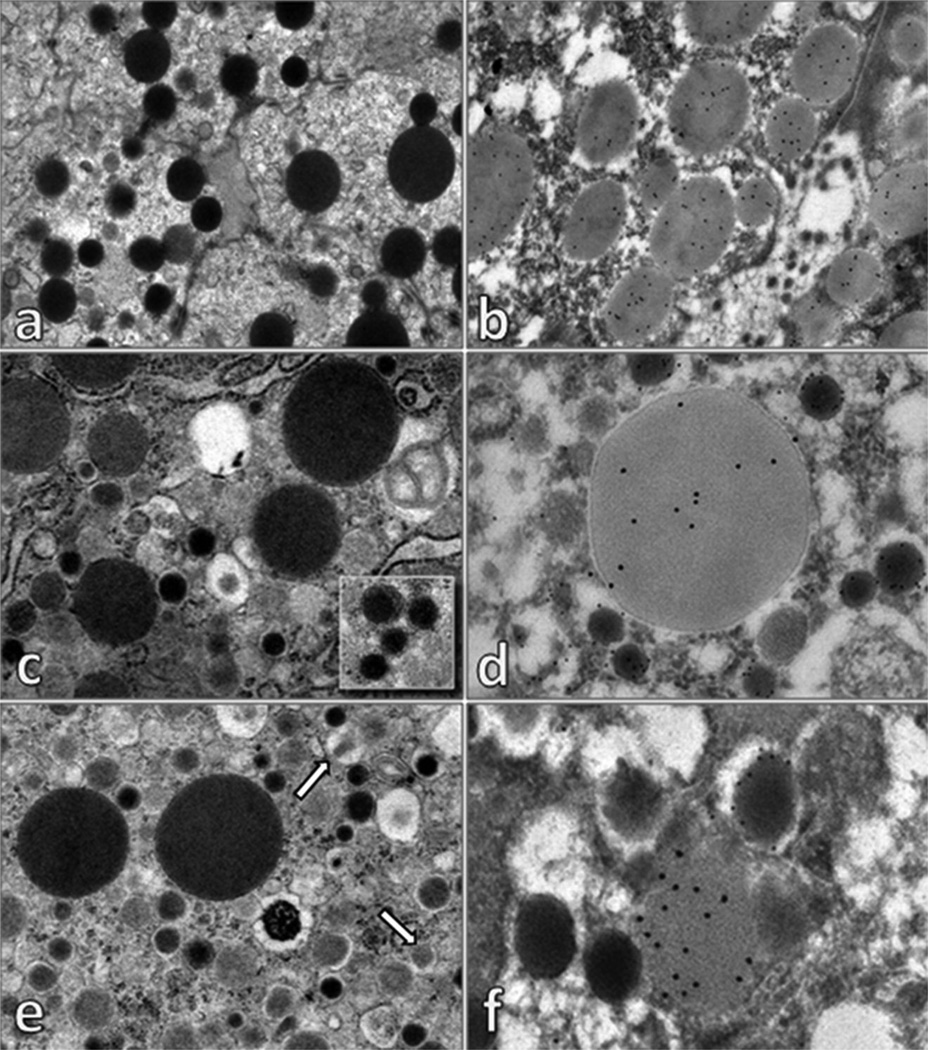
Interestingly, a subpopulation of cells showed both an endocrine and acinar phenotype as shown by colocalization of trypsin and glucagon in fetal baboons (Figure 4G) whereas neonatal baboons did not (Figure 4H). Immunoelectron microscopy: The above findings were confirmed by Immuno EM which demonstrated 2% of fetal cells with both glucagon-immunoreactive alpha-type secretory granules and trypsin-positive zymogen granules in the cytoplasm. Additionally, 1% of fetal cells contained both insulin-immunoreactive beta-type secretory granules and trypsin-positive zymogen granules in the cytoplasms (Figure 5). Conversely, this co-localization was not observed in adult cells (Supplemental Figure 3).
Figure 5. Electron microscopy.
The cytoplasm of acinar cells shows numerous electron dense zymogen granules (a), which are positive for trypsin (b). Some cells showed in the cytoplasm, zymogen granules and alpha-type secretory granules (c), with typical electron dense core and clear halo (inset). Double label electron microscopy immunocytochemistry (d) demonstrated that alpha-type secretory granules were glucagon-positive (glucagon immunoreactivity identified with 12 nm colloidal gold), while zymogen granules were positive for trypsin (trypsin-immunoreactivity is evidenced with 18 nm colloidal gold). Moreover, other cells presented in the cytoplasm zymogen granules were crystalline beta-type secretory granules (some indicated with arrows) (e). Double label electron microscopy immunocytochemistry (f) demonstrated that beta-type secretory granules were insulin-positive (insulin immunoreactivity identified with 12 nm colloidal gold), while zymogen granules were positive for trypsin (trypsin-immunoreactivity is evidenced with 18 nm colloidal gold). In tissues processed for electron microscopy immunocytochemistry (embedded in London White Resin, without post-fixation in 1% osmium tetroxide) zymogen granules appear clearer than that observed in tissue processed for conventional electron microscopy.
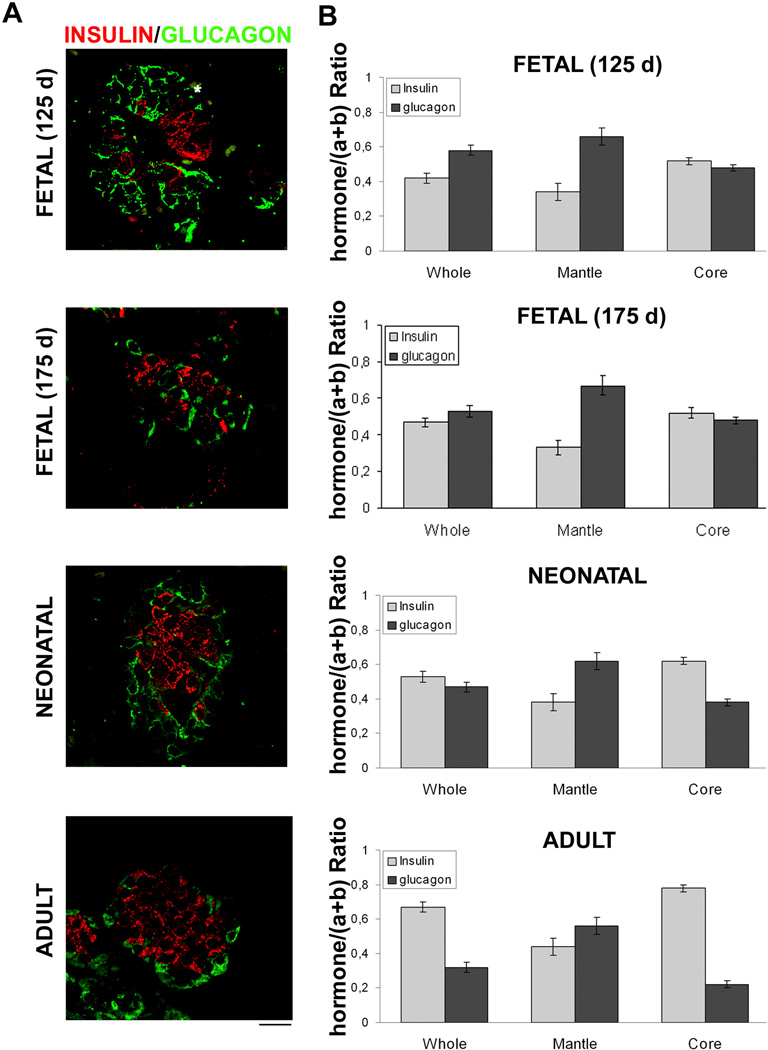
In the adult pancreas, the segregated cell type distribution was clearly evident with β-cells prevalently localized in the mantle and β-cell in the core islet (Figure 6). This observation was confirmed by a quantitative analysis. Areas labelled for glucagon (Figure 6A, green) and for insulin (Figure 6A, red) were quantified in the whole islet, in the islet mantle and in the islet core. The mantle region was defined as the region of 20-µm deep that followed the external perimeter of the islet (Bosco et al. 2010). The core region was the total islet area minus the mantle area. The results in a mature islet (adult) are expressed as b/(a+b) (a=glucagon, b=insulin), were the β-cells were 0.46+0.02 within the mantle and were 0.78+0.03 within the core, demonstrating that β-cells are prevalently segregated in the core region in the mature islet (Figure 6A and B). Pancreatic islets from fetal (125d and 175d) and neonatal baboons were analysed in the same fashion. The segregation of β-cells in the core region in the most immature fetal islets [b/(a+b)] were 0.52+0.03 and 0.30+0.02 in the core and mantle respectively; it progressively increased where neonatal islets [(b/(a+b)] β-cells were 0.62+0.03 and 0.38+0.03 in the core and mantle, respectively). However, the total β-cell ratios [b/(a+b)] within the islet were significantly lower in fetal and neonatal islets compared with adult islets and the ratios increased as gestational age increased (0.42+0.02, 0.46+0.02, 0.53+0.02 and 0.68+0.02 in the fetal 125d, fetal 145d, neonatal and adult, respectively. P<0.001).
Figure 6. Distribution of α- and β-cells in islet of Langerhans subregions during development.
A. Double immunofluorescence stainings. Pancreas sections from fetal (125d), fetal (175d), neonatal, and adult baboons were double stained with Insulin (red) and Glucagon (green), and images were analysed by means of confocal microscopy. Representative images are shown. White lines delimiting the islet mantle and core subregions are shown. Bar: 20 µm.
B. Analysis of α- and β-cells distribution in islet subregions. Areas labelled for glucagon (a) and insulin (b) were measured in the whole islets (Whole) and in different subregions (Mantle and Core) as described in material and methods. Results are expressed as insulin/(a+b) or glucagon/(a+b) ratio. Columns are means ± SEM. n=45 islets from three pancreas. ***P<0.01 vs adult baboon.
DISCUSSION
In this study, we demonstrated that fetal endocrine pancreas has no prevalence of a single cell type during fetal development and early newborn period. The true timing of endocrine differentiation is not well understood, but it is theorized that massive differentiation is contributory towards the increased percentage of endocrine pancreas seen during fetal development (Polak et al. 2000). Although fetal islets resembled postnatal baboon islets, the architectural structure became progressively more organized as gestational age advanced. Studies in humans have shown similar results, but these studies have involved fetuses from miscarriages and terminations of pregnancies, therefore the number of samples studied have been small and scattered through gestation (Piper et al. 2004; Brissova et al. 2005; Lukinius et al. 1992; Polak et al. 2000; Bouwens et al. 1997; Meier et al. 2010).
The percent areas of alpha, beta, and delta cells did not change over time in fetuses or in term animals after survival with or without exposure to feeds but remained larger that the adults(Figure 2); pancreas function, expressed as plasma insulin and glucose levels were similar between gestations and was within levels reported in preterm and newborn humans (Blanco et al. 2008; Committee on Fetus and Newborn 2011). The islet architectural, however, was clearly more organized in those animals who survived beyond birth (Figure 1). Data in newborn humans are lacking, in particular during the first week of life. Infant death during this period of life is usually due to sepsis, metabolic disorders or genetic conditions that would not provide a normal pancreas morphology (Iams & Creasy 2004). Therefore, we suggest that this model may provide appropriate insight into the postnatal changes in pancreas development in a healthy state.
Islet cell organization in the baboon is similar to that seen in humans. We demonstrated prevalent peripheral localization of glucagon-positive cells with a central localization of insulin-positive cells by confocal microscopy (Figure 4 and 6). Interestingly, the ratio of beta cell population within the islet and the percent ratio found in the core increased as gestational age increased resembling the organization of mature islets (adult baboons). Studies of fetal pancreas from rodent and non-human primates showed inconsistent results in regards to the core-mantle architecture (Bocian et al. 1999; Murtaugh 2007; Collombat et al. 2010). However, studies of pancreas from humans have demonstrated core-mantle organization, with insulin-producing cells localized centrally beginning in the mid-gestational period (Meier et al. 2010). Although some mouse/rat models show a typical core-mantle architecture similar to humans (Brissova et al. 2005), genetic similarity to mouse is only 80% (Waterston RH et al. 2002). On the other hand, the genetic similarity between humans and non human primates (Chimpanzees and Baboons) has been demonstrated to be in the range of 94–98% (Chavez et al. 2008; Cheng et al. 2005). Furthermore, adult baboons also develop insulin resistance and diabetes spontaneously, a rare feature in other animal models and protein expression of key insulin signaling in baboons have been shown to be 97–98% identical to humans (Chavez et al. 2008). Therefore, studying the morphogenesis of the pancreas during critical periods of development in baboons may be the key in the development of new treatments to prevent and treat diabetes, but most relevant to the present study is to provide new insights in the understanding of islet differentiation.
Surprisingly, a significant increase of the total pancreas weight was seen postnatally after 5 days of extrauterine life. Since there were no differences in the percent areas of each cell type in those newborns exposed to feeds, this postnatal increase in pancreas weight appears to be due to a large expansion of both endocrine and exocrine pancreas. In the rodent model, the postnatal period between birth and weaning is characterized by a massive expansion in beta cell mass with the increasing physiological demands but also balancing with beta cell death during pancreatic remodeling (Dhawan et al. 2007; Georgia & Bhushan 2004; Finegood 1995). Further studies targeting markers of cell death/regeneration will be performed to elucidate the cause/effect of these changes.
Other investigators have reported a 2–5% percent beta cell in humans and other animal models, (Limesand et al. 2005; Beringue et al. 2002; Bock et al. 2003; Meier et al. 2010) in contrast, in the current study we report a larger beta cell percent area throughout late gestation and early neonatal period (Figure 2 and Supplemental Figure 2). Unfortunately, the methodology utilized for pancreas measurements in different studies is largely variable, the gestational ages included have a wide range of age and most, have studied post-natal development. Moreover, most human studies have utilized aborted or infected fetuses and/or neonates who have died due to neonatal disease (Beringue et al. 2002; Meier et al. 2010); disease processes, particularly those of infectious origin, may have an impact on pancreas development due to potential effects on mitosis/apoptosis rates. Therefore, a non human primate model, where serial, consistent measurements are performed in an otherwise healthy animal may be more representative of normal development. Another potential explanation for the larger endocrine pancreas found during fetal development in this study may be species related differences or gender differences due to small sample size.
It has been established that the endocrine pancreas derives from epithelial (ductal) precursor cells (Bouwens et al. 1997). Ductal cell differentiation from putative precursor cells, sometimes referred to as “neogenesis”, has been suggested to occur in neonatal animals (Dhawan et al. 2007; Finegood 1995). It has not been determined, however, whether early pancreas cells are pluripotential with the capability of contributing to both endocrine and exocrine lineages, or if there is a subpopulation of cells that may be unipotent stem cells. In these experiments we demonstrate a subpopulation of cells that show both, an endocrine and acinar phenotype in the fetal baboon by immunohistochemistry and by colocalization of trypsin/glucagon and trypsin/insulin by confocal microscopy and Immuno EM (Figure 4 and 5). To the best of our knowledge, this is the first report of both exocrine and endocrine phenotypes occurring in the same pancreatic cell. The importance of this finding cannot be understated due to the potential long term implications of the effects of different interventions in a pancreatic cell that might be pluripotential at a particular period of development. Thus, the baboon provides a unique model where differentiation and different stages of development of endocrine and exocrine pancreas could be followed long term.
With prematurity being one of the two leading causes of perinatal morbidity and mortality and postnatal adaptation is associated with increasing incidences of type 2 diabetes mellitus, essential hypertension and coronary artery disease in surviving LBW babies (Whincup et al. 2008; Hovi et al. 2007; Hofman et al. 2006; Rotteveel et al. 2008; Bacchetta et al. 2009; Hack 2006), an increase in knowledge regarding fetal pancreas development will further our understanding of the disruptive glucose metabolism in these infants.
The ontogeny of the endocrine pancreas in the fetal/newborn baboon model provides insight into the normal pancreas development from late gestation through the neonatal period. Furthermore, the presence of pancreatic cells with both, endocrine and exocrine phenotypes during late gestation, may have long term implications if born during this critical period of development. A non-human primate model that survives prematurity and develops diabetes spontaneously would lead to further studies that might pave the way to the development of novel therapeutic strategies for patients with hyperglycemia and diabetes.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. David Han, MSc, Ph.D., Assistant Professor of Statistics, Department of Management Science & Statistic, University of Texas at San Antonio, for the statistical support. We thank the veterinarians and personnel at the University of Texas Health Science Center, the personnel at the Texas Biomedical Research Institute and the University of Oklahoma Health Sciences Center in Oklahoma of for their dedication and support for this project.
FUNDING
This study was supported by grants from the Robert Wood Johnson Foundation (67067, C.B.), CTSA (UL1RR025767 to C.B.), the National Institutes of Health (HL52636 to BPD resource Center & RO-1 DK080148 to F.F.), and NCRR (P51 RR013986 to the SNPRC, now TBRI).
Footnotes
DECLARATION OF INTEREST
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Reference List
- Bacchetta J, Harambat J, Dubourg L, Guy B, Liutkus Al, Canterino I, Kassa B, Putet G, Cochat P. Both extrauterine and intrauterine growth restriction impair renal function in children born very preterm. Kidney International. 2009;76:445–452. doi: 10.1038/ki.2009.201. [DOI] [PubMed] [Google Scholar]
- Beringue F, Blondeau B, Castellotti MC, Breant B, Czernichow P, Polak M. Endocrine Pancreas Development in Growth-Retarded Human Fetuses. Diabetes. 2002;51:385–391. doi: 10.2337/diabetes.51.2.385. [DOI] [PubMed] [Google Scholar]
- Blanco CL, Baillargeon JG, Morrison RL, Gong AK. Hyperglycemia in extremely low birth weight infants in a predominantly Hispanic population and related morbidities. Journal of Perinatology. 2006;26:737–741. doi: 10.1038/sj.jp.7211594. [DOI] [PubMed] [Google Scholar]
- Blanco CL, Liang H, Joya-Galeana J, DeFronzo RA, McCurnin D, Musi N. The Ontogeny of Insulin Signaling in the Preterm Baboon Model. Endocrinology. 2010;151:1990–1997. doi: 10.1210/en.2009-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco CLM, Falck AM, Green BKR, Cornell JEP, Gong AKM. Metabolic Responses to Early and High Protein Supplementation in a Randomized Trial Evaluating the Prevention of Hyperkalemia in Extremely Low Birth Weight Infants. [Article] Journal of Pediatrics. 2008;153:535–540. doi: 10.1016/j.jpeds.2008.04.059. [DOI] [PubMed] [Google Scholar]
- Bocian S, Zabel M, Wozniak W, Surdyk-Zasada J. Polyhormonal aspect of the endocrine cells of the human fetal pancreas. Histochemistry & Cell Biology. 1999;112:147–153. doi: 10.1007/s004180050401. [DOI] [PubMed] [Google Scholar]
- Bock T, Kyhnel A, Pakkenberg B, Buschard K. The postnatal growth of the beta-cell mass in pigs. Journal of Endocrinology. 2003;179:245–252. doi: 10.1677/joe.0.1790245. [DOI] [PubMed] [Google Scholar]
- Bosco D, Armanet M, Morel P, Niclauss N, Sgroi A, Muller YD, Giovannoni L, Parnaud G, Berney T. Unique Arrangement of α and β Cells in Human Islets of Langerhans. Diabetes. 2010;59:1202–1210. doi: 10.2337/db09-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwens L, Lu WG, Krijger RD. Proliferation and differentiation in the human fetal endocrine pancreas. Diabetologia. 1997;40:398–404. doi: 10.1007/s001250050693. [DOI] [PubMed] [Google Scholar]
- Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, Powers AC. Assessment of Human Pancreatic Islet Architecture and Composition by Laser Scanning Confocal Microscopy. Journal of Histochemistry and Cytochemistry. 2005;53:1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- Catalano PM, Kirwan JP, Haugel-de Mouzon S, King J. Gestational diabetes and insulin resistance: role in short- and long-term implications for mother and fetus. [Review] [87 refs] Journal of Nutrition. 2003;133:1674S–1683S. doi: 10.1093/jn/133.5.1674S. [DOI] [PubMed] [Google Scholar]
- Chavez A, Gastaldelli A, Guardado-Mendoza R, Lopez-Alvarenga J, Leland MM, Tejero ME, Sorice G, Casiraghi F, Davalli A, Bastarrachea R, Comuzzie A, DeFronzo R, Folli F. Predictive models of insulin resistance derived from simple morphometric and biochemical indices related to obesity and the metabolic syndrome in baboons. Cardiovascular Diabetology. 2009;8:22. doi: 10.1186/1475-2840-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AO, Lopez-Alvarenga JC, Tejero ME, Triplitt C, Bastarrachea RA, Sriwijitkamol A, Tantiwong P, Voruganti VS, Musi N, Comuzzie AG, DeFronzo RA, Folli F. Physiological and Molecular Determinants of Insulin Action in the Baboon. Diabetes. 2008;57:899–908. doi: 10.2337/db07-0790. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Ventura M, She X, Khaitovich P, Graves T, Osoegawa K, Church D, DeJong P, Wilson RK, Paabo S, Rocchi M, Eichler EE. A genome-wide comparison of recent chimpanzee and human segmental duplications. Nature. 2005;437:88–93. doi: 10.1038/nature04000. [DOI] [PubMed] [Google Scholar]
- Collombat P, Xu X, Heimberg H, Mansouri A. Pancreatic beta-cells: From generation to regeneration. Seminars in Cell & Developmental Biology. 2010;21:838–844. doi: 10.1016/j.semcdb.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Fetus and Newborn. Postnatal Glucose Homeostasis in Late-Preterm and Term Infants. Pediatrics. 2011;127:575–579. doi: 10.1542/peds.2010-3851. [DOI] [PubMed] [Google Scholar]
- Darendeliler F, Bas F, Bundak R, Coban A, Sancakli O, Eryilmaz SK, Kucukemre B, Disci R, Gokcay G, Aki S, Ince Z, Eskiyurt N. Insulin resistance and body composition in preterm born children during prepubertal ages. Clinical Endocrinology. 2008;68:773–779. doi: 10.1111/j.1365-2265.2007.03119.x. [DOI] [PubMed] [Google Scholar]
- Dhawan S, Georgia S, Bhushan A. Formation and regeneration of the endocrine pancreas. Current Opinion in Cell Biology. 2007;19:634–645. doi: 10.1016/j.ceb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici M, Hribal M, Perego L, Ranalli M, Caradonna Z, Perego C, Usellini L, Nano R, Bonini P, Bertuzzi F, Marlier LNJL, Davalli AM, Carandente O, Pontiroli AE, Melino G, Marchetti P, Lauro R, Sesti G, Folli F. High Glucose Causes Apoptosis in Cultured Human Pancreatic Islets of Langerhans. Diabetes. 2001;50:1290–1301. doi: 10.2337/diabetes.50.6.1290. [DOI] [PubMed] [Google Scholar]
- Finegood. Dynamics of beta-Cell Mass in the Growing Rat Pancreas: Estimation With a Simple Mathematical Model. Diabetes. 1995;44:249–256. doi: 10.2337/diab.44.3.249. [DOI] [PubMed] [Google Scholar]
- Garg R, Agthe AG, Donohue PK, Lehmann CU. Hyperglycemia and retinopathy of prematurity in very low birth weight infants. Journal of Perinatology. 2003;23:186–194. doi: 10.1038/sj.jp.7210879. [DOI] [PubMed] [Google Scholar]
- Georgia, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. Journal of Clinical Investigation. 2004;114:963–968. doi: 10.1172/JCI22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardado M, Davalli AM, Chavez AO, Hubbard GB, Dick EJ. Pancreatic islet amyloidosis, betacell apoptosis, and alpha-cell proliferation are determinants of islet remodeling in type-2 diabetic baboons. Proceedings of the National Academy of Sciences of the United States of America. 2009a;106:13992–13997. doi: 10.1073/pnas.0906471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardado M, Dick EJ, Jr, Jimenez-Ceja LM, Davalli A, Chavez AO. Spontaneous pathology of the baboon endocrine system. Journal of Medical Primatology. 2009b;38:383–389. doi: 10.1111/j.1600-0684.2009.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack M. Young adult outcomes of very-low-birth-weight children. Seminars in Fetal and Neonatal Medicine. 2006;11:127–137. doi: 10.1016/j.siny.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Halban, German MS, Kahn SE, Weir GC. Current status of islet cell replacement and regeneration therapy. Journal of Clinical Endocrinology & Metabolism. 2010;95:1034–1043. doi: 10.1210/jc.2009-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall NJ, Peters M, Eaton S, Pierro A. Hyperglycemia is associated with increased morbidity and mortality rates in neonates with necrotizing enterocolitis. Journal of Pediatric Surgery. 2004;39:898–901. doi: 10.1016/j.jpedsurg.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Hofman PL, Regan F, Cutfield WS. Prematurity G – Another Example of Perinatal Metabolic Programming? Hormone Research. 2006;66:33–39. doi: 10.1159/000093230. [DOI] [PubMed] [Google Scholar]
- Hovi P, Andersson S, Eriksson JG, Järvenpää AL, Strang-Karlsson S, Mäkitie O, Kajantie E. Glucose Regulation in Young Adults with Very Low Birth Weight. New England Journal of Medicine. 2007;356:2053–2063. doi: 10.1056/NEJMoa067187. [DOI] [PubMed] [Google Scholar]
- Iams JD, Creasy RK. Preterm Labor and Delivery. In: Creasy RK, Resnik R, editors. Maternal-Fetal Medicine. edn 5th. Philadelphia, Saunders: 2004. pp. 623–628. [Google Scholar]
- Kahan BW, Jacobson LM, Hullett DA, Ochoada JM, Oberley TD, Lang KM, Odorico JS. Pancreatic Precursors and Differentiated Islet Cell Types From Murine Embryonic Stem Cells. Diabetes. 2003;52:2016–2024. doi: 10.2337/diabetes.52.8.2016. [DOI] [PubMed] [Google Scholar]
- Kamath S, Chavez AO, Gastaldelli A, Casiraghi F, Halff GA, Abrahamian GA, Davalli AM, Bastarrachea RA, Comuzzie AG, Guardado-Mendoza R, Jimenez-Ceja LM, Mattern V, Paez AM, Ricotti A, Tejero ME, Higgins PB, Rodriguez-Sanchez IP, Tripathy D, DeFronzo RA, Dick EJ, Cline GW, Folli F. Coordinated Defects in Hepatic Long Chain Fatty Acid Metabolism and Triglyceride Accumulation Contribute to Insulin Resistance in Non-Human Primates. PLoS ONE. 2011;6:e27617. doi: 10.1371/journal.pone.0027617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaca M, Magnan C, Kargar C. Functional pancreatic beta-cell mass: Involvement in type 2 diabetes and therapeutic intervention. Diabetes & Metabolism. 2009;35:77–84. doi: 10.1016/j.diabet.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Liechty EA. The Resistant Premie: Documenting the Prevalence of Hyperglycemia in the Extremely Low Birth Weight Infant. The Journal of Pediatrics. 2010;157:699–700. doi: 10.1016/j.jpeds.2010.05.028. [DOI] [PubMed] [Google Scholar]
- Limesand SW, Jensen J, Hutton JC, Hay WW. Diminished beta cell replication contributes to reduced beta cell mass in fetal sheep with intrauterine growth restriction. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2005;288:R1297–R1305. doi: 10.1152/ajpregu.00494.2004. [DOI] [PubMed] [Google Scholar]
- Lukinius A, Ericsson JLE, Grimelius L, Korsgren O. Ultrastructural studies of the ontogeny of fetal human and procine endocrine pancreas, with special reference to colocalization of the four major islet hormones. Developmental Biology. 1992;153:376–385. doi: 10.1016/0012-1606(92)90122-w. [DOI] [PubMed] [Google Scholar]
- Mandarim-de-Lacerda CA. Stereological tools in biomedical research. Anais da Academia Brasileira de Ciencias. 2003;75:469–486. doi: 10.1590/s0001-37652003000400006. [DOI] [PubMed] [Google Scholar]
- Meier JJ. Linking the Genetics of Type 2 Diabetes With Low Birth Weight. Diabetes. 2009;58:1255–1256. doi: 10.2337/db09-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JJ, Kohler CU, Alkhatib B, Sergi C, Junker T, Klein HH, Schmidt WE, Fritsch H. {beta}-cell development and turnover during prenatal life in humans. European Journal of Endocrinology. 2010;162:559–568. doi: 10.1530/EJE-09-1053. [DOI] [PubMed] [Google Scholar]
- Mena P, Llanos A, Uauy R. Insulin homeostasis in the extremely low birth weight infant. Seminars in Perinatology. 2001;25:436–446. doi: 10.1053/sper.2001.30349. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC. Pancreas and beta-cell development: from the actual to the possible. Development. 2007;134:427–438. doi: 10.1242/dev.02770. [DOI] [PubMed] [Google Scholar]
- Orci L. Macro-and-micro-domains in the endocrine pancreas. Diabetes. 1982;31:538–565. doi: 10.2337/diab.31.6.538. [DOI] [PubMed] [Google Scholar]
- Pildes RS. Neonatal hyperglycemia. [Review] [20 refs] Journal of Pediatrics. 1986;109:905–907. doi: 10.1016/s0022-3476(86)80725-9. [DOI] [PubMed] [Google Scholar]
- Pilgaard K, Frærch K, Carstensen B, Poulsen P, Pisinger C, Pedersen O, Witte D, Hansen T, Jargensen T, Vaag A. Low birthweight and premature birth are both associated with type 2 diabetes in a random sample of middle-aged Danes. Diabetologia. 2010;53:2526–2530. doi: 10.1007/s00125-010-1917-3. [DOI] [PubMed] [Google Scholar]
- Piper K, Brickwood S, Turnpenny LW, Cameron IT, Ball SG, Wilson DI, Hanley NA. Beta cell differentiation during early human pancreas development. Journal of Endocrinology. 2004;181:11–23. doi: 10.1677/joe.0.1810011. [DOI] [PubMed] [Google Scholar]
- Polak M, Bouchareb-Banaei L, Scharfmann R, Czernichow P. Early pattern of differentiation in the human pancreas. Diabetes. 2000;49:225–232. doi: 10.2337/diabetes.49.2.225. [DOI] [PubMed] [Google Scholar]
- Rotteveel J, van Weissenbruch MM, Delemarre-Van de Waal HA. Decreased insulin sensitivity in small for gestational age males treated with GH and preterm untreated males: a study in young adults. European Journal of Endocrinology. 2008;158:899–904. doi: 10.1530/EJE-08-0152. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Kobberup S, Wong R, Lopez A, Quayum N, Still T, Kutchma A, Jensen J, Gianani R, Beattie G, Jensen J, Hayek A, Hutton J. Global gene expression profiling and histochemical analysis of the developing human fetal pancreas. Diabetologia. 2008;51:285–297. doi: 10.1007/s00125-007-0880-0. [DOI] [PubMed] [Google Scholar]
- Svenstrup, Skau M, Pakkenberg B, Buschard K, Bock T. Postnatal development of beta-cells in rats. Proposed explanatory model. APMIS. 2002;110:372–378. doi: 10.1034/j.1600-0463.2002.100502.x. [DOI] [PubMed] [Google Scholar]
- Van Noorden S. Tissue preparation and immunostaining techniques for light microscopy. In: Polak JM, Van Noorden S, editors. Immunocytochemistry. Modern Methods and Applications. Bristol: 1986. pp. 22–53. [Google Scholar]
- Waterston RH, Lindblad-Toh K, Birney E. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, Barrett-Connor E, Bhargava SK, Birgisdottir BsE, Carlsson S, de Rooij SR, Dyck RF, Eriksson JG, Falkner B, Fall C, Forsön T, Grill V, Gudnason V, Hulman S, Hyppönen E, Jeffreys M, Lawlor DA, Leon DA, Minami J, Mishra G, Osmond C, Power C, Rich-Edwards JW, Roseboom TJ, Sachdev HS, Syddall H, Thorsdottir I, Vanhala M, Wadsworth M, Yarbrough DE. Birth Weight and Risk of Type 2 Diabetes. JAMA: The Journal of the American Medical Association. 2008;300:2886–2897. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- Zoungas, Patel A. Cardiovascular outcomes in type 2 diabetes: the impact of preventative therapies. Annals of the New York Academy of Sciences. 2010;1212:29–40. doi: 10.1111/j.1749-6632.2010.05837.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.