Abstract
Development and maintenance of whisker-specific patterns along the rodent trigeminal pathway depends on an intact sensory periphery during the sensitive/critical period in development. Barrelette cells of the brain stem trigeminal nuclei are the first set of neurons to develop whisker-specific patterns. Those in the principal sensory nucleus (PrV) relay these patterns to the ventrobasal thalamus, and consequently, to the somatosensory cortex. Thus PrV barrelette cells are among the first group of central neurons susceptible to the effects of peripheral damage. Previously we showed that membrane properties of barrelette cells are distinct as early as postnatal day 1 (PND 1) and remain unchanged following peripheral denervation in newborn rat pups (Lo and Erzurumlu 2001). In the present study, we investigated the changes in synaptic transmission. In barrelette cells of normal PND 1 rats, weak stimulation of the trigeminal tract (TrV) that was subthreshold for inducing Na+ spikes evoked an excitatory postsynaptic potential–inhibitory postsynaptic potential (EPSP-IPSP) sequence that was similar to the responses seen in older rats (Lo et al. 1999). Infraorbital nerve transection at birth did not alter excitatory and inhibitory synaptic connections of the barrelette cells. These observations suggested that local neuronal circuits are already established in PrV at birth and remain intact after deafferentation. Strong stimulation of the TrV induced a sustained depolarization (plateau potential) in denervated but not in normal barrelette neurons. The plateau potential was distinct from the EPSP-IPSP sequence by 1) a sustained (>80 ms) depolarization above −40 mV; 2) a slow decline slope (<0.1 mV/ms); 3) partially or totally inactivated Na+ spikes on the plateau; and 4) a termination by a steep decay (> 1 mV/ms) to a hyperpolarizing membrane level. The plateau potential was mediated by L-type Ca2+ channels and triggered by a N-methyl-d-aspartate (NMDA) receptor-mediated EPSP. γ-aminobutyric acid-A (GABAA) receptor-mediated IPSP dynamically regulated the latency and duration of the plateau potential. These results indicate that after neonatal peripheral damage, central trigeminal inputs cause a large and longlasting Ca2+ influx through L-type Ca2+ channels in barrelette neurons. Increased Ca2+ entry may play a key role in injury-induced structural remodeling, and/or transsynaptic cell death.
INTRODUCTION
Afferent terminal arbors and dendritic fields of postsynaptic neurons of the rodent trigeminal pathway develop discrete patterns that replicate the distribution of the whisker and sinus hair follicles on the snout. These patterns are first established in the brain stem trigeminal complex where they are called “barrelettes,” and sequentially in the ventroposteromedial nucleus (VPM) of the dorsal thalamus (“barreloids”) and in layer IV of the primary somatosensory cortex (“barrels”) (for reviews, see Erzurumlu and Kind 2001; Killackey et al. 1995; O’Leary et al. 1994; Woolsey 1990). In the rat, barrelette patterns in the brain stem trigeminal nuclei develop before birth (Chiaia et al. 1992) and are dependent on the infraorbital (IO) nerve, which innervates the whiskers and sinus hairs. Trigeminothalamic projection cells (barrelette neurons) of the principal sensory nucleus (PrV) convey this pattern to the VPM, and VPM neurons in turn relay the patterns to the barrel cortex (Erzurumlu and Jhaveri 1990; Erzurumlu and Kind 2001; Killackey and Fleming 1985). Thus along the pathway to neocortex, PrV barrelette neurons are the first set of neurons to detect incoming patterned trigeminal afferents, or any disruption of them.
Previously we showed that barrelette neurons have distinct membrane properties that can be identified between PND 1 and PND 13, and these properties are not altered by IO nerve transection at birth (Lo and Erzurumlu 2001; Lo et al. 1999). This finding was surprising, because following peripheral deafferentation, dramatic changes take place in the PrV: whiskerrelated patterns are lost and a considerable degree of transsynaptic cell death occurs (Bates and Killackey 1985; Miller and Kuhn 1997). In the present study we investigated the development of synaptic transmission in normal PrV, dendritic field alterations in physiologically identified barrelette cells, and their synaptic transmission following denervation. We show that in normal rats, excitatory and inhibitory circuits in the barrelette region of the PrV are established by postnatal day (PND) 1 and IO nerve transection does not change this circuitry. In addition, along with dendritic remodeling, a conspicuous plateau potential emerges in deafferented barrelette neurons. This response is mediated by L-type Ca2+ channels. The generation of the plateau depends on activation of N-methyl-d-aspartate (NMDA)-type glutamate receptors. γ-Aminobu-tyric acid-A (GABAA) receptor-mediated inhibitory inputs regulate the latency and duration of the plateau potential.
METHODS
Morphological verification of IO nerve transection
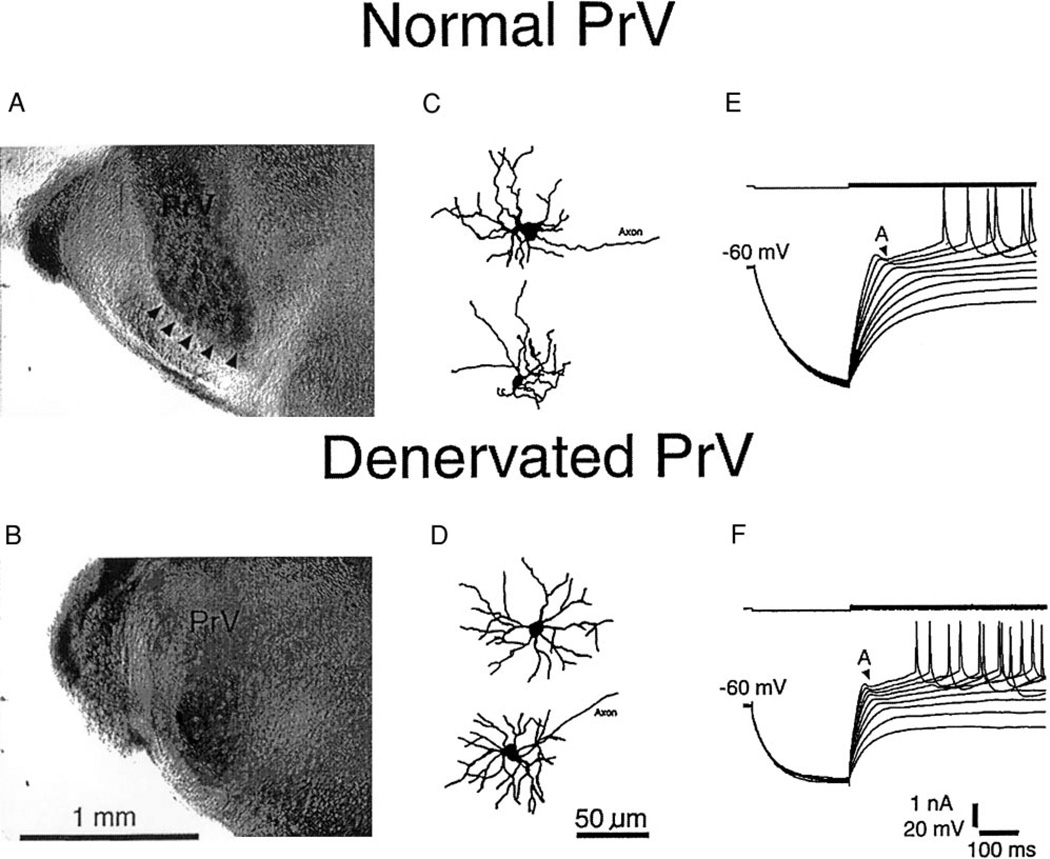
We tested dissolution of whisker-specific axon terminal patches in the ventral PrV by biocytin bulk fills. PND 8 Sprague-Dawley rat pups (n = 6) which had undergone unilateral IO nerve transection were anesthetized with Fluothane and killed by decapitation. After opening the skull, a cut was made at the midtectal level. Brain tissue rostral to the cut was removed to expose the trigeminal ganglia on both sides. The ganglia were punctured with a hypodermic needle, and biocytin crystals were inserted into the ganglia. Then the brain stem and trigeminal ganglia were dissected out and rinsed thoroughly to wash out biocytin crystals on the surface. Finally the preparation was incubated in artificial cerebrospinal fluid (ACSF) at 33°C for 6–8 h. After fixation in paraformaldehyde for 7 days, the brain stem was sectioned coronally at 500 µm and processed for biocytin reaction. On the normal side, biocytin-reaction product showed clear patches of axon terminals arranged in curvilinear rows reflecting the distribution of whiskers on the snout (Fig. 1A). These patterns were similar to those reported with other axonal and histochemical markers (e.g., Bates and Killackey 1985). On the denervated side, axonal patterns were no longer visible (Fig. 1B).
FIG. 1.
Morphological changes in denervated principal sensory nucleus (PrV). A: whisker-specific trigeminal afferent arbor patches in normal PrV revealed by ganglionic injection of biocytin. B: disappearance of afferent terminal patches in denervated PrV. C: examples of biocytin-labeled barrelette cells in normal PrV showing polarized dendritic trees. D: examples of biocytin-labeled barrelette cells in denervated PrV showing symmetrical dendritic trees. Both normal (E) and denervated (F) barrelette cells show a prominent A-type K+ conductance (IA) (denoted by A).
We also verified the effects of IO nerve transection by intracellular biocytin labeling. We filled the patch electrodes with 1% biocytin dissolved in potassium-based solution. Once membrane properties and synaptic responses were characterized, the cells were filled intracel-lularly with biocytin by passing AC pulses (±1 nA, 60 ms for each cycle, 100 cycles). One hour after biocytin injection, the slice was immersion fixed in 4% paraformaldehyde in 0.1 M phosphate buffer. The effects of nerve section were reflected in the orientation of dendritic fields of barrelette cells. In the normal PrV, barrelette cells showed polarized dendritic trees (n = 15, Fig. 1C). As demonstrated previously by others (Arends and Jacquin 1993), in the denervated PrV, barrelette cells with distinct IA lost their dendritic orientation, and their dendritic trees became symmetrical (n = 8, Fig. 1D).
Histological methods
The fixed slice was transferred into phosphate-buffered saline (PBS) at 4°C and then incubated in 10% methanol + 3% H2O2 overnight. After several rinses in PBS, the slice was reacted with avidin-biotin complex (ABC Elite kit, Vector Laboratories) overnight at 4°C (1:100 in PBS with 1.8% NaCl and 0.5% Triton X-100). The next day, the slice was rinsed again in PBS and 0.1 M acetate buffer (pH 6.0) and incubated in glucose oxidase–nickel ammonium sulfate and diaminobenzidine until the biocytin labeling could be visualized. The slice was rinsed in acetate buffer and PBS, mounted on a slide, dehydrated, and coverslipped. Labeled cells were drawn with a drawing tube attached to a Nikon Labophot microscope.
Brain slice preparation
PND 0 Sprague-Dawley rat pups were anesthetized with Fluothane and underwent unilateral transection of the IO nerve, and the animals were allowed to survive 4–8 days. They were then overdosed with Fluothane and killed by decapitation. These procedures were approved by the IACUC and followed National Institutes of Health guidelines. The brain was removed and immersed in cold (4°C) sucrose-based ACSF (in mM: 234 sucrose; 2.5 KCl; 1.25 NaH2PO4; 10 MgSO4; 24 NaHCO3; 11 glucose; 0.5 CaCl2) bubbled with 95% O2 −5% CO2 (pH 7.4). The brain stem was embedded in 2% agar and cut into 400-µm-thick transverse sections with a vibratome (Electron Microscopy Sciences) in the sucrose-based ACSF. Slices containing the PrV were selected, and the normal and denervated side of each slice was marked. After 1 h incubation in normal ACSF (in mM: 124 NaCl; 2.5 KCl; 1.25 NaH2PO4; 2 MgSO4; 26 NaHCO3; 10 glucose; 2 CaCl2, pH 7.4) at room temperature, each slice was transferred into a submerged-type recording chamber and continuously perfused (2 ml/ min) with oxygenized normal ACSF at room temperature.
Electrophysiological methods
Whole-cell patch micropipettes were pulled horizontally in two stages from borosilicate glass (WPI, K150F-4) with a P-87 puller (Sutter Instrument Co.). The patch electrodes were backfilled with a potassium-based solution (in mM: 140 K-gluconate; 10 HEPES; 1.1 EGTA-Na; 0.1 CaCl2; 2 MgCl2; 2 ATP-Na; 0.2 GTP-Na, pH 7.25) with a tip resistance of 7–10 MΩ. Neurons in the ventral part of the PrV (barrelette region) were blindly patched with the techniques described by Blanton et al. (1989) and Ferster and Jagadeesh (1992). Patch-electrode resistance was monitored in Bridge Mode of Axoclamp 2B amplifier by measuring the voltage drop induced by a current pulse (−100 pA, 200 ms). An increase in resistance of 20–50 MΩ was taken as a sign that the tip of the electrode contacted the surface of a neuron. A steady negative pressure was applied with a 5-ml syringe to form a gigaohm seal. Then brief suction was used to break into the neuronal soma. The formation of whole-cell configuration was indicated by a sudden drop in seal resistance and a DC drop of >55 mV. After “break-in,” the serial resistance (20–30 MΩ) was completely compensated with bridge balance, and junction potential (Neher 1992) was not corrected. We only collected data from cells with resting membrane potential negative to −55 mV and input resistance >200 MΩ with an Instrutek ITC-16 interface unit and stored on a Pentium III PC with Pulses (HEKA) software program.
Different DC pulse protocols were used to induce active conductances of trigeminal neurons. As we reported before (Lo et al. 1999), Barrelette cells in normal PrV were identified by their prominent IA Following IO nerve transection, barrelette cells still possessed a prominent IA (Fig. 1, E vs. F, see also Lo and Erzurumlu 2001) that was specifically blocked by 1 mM 4-aminopyradine (4-AP, n = 5). A pair of fine-tip stimulating electrodes (0.5 MΩ, WPI, IRM33A05KT) was inserted at various points along the trigeminal tract (TrV) lateral to the ventral PrV (barrelette region). Current pulses (0.2- to 0.5-ms duration, 0.05–1.0 mA) were passed through the electrodes at 0.2 Hz to evoke postsynaptic potentials. Identification of excitatory postsynaptic potentials (EPSPs) and inhibitory postsynaptic potentials (IPSPs) was based on their voltage dependency and their responses to glutamate and GABA antagonists. The non-NMDA component of an EPSP increased in amplitude with membrane hyperpolarization. The NMDA component of an EPSP was identified by its nonlinear voltage dependency, slow decay time, and blockade by d-APV (100 µM). The GABAA receptor-mediated IPSP was identified by its reversal with membrane hyperpolarization (~70 mV) and blockade by bicuculline (10 µM). The synaptically activated L-type Ca2+ conductance was blocked by 10 µM nitrendipine. To compare postsynaptic responses among different cells, the membrane potential was held at −60 mV, except in cases where indicated.
RESULTS
If patterning of trigeminal inputs and dendritic orientation of barrelette cells change so dramatically following deafferentation, which aspects of electrophysiological properties of these cells are affected, and how do they relate to structural plasticity or transsynaptic cell death following deafferentation? To address these issues, we examined synaptic responses of deafferented barrelette cells.
Development of postsynaptic responses in barrelette neurons
Trigeminal inputs activate both NMDA and non-NMDA glutamate receptors of the barrelette cells. These cells also receive inhibitory interneuronal inputs mediated by GABAA receptors (Lo et al. 1999). Postsynaptic potentials in barrelette cells were evoked by stimulation of the TrV at a low intensity (weak stimulus, usually <50 µA) that was subthreshold for inducing postsynaptic spikes. Such stimulation induced an EPSP-IPSP sequence (Fig. 2A, top trace) as early as PND 1. The IPSPs were reversed in polarity when the membrane potential was hyperpolarized (Fig. 2A, bottom trace) and blocked by bicuculline (data not shown). This response pattern was also observed in five other cells tested at PND 1, suggesting that neuronal circuitry in PrV is established by birth. At PND 10 or later, the postsynaptic responses of seven barrelette neurons were similar to those at PND 1 (Fig. 2, A vs. B).
FIG. 2.
Postsynaptic responses to weak stimulation of trigeminal tract (TrV) in barrelette neurons during postnatal development and after infraorbital (IO) nerve transection. A: stimulation of TrV at a subthreshold intensity for postsynaptic Na+ spikes (<50 µA) induced an excitatory postsynaptic potential–inhibitory postsynaptic potential (EPSP-IPSP) sequence in a barrelette cell at PND 1. Note that the IPSP is in hyperpolarizing direction at resting potential (−60 mV). It is reversed into depolarizing direction at −105 mV. B: at PND 10, weak stimulation induced an EPSP-IPSP sequence. The IPSP is reversed by membrane hyperpolar-ization (lower trace). C: weak stimulation of TrV induced an EPSP-IPSP sequence in denervated barrelette cells at PND 5.
IO nerve transection does not change local neuronal circuitry
In the denervated PrV, stimulation of TrV could still elicit an EPSP-IPSP sequence in barrelette cells (Fig. 2C). We recorded synaptic responses from 20 barrelette neurons in denervated PrV at PND 4–8. All of them received excitatory and inhibitory inputs following stimulation of the TrV. Thus IO nerve transection does not alter basic synaptic connections that are normally formed at birth.
IO nerve transection alters synaptic responses to strong stimulation of TrV
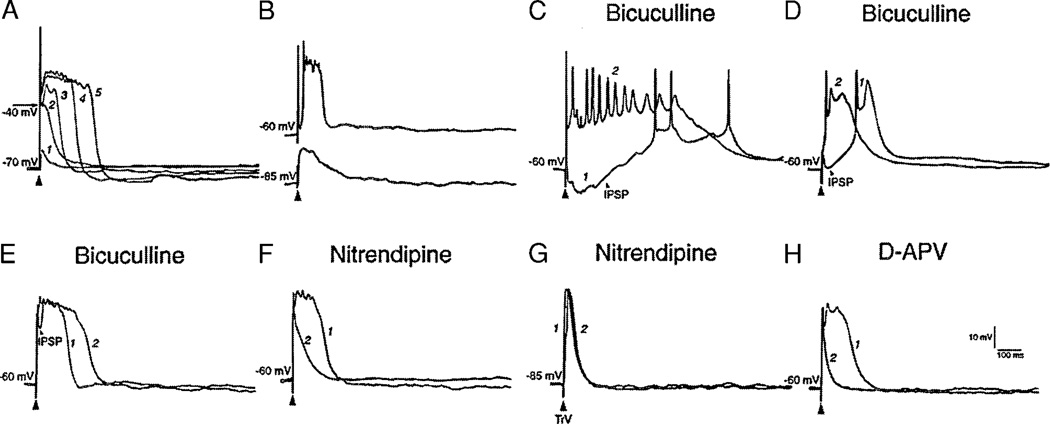
In normal barrelette cells, strong stimulation (300–500 µA) of the TrV induced an EPSP with a Na+ spike riding on it and was followed by a fast decay (>0.5 mV/ms) that resulted from an afterhyperpolarization (AHP) and IPSP (Fig. 3A). Stimulation of the TrV with three electrical shocks at 50 Hz evoked three EPSP-spike complexes and a compound hyperpolarization (Fig. 3D). In the presence of GABAA antagonist bicuculline (10 µM), a single shock elicited a long-lasting EPSP with multiple (2–5) spikes (Fig. 3G). This was the typical response pattern for most (27/32) barrelette cells in normal PrV. However, in almost all denervated barrelette cells (19/20), single electrical shock resulted in an EPSP-spike complex that is followed by a sustained depolarization (plateau potential). The plateau potential was distinct from the EPSP-IPSP sequence by 1) a sustained (>80 ms) depolarization above (positive to) −40 mV; 2) a slow decline slope (<0.1 mV/ms); 3) partially or totally inactivated Na+ spikes on the plateau (Fig. 3B); and 4) then, a steep decay to a hyperpolarizing membrane level (Fig. 4A). Since the barrelette cells receive a prominent IPSP (Lo et al. 1999), the plateau potential was sometimes delayed by the IPSP (Fig. 3C). Stimulation with three shocks always induced a much longer (>250 ms) plateau potential (Fig. 3, E and F). Application of bicuculline always prolonged the plateau potential (Fig. 3, B vs. H; C vs. I) and shortened the latency of the plateau (Fig. 3I).
FIG. 3.
Strong stimulation of TrV results in a plateau potential in denervated barrelette cells. A: single electrical shock at the maximal intensity (300–500 µA) induces an EPSP-IPSP sequence with a Na+ spike riding on it in a normal barrelette cell. B and C: denervated barrelette cells respond to strong stimulation with a plateau potential that appears either immediately after the Na+ spike (B) or after the IPSP (C). D: in the same normal barrelette cell, 3 shocks at 50 Hz evoked 3 Na+ spikes riding on 3 EPSP-IPSP sequences. E and F: in the same denervated barrelette cells, 3 shocks elicited long-lasting plateau potentials. Note that Na+ spikes are totally or partially inactivated during plateau potentials. G: in normal barrelette cell, single shock induces a prolonged EPSP with 2 spikes riding on it in the presence of bicuculline (10 µM) (E vs. A). H and I: in the same denervated barrelette cells, application of bicuculline either prolonged the duration of the plateau potential (H vs. B) or shortened its latency (I vs. C).
FIG. 4.
Mechanisms underlying the generation of the plateau potential. A: stimulation of TrV with increasing intensity increases the EPSP amplitude (trace 1 vs. trace 2) in a denervated barrelette neuron. When the late EPSP reaches above −40 mV, plateau potential is evoked (trace 3). Further increase of intensity causes an increase in amplitude and duration of the plateau potential (traces 4 and 5). B: when the membrane potential is held at −60 mV, strong stimulus results in a plateau potential (upper trace). However, at —85 mV, the same stimulus fails to induce a plateau potential (lower trace). C: single shock at moderate intensity (<250 µA) does not evoke the plateau potential (trace 1). In the presence of bicuculline, the same stimulus induces a plateau potential (trace 2). D: application of bicuculline shortens the latency of plateau potentials (trace 1: before; trace 2: after drug application). E: application of bicuculline prolongs the duration of the plateau potential (trace 1 vs. trace 2). Note that the IPSP is blocked in trace 2. F: nitrendipine blocks the plateau potential at −60 mV (trace 1 vs. trace 2). G: nitrendipine does not alter the size of the EPSPs in the same cell (trace 1 vs. trace 2). H: the plateau potential is blocked by d-APV (100 µM trace 1 vs. trace 2).
Voltage- and time-dependence for generation of plateau potential
The mechanism underlying the generation of plateau potentials could be revealed by stimulation of the TrV at different intensities (Fig. 4A). A weak stimulus (usually <50 µA) induced an EPSP-IPSP sequence (trace 1), while a moderate stimulus (usually <250 µA) evoked a Na+ spike riding on the EPSP (trace 2). A stronger stimulus (around 300 µA) resulted in a plateau potential that started right after the initial Na+ spike and AHP (trace 3). Further increase in stimulation intensity (to 500 µA) caused an increase in amplitude and duration of the plateau potential (traces 4 and 5). The threshold of the plateau potential was about −40 mV. Namely, an EPSP that depolarized the membrane above −40 mV would trigger a plateau potential. This voltage-dependency was confirmed by passing hyperpolarizing current. At resting membrane potential (−60 mV), strong stimulation of the TrV could evoke a plateau potential (Fig. 4B, top trace), whereas at a hyperpolarizing membrane potential (−85 mV), the same stimulus was not able to induce a plateau potential (Fig. 4B, bottom trace). In addition, the generation of plateau potential depended on the duration of depolarization. The plateau potential was not triggered by the depolarization of the Na+ spike, because the duration of the spike above −40 mV was very short (<2 ms). As shown in Fig. 4, A and B, the plateau potential was triggered by the late component of the EPSP, suggesting that its generation is time-dependent. When the EPSP depolarized the membrane above −40 mV for a certain period of time, the plateau potential was induced.
Regulatory effects of inhibitory input on plateau potential
Barrelette cells receive multiple inhibitory inputs (Lo et al. 1999). Because the generation of plateau potential required membrane depolarization caused by excitatory inputs, hyperpolarization from inhibitory inputs (IPSPs) can regulate the plateau potentials. We studied the regulatory effects of inhibition on plateau potentials by application of bicuculline (10 µM, n = 7). In the denervated PrV, when the IPSP evoked from stimulation of TrV was of sufficiently large amplitude and long duration, the late component of the EPSP depolarized the membrane below (negative to) −40 mV. Therefore the plateau potential was not induced (Fig. 4C, trace 1). Bicuculline blocked the IPSP and resulted in a plateau potential (Fig. 4C, trace 2). This phenomenon was observed in two barrelette cells. Generation of IPSP also delayed the plateau potential. When the IPSP was relatively small, the late component of EPSP could induce a plateau potential after the peak of the IPSP (Fig. 4D, trace 1). Bicuculline blocked the IPSP and shortened the latency of plateau potential (Fig. 4D, trace 2). When the IPSP was much smaller in amplitude than the EPSP, strong stimulation induced the plateau potential soon after the onset of IPSP. The IPSP was expressed as a hyperpolarizing notch before the plateau potential (Fig. 4E, trace 1). Bicuculline abolished the notch and shortened the latency of the plateau (Fig. 4E, trace 2). Therefore the latency of the plateau varied from 7 to 139 ms (n = 10), depending on the algebraic summation of EPSP and IPSP. As soon as the summated potential reached −40 mV, a plateau potential was induced. Bicuculline always shortened the latency of the plateau by 62 ± 6% (mean ± SE, n = 6). In addition, generation of IPSP regulated the duration of the plateau potential, because bicuculline prolonged the duration of the plateau (Fig. 4, D and E, trace 1 vs. trace 2). On average, bicuculline increased the duration of the plateau by 51 ± 12% (n = 6).
The plateau potential is mediated by L-type Ca 2+ channels
The waveform of plateau potential was clearly different from EPSP-IPSP sequence, suggesting some voltage-dependent ion channels were involved in the generation of plateau potential. To test this possibility, we used nitrendipine, an L-type Ca2+ channel blocker. Application of nitrendipine (10 µM) completely blocked the plateau potential (n = 8) and disclosed the underlying EPSP (Fig. 4F, trace 1 vs. trace 2). Before nitrendipine application, the duration of sustained depolarization above −40 mV was 142.9 ± 13.5 ms (n = 8). After application of nitrendipine, the duration of depolarization above −40 mV became 12.8 ± 3.1 ms (n = 8). The difference in depolarization duration was extremely significant (P < 0.0001), indicating that the plateau potential was completely blocked by nitrendipine. This blockade of plateau potential was not caused by a change in synaptic transmission, because at a hyperpolarizing membrane potential (−85 mV), nitrendipine did not affect the size of EPSPs (Fig. 4G, trace 1 vs. trace 2).
The plateau potential is triggered by NMDA receptor-mediated EPSP
Since the plateau potential was induced by the late component of the EPSP rather than the Na+ spike, we bath applied d-APV (100 µM) to block NMDA receptors. d-APV also blocked the plateau potential (n = 8) and disclosed the Na+ spike and non-NMDA component of the EPSP (Fig. 4H, trace 1 vs. trace 2). Before application of d-APV, the duration of depolarization above −40 mV was 129.5 ± 11.7 ms (n = 8), while after d-APV application, the duration dropped to 6.8 ± 1.3 ms (n = 8). This significant change in depolarization duration (P < 0.0001) indicated that d-APV completely blocked the plateau potential.
These observations further supported the time-dependency for the generation of the plateau potential. By inference, regenerative activation of L-type Ca2+ channels requires >10-ms depolarization above −40 mV, as shown by nitrendipine application. Application of d-APV prevents the plateau potential by shortening the depolarization to < 10 ms. Therefore neither Na+ spike nor non-NMDA receptor-mediated EPSP can trigger the plateau potential.
DISCUSSION
Following neonatal denervation of the whisker pad, PrV neurons undergo dramatic changes. First, whisker-specific patterning of trigeminal afferent terminals is lost and barrelette cells reorient their dendritic trees from focalized to nonspecific symmetric distribution. These prominent structural changes are easily detected with histochemical stains reflecting synaptic modifications (see Woolsey 1990 for a review). Second, substantial cell death occurs within the denervated PrV through apoptotic mechanisms (Miller and Kuhn 1997). Presently the intracellular signaling pathways and molecular mechanisms underlying structural plasticity of barrelette neurons and apoptotic events are not known. Here we show that, following denervation of the PrV at birth, barrelette neurons display a sustained depolarizing potential (plateau potential) mediated by L-type Ca2+ channels. The generation of plateau potential requires activation of NMDA receptors. Furthermore, GABAA receptor-mediated IPSPs modify the plateau potential in different ways. Presently we do not know to what extent this elevated Ca2+ entry into the barrelette cells participates in dendritic remodeling or deafferentation-induced apoptotic events.
Patterned central arbors of single primary afferent fibers in the denervated PrV diffuse after a week following peripheral injury (Bates and Killackey 1985). These changes in afferent organization are accompanied by the rearrangement of barrelette cell dendritic trees as described in the present study, and previously (Arends and Jacquin 1993). Presumably, during structural modification, single barrelette neurons end up receiving more synaptic inputs than normal PrV. The spatial summation of excitatory inputs results in a compound EPSP that might lead to activation of L-type Ca2+ channels. This is supported by the results of a previous study which showed larger and more complex receptive fields in denervated PrV neurons (Waite 1984). The plateau potential most likely results from this increased synaptic activation of barrelette neurons. The L-type Ca2+ channel-mediated plateau potential in the denervated PrV is a sustained depolarization with a slow decay, suggesting that it results from regenerative activation of L-type Ca2+ channels. Such activation of L-type Ca2+ channels requires membrane depolarization above −40 mV for >10 ms; therefore, NMDA receptor-mediated EPSP plays a pivotal role in the generation of plateau potential. In other brain structures, NR2B subunit of the NMDA receptors is gradually replaced by NR2A subunit during postnatal development, so that the duration of NMDA synaptic current is shortened in older animals (see review by Cull-Candy et al. 2001). In the visual cortex, the subunit composition of NMDA receptors is bidirectionally modified by visual experience and deprivation (Philpot et al. 2001; Quinlan et al. 1999a,b). This may also be true for the trigeminal system. In the present study we show that IO nerve transection does not disrupt local neuronal circuits in PrV. However, the subunit composition of NMDA receptors may be changed after IO transection. If denervation delays the replacement of NR2B subunit as shown in the visual cortex, NMDA receptor-mediated EPSP in denervated barrelette cells may have a longer duration than normal ones, that results in a plateau potential. Ongoing studies investigate the possible changes in subunit composition of NMDA receptors after IO transection.
GABAA receptor-mediated IPSP may delay, shorten, or prevent the generation of the plateau potential. Thus the plateau potential in denervated barrelette neurons may be caused by an absent or diminished GABAA receptor activity after IO nerve transection. However, this possibility seems unlikely, because our whole-cell recordings from denervated PrV did not reveal a diminished IPSP (see Fig. 2C). In addition, application of bicuculline modified the latency or the duration of the plateau potential, suggesting that GABAA-mediated IPSP in denervated PrV is still functional. Another possibility for the induction of plateau potential in denervated PrV is that IO nerve transection increases the expression of L-type Ca2+ channels. Immunohistochemical examination of L-type calcium channel expression in normal versus denervated PrV may shed light on this issue.
Synaptically activated plateau potentials have previously been described in a few types of neurons in invertebrates (Dicaprio 1997; Kiehn and Harris-Warrick 1992) and vertebrates (Campbell and Hesslow 1984; Di Prisco et al. 1997; Hounsgaard and Kiehn 1993; Morisset and Nagy 1998; Rekling and Feldman 1997; Russo and Houndgaard 1996). Recently, it was noted that such potentials are prominent during refinement of the rodent visual pathways both in the developing superior colliculus (Lo and Mize 1999, 2000) and in the lateral geniculate nucleus (Lo et al. 2002). Since the rat trigeminal pathway develops much earlier than the visual pathway, we do not know if such potentials are also prominent during the establishment of the barrelettes in late embryonic stages. In most types of neurons, the generation of the plateau potential requires Ca2+ influx through L-type Ca2+ channel. However, a Ca2+-activated, nonselective cationic conductance (ICAN) may also be involved (Kiehn and Eken 1998; Morisset and Nagy 1999; Pearlstein and Dubuc 1998; Rekling and Feldman 1997; Zhang et al. 1995). The role of L-type Ca2+-mediated plateau potentials in structural and functional plasticity events is not fully understood. In spinal cord dorsal horn neurons, Ca2+ influx through L-type channels is a critical component of short-term synaptic plasticity (Morisset and Nagy 2000; Russo and Hounsgaard 1994). In the superior colliculus of neonatal rats, it is associated with the induction of long-term depression of retino-collicular transmission (Lo and Mize 2000). Now, we show that the plateau potential is present mainly in denervated PrV; it is possible that these potentials play a role in injury-induced plastic rearrangements within the PrV.
Synaptic plasticity and reorganization require an elevation of intracellular concentration of Ca2+ through Ca2+ release from intracellular stores or Ca2+ influx via NMDA receptors or L-type Ca2+ channels (Chittajallu et al. 1988; Zuker 1999). Furthermore, activation of NMDA receptors has been shown to play a crucial role in the formation of whisker-specific patterns in the brain (Iwasato et al. 1997, 2000; Li et al. 1994). However, the role of Ca2+ influx via L-type channel in pattern formation and injury-induced structural plasticity is still unknown. As judged by membrane potential changes, the Ca2+ influx through L-type Ca2+ channels is much larger and longer lasting than that through NMDA receptors. L-type Ca2+ channels exhibit significantly slower activation kinetics (Fox et al. 1987). Ca2+ influx via L-type Ca2+ channels could play a particularly important role in signaling pathways for synaptic plasticity, e.g., promoting calmodulin translocation (Deisseroth et al. 1998) and cAMP response element-binding protein (CREB) phosphorylation (Rajadhyaksha et al. 1999), regulating brain-derived neurotrophic factor (BDNF) mRNA expression (Shieh et al. 1998; Tao et al. 1998). A recent study demonstrated that Ca2+ influx via L-type channels induces BDNF gene activation more effectively than those evoked via NMDA receptors (Tabuchi et al. 2000). The role of this neurotrophin in dendritic differentiation has been noted (Hirai and Launey 2000; Horch et al. 1999; Mertz et al. 2000). Thus emergence of plateau potentials in denervated barrelette neurons may be linked to their dendritic plasticity.
Synaptic activation of L-type Ca2+ channels in denervated PrV could also be associated with the ensuing cell death. During normal development, programmed cell death in the PrV begins at E19 and continues until PND10 (Ashwell and Waite 1991; Miller and Al-Ghoul 1993). Neonatal IO nerve section leads to death of an additional one-third of the PrV neurons through apoptosis (Miller and Kuhn 1997). In light of evidence that several forms of excitotoxicity events are mediated by increased levels of Ca2+ influx via L-type Ca2+ channels (Freund and Reddig 1994; Leski et al. 1999), the emergence of plateau potentials in denervated barrelette cells could reflect ongoing cell death induced by deafferentation.
Acknowledgments
This research was supported by National Institute of Neurological Disorders and Stroke Grant NS-3707.
REFERENCES
- Arends JJ, Jacquin MF. Lucifer Yellow staining in fixed brain slices: optimal methods and compatibility with somatotopic markers in neonatal brain. J Neurosci Methods. 1993;50:321–339. doi: 10.1016/0165-0270(93)90039-t. [DOI] [PubMed] [Google Scholar]
- Ashwell KW, Waite PME. Cell death in the developing trigeminal nuclear complex of the rat. Dev Brain Res. 1991;63:291–295. doi: 10.1016/0165-3806(91)90089-2. [DOI] [PubMed] [Google Scholar]
- Bates CA, Killackey HP. The organization of the neonatal rat’s brainstem trigeminal complex and its role in the formation of central patterns. J Comp Neurol. 1985;240:265–287. doi: 10.1002/cne.902400305. [DOI] [PubMed] [Google Scholar]
- Blanton MG, Turco JJL, Kriegstein AR. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Campbell NC, Hesslow G. Plateau potentials evoked by climbing-fibre stimulation are restricted to the Purkinje cell dendrites of the cat. Neurosci Lett. 1984;45:187–192. doi: 10.1016/0304-3940(84)90097-1. [DOI] [PubMed] [Google Scholar]
- Chiaia NL, Bennett-Clarke CA, Eck M, White FA, Crissman RS, Rhoades RW. Evidence for prenatal competition among the central arbors of trigeminal primary afferent neurons. Neuroscience. 1992;12:62–76. doi: 10.1523/JNEUROSCI.12-01-00062.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Alford S, Collingridge GL. Ca2+ and synaptic plasticity. Cell Calcium. 1988;24:377–385. doi: 10.1016/s0143-4160(98)90061-6. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Heist EK, Tsien RW. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392:198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- Dicaprio R. Plateau potentials in motor neurons in the ventilatory system of the crab. J Exp Biol. 1997;200:1725–1736. doi: 10.1242/jeb.200.12.1725. [DOI] [PubMed] [Google Scholar]
- Di Prisco GV, Pearlstein E, Robitaille R, Dubuc R. Role of sensory-evoked NMDA plateau potentials in the initiation of locomotion. Science. 1997;278:1122–1125. doi: 10.1126/science.278.5340.1122. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S. Thalamic axons confer a blueprint of the sensory periphery onto the developing rat somatosensory cortex. Dev Brain Res. 1990;56:229–234. doi: 10.1016/0165-3806(90)90087-f. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Kind PC. Neural activity: sculptor of “barrels” in the neocortex. Trends Neurosci. 2001;24:589–595. doi: 10.1016/s0166-2236(00)01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D, Jagadeesh B. EPSP-IPSP interactions in cat visual cortex studied with in vivo whole-cell patch recording. Neuroscience. 1992;12:1262–1274. doi: 10.1523/JNEUROSCI.12-04-01262.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AP, Nowycky MC, Tsien RW. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol (Lond) 1987;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund WD, Reddig S. AMPA/Zn (2+)-induced neurotoxicity in rat primary cortical cultures: involvement of L-type calcium channels. Brain Res. 1994;22:257–264. doi: 10.1016/0006-8993(94)90487-1. [DOI] [PubMed] [Google Scholar]
- Hirai H, Launey T. The regulatory connection between the activity of granule cell NMDA receptors and dendritic differentiation of cerebellar Purkinje cells. Neuroscience. 2000;15:5217–5224. doi: 10.1523/JNEUROSCI.20-14-05217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch HW, Kruttgen A, Portbury SD, Katz LC. Destabilization of cortical dendrites and spines by BDNF. Neuron. 1999;23:353–364. doi: 10.1016/s0896-6273(00)80785-0. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Calcium spikes and calcium plateaux evoked by differential polarization in dendrites of turtle motoneurones in vitro. J Physiol (Lond) 1993;468:245–259. doi: 10.1113/jphysiol.1993.sp019769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasato T, Datwani A, Wolf AM, Nishiyama H, Taguchi Y, Tonegawa S, Knopfel T, Erzurumlu RS, Itohara S. Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in barrel cortex. Nature. 2000;406:726–731. doi: 10.1038/35021059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasato T, Erzurumlu RS, Huerto PT, Sasaoka T, Ulupinar E, Tonegawa S. NMDA receptor-dependent refinement of somatotopic maps. Neuron. 1997;19:1–20. doi: 10.1016/s0896-6273(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Eken T. Functional role of plateau potentials in vertebrate motor neurons. Curr Opin Neurosci. 1998;8:746–752. doi: 10.1016/s0959-4388(98)80117-7. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Harris-Warrick RM. Serotonergic stretch receptors induce plateau properties in a crustacean motor neuron by a dual-conductance mechanism. J Neurophysiol. 1992;68:485–495. doi: 10.1152/jn.1992.68.2.485. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Bennett-Clarke CA, Rhoades RW. The formation of a cortical somatotopic map. Trends Neurosci. 1995;18:402–407. doi: 10.1016/0166-2236(95)93937-s. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Fleming K. The role of the principal sensory nucleus in central trigeminal pattern formation. Brain Res. 1985;354:141–145. doi: 10.1016/0165-3806(85)90077-x. [DOI] [PubMed] [Google Scholar]
- Leski ML, Valentine SL, Coyle JT. L-type voltage-gated calcium channels modulate kainic acid neurotoxicity in cerebellar granule cells. Brain Res. 1999;828:27–40. doi: 10.1016/s0006-8993(99)01270-6. [DOI] [PubMed] [Google Scholar]
- Li Y, Erzurumlu RS, Chen C, Jhaveri S, Tonegawa S. Whisker-related neuronal patterns fail to develop in the brainstem trigeminal nuclei of MDAR1 knockout mice. Cell. 1994;76:427–437. doi: 10.1016/0092-8674(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Lo F-S, Erzurumlu RS. Neonatal deafferentation does not alter membrane properties of trigeminal nucleus principalis neurons. J Neurophysiol. 2001;85:1088–1098. doi: 10.1152/jn.2001.85.3.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo F-S, Guido W, Erzurumlu RS. Electrophysiological properties and synaptic responses of cells in the trigeminal principal sensory nucleus of postnatal rats. J Neurophysiol. 1999;82:2765–2775. doi: 10.1152/jn.1999.82.5.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo F-S, Mize RR. Retinal input induces three firing patterns in neurons of the superficial superior colliculus of neonatal rats. J Neurophysiol. 1999;81:54–958. doi: 10.1152/jn.1999.81.2.954. [DOI] [PubMed] [Google Scholar]
- Lo F-S, Mize RR. Synaptic regulation of L-type Ca2+ channel activity and long-term depression during refinement of the retinocollicular pathway in developing rodent superior colliculus. Neuroscience. 2000;20 doi: 10.1523/JNEUROSCI.20-03-j0003.2000. RC58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo F-S, Ziburkus J, Guido W. Synaptically evoked Ca2+-mediated plateau potentials in LGN. J Neurophysiol. 2002;87:1175–1185. doi: 10.1152/jn.00715.1999. [DOI] [PubMed] [Google Scholar]
- Mertz K, Koscheck T, Schilling K. Brain-derived neurotrophic factor modulates dendritic morphology of cerebellar basket and stellate cells: an in vitro study. Neuroscience. 2000;97:303–310. doi: 10.1016/s0306-4522(99)00585-0. [DOI] [PubMed] [Google Scholar]
- Miller MW, Al-Ghoul WM. Numbers of neurons in the developing principal sensory nucleus of the trigeminal nerve: enhanced survival of early-generated neurons over late-generated neurons. J Comp Neurol. 1993;330:491–501. doi: 10.1002/cne.903300405. [DOI] [PubMed] [Google Scholar]
- Miller MW, Kuhn PE. Neonatal transection of the infraorbital nerve increases the expression of proteins related to neuronal death in the principal sensory nucleus of the trigeminal nerve. Brain Res. 1997;769:233–244. doi: 10.1016/s0006-8993(97)00713-0. [DOI] [PubMed] [Google Scholar]
- Morisset V, Nagy F. Nociceptive integration in the rat spinal cord: role of nonlinear membrane properties of deep dorsal horn neurons. Eur J Neurosci. 1998;10:3642–3652. doi: 10.1046/j.1460-9568.1998.00370.x. [DOI] [PubMed] [Google Scholar]
- Morisset V, Nagy F. Ionic basis for plateau potentials in deep dorsal horn neurons of rat spinal cord. Neuroscience. 1999;19:7309–7316. doi: 10.1523/JNEUROSCI.19-17-07309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisset V, Nagy F. Plateau potential-dependent windup of the response to primary afferent stimuli in rat dorsal horn neurons. Eur J Neurosci. 2000;12:3087–3095. doi: 10.1046/j.1460-9568.2000.00188.x. [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- O‘Leary DDM, Ruff NL, Dyck RH. Development, critical period plasticity, and adult reorganizations of mammalian somatosensory systems. Curr Opin Neurobiol. 1994;4:535–544. doi: 10.1016/0959-4388(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Pearlstein E, Dubuc R. Cellular mechanisms underlying plateau potentials in lamprey reticulospinal neurons. Soc Neurosci Abstr. 1998;24:1669. [Google Scholar]
- Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-d-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci USA. 1999a;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nature Neurosci. 1999b;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- Rajadhyaksha A, Barczak A, Macias W, Leveque J-C, Lewis LS, Konradi C. L-type Ca2+ channels are essential for glutamate-mediated CREB phosphorylation and c-fos gene expression in striatal neurons. Neuroscience. 1999;19:6348–6359. doi: 10.1523/JNEUROSCI.19-15-06348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. Calcium-dependentplateau potentials in rostral ambiguous neurons in the newborn mouse brain stem in vitro. J Neurophysiol. 1997;78:2483–2492. doi: 10.1152/jn.1997.78.5.2483. [DOI] [PubMed] [Google Scholar]
- Russo RE, Hounsgaard J. Short-term plasticity in turtle dorsal horn neurons mediated by L-type Ca2+ channels. Neuroscience. 1994;61:191–197. doi: 10.1016/0306-4522(94)90222-4. [DOI] [PubMed] [Google Scholar]
- Russo RE, Hounsgaard J. Plateau-generating neurons in the dorsal horn in an in vitro preparation of the turtle spinal cord. J Physiol (Lond) 1996;493:39–54. doi: 10.1113/jphysiol.1996.sp021363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh PB, Hu S-C, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Tabuchi A, Nakaoka R, Amano K, Yukimine M, Andoh T, Kuraishi Y, Tsuda M. Differential activation of brain-derived neurotrophic factor gene promoters I and III by Ca2+ signals evoked via L-type voltage-dependent and N-methyl-d-aspartate receptor Ca2+ channels. J Biol Chem. 2000;275:17269–17275. doi: 10.1074/jbc.M909538199. [DOI] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greeenberg ME. Ca influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Waite PME. Rearrangement of neuronal responses in the trigeminal system of the rat following peripheral nerve section. J Physiol (Lond) 1984;352:425–445. doi: 10.1113/jphysiol.1984.sp015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA. Peripheral alteration and somatosensory system development. In: Coleman EJ, editor. Development of Sensory Systems in Mammals. New York: Wiley; 1990. pp. 461–516. [Google Scholar]
- Zhang B, Wootton JF, Harris-Warrick RM. Calcium-dependent plateau potentials in a crab stomatogastric ganglion motor neuron II. Calcium activated slow inward current. J Neurophysiol. 1995;74:1938–1946. doi: 10.1152/jn.1995.74.5.1938. [DOI] [PubMed] [Google Scholar]
- Zuker RS. Calcium- and activity-dependent synaptic plasticity. Curr Opin. 1999;9:305–313. doi: 10.1016/s0959-4388(99)80045-2. [DOI] [PubMed] [Google Scholar]