Abstract
Optimistic bias (OB) is seen when individuals underestimate their probability of experiencing negative life events and overestimate their probability of experiencing positive life events. A reduced OB has been linked with increased depression symptoms . However, given the relevance of this information to mood and anxiety disorders, little is currently known regarding the neurobiology of OB. In the current study, we examine the neural basis of OB in healthy individuals (n=33) during probability estimation of future positive and negative events occurring to themselves relative to other, comparable individuals. In line with previous work, subjects showed significant OB; they considered themselves significantly more likely to experience future positive and significantly less likely to experience future negative events relative to comparable others. Positive, relative to negative events, un-modulated by subjects’ probability estimates, were associated with significantly greater activity within ventromedial prefrontal cortex (vmPFC) and posterior cingulate cortex (PCC). Moreover, responses within both regions to positive events negatively related to the healthy subjects’ self reports of depression symptoms. However, there was no significant modulation of activity in either region by the subject’s OB, objectified as the level to which they thought the event was more likely [positive events] or less likely [negative events] to occur to them relative to comparable others. In contrast, activity within rostral anterior cingulate cortex (rACC) was positively modulated by OB for positive events and activity within anterior insula and dorsomedial prefrontal cortex (dmPFC) was negatively modulated by OB for negative events. However, there was no significant relationship between responsiveness within these regions and self reports of depression symptoms. The data are discussed with reference to current models of vmPFC, rACC and anterior insula functioning.
Keywords: fMRI, ventromedial prefrontal prefrontal cortex, rostral anterior cingulate cortex, optimistic bias
1. INTRODUCTION
Optimistic bias (OB) is the tendency to believe that negative events are less likely, and positive events more likely, to happen to oneself than to others (Weinstein, 1980). It can lead to serious underestimations of health and economic risks. Biases can be resistant to change and contribute to an unwillingness to take preventative action. Significantly, reduced OB is seen in patients with depression where the extent of reduction is related to symptom severity (Strunk et al., 2006). Thus, an understanding of the neuro-cognitive systems mediating OB is important.
Very little work has considered the neural systems mediating OB/optimism (Sharot et al., 2012; Sharot et al., 2011; Sharot et al., 2007). However, two potentially separable functional systems have been implicated. Sharot et al. (2007) asked subjects to think about events that had occurred in the past or might occur in the future (e.g., ‘winning an award’ or ‘the end of a romantic relationship’) and the Blood Oxygen Level Dependent (BOLD) response to positive and negative events was contrasted. Within both the amygdala and ventromedial prefrontal cortex (vmPFC including subcallosal anterior cingulate cortex; sACC), BOLD responses were reduced when subjects imagined future negative relative to future positive events. Moreover, the degree of this difference in BOLD response within sACC correlated positively to a self-report measure of trait optimism. Sharot et al. (2011) examined BOLD responses to information updating the individual’s probability estimates of potential future negative events occurring to the self. They reported that estimation errors calling for a positive update (i.e., the undesirable event was less likely to occur than the subject’s estimate) were tracked within a rather more rostral and bilateral region of medial prefrontal cortex (as well as left inferior frontal cortex and right cerebellum). In contrast, a region of right inferior frontal cortex extending into the insula tracked estimation errors calling for a negative update (i.e., the undesirable event was more likely to occur than the subject’s estimate). In Sharot et al. (2011), more optimistic individuals showed reduced negative (but not positive) update tracking relative to less optimistic individuals. Finally, Sharot et al. (2012) reported that transcranial magnetic stimulation (TMS) of left, but not right, inferior frontal cortex (IFG) increased updating of the probability of negative events following information that the event was more likely than expected. TMS of either nor right IFG had a significant impact on updating subject estimations on the probability of negative events following information that the event was less likely than expected.
In the current study, we examined subjects’ BOLD responses when they estimated the probability that a positive/negative event would occur to them in the future relative to a similar other individual. Moreover, we examined BOLD responses to positive and negative events with and without parametric modulation by the subjects’ estimate of the likelihood of that action occurring to them in the future (i.e., their level of OB). Our aim was to address three issues:
First, what are the systems mediating the OB? We predicted that those regions related to optimism for future events (i.e., vmPFC; Sharot, et al., 2007) and/or tracking estimation errors calling for positive or negative updates (i.e., rostral medical frontal cortex (rMFC) and inferior frontal gyrus (IFG)/insula; Sharot, et al., 2011) would be modulated by the subjects’ OB.
Second, is there dissociation in the regions mediating OB for positive relative to negative future events? Notably, Sharot et al. (2011) reported that estimation errors calling for a positive update were tracked within bilateral rMPFC (as well as left inferior frontal cortex and right cerebellum) while a region of right IFG/insula tracked estimation errors calling for a negative update. Moreover, disruption of left insula functioning by TMS reduced the negative updating of negative event probabilities following new information (Sharot, et al., 2012). Interestingly, the insula has been implicated in the anticipation of aversive reinforcement expectancies during decision making tasks (Kuhnen and Knutson, 2005; Liu et al., 2007; Preuschoff et al., 2008; Wu et al., 2011). Given this and the findings of Sharot et al. (2011; 2012), we predicted that IFG/insula might be relatively more involved in a subject’s OB for future negative events.
Third, we also examined the relationship between OB-related BOLD responses and self-reported level of depressive symptomatology. As noted above, reduced OB is seen in patients with depression where the extent of reduction is related to symptom severity (Strunk, et al., 2006). As optimism can, in some respects, be considered the inverse of depression, we predicted, following Sharot et al. (2007) that self-reported depression symptomatology might be related to value representations within vmPFC. The current study tests these predictions.
2. MATERIAL AND METHODS
2.1. Subjects
Thirty-three right-handed subjects (eighteen males, fifteen females; aged 22–48, mean age = 29.15) volunteered for the study and were paid for their participation. Subjects were in good physical health as confirmed by a complete physical exam, with no history of any psychiatric illness as assessed by the DSM-IV (1994) criteria based on the Structural Clinical interview for DSM-IV Axis I disorders (SCID) (First et al., 1997). All subjects gave written informed assent/ consent to participate in the study, which was approved by the National Institute of Mental Health Institutional Review Board.
2.2. Optimistic Bias (OB) Task
The stimuli consisted of 40 high negative (e.g., having a heart attack; being sentenced to jail), 40 low negative (e.g., getting seasick; losing your spot in a long line), 40 high positive (e.g., finding the cure for AIDS; living past 80) and 40 low positive (e.g., getting a hug; having a perfect hair day) possible future events. These 160 stimuli were selected from a larger set of stimuli given to a different group of 35 healthy adults who were asked to rate potential future events according to their pleasantness/ unpleasantness. The high pleasant and unpleasant items and low pleasant and unpleasant items were matched according to their level of differentiation from neutral (though in opposite directions). In addition, the four different future event types were matched on number of letters and words.
Prior to scanning, subjects were told that they would read different possible future events. For each event, they were told to rate the likely probability of the event happening to them across their lifetime, compared to other people of their same gender and age. The subjects rated their likelihood according to a 4 point scale where 1=much below average; 2=below average; 3=above average; or 4=much above average, using the second and third digit of both hands. Each event was presented for 5500 ms following a 500 ms fixation point. In addition, for each experimental run, 48 3000 ms fixation points were presented between the stimuli (4 at beginning of run, 4 at end of run and 40 randomized throughout the run), serving as an implicit baseline. The fMRI scan acquisition followed an event-related design, and consisted of four runs.
Following EPI acquisition, subjects rated each of the 160 events on a 5-point Likert scale according to how much experience they had with each event, where 1=don’t know anybody this has happened to and 5=it has happened to me. The paradigm was programmed in E-Studio. Stimuli were presented on a computer display that was projected onto a mirror in the MRI scanner. Subjects were placed in a light head restraint within the scanner to limit movement during acquisition.
2.3. Inventory of Depressive Symptomatology (IDS)
Because we were interested in the relationship between OB and depressive symptoms, subjects also completed the Inventory of Depressive Symptomatology (IDS) (Rush et al., 1986). This scale assesses the level of depressive symptomatology by means of 30 items that each describes a symptom of depression. Two sets of symptoms are mutually exclusive (weight loss/gain and appetite loss/gain), resulting in 28 scored items. Each item is scored according to best fit (0 through 3).
2.4. MRI parameters
Whole-brain blood oxygen level dependent (BOLD) fMRI data were acquired using a 1.5 Tesla GE MRI scanner. Following sagital localization, functional T2* weighted images were acquired using an echo-planar single-shot gradient echo pulse sequence (matrix = 64 × 64 mm, repetition time (TR) = 3000 ms, echo time (TE) = 30 ms, field-of-view (FOV) = 240 mm (3.75 × 3.75 × 4 mm voxels). Images were acquired in 31 contiguous 4 mm axial slices per brain volume, with each run lasting 6 minutes 24 seconds. In the same session, a high-resolution T1-weighed anatomical image was acquired to aid with spatial normalization (three-dimensional Spoiled GRASS; TR = 8.1 ms; TE = 3.2 ms, flip angle = 20°; FOV = 240 mm, 124 axial slices, thickness = 1.0 mm; 256 × 256 acquisition matrix).
2.5. Imaging data preprocessing
Data were analyzed within the framework of the general linear model using Analysis of Functional Neuroimages (AFNI) (Cox, 1996). Both individual and group-level analyses were conducted. The first four volumes in each scan series, collected before equilibrium magnetization was reached, were discarded. Motion correction was performed by registering all volumes in the EPI dataset to a volume collected close to acquisition of the high resolution anatomical dataset.
The EPI datasets for each subject were spatially smoothed (isotropic 6 mm kernel) to reduce variability among individuals and generate group maps. Next, the time series data were normalized by dividing the signal intensity of a voxel at each time point by the mean signal intensity of that voxel for each run and multiplying the result by 100, producing regression coefficients representing percent-signal change.
Following this, the following regressors were generated: indicator functions for high intensity positive events, low intensity future positive events, high intensity future negative events and low intensity future negative events; indicator functions for high intensity positive events, low intensity future positive events, high intensity future negative events and low intensity future negative events multiplied by the subject’s judgment of event likelihood. These regressors were created by convolving the train of stimulus events with a gamma-variate hemodynamic response function to account for the slow hemodynamic response. In other terms, the first set regressors modeled the average response to each of the four categories (unmodulated), and the second set modeled the deviation from the average response explained by the subject’s judgment of event likelihood (modulated). Linear regression modeling was performed using the 8 regressors described above plus 6 head motion regressors. This produced a modulated and unmodulated β coefficient and associated t statistic for each voxel and regressor. There was no significant collinearity between the regressors as detected by AFNI. Voxel-wise group analyses involved transforming single subject beta coefficients into standard coordinate space (Talairach and Tournoux, 1988).
2.6: fMRI data analysis
Three analyses were then performed on regression coefficients from individual subject analyses. The first analysis on the BOLD responses to the un-modulated regressors involved a 2 (Emotion: Negative, Positive) by 2 (Intensity: High, Low) ANOVA. The aim of this ANOVA was to determine regions responsive to two aspects of stimuli: 1) stimulus class, defined as regions that differentially respond to future positive, relative to future negative events, irrespective of the subject’s ratings of probability of these events, and 2) stimulus intensity.
The second and third analyses involved used the modulation regressors for positive and negative events, respectively. Thus, the second analysis involved the averaged response to positive future events of either high or low intensity, modulated by the subject’s estimate of each individual event’s likelihood, relative to baseline. Conversely, the third analysis involved the averaged response to negative future events of either high or low intensity, modulated by the subject’s estimate of event likelihood relative to baseline.
Statistical maps were created for each analysis by thresholding at a single-voxel p value of p<0.005. To correct for multiple comparisons, we performed a spatial clustering operation using AlphaSim (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf) with 1,000 Monte Carlo simulations taking into account the EPI matrix covering the entire brain. This procedure yielded a minimum cluster size with a map-wise false-positive probability of p<0.05, corrected for multiple comparisons.
After observing hypothesized group differences, post-hoc analyses were performed to determine the source of significant interactions. For these analyses, average percent signal change was taken from all voxels within each ROI generated from the functional mask, and t-tests carried out within SPSS to pinpoint the nature of interaction effects.
3. RESULTS
3.1. Behavioral Results
Presence of OB
Subjects displayed OB for both positive and negative events. They considered themselves significantly more likely than same gender individuals their own age to experience both high and low intensity positive events (t[32]=7.28 & 6.60 respectively, p<0.001 in both cases). In addition, they considered themselves significantly less likely than same gender individuals of same age to experience both high and low intensity negative events (t[32]=11.67 & 3.40; p<0.001 & 0.005 respectively).
With respect to the subjects’ past experience with the events, a 2 (Emotion: Negative, Positive) by 2 (Intensity: High, Low) ANOVA revealed highly significant main effects of emotion and intensity as well as a significant emotion-by-intensity interaction (F(1,32)=409.63, 608.70, 319.80 respectively; all p< 0.001); see Table 1. Subjects reported more experience with positive events relative to negative events (M[Pos]=4.13, s.e.=0.05; M[Neg]= 3.06; s.e.=0.06) and less experience with high relative to low intensity events (M[high]=2.96, s.e.=0.05; M[Low]=4.23, s.e.=0.06). With respect to the interaction, subjects reported significantly more experience with highly positive events relative to mildly negative events (t(33)=2.44; p<0.05), more experience with mildly negative events relative to mildly positive events (t(33)=6.18; p<0.001) and more experience with mildly positive events relative to highly negative events (t(33)=33.04; p<0.001).
Table 1.
Ratings, RTs and experience for the four event categories. S.D. in Brackets ().
| Event | Ratings† | RTs | Experience |
|---|---|---|---|
| High negative | 1.7 (0.39) | 2487.2 (348.39) | 2.1 (0.33) |
| Low negative | 2.3 (0.34) | 2544.7 (340.47) | 4.0 (0.42) |
| Low positive | 2.8 (0.29) | 2630.5 (386.72) | 4.4 (0.30) |
| High positive | 2.8 (0.24) | 2656.6 (391.22) | 3.9 (0.40) |
OB Behavioral Ratings and Self Reports of Depression Symptomatology
Using correlational analysis we examined whether the OB ratings of our healthy subjects related to their self-reported levels of depression symptomatology as assessed by the IDS. In line with predictions, we found that the level of OB for negative items correlated significantly and negatively with level of depressive symptomatology (Pearson’s r(33) =−.35; p<0.05). There was however no significant correlation between OB ratings for positive items and IDS scores.
3.2. fMRI Results
3.2.1. Non-parametric Data (the Response to Stimulus Class)
An initial whole-brain 2 (Emotion: Negative, Positive) by 2 (Intensity: High, Low) ANOVAs was conducted on the subjects’ BOLD responses to the events (un-modulated by their probability estimates). This initial analysis revealed regions showing main effects for both emotion and intensity, as well as an emotion-by-intensity interaction.
Main Effect of Emotion
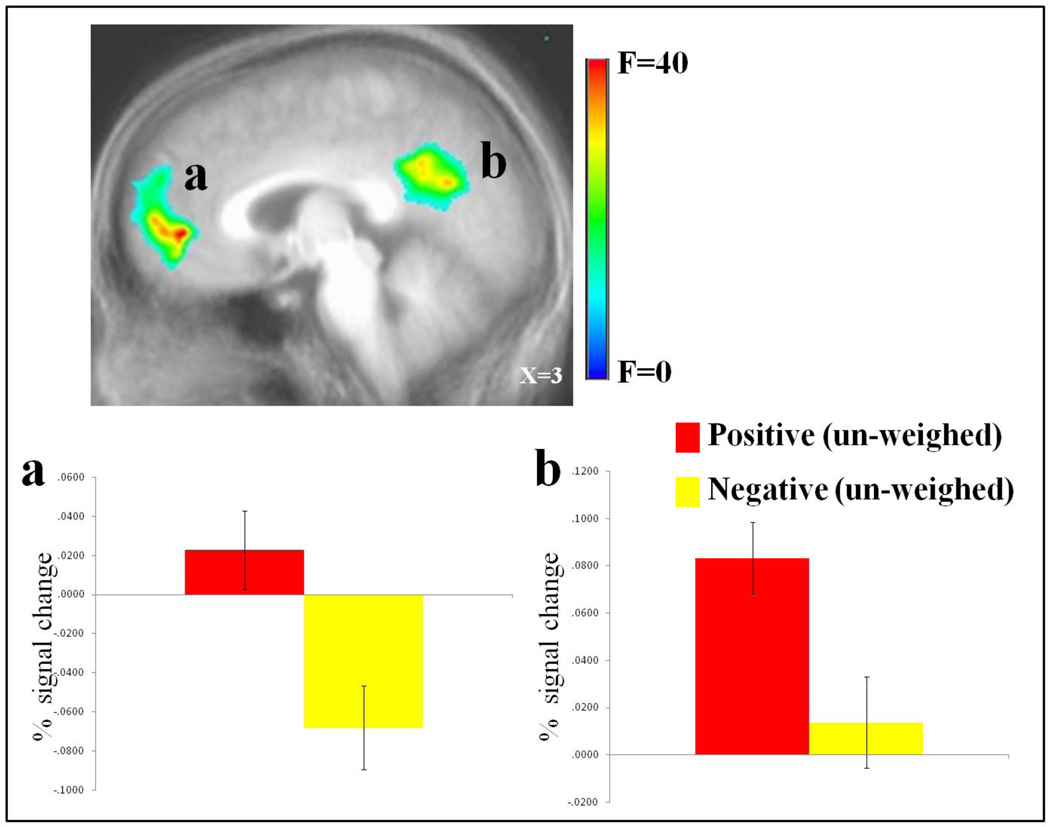
Regions showing a main effect for emotion included vmPFC, posterior cingulate cortex (PCC) and left dorsolateral frontal and inferior parietal cortices (BA 46 & 40). vmPFC and PCC showed greater activity for positive relative to negative events; see Figure 1. In contrast, left lateral frontal and parietal cortices showed greater activity for negative relative to positive events.
Figure 1. Main effect of emotion un-weighed data.
Increased BOLD responses to positive relative to negative events in (a) right vmPFC (8, 48, −3) , and (b) left PCC (−5, −54, 30).
Main Effect of Intensity
Regions showing a main effect for intensity included inferior frontal cortex (BA 47) and right superior parietal cortex (BA 7). In all cases, the BOLD responses were significantly stronger for high intensity, relative to low intensity, positive and negative events.
Emotion-by-intensity Interaction
A large region of postcentral gyrus (BA 40) showed a significant emotion-by-intensity interaction. Within this region, high and low intensity positive events elicited significantly greater activity than high and low intensity negative events (t(32) = 9.81 to 3.72; p<0.001). However, while the response to high and low intensity positive events did not differ (t(32)=1.46; n.s.), the response to low intensity negative events was significantly greater than that to high intensity negative events (t(32) = 7.39; p<0.001).
3.2.2. Parametric Data (BOLD Responses Modulated by OB)
Following this initial analysis, a second set of analyses identified brain regions where activity varied as a function of subjects’ event-probability estimates for specific events. In other words, these were regions specifically supporting individual variability in OB. For this purpose, in two sets of analyses, we considered the parametric regressors for the positive and negative events, respectively, each considered against baseline using t tests. As our interest was in identifying regions implicated in individual variability for OB for positive and negative items rather than in generating differential variability for positive and negative items of differing intensity levels (unlike our goal for the non-parametric analysis data above), we did not use an ANOVA analysis design for the parametric data.
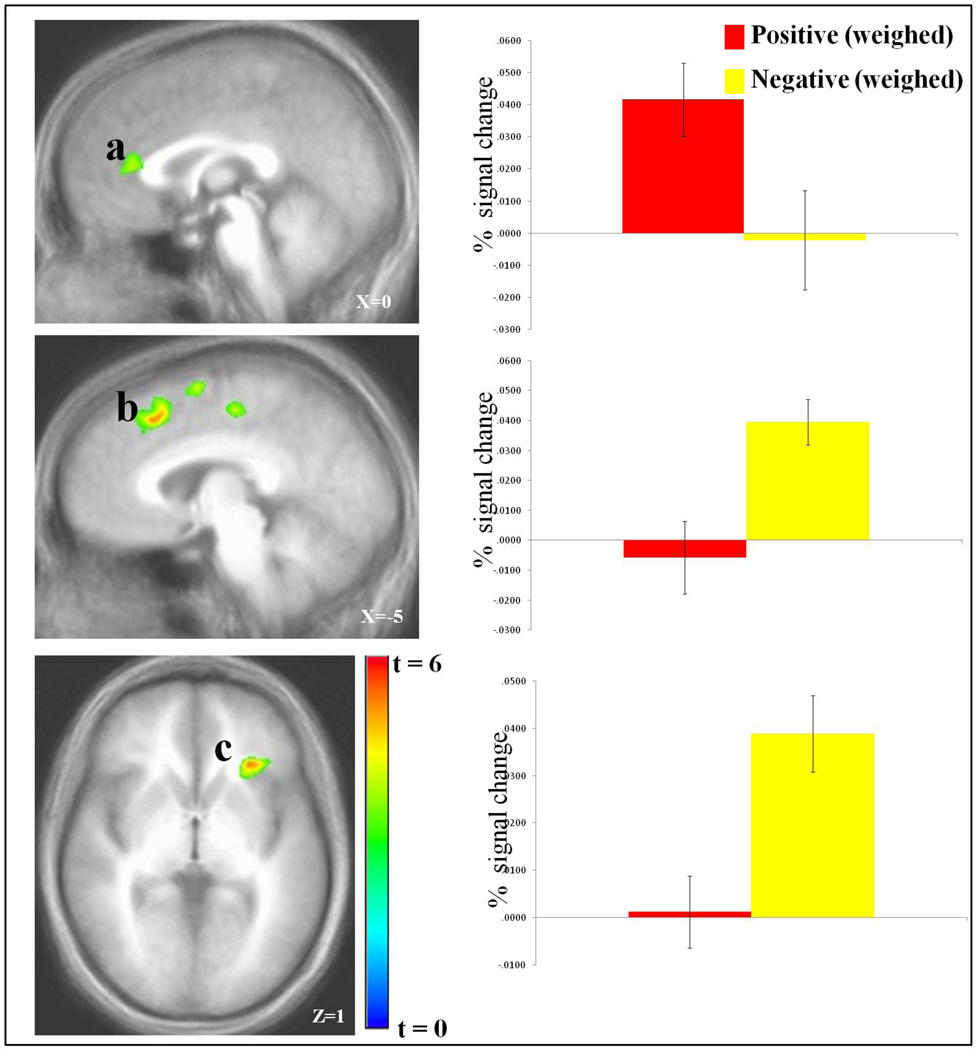
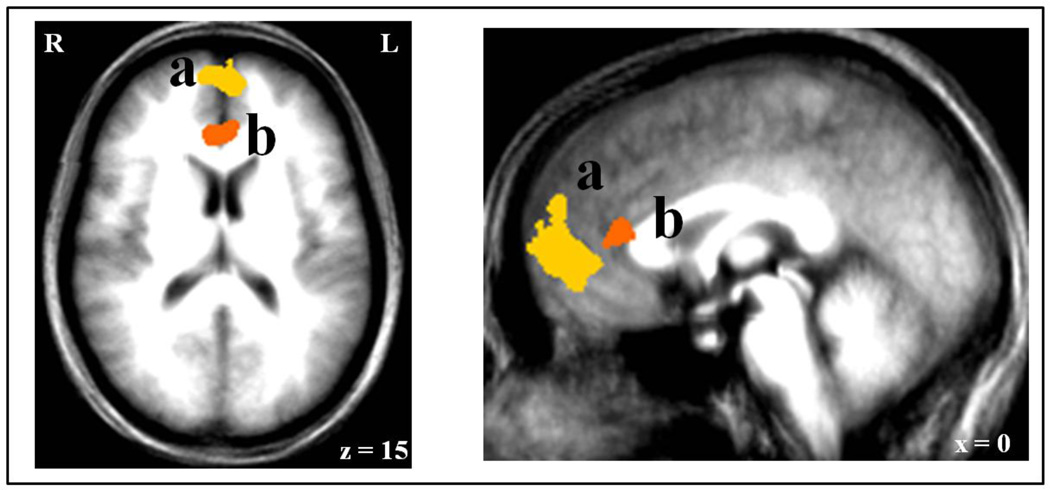
As can be seen in Figure 2, activity in an extensive region of rACC was significantly positively correlated with the likelihood estimates for positive events. Notably, a conjunction analysis revealed that this region of rACC showed no overlap with the extensive region of vmPFC that showed significantly greater activity to positive relative to negative events; see Figure 3. Indeed, there was no differential activity within this region to positive relative to negative events un-modulated by the subjects’ subjective probabilities (t[32]=1.27; p=0.215). Moreover, there was no significant modulation within the vmPFC region identified in the first analysis by the subject’s probability estimation for positive events happening to themselves relative to others (t[32]=0.82; p=0.418).
Figure 2. Weighed BOLD response to the events.
Increased BOLD responses as a function of the subject’s increased probability estimates to positive events in (a) right rACC (5, 32, 12), and to negative events in (b) left dmPFC (−6, 22, 43) , and (c) left insula (−27, 26, 3).
Figure 3. Regions of vmPFC identified by the analyses.
For comparison, the vmPFC showing an increased BOLD response to (a) positive relative to negative events (8, 48, −3), and the rACC showing an increased BOLD response to (b) positive events as a function of the subject’s increased probability estimates (5, 32, 12).
As can also be seen in Figure 2, activity within left insula and dmPFC negatively correlated with the likelihood estimates of negative events happening. However, as with the rACC-positive event association, neither of the two regions identified for negative-event-specific OB showed differential activity for negative relative to positive events unmodulated by the subjects’ subjective probabilities (t[32]<1, p=0.453 & 0.848 respectively).
3.2.4. EPI Correlational Analysis
Using correlational analysis we examined whether the BOLD response within our core regions of interest related to level of depression as assessed by the IDS. The first analysis considered the non-parametric ANOVA, where average response was examined, independent of subjects’ event-specific ratings. Here, the correlational analyses examined associations with the vmPFC and PCC regions identified by the main effect of emotion. This revealed significant negative correlations between BOLD responses within both vmPFC and PCC to positive events and within vmPFC to negative events and depression symptomatology (Pearson’s r(33) =−.414, −.442 & −.367 respectively; all p<0.05). The second set of analyses considered the OB-modulated ANOVA where the data were modulated by subjects’ estimates. Here, the correlational analyses examined associations with the rACC identified for positive events as well as the insula and dACC regions identified for negative events. This second set of correlations revealed no relationships between BOLD responses within either rACC, insula or dmPFC and depression scores (Pearson’s r(33) range =−.014 to .068 p range = .939 to .707).
4. DISCUSSION
The current study investigated the neural systems mediating OB. There were four main findings: First, positive events were associated with significantly greater activity within vmPFC and PCC than negative events. Second, subjects’ OBs for positive events (i.e., increased probability estimates of positive events occurring to themselves relative to others) were associated with increased activity within rACC. Third, subjects’ OBs for negative events (i.e., decreased probability estimates of negative events occurring to themselves relative to others) were associated with decreased activity within left insula and dmPFC. Fourth, depression symptomatology, as measured by the IDS, was inversely related to responsiveness within vmPFC and PCC to the presentation of potential future positive events. However, level of depression symptoms did not relate to activity in OB-specific regions identified by the parametric modulation.
This study considered three issues: First, would systems related to optimism for future events (i.e., vmPFC; Sharot, et al., 2007) and/or tracking estimation errors calling for positive or negative updates (i.e., rMFC and IFG/insula; Sharot, et al., 2011) be modulated by the subjects’ OB? With respect to vmPFC, similar to Sharot et al. (2007), we observed increased activity within vmPFC in response to positive relative to negative potential future events. We also observed such activity in the PCC. Given the considerable previous work indicating that both vmPFC and PCC are involved in the representation of the subjective value of a stimulus (Ballard and Knutson, 2009; Blair et al., 2006; Kable and Glimcher, 2007; Levy and Glimcher, 2011; McClure et al., 2007), greater activity within these two regions elicited by positive relative to negative potential future events may reflect relative subjective value ratings for these two event classes. Notably though, activity within vmPFC (or PCC) was not modulated by the subjects’ OB. Subjects did not show greater activity within either region as they considered a positive event more likely to occur to the self than another or a negative event less likely to occur to the self than another. Thus, biased subjective value representations within vmPFC (and PCC; i.e., increased responsiveness to positive items) may contribute to optimism (and protect from depression; see also below). However, these representations may be less involved in generating an individual’s OB.
With respect to rMFC, there was a region of rACC that was parametrically modulated by the individual’s OB for positive items. This region showed no overlap with the vmPFC region showing significantly greater responding to positive relative to negative events. As such these data suggest functional specificity between these relatively proximal regions. As mentioned above, considerable work from the decision making literature has implicated vmPFC in the representation of the subjective value of a stimulus, consistent with the current results. Rather less work has attributed such a role to the rACC - though a recent study reported modulation within this region by the subjective value of peers providing self-referential social reinforcement to the subject (Jones et al., 2011). Indeed, this region of rACC has more typically been implicated in self-referential processing, potentially particularly emotional aspects of self-referential processing (Ganesh et al., 2011; Gusnard et al., 2001; Northoff et al., 2006; Pizzagalli, 2011). It is possible that rACC’s role in self-referential processing is contributing to its modulation by the subject’s judgment that a positive event is more likely to occur to the self rather than to another individual. Alternatively, it is worth remembering that Sharot et al. (2011) reported that rMPFC tracked estimation errors calling for a positive update (the situation was better than expected). It can be speculated that is some degree of computational overlap with the process here, where a region of rMPFC tracked the level to which the subject thought positive events were more likely to occur to them relative to comparable others. It is certainly interesting to note that in both studies rMPFC was tracking a difference between a good occurrence for the self and an alternative condition. In the current study, it was the difference between the likelihood of the positive event occurring to the self relative to another. In Sharot et al. (2011), it was the difference between the “real” likelihood of an event occurring and what the subject thought the likelihood was of the event occurring when this called for a positive update (i.e., the more the situation was better than expected).
With respect to IFG/ insula, there was a region of left insula that was parametrically modulated by the individual’s OB for negative items. This finding, together with the rACC finding, relates directly to our second question regarding whether regions mediating OB for future positive events are dissociable from those mediating OB for future negative events. We found that rACC was modulated by subject’s OB for future positive events while left insula (and dorsomedial frontal cortex; dmFC) was modulated by subject’s OB for future negative events. In short, subjects’ OB for negative events did not modulate rACC activity but it did modulate insula (and dmFC) activity; as subjects considered a negative future event to be less likely to happen to the self relative to others, there was a reduction in activity in these regions. Interestingly, these latter two regions are consistently implicated in the anticipatory response to impending negative events (Knutson and Greer, 2008; Knutson et al., 2007), and choosing actions to avoid negative consequences engaging these regions, particularly anterior insula (Kuhnen and Knutson, 2005; Liu, et al., 2007). The current data extend this prior work by showing that as subjects consider future negative events more likely to occur to them, regions representing the negative expected value of the potential negative event increase in activity. The OB for negative events (considering negative events less likely to occur to the self than others) may reflect the proposed function of this signal to influence the individual away from undesirable options (Knutson and Greer, 2008).
The third issue we wished to address in this study was the relationship between OB related BOLD responses and self-reported level of depressive symptomatology. Sharot et al. (2007) reported that the degree of the difference in BOLD response within sACC to positive relative to negative future events correlated positively to a self-report measure of trait optimism. Sharot et al. (2011) reported that more optimistic individuals showed reduced negative (but not positive) update tracking within right IFG/insula relative to less optimistic individuals. While we did not specifically use a self-report of optimism in the current study, we did find that self-reported depression symptomatology was inversely associated with responsiveness within vmPFC (and PCC) to the presentation of potential future positive events. These data suggest that biased subjective value representations within vmPFC and PCC (i.e., increased responsiveness to positive items) may contribute to optimism/ protect from depression symptomatology. However, with respect to this last point it is important to note a caveat. The mechanisms mediating mild depression symptoms in healthy individuals can be different from mediating clinical MDD. As such, any implications of these data for clinical MDD should be evaluated with considerable caution.
In conclusion, we found evidence for different roles of vmPFC and rACC. VmPFC was involved in the representation of subjective value of future events and this related inversely to level of depression symptoms (and probably, though untested here, positively to optimism; cf. Sharot et al., 2008). rACC was involved in computations underpinning the OB for positive future events. Potentially, rACC’s role in self-referential processing (e.g., Northoff, et al., 2006; Pizzagalli, 2011) may relate to its role in OB for positive events occurring to the self. Finally, left insula and dmFC showed sensitivity to the OB for negative future events.
Table 2.
Significant areas of activation from the un-weighed analysis for the emotion and intensity main effect and emotion-by-intensity interaction †
| REGION | BA | Mm3 | X | Y | Z | F-value | ††P< |
|---|---|---|---|---|---|---|---|
| Emotion main effect | |||||||
| Positive > negative | |||||||
| R vmPFC | 10 | 7388 | 8 | 48 | −3 | 51.19 | 5.0E-8 |
| L PCC | 31 | 8558 | −5 | −54 | 30 | 63.17 | 5.0E-9 |
| L superior frontal gyrus | 8 | 4492 | −16 | 24 | 47 | 47.19 | 1.0E-7 |
| L postcentral gyrus | 3 | 28811 | −36 | −29 | 47 | 63.01 | 5.0E-9 |
| L middle temporal gyrus | 22 | 12543 | −53 | −39 | 1 | 61.41 | 1.0E-8 |
| L middle temporal gyrus | 22 | 4826 | −53 | −8 | −8 | 57.96 | 5.0E-8 |
| R middle temporal gyrus | 21 | 2696 | 54 | −5 | −13 | 80.17 | 5.0E-10 |
| R middle occipital gyrus | 18/19 | 18828 | 30 | −80 | 8 | 73.99 | 1.0E-9 |
| L middle occipital gyrus | 17 | 5622 | −25 | −79 | 13 | 56.09 | 5.0E8 |
| Negative > positive | |||||||
| R superior frontal gyrus | 8 | 1861 | 33 | −20 | −12 | 26.02 | 5.0E5 |
| L middle frontal gyrus | 46 | 2279 | −43 | 31 | 24 | 36.81 | 1.0E-6 |
| R precentral gyrus | 4 | 16378 | 35 | −25 | 61 | 72.33 | 1.0E −9 |
| L supramarginal gyrus | 40 | 2186 | −54 | −40 | 34 | 36.02 | 5.0E-6 |
| L culmen | 2344 | −15 | −54 | −15 | 52.49 | 5.0E-8 | |
|
Intensity main effect (High > Low intensity items) |
|||||||
| L inferior frontal gyurs* | 47 | 511 | −46 | 22 | 3 | 23.87 | 5.0E-5 |
| R superior parietal gyrus | 7 | 850 | 35 | −72 | 49 | 33.27 | 5.0E-6 |
| R precuneus | 7 | 2216 | 16 | −64 | 40 | 28.44 | 1.0E-5 |
| R cuneus | 788 | 28 | −74 | 27 | 18.78 | 5.0E-4 | |
| R precentral gyrus | 4 | 4724 | 35 | −12 | 53 | 36.41 | 1.0E-6 |
| L superior temporal gyrus | 2166 | −43 | −50 | 21 | 24.64 | 5.0E-5 | |
|
Emotion-by-Intensity Interaction |
|||||||
| L postcentral gyrus | 40 | 8265 | −38 | −34 | 57 | 78.74 | 5.0E-10 |
All activations are effects observed in whole brain analyses significant at p < 0.005 corrected for multiple comparisons (significant at p < 0.01),
except for * significant at p < 0.005 uncorrected.
Uncorrected values
Table 3.
Significant areas of activation for the analysis weighed according to subject probability estimates for positive and negative events †
| REGION | BA | Mm3 | X | Y | Z | t- value |
P<†† |
|---|---|---|---|---|---|---|---|
| Positive events: | |||||||
|
Positive association with increased estimate of event |
|||||||
| R rACC | 24 | 1171 | 5 | 32 | 12 | 4.10 | 5.0E-4 |
| L postcentral gyrus | 3 | 21386 | −37 | −31 | 55 | 10.76 | 5.0E-11 |
| R culmen | 4570 | 22 | −52 | −17 | 7.08 | 5.0E-7 | |
|
Negative association with increased estimate of event |
|||||||
| R medial frontal gyrus | 6 | 2103 | 6 | −19 | 48 | 5.42 | 1.0E-5 |
| R postcentral gyrus | 3 | 33617 | 41 | −26 | 51 | 14.78 | 5.0E-14 |
| L culmen | 3870 | −16 | −52 | −14 | 6.96 | 5.0E-7 | |
| R posterior cingualate | 13 | 5277 | 40 | −24 | 20 | 6.16 | 5.0E-6 |
| Negative events: | |||||||
|
Positive association with increased estimate of event |
|||||||
| L dmPFC | 32 | 2789 | −6 | 22 | 43 | 5.25 | 5.0E-5 |
| L insula | 1143 | −27 | 26 | 3 | 5.15 | 5.0E −5 | |
| L precentral gyrus | 6/9 | 1119 | −40 | 4 | 35 | 4.11 | 5.0E-4 |
| R precentral gyrus | 3 | 12195 | −31 | −25 | 56 | 6.88 | 5.0E-7 |
|
Negative association with increased estimate of event |
|||||||
| L precentral gyrus | 3 | 21759 | 36 | −26 | 49 | 8.54 | 5.0E-9 |
| L culmen | 2207 | −20 | −51 | −18 | 5.89 | 5.0E-6 |
All activations are effects observed in whole brain analyses significant at p < 0.005 corrected for multiple comparisons (significant at p < 0.01).
Uncorrected values
Highlights.
fMRI is used to examined the neural mechanism of Optimistic Bias (OB)
OB for positive items was associated with increased rACC activation
However, positive items per se was associated with increased vmPFC activation
Depression scores correlated with vmPFC, but not rACC activation
This suggests dissociable roles for vmPFC and rACC in OB and value representation and depression
ACKNOWLEDGEMENTS
This research was supported by the Intramural Research Program of the NIH: NIMH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest or financial disclosures to report.
REFERENCE LIST
- Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage. 2009;45:143–150. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Marsh AA, Morton J, Vythilingham M, Jones M, Mondillo K, Pine DS, Drevets WC, Blair RJR. Choosing the lesser of two evils, the better of two goods: Specifying the roles of ventromedial prefrontal cortex and dorsal anterior cingulate cortex in object choice. Journal of Neuroscience. 2006;26(44):11379–11386. doi: 10.1523/JNEUROSCI.1640-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Ganesh S, van Schie HT, de Lange FP, Thompson E, Wigboldus DH. How the Human Brain Goes Virtual: Distinct Cortical Regions of the Person-Processing Network Are Involved in Self-Identification with Virtual Agents. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr227. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Somerville LH, Li J, Ruberry EJ, Libby V, Glover G, Voss HU, Ballon DJ, Casey BJ. Behavioral and neural properties of social reinforcement learning. J Neurosci. 2011;31(37):13039–13045. doi: 10.1523/JNEUROSCI.2972-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Greer SM. Anticipatory affect: neural correlates and consequences for choice. Philos Trans R Soc Lond B Biol Sci. 2008;353:3771–3786. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G. Neural predictors of purchases. Neuron. 2007;53:147–157. doi: 10.1016/j.neuron.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk-taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW. Comparing apples and oranges: using reward-specific and reward-general subjective value representation in the brain. J Neurosci. 2011;31(41):14693–14707. doi: 10.1523/JNEUROSCI.2218-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. J Neurosci. 2007;27(17):4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. J Neurosci. 2007;27(21):5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36(1):183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28(11):2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C. The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Research. 1986;18(1):65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- Sharot T, Kanai R, Marston D, Korn CW, Rees G, Dolan RJ. Selectively altering belief formation in the human brain. Proc Natl Acad Sci U S A. 2012;109:17058–17062. doi: 10.1073/pnas.1205828109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T, Korn CW, Dolan RJ. How unrealistic optimism is maintained in the face of reality. Nature Neuroscience. 2011;14(11):1475–1479. doi: 10.1038/nn.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T, Riccardi AM, Raio CM, Phelps EA. Neural mechanisms mediating optimism bias. Nature. 2007;450:102–106. doi: 10.1038/nature06280. [DOI] [PubMed] [Google Scholar]
- Strunk DR, Lopez H, DeRubeis RJ. Depressive symptoms are associated with unrealistic negative predictions of future life events. Behavior Research and Therapy. 2006;44:861–882. doi: 10.1016/j.brat.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart: Thieme. 1988 [Google Scholar]
- Weinstein ND. Unrealistic optimism about future life events. Journal of Personality and Social Psychology. 1980;39:806–820. [Google Scholar]
- Wu CC, Bossaerts P, Knutson B. The affective impact of financial skewness on neural activity and choice. PLoS One. 2011;6(2):e16838. doi: 10.1371/journal.pone.0016838. [DOI] [PMC free article] [PubMed] [Google Scholar]