Abstract
Background
Previous studies indicate that negative symptoms reflect a separable domain of pathology from other symptoms of schizophrenia. However, it is currently unclear whether negative symptoms themselves are multi-faceted, and whether sub-groups of patients who display unique negative symptom profiles can be identified.
Methods
A data-driven approach was used to examine the heterogeneity of negative symptom presentations in two samples: Study 1 included 199 individuals with schizophrenia assessed with a standard measure of negative symptoms and Study 2 included 169 individuals meeting criteria for deficit schizophrenia (i.e., primary and enduring negative symptoms) assessed with a specialized measure of deficit symptoms. Cluster analysis was used to determine whether different groups of patients with distinct negative symptoms profiles could be identified.
Results
Across both studies, we found evidence for two distinctive negative symptom sub-groups: one group with predominantly Avolition-Apathy (AA) symptoms and another with a predominantly Diminished Expression (DE) profile. Follow-up discriminant function analyses confirmed the validity of these groups. AA and DE negative symptom sub-groups significantly differed on clinically relevant external validators, including measures of functional outcome, premorbid adjustment, clinical course, disorganized symptoms, social cognition, sex, and ethnicity.
Conclusions
These results suggest that distinct subgroups of patients with elevated AA or DE can be identified within the broader diagnosis of schizophrenia and that these subgroups show clinically meaningful differences in presentation. Additionally, AA tends to be associated with poorer outcomes than DE, suggesting that it may be a more severe aspect of psychopathology.
Keywords: Schizophrenia, Negative Symptoms, Avolition, Apathy, Emotion, Blunted Affect
Introduction
Studies examining the factor structure of psychiatric symptoms in schizophrenia have consistently indicated that negative symptoms represent a domain of psychopathology that is distinct from other aspects of the illness (e.g., psychosis and disorganization) (Keefe et al., 1992; Mueser et al., 1994; Peralta & Cuesta, 1995; Sayers et al., 1996; Kelley et al., 1999). However, negative symptoms may not be a unitary construct, as studies examining the factor structure of items within negative symptom scales typically find evidence for two distinct factors: one reflecting diminished expression (DE) consisting of alogia and blunted affect, and the other representing volitional pathology, including asociality, avolition, and anhedonia (“avolition-apathy”, AA) (Keefe et al., 1992; Horan et al., 2011; Mueser et al., 1994; Peralta & Cuesta, 1995; Sayers et al., 1996; Kelley et al., 1999; Kimhy et al., 2006; Kirkpatrick et al., 2011; Nakaya & Ohmori, 2008; Strauss et al., 2012; for review see Blanchard & Cohen, 2006). Studies reporting the factor structure of negative symptom rating scales are important in that they indicate the degree of shared variance among specific items, i.e., how negative symptom sub-domains group together. However, factor analytic studies do not speak to the important issue of heterogeneity of symptom presentation in schizophrenia, i.e., how individuals group together based upon their negative symptom profiles.
Although attention has been given to explaining clinical heterogeneity of schizophrenia (Strauss, Carpenter, Bartko, 1974; Crow, 1985; Andreasen, 1989), and identifying more homogeneous clinical sub-groups within the broader diagnosis of schizophrenia (Carpenter et al., 1988; Kirkpatrick et al., 2001), there has been little data-driven exploration of whether people with schizophrenia can be meaningfully separated based upon their negative symptom profiles. In the current study, we took a data-driven approach to examining the heterogeneity of negative symptoms in people with schizophrenia across two studies. The first study examined a sample of outpatients with schizophrenia to test the possibility that separable sub-groups of people with schizophrenia could be indentified based upon their AA and DE profiles. The second study aimed to validate results of the first in a sample that was enriched for primary and enduring negative symptoms (i.e., deficit schizophrenia). Based upon prior factor analytic work, we hypothesized that two distinct negative symptom profiles (AA and DE) would exist, and that these subgroups would differ on external validators known to correlate with negative symptoms. In particular, we expected AA to be generally associated with poorer outcomes than DE (e.g., social outcome, vocational outcome, premorbid adjustment, social cognition).
Study 1
In Study 1, we tested the hypothesis of separate AA and DE negative symptom subgroups using a standard negative symptom scale in a large sample of individuals with schizophrenia, and evaluated whether these subgroups differed on relevant external validators using data come from a clinical research project based at the VA Greater Los Angeles Healthcare System (Green et al., in press). External validators included measures of symptom severity, functional outcome, functional capacity, anhedonia, defeatist attitudes, and social cognition.
Method
Participants
Participants included 199 individuals meeting DSM-IV criteria for schizophrenia as determined via the Structured Clinical Interview for DSM-IV (SCID: First et al., 1997). The sample was on average 46.6 (9.8) years old, with 12.7 (1.8) years of participant education, 13.5 (3.6) years of parental education, 63.9% were male, and 30.7% were Caucasian, 10.4% Latino, 43.1% African-American, 4.5% Asian, 5.4% Other. Participants were recruited from outpatient treatment clinics at the Veterans Affairs Greater Los Angeles Healthcare System and the community for a larger study on visual perception and social cognition. Participants were excluded if they had an active substance use disorder in the past 6 months, identifiable neurological disorder, mental retardation, history of loss of consciousness for more than 1 hour, or insufficient fluency in English.
Measures
Measures of functional outcome, symptom severity, defeatist attitudes, anhedonia, functional capacity, and social cognition were examined as external validators. Functional outcome was assessed via the Comprehensive Assessment of Functioning Interview (CAF; Brekke & Aisley, 1995) (collateral informants were not used), which was used to complete the Role Functioning Scale (RFS; McPheeters, 1984) and the Strauss-Carpenter Scale (Strauss & Carpenter, 1972). Symptom severity was assessed via the Scale for the Assessment of Negative Symptoms (SANS: Andreasen, 1982; attention items were excluded) and the expanded 24-item UCLA version of the Brief Psychiatric Rating Scale (BPRS: Overall & Gorham, 1962; Lukoff et al., 1986). Multiple external validators known to be associated with negative symptoms were examined including: defeatist performance beliefs using the Defeatist Performance Belief subscale (Grant and Beck, 2009) of the Dysfunctional Attitudes Scale (DAS: Weissman, 1978) (which also includes the Need for Approval subscale), consummatory and anticipatory pleasure using the Temporal Experience of Pleasure Scale (TEPS: Gard et al., 2006; see also Gard et al., 2007; Strauss et al., 2011), the revised Chapman Scales for Physical and Social Anhedonia (Chapman & Chapman, 1978; Eckblad et al., 1982), functional capacity using the UCSD Performance-based Skills Assessment (UPSA; Patterson et al., 2001), and social cognition using the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT; Mayer et al., 2003), The Awareness of Social Inference Test (Part-III) (TASIT; McDonald, Flanagan, Rollins, 2002), and the Profile of Nonverbal Sensitivity (PONS; Rosenthal et al., 1979).
Procedures
Participants completed the battery of assessments over multiple sessions (i.e., 2-3 visits each separated by 1 week). All SCID, SANS, and BPRS interviewers were trained through the Treatment Unit of the Department of Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center (MIRECC). The process included formal didactics, achieving a minimal level of reliability, and co-rated interviews with faculty members. After initial certification all raters participated in a continuous quality assurance program.
Data Analysis
The following series of analytical techniques were performed to achieve the aims of the study. First, Principal Component Analysis (PCA) using varimax rotation and Kaiser normalization was performed on the severity scores for each of the 4 SANS global scores. The 4 global scores were selected rather than all 25 SANS items to meet rule-of-thumb recommendations for ratio of subjects per number of items entered to factor analysis. Varimax rotation was selected based upon a priori considerations, with the aim of identifying distinct domains of negative symptoms. The number of factors extracted was based on the eigen value >1 criterion. Individual factor scores were then saved as variables for each subject.
Second, mean factor scores for each of the factors obtained were submitted to cluster analysis using Ward's method and squared Euclidean distance to determine whether subgroups could be identified based upon negative symptom profiles. Cluster analysis is a technique used to group cases together based upon similar characteristics. Ward's method was selected because it generates results that are consistent with other agglomerative clustering methods, and because it is less affected by outliers than other clustering methods. Squared Euclidian distance was selected as the dissimilarity measure because it is the most widely used dissimilarity measure applied in cluster analysis (Everitt et al., 2001). In the absence of objective methods for determining the definitive number of clusters, we followed recommendations of Aldenderfer and Blashfield (1984) and Everitt, Landau, & Leese, (2001) to evaluate the optimal number of clusters using multiple methods. We initially conducted preliminary cluster analyses with varying numbers of cluster solutions to identify trivial clusters in a manner similar to “scree testing”. Next, we examined the distance between group centroids to determine whether there was adequate separation among clusters identified in the various solutions. These heuristic strategies do not rule out alternative cluster solutions, but provide an indication of the point at which further clustering is not productive because of reduced distance among the cluster spaces. Next, we examined the stability of the cluster solution structure by comparing a hierarchical agglomerative method (i.e., Ward's) and an iterative portioning method (i.e., K-means). Cohen's Kappa was used to determine the agreement between the K-means and Ward's method solutions. This analysis allowed us to determine if similar clusters were present regardless of the algorithm used to derive them. Finally, visual inspection of the dendogram plot resulting from cluster analysis was also used to confirm the optimal number of clusters. Mean factor score loadings were then converted to Z-scores for each subject, and mean Z-scores were plotted for the clusters identified to determine meaning for the clusters obtained.
Third, to confirm differences between the clusters, SANS symptom severity scores for the two factors were entered into discriminant function analysis, where clusters identified in the cluster analysis served as the grouping variable. Discriminant function analysis allowed for a determination of the extent to which clusters were separated by the severity of specific negative symptoms. An iterative partitioning method was applied within the discriminant function analysis to determine cluster stability. Using this iterative method, a large number of cluster reassignments would suggest poor stability of cluster classification.
Finally, ANOVAs and Chi-square analyses were calculated to examine potential differences among patient clusters on relevant external validators. Similar cluster analysis and discriminant function procedures have been used in other studies examining heterogeneity in schizophrenia (e.g., Goldstein et al., 2002, 2005; Horan & Goldstein, 2003a,b; Strauss & Herbener, 2011).
Results
Are there Distinct Dimensions of Negative Symptoms?
PCA indicated two factors explaining 76.8% of the variance. Factors represented a DE factor, consisting of the restricted affect and alogia items, and an AA factor, consisting of the avolition and anhedonia-asociality items. None of the subscales loaded highly (> 0.45) on more than one factor. These results are consistent with previous studies examining the factor structure of the SANS (Keefe et al., 1992; Mueser et al., 1994; Peralta & Cuesta, 1995; Sayers et al., 1996; Kelley et al., 1999) (see Table 1).
Table 1. Factor Loadings for Symptom Severity Scores on the Scale for the Assessment of Negative Symptoms (Study 1) and Schedule for the Deficit Syndrome (Study 2).
| Study 1: SANS | ||
|---|---|---|
|
| ||
| Diminished Expression Factor | Avolition-Apathy Factor | |
| Affective Flattening | 0.90 | 0.15 |
| Alogia | 0.90 | 0.14 |
| Avolition | 0.21 | 0.80 |
| Anhedonia-Asociality | 0.07 | 0.86 |
|
| ||
| Eigen values | 2.04 | 1.03 |
| % of Variance | 50.9% | 25.8% |
|
| ||
| Study 2: SDS | Diminished Expression Factor | Avolition-Apathy Factor |
|
| ||
| Restricted Affect | 0.91 | 0.05 |
| Diminished Emotional Range | 0.77 | 0.19 |
| Poverty of Speech | 0.67 | 0.43 |
| Curbed Interests | 0.16 | 0.82 |
| Diminished Sense of Purpose Diminished Social | 0.08 | 0.89 |
| Drive | 0.40 | 0.65 |
|
| ||
| Eigen values % of Variance | 1.14 | 3.02 |
| 19.0% | 50.3% | |
Note. Bold = primary symptom loadings for each factor
Do Sub-Groups of Patients with Different Negative Symptom Profiles Exist?
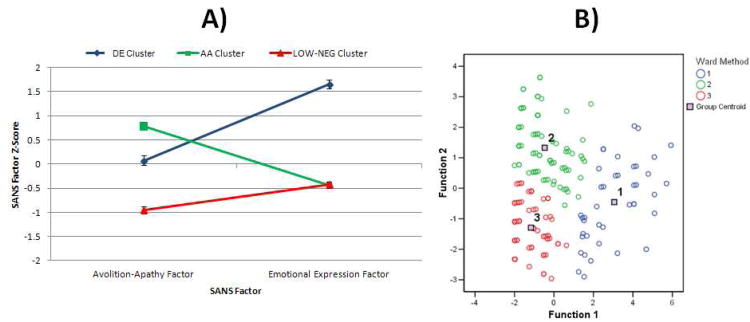
Visual inspection of the dendogram indicated that a three-cluster solution was optimal. The selection of the three-cluster solution was supported by analyses indicating good agreement between Ward's method and the K-means clustering. Support for a three-cluster solution was also found after clusters were plotted in multidimensional space, as separation among cluster centroids was clear for the three-cluster solution, but not as clear for other solutions. These multiple methods thus converged to indicate that a three-cluster solution was optimal.
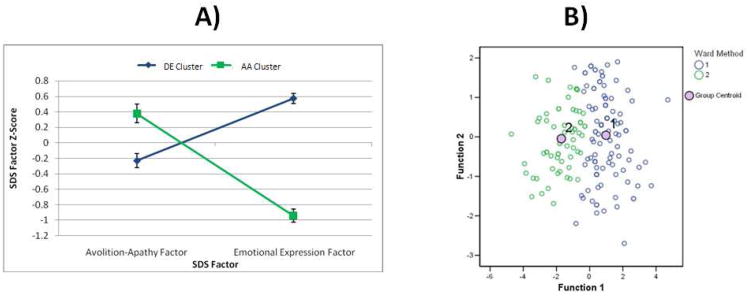
After plotting the means and SEs of the Z-scores for the SANS dimensions, it was clear that the three groups reflected patients with: 1) higher DE and lower AA (DE group: n = 41), 2) lower DE and higher AA (AA group: n = 85), and 3) low scores on both DE and AA (a low negative symptom group, LOW-NEG: n = 73) (see Figure 2A).
Figure 2. Means and SEs for SDS Factor Scores in AA and DE Clusters (Panel A) and Two-Cluster Solution Plotted in Discriminant Function Space (Panel B) for Study 2.
Note. Panel A: Values represent Means and SEs for Z-scores of standardized factor scores calculated for each subject. Higher values represent greater symptom severity. Panel B: Cluster 1 = Diminished Expression; Cluster 2 = Avolition-Apathy.
Discriminant function analysis indicated that the three clusters were adequately separated in discriminant function space (see Figure 2B), and the iterative partitioning indicated high cluster stability, as 97.5% of cases were correctly classified (Wilks' Lambda = 0.41, p < .001). Thus, there was little overlap among negative symptom scores of the three groups.
Do Negative Symptom Subgroups differ on External Validators?
As shown in Table 2, the three groups did not differ in demographics. Post hoc LSD contrasts indicated that in comparison to the LOW-NEG group, both AA and DE had more severe BPRS total, BPRS negative symptoms, and dysfunctional attitudes, as well as poorer work functioning, social functioning, family role functioning, and independent living skills (ps < 0.05). The DE group did not differ from the LOW-NEG group on conceptual disorganization, psychosis, social anhedonia, Strauss-Carpenter Scale hospitalization, or MSCEIT total score. Additionally, the AA patients had worse psychosis, higher social anhedonia, and more frequent hospitalizations (Strauss-Carpenter Scale) compared to the LOW-NEG group (ps < 0.05).
Table 2. Demographic and Characteristics of AA, DE, and LOW-NEG Groups in Study 1.
| Diminished Expressivity | Avolition Apathy | Low Negative | Test Statistic and Significance | |
|---|---|---|---|---|
| Demographics | ||||
| Age | 45.3 (12.0) | 47.4 (9.1) | 46.3 (9.4) | F = 0.64, p = n.s. |
| Education | 12.5 (1.3) | 12.7 (1.9) | 12.7 (1.9) | F = 0.23, p = n.s. |
| Parental Education | 13.7 (4.3) | 13.1 (3.4) | 13.8 (3.6) | F = 0.76, p = n.s. |
| % Male | 78.9% | 66.3% | 63.2% | χ2 = 2.90, p = n.s. |
| % Caucasian | 36.8% | 26.5% | 38.8% | χ2 = 4.45, p = n.s. |
| % Never Married | 63.4% | 59.5% | 57.5% | χ2 = 4.94, p = n.s. |
| General Symptoms | ||||
| BPRS Positive | 2.2 (0.9) | 2.6 (0.9) | 2.0 (0.7) | F = 8.61, p < 0.001 |
| BPRS Negative | 3.1 (0.8) | 1.6 (0.6) | 1.4 (0.5) | F = 94.61, p < 0.001 |
| BPRS Depression | 1.9 (0.8) | 2.1 (0.8) | 1.9 (0.7) | F = 2.30, p = n.s. |
| BPRS Agitation | 1.2 (0.3) | 1.3 (0.4) | 1.2 (0.4) | F = 1.55, p = 0.22 |
| BPRS Conceptual Disorganization Item | 1.71 (1.2) | 2.33 (1.6) | 1.93 (1.1) | F = 3.26, p = 0.04 |
| BPRS Total | 46.7 (10.3) | 47.5 (11.0) | 39.8 (8.4) | F = 12.91, p < 0.001 |
| Anhedonia | ||||
| TEPS Consummatory | 35.1 (7.8) | 34.8 (7.8) | 36.1 (6.6) | F = 0.56, p = n.s. |
| TEPS Anticipatory | 44.2 (9.0) | 43.4 (10.0) | 45.1 (7.3) | F = 0.59, p = n.s. |
| Chapman Physical | 19.8 (9.6) | 19.6 (9.1) | 17.5 (9.0) | F = 1.3, p = n.s. |
| Chapman Social | 16.5 (8.1) | 18.7 (8.2) | 14.0 (7.5) | F = 6.9, p = 0.001 |
| Defeatist Attitudes | ||||
| Defeatist Performance Beliefs | 53.4 (16.1) | 55.8 (18.9) | 45.6 (16.3) | F = 6.8, p = 0.001 |
| Need for Acceptance | 31.3 (9.7) | 32.4 (12.4) | 27.3 (10.6) | F = 4.1, p = 0.02 |
| Functional Outcome | ||||
| S-C Hospitalization | 4.0 (0.2) | 3.8 (0.4) | 4.0 (0.2) | F = 3.9, p < 0.02 |
| S-C Social | 2.3 (1.5) | 1.4 (1.4) | 3.3 (1.2) | F = 39.3, p < 0.001 |
| S-C Work | 0.6 (1.0) | 0.3 (0.9) | 1.2 (1.5) | F = 10.6, p < 0.001 |
| RFS Work | 2.2 (1.4) | 1.9 (1.2) | 3.1 (1.9) | F = 14.1, p < 0.001 |
| RFS Independent Living | 4.0 (1.5) | 4.0 (1.7) | 5.1 (1.4) | F = 8.6, p < 0.001 |
| RFS Family | 4.4 (1.8) | 3.9 (1.9) | 5.4 (1.4) | F = 14.7, p < 0.001 |
| RFS Social | 3.4 (2.0) | 2.6 (1.6) | 5.0 (1.5) | F = 42.3, p < 0.001 |
| Functional Capacity | ||||
| UPSA Total | 73.9 (10.2) | 73.5 (12.2) | 76.0 (10.4) | F = 0.99, p = n.s. |
| Social Cognition | ||||
| MSCEIT total | 86.5 (11.9) | 79.9 (14.6) | 85.2 (12.1) | F = 4.6, p = 0.01 |
| TASIT | 47.3 (6.1) | 45.2 (8.1) | 45.4 (6.9) | F = 1.2, p = n.s. |
| PONS | 77.5 (7.5) | 78.2 (7.8) | 80.0 (5.4) | F = 1.8, p = n.s. |
Regarding contrasts between the DE and AA groups, post hoc tests indicated that the AA group had significantly more severe BPRS conceptual disorganization and psychosis, higher rates of hospitalization (Strauss-Carpenter Scale), poorer social functioning (Strauss-Carpenter Scale), and greater social cognition impairment (MSCEIT). The difference in total MSCEIT scores was primarily accounted for by the perceiving emotions branch, as differences between AA and DE on all other MSCEIT subtests were nonsignificant.
Study 2
In Study 2 we attempted to validate results of the initial study in a large sample of people meeting criteria for deficit schizophrenia (Carpenter et al., 1988) using archival data from the Maryland Psychiatric Research Center (MPRC). Individuals meet criteria for “deficit schizophrenia” if they have 2 or more negative symptoms that are clinically significant, and those symptoms are considered primary (i.e., not secondary to factors such as depression, anxiety, disorganization) and enduring (i.e., stable >1 year). Deficit patients were selected to provide a more definitive test of the existence of distinct subgroups apart from the influence of secondary or transitory negative symptoms. The deficit/nondeficit categorization has demonstrated validity in a variety of ways and good reliability across multiple studies (e.g., Kirkpatrick et al., 2001; Strauss et al., 2008, 2010a,b, 2012). External validators with known associations to negative/deficit symptoms were examined, including sex, marital status, premorbid adjustment, course, functional outcome, and Extrapyramidal Symptoms (EPS).
Method
Participants
Participants included 169 individuals with a DSM diagnosis of schizophrenia who met criteria for deficit schizophrenia (Carpenter et al., 1988). Structured clinical interviews, including the SCID (First et al., 1997), information from informants and medical records, and a diagnostic conference were used to establish best estimate diagnosis. The final sample was on average 39.4 (12.48) years old, had 11.7 (2.69) years of education, 79.3% were male, and 56.2% were Caucasian, 38.5% African-American, 1.8% Asian, 3.0% Other, and 0.6% Mixed race. Approximately 55% were outpatients and 45% inpatients at the time of assessment.
Measures
Measures of symptom severity, functional outcome, EPS, premorbid adjustment, clinical course, and demographics were examined as external validators. Positive and Disorganized symptoms were assessed using the Scale for the Assessment of Positive Symptoms (SAPS; Andreason, 1984); negative symptoms and deficit categorization were determined using the Schedule for the Deficit Syndrome (SDS; Kirkpatrick et al., 1989). The Level of Function Scale (LOF; Hawk et al., 1975) allowed for ratings on 8 domains of functioning based upon the most usual level of functioning experienced over the past 6 months (except symptoms which are rated over the past month): Duration of Hospitalization, Frequency of Social Contacts, Quality of Social Relations, Usefully Employed, Quality of Useful Work, Symptoms, Ability to Meet own Basic Needs, and Fullness of Life. EPS was assessed via the MPRC Involuntary Movement Scale (MIMS; Cassady et al., 1997). The Premorbid Adjustment Scale (PAS) (Cannon-Spoor et al., 1982) was administered to assess premorbid functioning in relation to childhood (up to 11 years), early adolescence (12 to 15 years), late adolescence (16 to 18 years), and adulthood (19 years and above). Social and academic premorbid domain scores were calculated for childhood, early adolescence, and late adolescence based upon results of factor analytic studies (Allen et al., 2005; Cannon et al., 1997). Information regarding demographics, onset of psychosis, and clinical course were obtained using a standard clinical interview.
Procedures
Tests were administered as part of each patient's intake evaluation at the MPRC. Testing procedures generally took place over multiple days (i.e., 2-3 visits each separated by 1 week), with each session typically lasting from 1-3 hours and breaks allowed as needed to diminish fatigue and maintain motivation. For all subjects, demographic, diagnostic, and symptom ratings were administered by clinicians trained to administer these measures reliably. The SDS was administered by originators of the instrument (BK, RWB) or staff trained by these individuals to administer the measure reliably.
Data Analysis
Statistical procedures were similar to Study 1, with the exception that factor analysis was performed on the 6 SDS subscale severity scores: restricted affect; poverty of speech; diminished emotional range; curbed interests; diminished sense of purpose; diminished social drive.
Results
Are there Distinct Dimensions of Negative Symptoms?
PCA indicated two distinct and interpretable factors explaining 69% of the variance, reflecting an AA factor (Curbed Interests, Diminished Sense of Purpose, and Diminished Social Drive items) and a DE factor (Restricted Affect, Diminished Emotional Range, and Poverty of Speech items). None of the subscales loaded highly (> 0.45) on more than one factor.
Replication of Negative Symptom Sub-Groups
Visual inspection of the dendogram indicated that a two-cluster solution was optimal. The multiple cluster validation methods described under Study 1 were also conducted here, and indicated that the two-cluster solution was optimal. Means and standard errors were calculated for AA and DE factors for each group to determine the meaning of clusters obtained (see Figure 2). As can be seen in Figure 2A, cluster 1 (n =105) reflected a group rated as having more severe scores on DE items and relatively lower scores on AA items. Cluster 2 (n = 64) reflected a group with more severe scores on AA items and relatively lower scores on DE items.
When AA and DE factor scores were entered into discriminant function analysis, results indicated the two cluster solution was adequately separated in discriminant function space (see Figure 2B). An iterative partitioning method was applied to examine cluster stability. Results of this iterative method indicated that 96.4% of cases were correctly classified (Wilks' Lambda = 0.368, p < .001). These results indicate that there is little overlap in SDS factor scores among the patient clusters identified.
Do Negative Symptom Sub-groups differ on External Validators?
Demographic and clinical characteristics of the two groups are presented in Table 3. Groups did not differ on age, education, or marital status. However, groups did differ on sex and ethnicity, such that the DE group had a lower proportion of Caucasian subjects than the AA, and AA had a greater proportion of males than the DE.
Table 3. Demographic and Clinical Characteristics of AA and DE Groups in Study 2.
| Diminished Expressivity | Avolition Apathy | Test Statistic and Significance | |
|---|---|---|---|
| Demographics | |||
| Age | 38.0 (12.48) | 41.69 (12.25) | F = 3.52, p = n.s. |
| Education | 11.75 (2.73) | 11.65 (2.63) | F = 0.06, p = n.s. |
| % Male | 74.3% | 87.5% | χ2 = 4.23, p = 0.05 |
| % Caucasian | 50.5% | 65.6% | χ2 = 5.27, p = 0.04 |
| % Never Married | 82.9% | 82.8% | χ2 = 2.29, p = n.s. |
| Clinical | |||
| Age of Onset | 21.78 (7.67) | 20.88 (4.91) | F = 2.29, p = n.s. |
| Type of Onset | χ2 = 3.85, p = 0.05 | ||
| Gradual with no clear date of onset of psychosis | 52% | 44% | |
| Gradual onset of psychosis over a 12-month period | 20% | 41% | |
| Clear and abrupt onset of psychosis | 28% | 15% | |
| Number of Previous Hospitalizations | 2.49 (4.22) | 2.23 (2.49) | F = 0.10, p = n.s. |
| % with any Family Member Hospitalized for Psych. | 31% | 52% | χ2 = 3.88, p = 0.05 |
| SAPS Positive Symptom Severity | |||
| Global Hallucinations | 1.69 (1.77) | 1.86 (1.97) | F = 0.14, p = n.s. |
| Global Delusions | 1.81 (1.57) | 2.32 (1.74) | F = 1.80, p = n.s. |
| Global Bizarre Behavior | 0.73 (1.08) | 1.29 (1.48) | F = 3.64, p = n.s. |
| Global Thought Disorder | 0.77 (1.13) | 1.46 (1.73) | F = 4.69, p = 0.03 |
| SAPS Total | 18.54 (19.94) | 26.57 (24.35) | F = 2.43, p = n.s. |
| MIMS EPS Severity | |||
| Dyskinesia | 1.39 (1.39) | 1.62 (1.44) | F = 0.93, p = n.s. |
| Parkinsonism | 0.88 (1.43) | 0.77 (1.69) | F = 0.18, p = n.s. |
| Akathesia | 0.52 (1.72) | 0.29 (0.94) | F = 0.77, p = n.s. |
| Premorbid Adjustment | |||
| CH Social | 1.99 (1.40) | 2.79 (1.50) | F = 8.45, p = 0.01 |
| CH Academic | 1.98 (1.29) | 2.25 (1.26) | F = 1.27, p = n.s. |
| EA Social | 2.20 (1.51) | 2.45 (1.60) | F = 0.72, p = n.s. |
| EA Academic | 2.35 (1.52) | 2.61 (1.30) | F = 0.91, p = n.s. |
| LA Social | 2.71 (1.78) | 3.02 (1.67) | F = 0.86, p = n.s. |
| LA Academic | 2.81 (1.79) | 2.75 (1.47) | F = 0.04, p = n.s. |
| AD Social | 3.21 (1.67) | 3.58 (1.44) | F = 1.00, p = n.s. |
| Functional Outcome | |||
| Duration of Hospitalization | 2.09 (1.84) | 2.73 (1.69) | F = 4.01, p = 0.05 |
| Frequency of Social Contacts | 1.43 (1.42) | 1.38 (1.25) | F = 0.05, p = n.s. |
| Quality of Social Relations | 1.28 (1.47) | 1.04 (1.20) | F = 1.04, p = n.s. |
| Usefully Employed | 0.68 (0.91) | 0.24 (0.52) | F = 9.80, p = 0.01 |
| Quality of Useful Work | 0.80 (0.95) | 0.36 (0.66) | F = 8.32, p = 0.01 |
| Symptoms | 1.66 (1.18) | 1.67 (1.18) | F = 0.01, p = n.s. |
| Ability to Meet Own Basic Needs | 2.96 (1.03) | 3.09 (1.01) | F = 0.61, p = n.s. |
| Fullness of Life | 1.26 (0.67) | 1.34 (0.55) | F = 0.56, p = n.s. |
| Overall Level of Function | 1.11 (0.86) | 1.06 (0.84) | F = 0.12, p = n.s. |
With regard to onset, the DE group had a greater likelihood of abrupt onset of psychosis compared to the gradual onset observed in the AA group. The DE group was also hospitalized for longer durations compared to the AA group. However, AA patients were more likely to have a family member who had been hospitalized for psychiatric reasons. Groups also significantly differed on clinical symptom severity as measured by the SAPS. Specifically, AA individuals had more severe thought disorder than the DE; however, AA and DE groups did not significantly differ on the severity of hallucinations, delusions, or bizarre behavior. Groups did not differ on EPS. AA patients were also significantly more likely to have poorer premorbid social adjustment in childhood than DE patients. However, no differences were found between groups for social functioning in early adolescence, late adolescence, or adulthood. Groups did not differ in premorbid academic function at any of the PAS time intervals.
On the LOF scale, AA patients were less likely to be gainfully employed and less likely to produce high quality work than DE patients. Groups did not differ on other LOF domains or overall level of function. Thus, both groups evidence significant functional impairments; however, the AA group displayed greater impairment on occupational functioning.
General Discussion
In both studies, we replicated previous factor analytic results indicating that AA and DE represent separable dimensions that exist within the broader construct of negative symptoms (see Blanchard & Cohen, 2006). More importantly, cluster and discriminant function analyses conducted in Study 1 supported our hypothesis that individual patients can be identified who have a clinical presentation that is predominantly characterized by either AA or DE. These findings were replicated in a separate sample of “deficit schizophrenia” patients with primary and enduring negative symptoms in Study 2.
In Study 1, there were important similarities and differences between the AA and DE groups with regard to external validators other than those intrinsic to the classification process. As might be expected, the AA and DE clusters displayed more pathological scores than low negative symptom patients on measures of dysfunctional attitudes, work functioning, social functioning, family role functioning, and independent living. Although AA and DE groups did not differ from each other on the aforementioned variables, there were several notable differences between these groups. In comparison to DE subjects, the AA group had more severe disorganization and psychosis, poorer social functioning, and greater social cognition deficits.
In Study 2, the importance of the AA and DE sub-groups was confirmed by evidence indicating that the groups differed on relevant external validators Specifically, results indicated that in comparison to the DE group, the AA group was: 1) more likely to have a family member who has been hospitalized for psychiatric reasons, 2) less likely to be gainfully employed, 3) less likely to complete high quality work, 4) more likely to manifest symptoms of formal thought disorder, 5) more likely to have poorer premorbid social adjustment in childhood, 6) more likely to have a gradual onset of psychosis, and 7) more likely to be male. In comparison to the AA group, individuals in the DE group had a greater likelihood of: 1) being African-American, 2) having an abrupt onset of psychosis, and 3) having a longer duration of hospitalization.
Across both studies, we found support for the existence of separable DE and AA clusters, and the AA sub-group demonstrated greater impairment on measures of functional outcome, psychosis, and disorganization; however, the unique predictors of the DE group were not consistent in both studies. The pattern of findings across both studies suggests that the AA presentation represents a phenomenologically distinct and more pathological negative symptom profile than DE, which is interesting given that DE has more severe total negative symptom scores.
These results extend previous studies indicating that AA has several unique associations that are not true of DE. For example, smell identification deficits have been strongly associated with AA, particularly social drive, but not other negative symptom domains such as restricted affect (Malaspina & Coleman, 2003; Goudsmit et al 2003; Seckinger et al 2004; Strauss et al., 2010a). AA has also been associated with longer duration of untreated psychosis, while DE is not (Malla et al., 2002), and AA is more strongly associated with reduced medication treatment compliance than DE (Tattan & Creed, 2001). Neuropsychological studies also indicate that AA is dissociable from DE in that it is uniquely associated with executive functioning and abstraction-flexibility deficits (Suslow et al., 1998) (see Messinger et al., 2011 for a review). Our findings contradict evidence that DE is more strongly associated with worse premorbid function, quality of life, functional outcome, and social cognition than AA (Gur et al., 2006, 2007).
Several limitations should be noted in relation to these studies. First, the datasets analyzed were archival, and parallel measures were not used across studies. Second, measures of neuropsychological functioning were not examined, and we were thus unable to determine whether impairments in social cognition reflect generalized cognitive deficits. Third, it is possible that environmental factors have differential contributions to AA relative to DE, and we were unable to evaluate such possibilities. For example, disability compensation may be more strongly associated with AA than DE, disproportionately affecting ratings of avolition and asociality more so than restricted affect or alogia. Finally, the samples consisted of chronic patients, and it is possible that different results would emerge with patients earlier in the course of illness. Future studies should consider these issues in relation to negative symptom heterogeneity.
The current findings have several important implications for understanding negative symptoms of schizophrenia. First, results have relevance to the consideration of which psychological dimensions should be considered in the DSM and ICD diagnostic criteria for schizophrenia. The current findings, along with previous research reviewed in this manuscript, suggest that AA and DE should be considered distinct domains of psychopathology to be rated separately by clinicians. By combining DE and AA into a more general domain of “negative symptoms”, valuable information related to the cause and predictive outcome of individual negative symptoms might be lost. Second, when new negative symptom rating scales are developed, these should include an adequate sampling of AA and DE items. Two such scales were developed in response to the NIMH consensus conference on negative symptoms, the Brief Negative Symptom Scale (BNSS: Kirkpatrick et al., 2011) and the Clinical Assessment Interview for Negative Symptoms (CAINS: Kring et al., in press). Finally, pharmacological studies could benefit from targeting AA and DE groups or dimensions separately. Given differences in neural processes thought to underlie these symptoms (Foussias & Remington, 2010), it is possible that AA and DE necessitate different therapeutic targets. Without reducing heterogeneity within negative symptoms, attempts to successfully remediate negative symptoms will continue be met with difficulty, given that negative symptoms do not reflect a unified construct (Blanchard & Cohen, 2006; Kirkpatrick & Fischer, 2006).
Figure 1. Means and SEs for SANS Factor Scores in AA, DE, and LOW-NEG Clusters (Panel A) and Three-Cluster Solution Plotted in Discriminant Function Space (Panel B) for Study 1.
Note. Panel A: Values represent Means and SEs for Z-scores of standardized factor scores calculated for each subject. Higher values represent greater symptom severity. Panel B: Cluster 1 = Diminished Expression; Cluster 2 = Avolition-Apathy; Cluster 3 = Low Negative Symptoms.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldenderfer MS, Blashfield RK. Cluster analysis. Sage; Beverly Hills, CA: 1984. [Google Scholar]
- Allen DN, Frantom LV, Strauss GP, van Kammen DP. Differential patterns of premorbid academic and social deterioration in patients with schizophrenia. Schizophrenia Research. 2005;75:389–397. doi: 10.1016/j.schres.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive symptoms (SAPS) University of Iowa; Iowa City: 1984. [Google Scholar]
- Andreasen NC. Negative symptoms in schizophrenia: Definition and reliability. Archives of General Psychiatry. 1982;39:784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. British Journal of Psychiatry. 1989;S7:49–58. [PubMed] [Google Scholar]
- Blanchard JJ, Horan WP, Collins LM. Examining the latent structure of negative symptoms: Is there a distinct subtype of negative symptom schizophrenia? Schizophrenia Research. 2005;77(2–3):151–65. doi: 10.1016/j.schres.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Kring AM, Horan WP, Gur R. Toward the Next Generation of Negative Symptom Assessments: The Collaboration to Advance Negative Symptom Assessment in Schizophrenia. Schizophrenia Bulletin. 2011;37(2):291–99. doi: 10.1093/schbul/sbq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard JJ, Cohen AS. The Structure of Negative Symptoms within Schizophrenia: Implications for Assessment. Schizophrenia Bulletin. 2006;32:238–245. doi: 10.1093/schbul/sbj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Kirkpatrick B, Davis OR, Irish D, Summerfelt A, Carpenter WT., Jr Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. American Journal of Psychiatry. 1994;151:20–6. doi: 10.1176/ajp.151.1.20. [DOI] [PubMed] [Google Scholar]
- Brekke JS, Aisley RA. Community adjustment form-revised. Unpublished Instrument. 1995 [Google Scholar]
- Buchanan RW, Breier A, Kirkpatrick B, Ball P, Carpenter WT., Jr Positive and negative symptom response to clozapine in schizophrenic patients with and without the deficit syndrome. American Journal of Psychiatry. 1998;155:751–60. doi: 10.1176/ajp.155.6.751. [DOI] [PubMed] [Google Scholar]
- Cannon-Spoor HE, Potkin SG, Wyatt RJ. Measurement of premorbid adjustment in chronic schizophrenia. Schizophrenia Bulletin. 1982;8:470–84. doi: 10.1093/schbul/8.3.470. [DOI] [PubMed] [Google Scholar]
- Cannon M, Jones P, Gilvarry C, Rifkin L, McKenzie K, Foerster A, Murray RM. Premorbid social functioning in schizophrenia and bipolar disorder: Similarities and differences. American Journal of Psychiatry. 1997;154:1544–50. doi: 10.1176/ajp.154.11.1544. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: The concept. American Journal of Psychiatry. 1988;145(5):578–83. doi: 10.1176/ajp.145.5.578. [DOI] [PubMed] [Google Scholar]
- Cassady SL, Thaker GK, Summerfelt A, Tamminga CA. The Maryland Psychiatric Research Center scale and the characterization of involuntary movements. Psychiatry Research. 1997;70:21–37. doi: 10.1016/s0165-1781(97)03031-x. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Unpublished test. University of Wisconsin; Madison: 1978. The Revised Physical Anhedonia Scale. [Google Scholar]
- Crow TJ. The two-syndrome concept: origins and current status. Schizophrenia Bulletin. 1985;11:471–86. doi: 10.1093/schbul/11.3.471. [DOI] [PubMed] [Google Scholar]
- Eckblad ML, Chapman LJ, Chapman JP, Mishlove M. Unpublished test. University of Wisconsin; Madison: 1982. The Revised Social Anhedonia Scale. [Google Scholar]
- Everitt SE, Landau S, Leese M. Cluster analysis. Arnold; London: 2001. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structural Clinical Interview for DSM-IV Axis I Disorders (SCID-IV) New York: New York State Psychiatric Institute, Biometrics Research; 1997. [Google Scholar]
- Foussias G, Remington G. Negative symptoms in schizophrenia: Avolition and Occam's razor. Schizophrenia Bulletin. 2010;36(2):359–369. doi: 10.1093/schbul/sbn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory pleasure components of the experiences of pleasure: A scale development study. Journal of Research & Personality. 2006;40:1086–102. [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophrenia Research. 2007;93:253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G, Minshew NJ, Allen DN, Seaton BE. High-functioning autism and schizophrenia: A comparison of an early and late onset neurodevelopmental disorder. Archives of Clinical Neuropsychology. 2002;17(5):461–75. [PubMed] [Google Scholar]
- Goldstein G, Sanders RD, Forman SD, Tarpey T, Gurklis JA, Van Kammen DP, Keshavan MS. The effects of antipsychotic medication on factor and cluster structure of neurologic examination abnormalities in schizophrenia. Schizophrenia Research. 2005;75(1):55–64. doi: 10.1016/j.schres.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Goudsmit N, Coleman E, Seckinger RA, Wolitzky R, Stanford AD, Corcoran C, Goetz RR, Malaspina D. A brief smell identification test discriminates between deficit and non-deficit schizophrenia. Psychiatry Research. 2003;120(2):155–64. doi: 10.1016/s0165-1781(03)00194-x. [DOI] [PubMed] [Google Scholar]
- Grant PM, Beck AT. Defeatist Beliefs as a Mediator of Cognitive Impairment, Negative Symptoms, and Functioning in Schizophrenia. Schizophrenia Bulletin. 2009;35(4):798–806. doi: 10.1093/schbul/sbn008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. From perception to functional outcome in schizophrenia: Modeling the role of ability and motivation. Archives of General Psychiatry. doi: 10.1001/archgenpsychiatry.2012.652. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Loughead J, Kohler CG, Elliott MA, Lesko K, Ruparel K, Wolf DH, Bilker WB, Gur RC. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Archives of General Psychiatry. 2007;64:1356–1366. doi: 10.1001/archpsyc.64.12.1356. [DOI] [PubMed] [Google Scholar]
- Hawk AB, Carpenter WT, Strauss JS. Diagnostic criteria and five-year outcome in schizophrenia: a report from the International Pilot Study of Schizophrenia. Archives of General Psychiatry. 1975;32:343–347. doi: 10.1001/archpsyc.1975.01760210077005. [DOI] [PubMed] [Google Scholar]
- Horan WP, Goldstein GA. A retrospective study of premorbid ability and aging differences in cognitive subtypes of schizophrenia. Psychiatry Research. 2003;118:209–221. doi: 10.1016/s0165-1781(03)00078-7. [DOI] [PubMed] [Google Scholar]
- Horan WP, Goldstein G. Differences in the course of cognitive impairments among cognitively-based schizophrenia subtypes. Schizophrenia Research. 2003;61(2-3):325–326. doi: 10.1016/s0920-9964(02)00313-4. [DOI] [PubMed] [Google Scholar]
- Horan WP, Kring AM, Gur RE, Reise SP, Blanchard JJ. Development and psychometric validation of the Clinical Assessment Interview for Negative Symptoms (CAINS) Schizophrenia Research. 2011;132:140–145. doi: 10.1016/j.schres.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Harvey PD, Lenzenweger MF, Davidson M, Apter SH, Schmeidler J, Mohs RC, Davis KL. Empirical assessment of the factorial structure of clinical symptoms in schizophrenia: Negative symptoms. Psychiatry Research. 1992;44(2):153–65. doi: 10.1016/0165-1781(92)90049-9. [DOI] [PubMed] [Google Scholar]
- Kelley ME, van Kammen DP, Allen DN. Empirical validation of primary negative symptoms: Independence from effects of medication and psychosis. American Journal of Psychiatry. 1999;156(3):406–11. doi: 10.1176/ajp.156.3.406. [DOI] [PubMed] [Google Scholar]
- Kimhy D, Yale S, Goetz RR, McFarr LM, Malaspina D. The factorial structure of the schedule for the deficit syndrome in schizophrenia. Schizophrenia Bulletin. 2006;32(2):274–8. doi: 10.1093/schbul/sbi064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT., Jr The Schedule for the Deficit Syndrome: An instrument for research in schizophrenia. Psychiatry Research. 1989;30:119–23. doi: 10.1016/0165-1781(89)90153-4. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Fischer B. Subdomains within the negative symptoms of schizophrenia: Commentary. Schizophrenia Bulletin. 2006;32:246–49. doi: 10.1093/schbul/sbj054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT., Jr A separate disease within the syndrome of schizophrenia. Archives of General Psychiatry. 2001;58(2):165–71. doi: 10.1001/archpsyc.58.2.165. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT, Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophrenia Bulletin. 2006;32(2):214–9. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, Marder SR. The brief negative symptom scale: psychometric properties. Schizophrenia Bulletin. 2011;37(2):300–5. doi: 10.1093/schbul/sbq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The Clinical Assessment Interview for Negative Symptoms (CAINS): Final Development and Validation. American Journal of Psychiatry. doi: 10.1176/appi.ajp.2012.12010109. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukoff D, Nuechterlein KH, Ventura J. Manual for the expanded Brief Psychiatric Rating Scale (BPRS) Schizophrenia Bulletin. 1986;12:594–602. [Google Scholar]
- Malla AK, Takhar JJ, Norman RMG, Manchanda R, Cortese L, Haricharan R, Verdi M, Ahmed R. Negative symptoms in first episode non-affective psychosis. Acta Psychiatry Scandanavia. 2002;105:431–439. doi: 10.1034/j.1600-0447.2002.02139.x. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Coleman E. Olfaction and social drive in schizophrenia. Archives of General Psychiatry. 2003;60(6):578–84. doi: 10.1001/archpsyc.60.6.578. [DOI] [PubMed] [Google Scholar]
- Mayer JD, Salovey P, Caruso DR, Sitarenios G. Measuring emotional intelligence with the MSCEIT V2.0. Emotion. 2003;3(1):97–105. doi: 10.1037/1528-3542.3.1.97. [DOI] [PubMed] [Google Scholar]
- McDonald S, Flanagan S, Rollins J. The Awareness of Social Inference Test. Suffolk, UK: Thames Valley Test Company, Ltd; 2002. [Google Scholar]
- McPheeters HL. Statewide mental health outcome evaluation: a perspective of two southern states. Community Mental Health Journal. 1984;20:44–55. doi: 10.1007/BF00754103. [DOI] [PubMed] [Google Scholar]
- Messinger JW, Trémeau F, Antonius D, Mendelsohn E, Prudent V, Stanford AD, Malaspina D. Avolition and expressive deficits capture negative symptom phenomenology: implications for DSM-5 and schizophrenia research. Clinical Psychology Review. 2011;31(1):161–8. doi: 10.1016/j.cpr.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueser KT, Sayers SL, Schooler NR, Mance RM, Haas GL. A multisite investigation of the reliability of the Scale for the Assessment of Negative Symptoms. American Journal of Psychiatry. 1994;151(10):1453–62. doi: 10.1176/ajp.151.10.1453. [DOI] [PubMed] [Google Scholar]
- Nakaya M, Ohmori K. A two-factor structure for the Schedule for the Deficit Syndrome in schizophrenia. Psychiatry Research. 2008;158(2):256–9. doi: 10.1016/j.psychres.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:149–65. [Google Scholar]
- Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV. UCSD Performance-Based Skills Assessment: Development of a New Measure of Everyday Functioning for Severely Mentally Ill Adults. Schizophrenia Bulletin. 2001;27(2):235–45. doi: 10.1093/oxfordjournals.schbul.a006870. [DOI] [PubMed] [Google Scholar]
- Peralta V, Cuesta MJ. Negative symptoms in schizophrenia: A confirmatory factor analysis of competing models. American Journal of Psychiatry. 1995;152(10):1450–7. doi: 10.1176/ajp.152.10.1450. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Hall JA, DiMatteo MR, Rogers PL, Archer D. Sensitivity to nonverbal communication: The PONS test. Baltimore: John Hopkins University Press; 1979. [Google Scholar]
- Sayers SL, Curran PJ, Mueser KT. Factor structure and construct validity of the scale for the assessment of negative symptoms. Psychological Assessment. 1996;8(3):269–80. [Google Scholar]
- Seckinger RA, Goudsmit N, Coleman E, Harkavy-Friedman J, Yale S, Rosenfield PJ, Malaspina D. Olfactory identification and WAIS-R performance in deficit and nondeficit schizophrenia. Schizophrenia Research. 2004;69(1):55–65. doi: 10.1016/S0920-9964(03)00124-5. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Allen DN, Duke LA, Ross SA, Schwartz J. Automatic affective processing impairments in patients with deficit syndrome schizophrenia. Schizophrenia Research. 2008;102:76–87. doi: 10.1016/j.schres.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Allen DN, Ross SA, Duke LA, Schwartz J. Olfactory hedonic judgment in patients with deficit syndrome schizophrenia. Schizophrenia Bulletin. 2010a;36:860–868. doi: 10.1093/schbul/sbn178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Jetha SS, Duke LA, Ross SA, Allen DN. Impaired Facial Affect Labeling Discrimination in Patients with Deficit Syndrome Schizophrenia. Schizophrenia Research. 2010b;118:146–153. doi: 10.1016/j.schres.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Allen DN, Miski P, Kirkpatrick B, Buchanan RW, Carpenter WT. Differential patterns of premorbid academic and social deterioration in deficit and non-deficit schizophrenia. Schizophrenia Research. 2012;135:134–138. doi: 10.1016/j.schres.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Harrow M, Grossman LS, Rosen C. Periods of Recovery in Deficit Syndrome Schizophrenia: A 20-Year Multifollowup Longitudinal Study. Schizophrenia Bulletin. 2010;36(4):788–99. doi: 10.1093/schbul/sbn167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Herbener ES. Patterns of emotional experience in schizophrenia: Differences in emotional response to visual stimuli are associated with clinical presentation and functional outcome. Schizophrenia Research. 2011;128(1–3):117–23. doi: 10.1016/j.schres.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Hong LE, Keller WR, Buchanan RW, Gold JM, Fischer BA, McMahon RP, Catalano LT, Culbreth AJ, Carpenter WT, Kirkpatrick B. Factor Structure of the Brief Negative Symptom Scale. Schizophrenia Research. 2012;142:96–98. doi: 10.1016/j.schres.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Sandt AR, Catalano LT, Allen DN. Negative symptoms and depression predict psychological well-being in individuals with schizophrenia. Comprehensive Psychiatry. 2012;53:1137–1144. doi: 10.1016/j.comppsych.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Wilbur RC, Warren KR, August SM, Gold JM. Anticipatory vs. Consummatory Pleasure: What is the Nature of Hedonic Deficits in Schizophrenia? Psychiatry Research. 2011;187:36–41. doi: 10.1016/j.psychres.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J, Carpenter W. The prediction of outcome in schizophrenia: I. Characteristics of outcome. Archives of General Psychiatry. 1972;27:739–746. doi: 10.1001/archpsyc.1972.01750300011002. [DOI] [PubMed] [Google Scholar]
- Strauss JS, Carpenter WT, Jr, Bartko JJ. The diagnosis and understanding of schizophrenia. Part III. Speculations on the processes that underlie schizophrenic symptoms signs. Schizophrenia Bulletin. 1974;11:61–69. doi: 10.1093/schbul/1.11.61. [DOI] [PubMed] [Google Scholar]
- Suslow T, Junghanns K, Weitzsch C, Volker A. Relations between Neuropsychological Vulnerability Markers and Negative Symptoms in Schizophrenia. Psychopathology. 1998;31:178–187. doi: 10.1159/000029038. [DOI] [PubMed] [Google Scholar]
- Tattan TMG, Creed FH. Negativve symptoms of schizophrenia and compliance with medication. Schizophrenia Bulletin. 2001;27(1):149–155. doi: 10.1093/oxfordjournals.schbul.a006853. [DOI] [PubMed] [Google Scholar]
- Weissman A. The Dysfunctional Attitudes Scale: A validation study. Philadelphia, PA: University of Pennsylvania; 1978. [Google Scholar]