Abstract
The cuticular hairs and sensory bristles that decorate the adult Drosophila epidermis and the denticles found on the embryo have been used in studies on planar cell polarity and as models for the cytoskeletal mediated morphogenesis of cellular extensions. ZP domain proteins have recently been found to be important for the morphogenesis of both denticles and bristles. Here we show that the ZP domain protein Dusky-Like is a key player in hair morphogenesis. As is the case in bristles, in hairs dyl mutants display a dramatic phenotype that is the consequence of a failure to maintain the integrity of the extension after outgrowth. Hairs lacking dyl function are split, thinned, multipled and often very short. dyl is required for normal chitin deposition in hairs, but chitin is not required for the normal accumulation of Dyl, hence dyl acts upstream of chitin. A lack of chitin however, does not mimic the dyl hair phenotype, thus Dyl must have other targets in hair morphogenesis. One of these appears to be the actin cytoskeleton. Interestingly, dyl mutants also display a unique planar cell polarity phenotype that is distinct from that seen with mutations in frizzled/starry night or dachsous/fat pathway genes. Rab11 was previously found to be essential for Dyl plasma membrane localization in bristles. Here we found that the expression of a dominant negative Rab11 can mimic the dyl hair morphology phenotype consistent with Rab11 also being required for Dyl function in hairs. We carried out a small directed screen to identify genes that might function with dyl and identified Chitinase 6 (Cht6) as a strong candidate, as knocking down Cht6 function led to weak versions of all of the dyl hair phenotypes.
Keywords: dyl, Drosophila, wing hair, chitin, planar polarity
Introduction
The adult cuticle of Drosophila is decorated with a variety of polarized extensions. These include bristle sense organs and non-sensory hairs (trichomes) and arista laterals (Adler, 2002; He and Adler, 2001; Tilney et al., 2000; Wong and Adler, 1993). The larger of these structures (bristle shafts and arista laterals) are formed by polyploid cells while epidermal hairs are produced by outgrowths from the apical surface of diploid cells. All of these extensions involve both the actin and microtubule cytoskeletons (Geng et al., 2000; Tilney et al., 2000; Turner and Adler, 1998) and many genes have been found to be important in the morphogenesis of all 3-cell types. However, mutations in some genes do not produce equivalent phenotypes in these cell types or in the related denticles that decorate larvae (Fernandes et al., 2010; Price et al., 2006). This could be due to the great difference in size or in other extension specific properties.
The array of distally pointing hairs on the wing of Drosophila has been used extensively in studies on planar cell polarity (PCP)(Adler, 2002; Adler, 2012; Amonlirdviman et al., 2005; Goodrich and Strutt, 2011; Lawrence et al., 2007; Strutt, 2002; Wu and Mlodzik, 2009). As an aid to such studies some years ago we characterized changes in pupal wing gene expression around the time of hair morphogenesis (Ren et al., 2005). More than 1300 genes were identified where a greater than two fold change in expression was seen and 436 where a fivefold or greater change was detected. One of the genes that stood out was CG15013, which is also known as dusky-like (dyl). Dyl contains a ZP (zona pellucida) domain, which has been found in a number of transmembrane and secreted proteins that are thought to organize the apical extracellular domain and perhaps in some contexts link it to the actin cytoskeleton (Fernandes et al., 2010; Plaza et al., 2010). The expression of dyl increased 118 fold from the start to late in the process of hair mophogenesis. Recently dyl and other genes that encode ZP domain proteins were shown to play a key role in the morphogenesis of first larval instar denticles and different ZP proteins were found to localize to different parts of the denticle (Fernandes et al., 2010). Differences in mutant phenotypes reflected the differences in protein localization and suggested that at least some ZP domain proteins link cuticle to the apical plasma membrane. Further, we recently found that dyl function was required for the formation of normal cuticle in adult sensory bristles (Nagaraj and Adler, 2012). We additionally established that Dyl functioned as a Rab11 effector for chitin deposition and that Rab11 function was required for the localization of Dyl in the plasma membrane.
Here we report that dyl is also important for wing hair morphogenesis. dyl loss of function leads to thin, split and multipled hairs and the Dyl protein accumulated in hairs. Interestingly, the mutant phenotype was not due to an effect on hair growth. Rather, the abnormalities were first detected after hair outgrowth was largely complete. This was very similar to the phenotype seen in sensory bristles where the adult stub bristle phenotype was associated with bristle collapse and not a failure in growth (Nagaraj and Adler, 2012). We also saw abnormalities in chitin deposition in these wings consistent with the role for dyl in cuticle formation seen previously in both embryonic denticles and in sensory bristles (Fernandes et al., 2010; Nagaraj and Adler, 2012). In contrast chitin was not required for the normal accumulation of Dyl in growing hairs, thus Dyl acts upstream of chitin. In addition to the hair morphology abnormalities seen with a reduction in Dyl we also found that the normal parallel alignment of neighboring hairs was degraded. This late planar cell polarity phenotype was somewhat reminiscent of phenotypes seen in mutations in genes that encode septate junction components (Moyer and Jacobs, 2008; Venema et al., 2004), but unique in detail. We also found that the directed and premature expression of dyl led to multiple and branched wing hairs. This was seen in both growing pupal wings and in adult wings and appeared to be due to effects on the cytoskeleton and not on chitin/cuticle deposition.
Evidence for a role for Rab11 in hair morphogenesis was recently provided by several groups (Gault et al., 2012; Purvanov et al., 2010), although no evidence for a dyl like phenotype was reported. We found the directed expression of dominant negative Rab11 was able to produce phenotypes that mimicked the dyl hair phenotypes. We also observed that Rab11 is found in growing hairs and accumulated at the distal tip of the hair. This localization pattern is similar to what is seen in sensory bristles (Nagaraj and Adler, 2012). An interesting finding in our experiments was a frizzled/starry night (fz/stan) like PCP phenotype (Adler, 2002; Adler, 2012; Goodrich and Strutt, 2011; Lawrence et al., 2007; Wu and Mlodzik, 2009) in wings where the DN-Rab11 was expressed and that this was associated with a disruption of the normal asymmetric accumulation of the Stan and Inturned proteins. This may be due to effects on the intracellular transport needed to form the proximal and distal PCP protein complexes.
The dyl hair kd phenotype shared some characteristics with that seen for mutations in kkv, which encodes chitin synthase (Devine et al., 2005; Moussian et al., 2005; Ostrowski et al., 2002; Ren et al., 2005). However, kkv mutations did not result in the branching and multiple hairs produced by dyl mutants hence a lack of chitin deposition cannot explain the dyl mutant phenotype. The expression of kkv also changes dramatically during hair morphogenesis (Ren et al., 2005). In an attempt to identify additional genes that might function along with dyl and kkv we screened 103 genes whose expression is strongly modulated during the terminal differentiation of Drosophila epidermal cells (Ren et al., 2005). A knock down of 45 lead to a phenotype that was at least somewhat reminiscent of that seen in dyl or kkv mutants. Some of these are likely play a role in cuticle formation. The most notable was Chitinase 6 (Cht6), whose expression also dramatically increases late in hair morphogenesis (Ren et al., 2005). Reducing Cht6 activity in wing cells resulted in a weak version of all of the phenotypes seen with dyl.
Materials and methods
Fly Culture and Strains
All flies were grown on standard media. Oregon R was used as a wild type control. The kkv, Rab11 and dyl mutant and deficiency lines were obtained from the Bloomington Drosophila stock center at Indiana University unless stated otherwise. Most of the stocks used for generating flp/FRT somatic clones and the Gal4 driver lines were also obtained from the Bloomington stock center. The UAS-Rab11dsRNA pWIZ stock was provided by D. Ready; rab1193Bi/TM6, rab11ex1/TM6, Rab11 ΔFRT/TM6, and y w hs-flp; FRT5377 hrp-GFP/TM3 by R. Cohen; The UAS-dyl stocks and the anti-Dyl antibodies were kindly provided by F. Payre. Stocks used to induce RNAi were obtained from the Vienna Stock Center (V lines) and from the stock center in Bloomington (T lines from the Harvard collection). Key lines for RNAi experiments included V102166 (dyl) and V107916 (Cht-6). The line that carried a UAS dominant negative Rab11 transgene was obtained from the Bloomington stock center (B-23261). An alternative stock that carried a similar transgene did not express well (B-9790) in our hands.
A screen to identify additional genes that shared aspects of the dyl and/or kkv mutant phenotypes was done by crossing UAS-geneX-dsRNA females to UAS-dicer2; ptc-Gal4 males. This resulted in females where RNAi was enhanced by the dicer-2 expressing transgene and males where this was not the case. This allowed us in one cross to be able to see the enhanced phenotype and if this was lethal the unenhanced one. The larvae and pupae were cultured at 25°C. In cases where even the unenhanced males died under these conditions vials were moved to 21°C or 18°C, which results in lower Gal4 activity and hence a weaker RNAi effect. In several cases this resulted in viability that allowed us to score for a possible wing phenotype.
Generation of kkv, rab1, and dyl kd and oe clones
Clones where dyl was knocked down were generated by crossing w hs-flp; AyGal4 UAS-LacZ; UAS-dicer 2 females and UAS-dyl-RNAi males. Vials were heat shocked for 20 minutes to induce clones. A similar protocol was used to generate clones that over-expressed (oe) dyl by substituting males that carried UAS-dyl instead of UAS-dyl-RNAi. kkv mutant clones were generated by crossing w hs-flp; FRT82 Ubi-GFP/TM6 females to FRT82 kkv1 e/TM6 males (Xu and Rubin, 1993). Vials were heat shocked at 37°C for one hour to induce flp and clone formation. Rab11ex1 clones were induced in an analogous experiment. Rab11 ΔFRT clones were induced as described by (Bogard et al., 2007). To examine the consequences of a reduction in dyl function in cells that lacked kkv we generated flies that were w hs-flp; ptc-Gal4/UAS-dyl-RNAi; FRT82 kkv1 e/FRT82 flies. These flies were heat shocked to induce kkv clones and we compared the phenotypes of the clones inside and outside of the ptc domain where dyl was kd. Flies carrying kkv clones often died prior to or soon after eclosion. Others were unable to “pump out” their wings. These problems appear to be due to a failure in the cuticular barrier that separates the cells and hemolymph from the external world (Nagaraj and Adler, 2012). To minimize this we tried to limit clones to a very small size by delaying heat shocking until at least the wandering third instar stage. Perdurance is not a problem for kkv clones due to the dramatic increase in expression of the gene in wing cells during the second day of pupal life (Ren et al., 2005).
Immunostaining
Immunostaining was done by standard protocols (e.g. see (He et al., 2005) on paraformaldehyde fixed material. Primary antibodies were used at the following concentrations: Rabbit anti-GFP (1:4000 Molecular Probes), mouse anti-GFP (1:1000 Molecular Probes), mouse anti-B-galactosidase (Developmental Studies Hybrioma Lab (1:1000)(Mab 40–1) and anti-Dyl (1:1–2000 (kindly provided by F. Payre; or 1:5000 our independently isolated antibody, which is directed against a segment in the extra-cellular domain). Alexa 488 and Alexa 568 conjugated secondary antibodies (1:250) were purchased from Molecular Probes. For F-actin staining we used Alexa Flour Phalloidin (488, 568 or 647) (Molecular Probes).
Chitin Staining using CBD-Rhodamine probe
To visualize chitin, pupal wings were fixed and incubated in a 1:200 dilution of the Rhodamine conjugated chitin binding probe (New England Biolabs) (Gangishetti et al., 2009) in phosphate-buffered saline (PBS) containing 0.3% Triton X-100 for 2–4 hours at room temperature, rinsed in PBS and mounted. This staining procedure was combined serially with other staining protocols in a number of experiments (Nagaraj and Adler, 2012). The chitin staining (always done first) often lead to less than optimal results for the other staining (e.g. phalloidin or antibody), but adequate results were usually possible.
Image analysis
Confocal images were obtained using a Zeiss Meta laser scanning confocal scope at the Keck Center for Cellular Imaging at the University of Virginia or a CARV spinning disc unit on a Nikon Eclipse TE200 microscope controlled by Metamorph software. Cuticle images were obtained using a Spot digital camera (National Diagnostics) on a Zeiss Axioskop 2 microscope. Images were analyzed using Image J and processed using Adobe Photoshop.
Analysis of Hair Polarity
We first attempted to quantify the PCP phenotype of dyl by examining hair polarity using an automated approach with the Metamorph or ImageJ software. Both of these programs did a good job in recognizing and measuring the polarity of wild type hairs but they had difficulties with the thinner, branched and occasionally crossed dyl hairs. Hence we manually measured hair orientation for both wild type and dyl mutant hairs using ImageJ. We did not score mutant hairs that were branched or otherwise highly abnormal in structure. The data presented is all from the middle of the D cell of the wing (distal to the posterior cross vein) however qualitatively similar results were obtained from the other two regions we examined in preliminary experiments. qualitatively similar results were also obtained from examining wings both by light microscopy (when mounted) and SEM. The data was analyzed using the Oriana software package (Kovach Computing Services).
Results
dyl functions cell autonomously in hair morphogenesis
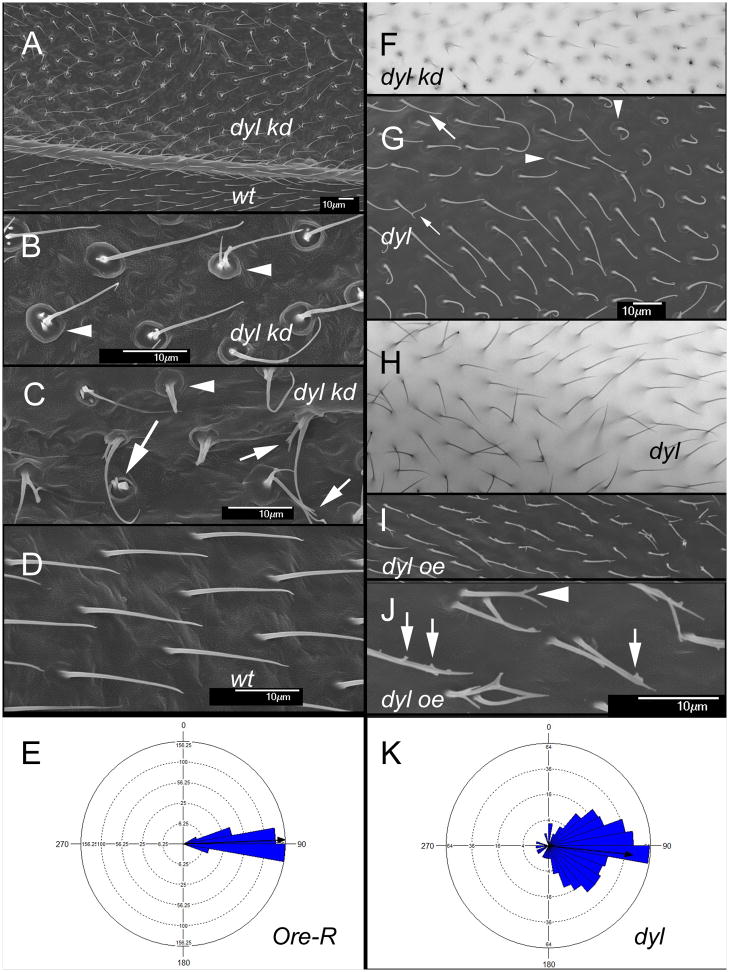
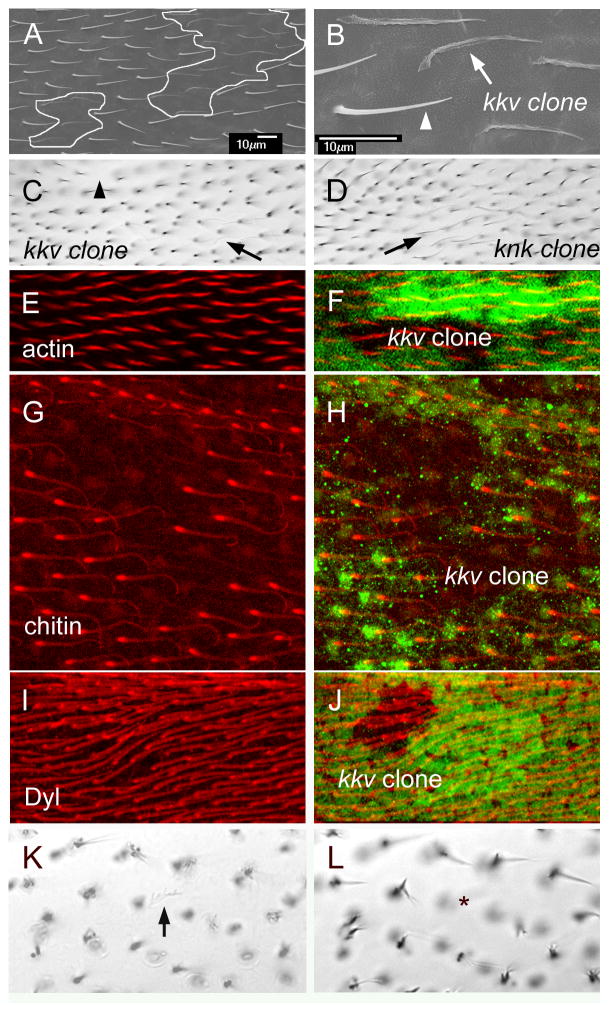
To examine the role of dyl in hair morphogenesis we used transgene mediated RNAi to knock down (kd) dyl function. When ptc-Gal4 was used to kd expression in a stripe down the middle of the wing we observed the hairs were more upright than normal and often much thinner. Many of the hairs were multipled and/or split and there were stubs that likely represented collapsed hairs (Fig 1A,B, C,F). Some hairs also appeared curved. These were somewhat difficult to image in the light microscope and more detail could be seen using scanning electron microscopy (Fig 1A,B,C). In the SEM we could also see prominent “cups” surrounding the hairs (Fig 1B,C arrowheads). Less prominent “cups” could often be seen in wild type wings. These may represent a remnant of the hair pedestals identified many years ago by Mitchell, Petersen and colleagues (Mitchell et al., 1990). We refer to this collection of hair morphology phenotypes as the “dyl hair phenotype”.
Fig. 1.
Dyl functions in hair morphogenesis. (A) An SEM of an adult ptc-Gal4 UAS-dyl RNAi wing. The ptc domain includes the region above the vein shown in the image (marked by dyl kd). Note the hair polarity phenotype. (B) A high mag SEM of an adult ptc-Gal4 UAS-dyl wing. The arrowheads point to the hair “cups”. (C) A high mag SEM of an adult ptc-Gal4 UAS-dyl wing. The arrowhead points to a hair “cups”. The arrows point to thin and/or branched dyl kd hairs. (D) An SEM of phenotypically wild type hairs from outside of the ptc domain of the ptc-Gal4 UAS-dyl RNAi wing in (A). (E) A Rose diagram showing the distribution of hair orientation for Ore-R wings. The arrow shows the mean orientation. (F). A bright field micrograph of the phenotype associated with a dyl kd (ptc-Gal4 UAS-dyl RNAi). (G) An SEM of a dylMI02088/Df wing. Note the abnormal hair polarity. The larger arrow points to a thin hair, the smaller arrow to a split hair and the arrowheads to “cups” at the base of dyl mutant hairs. (H). A bright field micrograph of a dylMI02088/Df wing. Note the poor alignment of neighboring hairs. (I) An SEM of a ptc-Gal4 UAS-dyl wing. (J) A higher mag SEM of a ptc-Gal4 UAS-dyl wing where the small branches (arrows) and split (arrowheads) can be seen. (K). A Rose diagram showing the distribution of hair orientation for dylMI02088/Df wings. The arrow shows the mean orientation. Note how much broader the distribution is than in Ore-R.
In experiments on both embryonic denticles and on adult bristles the dyl kd phenotype was found to be specific to the dyl gene (Fernandes et al., 2010; Nagaraj and Adler, 2012). To confirm that the kd phenotype was also specific for the wing hair phenotype we examined wings of adult escapers that were either homo or hemizygous for a hypomorphic allele of dyl (dylMI02088). The wings of these flies showed a weak version of the kd phenotypes with somewhat thinned and occasionally split hairs and hair cups confirming the specificity of the kd phenotype (Fig 1G,H arrows and arrowheads respectively).
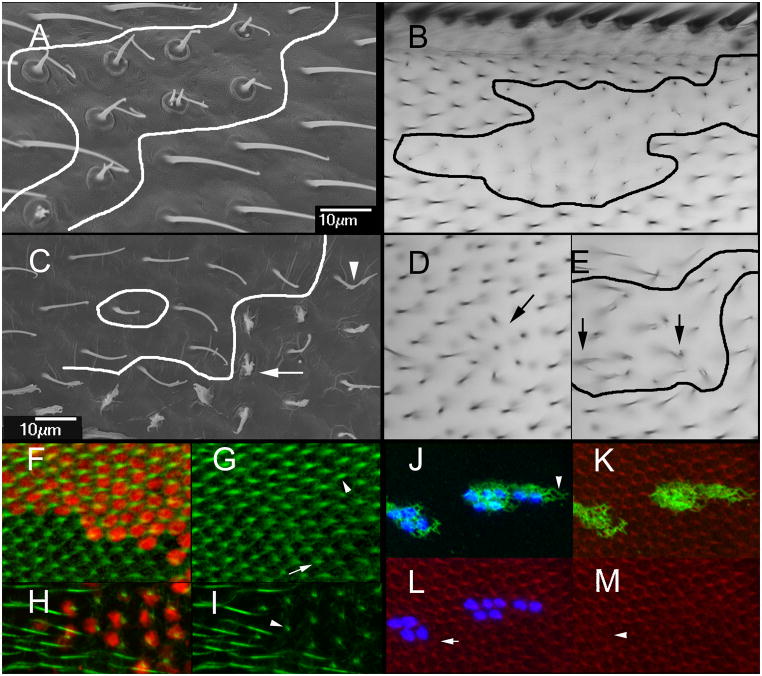
The phenotype of the ptc-Gal4 driven kd wings indicated that dyl did not act systemically. However, because ptc expression levels decrease in a gradient as one moves laterally away from the midline those observations did not rule out short distance non-autonomy for dyl. To examine the cell autonomy of the dyl kd we induced flip out clones (Struhl and Basler, 1993). In adult wings examined by either SEM or light microscopy the clones could be identified by groups of cells that had the dyl hair phenotype that were directly juxtaposed to normal hairs (Fig 2A,B). These observations suggested that the kd acted cell autonomously. To test this more rigorously we examined pupal wing clones marked by the expression of LacZ (Fig 2F–I). In these experiments the dyl mutant phenotype was only seen in the marked cells and there was no evidence of rescue by neighboring wild type cells. We concluded that the dyl hair phenotype is cell autonomous.
Fig. 2.
Cell autonomy of dyl. (A) An SEM of a dyl kd flip out clone. The putative clone boundary is outlined. Note the strong dyl phenotype of the putative clone cells juxtaposed to wild type hairs. (B). A light micrograph showing of of a dyl kd flip out clone. Once again note the wild type cells that are juxtaposed next to cells that show a strong dyl mutant phenotype. (C). An SEM of a dyl oe flip out clone. Note some cells show a very strong phenotype (arrow), some a weak phenotype (arrowhead) and others appear wild type. (D) A bright field micrograph of a small dyl oe clone. A small group of hairs appear to point inward (arrow). (E) A bright field micrograph of a larger dyl oe clone. The putative clone is outlined. As was observed in the SEM such clones contain cells that display a range of phenotypes. The arrows point to abnormal hairs. (F) A dyl kd flip out clone in a 33 hr pupal wing marked by the expression of LacZ (red). F-actin (green) shows the growing hairs. (G) The same wing as in F but with only the green (F-actin) channel. Note the clone hairs (arrowhead) are of wild type morphology at this stage and appear longer on average than the wild type neighbors (arrow). (H) A dyl kd flip out clone in a 46 hr pupal wing marked by the expression of LacZ (red). F-actin (green) shows the growing hairs. (I) The same wing as in H but with only the green channel shown. The phenotype of the dyl hairs is dramatic while neighboring wild type hairs show no phenotype. At this late stage F-actin staining is less vigorous and consistent from hair to hair than in younger wings particularly when combined with antibody staining. (J) A flip out dyl oe clone marked by the expression of LacZ (blue). Note the accumulation of Dyl is fibrous and extends beyond the clone cells (arrowhead). Note that the endogenous Dyl found in hairs is not visible at this level of exposure. (K). The same cells as in J with F-actin staining shown. (L). The same cells as in J with actin and LacZ shown. Note that some wild type cells show abnormal hair F-actin (arrow). (M). The same cells showing only F-actin staining. NBote that some kd cells also display abnormal hair F-actin (arrowhead).
dyl is essential for the maintenance of hair integrity
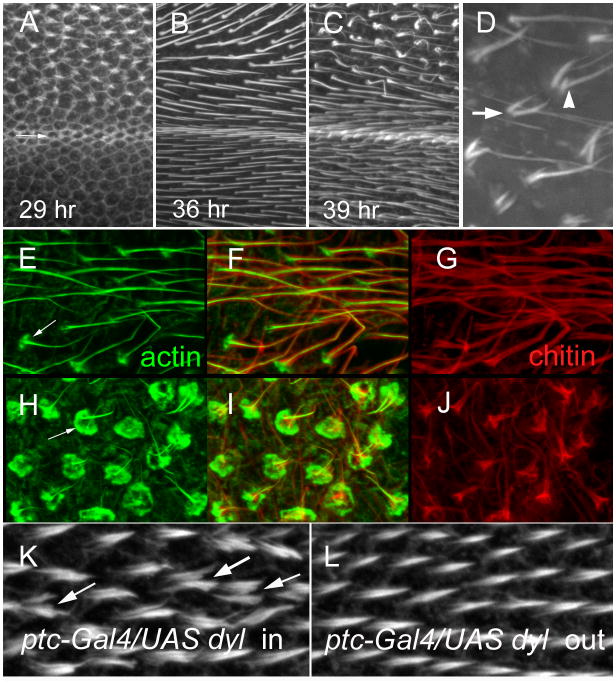
To determine if the dyl hair phenotype was due to a direct effect on hair growth we examined pupal wings where dyl was kd in the ptc domain (Fig 3A–C). We observed that hair outgrowth began precociously in the kd cells (Fig 3A) and that for several hours those hairs appeared morphologically normal although more advanced/substantial than neighboring control hairs (Fig 2F,G; Fig 3B). Around 38–41 hr awp, when hair growth is largely complete the dyl kd hairs became thinned, bent, split, collapsed and multipled and began to take on the morphology of the adult kd hairs (Fig 3C,D). We similarly examined dyl kd flip out clones and also found the morphological abnormalities were not seen in growing hairs - only in older hairs (Fig 2F–I). Thus, the adult dyl hair phenotype is not due to a defect in growth, rather one in the maintenance of hair morphology.
Fig. 3.
Dyl and hair morphogenesis. (A) A 29 hr ptc-Gal4 UAS-dyl RNAi pupal wing stained to show F-actin. The arrow shows the boundary between the ptc domain and the wild type wing posterior to it (below). Note the hairs forming inside the ptc domain and not outside it. (B) A 36 hr ptc-Gal4 UAS-dyl RNAi pupal wing stained for F-actin. Hairs are seen both in and outside of the ptc domain, but note the hairs inside the ptc domain appear longer, thicker and are stained more brightly. (C). A 39 hr ptc-Gal4 UAS-dyl RNAi pupal wing stained for F-actin. Note hairs inside the ptc domain are starting to appear abnormal. (D). A higher magnification view of abnormal ptc-Gal4 UAS-dyl RNAi hairs in a 39 hr wing. The arrow points to a multiple hair cell. The arrowhead points to a split hair. (E) A 43 hr ptc-Gal4 UAS-dyl RNAi pupal wing stained for F-actin. This image is for a region outside of the ptc domain. Note the F-actin (green) in the hair is central to chitin (red). Relatively weak staining of hair cups is visible (arrow). (F) A merged image of E and G. (G). The same wing region shown in E, but stained for chitin in red. (H) A 43 hr ptc-Gal4 UAS-dyl RNAi pupal wing stained for F-actin. This image is for a region inside of the ptc domain. The arrow points to the large accumulation of F-actin at the base of the hair and the abnormal structure of the hairs. (I) A merge of H and J. (J) The same wing region shown in H, but stained for chitin in red. Note the staining is far brighter in the proximal part of the hair. This is not seen in wt. (K). A 33 hr ptc-Gal4 Tub-Gal80ts UAS-dyl pupal wing inside of the ptc domain. The arrow points to a multiple/split hair cell. (L). A 33 hr ptc-Gal4 Tub-Gal80ts UAS-dyl pupal wing outside of the ptc domain. Note that the relative total hair F-actin staining is on average slightly stronger in K than L.
Since the dyl bristle phenotype is associated with altered chitin deposition (Nagaraj and Adler, 2012) we examined chitin deposition in both wild type and dyl kd wings. In wild type wings hair outgrowth starts around 32 hr awp and by about 40hr awp it is largely complete (Mitchell et al., 1990; Wong and Adler, 1993). Around this time wing expansion begins (Mitchell et al., 1990). This process leads to a flattening of the cuboidal pupal wing cells and a large increase in the apical surface of the wing (Fig S1). The first sign of this is a slight curving in the proximal posterior region of the wing. As the wing expands and lengthens along the proximal distal axis two folds appear on the distal posterior region of the wing. The continued expansion leads to the wing folding back so that the distal tip is pointed proximally. At the early stages of this process we observed a dramatic accumulation of F-actin foci just under the apical plasma membrane (in the apical 1 um of the wing cells) (Fig S1). Once expansion had proceeded to the extent that the start of folding was seen we were able to detect chitin by staining with a fluorescent chitin binding protein (Fig S1). We suggest that the increase in F-actin foci is associated with exocytosis and the deposition of cuticle. This did not appear to be dramatically altered in the dyl kd. A few hrs later when in wild type a small disc of F-actin is seen at the base of wild type hairs (Fig 3E)(Roch et al., 2003) the dyl kd hairs showed a dramatically enhanced F-actin disc (Fig 3H). The enhanced actin staining was seen at all stages where basal discs were present. We suggest this is the cause of the cup-like structure seen in the SEM of kd wings. We found that chitin staining was present but abnormal in the dyl kd hairs (Fig 3G, J). In wild type hairs at this time the hair is long and gently tapered. The hair still stains strongly for F-actin and chitin staining is seen smoothly and relatively evenly outlining the F-actin staining all along the hair. In the mutant hairs both F-actin and chitin staining were abnormal. Hair F-actin staining was reduced distally and chitin staining was both reduced distally and much stronger proximally.
Dyl and PCP
In adult wings where dyl expression was knocked down during hair morphogenesis we noted that the orientation of hairs was abnormal with many hairs pointing 30° or more from distal (Fig 1A). Hairs often stood more erect than normal and neighboring hairs were not well aligned. This was not due to a disturbance of hair polarity during mounting as the abnormal polarity was restricted to the ptc domain and the SEM samples were not mounted between a cover slip and a microscope slide. Further, the polarity disruption was also observed in the light microscope in wings simply placed on a slide without mounting media or a cover slip (Fig S2A). We also observed mutant hairs with abnormal polarity next to wild types hairs of normal polarity in flip out kd clones observed without mounting (Fig S2BC). Hence, mounting is unrelated to the polarity phenotype. Since the kd hairs showed normal polarity at early and mid-stages of growth in the pupal wing the kd is not altering the subcellular site for hair initiation as do mutations in fz/stan pathway (Wong and Adler, 1993) and ds/ft pathway genes (Adler et al., 1998). The disruption of local alignment phenotype was quite prominent in parts of the wings of dylMI02088 /Df flies (Fig 1G,H). We took advantage of these wings to quantify the abnormal hair polarity as described in the methods. As expected from simple observation wild type wings showed a tight distribution of hair orientations (Fig 1E), while the distribution was markedly broader in the mutant (Fig 1K). For example, less than 1% (3/331) Ore-R hairs were oriented more than 20° from distal, while almost 50% (164/330) of dylMI02088 /Df hairs were. The two populations of hairs were significantly different in terms of orientations (p < 10−12 Marida-Watson-Wheeler; p<0.001 Watson U2).
The poor alignment of neighboring hairs and the lack of an effect on the site of prehair initiation is reminiscent of the phenotype seen with mutations in genes that encode components of the septate junction such as Gliotectin (Moyer and Jacobs, 2008; Venema et al., 2004). As was described previously this abnormal polarity is associated with the hair pedestal/base not being parallel to the blade surface (Fig S3). Thus, this polarity phenotype is not a mimic of the dyl phenotype, which represents a novel planar cell polarity phenotype.
Dyl accumulates in growing hairs
Based on what is known about ZP domain proteins it seemed likely that Dyl functioned in the hair to mediate chitin deposition and to insure the maintenance of hair structure (Fernandes et al., 2010; Nagaraj and Adler, 2012; Plaza et al., 2010). We immunolocalized Dyl in developing pupal hairs and found that Dyl accumulated in extending hairs (Fig 4) consistent with it acting locally as expected. Dyl levels remained high throughout hair outgrowth and the period of chitin deposition and at late stages appeared more prominent at the base of the hair (Fig S4). We did not see substantial Dyl immunostaining prior to hair initiation consistent with the dramatic increase in Dyl mRNA levels from 32hr to 40hr awp. Based on the precocious hair initiation kd phenotype Dyl is expected to be present earlier than 32hr awp. Our failure to detect it is likely due to our antibody reagent not being sensitive enough. It is also possible that Dyl is not localized to a particular subcellular region prior to hair outgrowth making its detection more difficult. As a control for the specificity of the antibodies we immunostained pupal wings where dyl was kd in the ptc domain. There was a dramatic loss of immunostaining in this region confirming the specificity of the antibody reagent for immunostaining pupal wings (Fig S4).
Fig. 4.
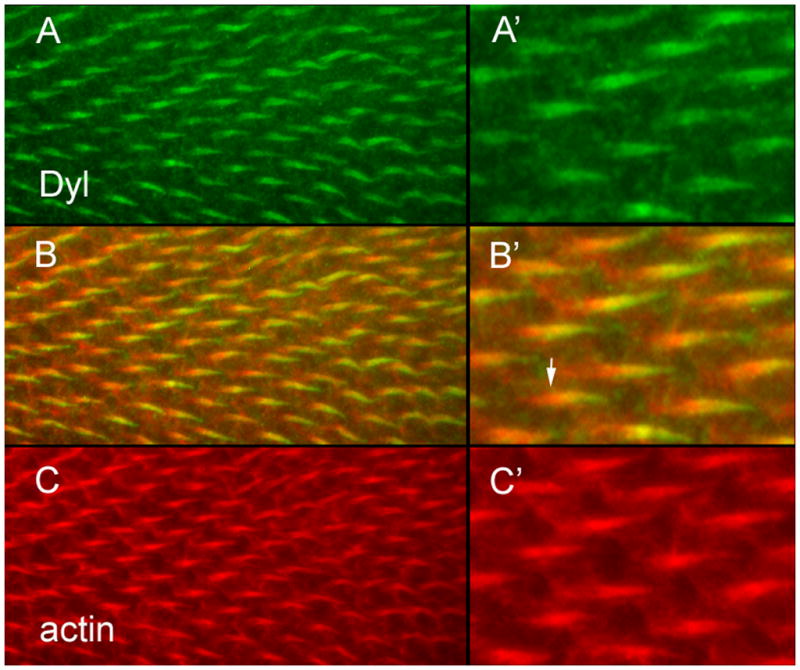
Dyl accumulates in growing hairs. (A) Dyl antibody staining shows the protein accumulates in growing hairs. (B) A merge of A and C. (C) F-actin staining. (A’) A higher magnification image of part of the field in A. (B’) A higher magnification image of part of the field in B. The arrow points to the proximal “root” of the hair that stains for actin but not the plasma membrane localized Dyl. (C’) A higher magnification image of part of the field in C.
A dyl gain of function hair morphology phenotype
We carried out complementary experiments where we over expressed (oe) dyl using ptc-Gal4. In such experiments dyl is also expressed throughout development as opposed to be strongly modulated as a function of time in wing cells. For simplicity we will use oe in discussing such experiments. We found extensive lethality among ptc>dyl animals and needed to restrict the time of expression by co-expressing a temperature sensitive Gal80 protein and utilizing a temperature shift. Under these conditions we found that the over expression of dyl during the time between wpp formation and hair outgrowth resulted in multipled and branched hairs. These were not thinned as we observed in the dyl kd hairs. The pattern of branching was quite dramatic in some cases. Due to the small size of many of the branches they were best seen by the use of scanning electron microscopy (Fig 1I,J). We also examined in pupal wings the consequences of dyl over expression (Fig 3KL). We observed multiple and split hairs at all stages of growth (Fig 3KL), hence this phenotype is not due to an effect on hair maintenance or cuticle deposition, which begins about 10 hours after hair initiation. Rather the multiple/split hair cell phenotype is likely due to effects on the cytoskeleton. The dyl oe pupal hairs often appeared thicker than their wild type neighbors particularly if one adds together the multiple and split hairs. Hence there appears to be more “hair volume” and more total hair F-actin associated with dyl oe. In some of these experiments we noticed what appeared to be a slight delay in hair initiation. This gain of function phenotype interfered with transgene rescue experiments.
We also examined flip out clones where dyl was over expressed. Surprisingly, the phenotypes of cells in the center of the clones appeared to be generally more severe than hairs at the periphery (Fig 2C–E). Since there was no cell marker in this experiment we could not determine if the clone cells were influencing their wild type neighbors or if the wild type cells were partly rescuing juxtaposed oe cells. We examined flip out clones in pupal wings and observed by immunostaining that the oe Dyl extended beyond the clone, which was marked by the expression of LacZ (Fig 2J arrow, Fig S5AB). In most cases this effect was modest but in some clones the oe Dyl extended many cells beyond the clone (Fig S5AB). Interestingly, there appeared to be a bias for the oe Dyl to be found distal to the clone. The oe Dyl was apical to the cell (Fig S5), appeared to be in a fibrous network, and in some cases it appeared to outline cells (Fig 2J–M, Fig S5A,D,H). We also noticed that clone cells hair growth often appeared to be retarded and abnormal (Fig 2J–M arrowhead). We also could detect abnormal hairs in nearby wild type cells, thus dyl oe can act cell non-autonomously ((Fig 2J–M arrow, Fig S5A–C arrows). This non-autonomy was usually associated with obvious non-autonomous Dyl accumulation.
Rab11 and hair morphogenesis
In developing bristles Dyl acts as a Rab11 effector for chitin deposition and Rab11 function is required for the plasma membrane localization of Dyl (Nagaraj and Adler, 2012). To determine if this relationship was conserved in hairs we attempted to determine if a lack of Rab11 function lead to a similar hair morphology phenotype. We did not see any phenotype associated with a kd of Rab11 in hairs. Nor did we see any hair phenotype in Rab11 hypomorphs that survived to adulthood and showed a mutant phenotype in macrochaetae. We also generated clones of wing cells that were homozygous for two independent Rab11 null alleles (Bogard et al., 2007; Dollar et al., 2002). We did not see any hair phenotype in such wings but a limitation of these experiments was that we only obtained small clones. A failure to recover Rab11 null clones in imaginal discs was reported recently (Xu et al., 2011) and is consistent with our observations. This could be due to Rab11 having an imaginal disc cell essential function (e.g. cytokinesis) and this function being more sensitive to a lack of Rab11 than Dyl insertion into the plasma membrane. This would result in cells with low levels of Rab11 being lost and explain our failure to see a Rab11 loss of function phenotype.
As an alternative to examining mutants in or a kd of the endogenous Rab11 gene we examined the consequences of the expression of either a dominant negative (DN) or constitutively active (CA) Rab11. Driving the expression of DN-Rab11 mutant protein using ptc-Gal4 was lethal. To get around this lethality we used a temperature sensitive Gal80 protein and a variety of temperature (shift) regimens. As a first check that the DN-Rab11 was able to induce a Rab11 loss of function like phenotype we examined the scutellar bristles where ptc-Gal4 drives transgene expression. Rab11 is known to be essential for the determinative cell divisions that give rise to the bristle sense organ and for morphogenesis of the bristle shaft (Emery et al., 2005; Jafar-Nejad et al., 2005; Nagaraj and Adler, 2012). In flies that were raised at 27.5°C (which allows a low level of expression in the presence of the ts Gal80 protein) several phenotypes were seen including a complete loss of the scutellar bristles, the loss of one or more but not all of the scutellar bristles and bristles that were short, or curved or mis-oriented. We also saw flies with extra scutellar bristles. In flies raised at 21°C no bristle phenotype was observed. We thus concluded the expression of DN-Rab11 by ptc-Gal4 was able to produce loss of function like phenotypes. In flies that were raised as larvae at 27.5°C and then shifted to 29°C at white prepuae the scutellum was greatly reduced in size suggesting that a high level of expression of DN-Rab11 either reduced cell proliferation or caused cell death (Fig S6C).
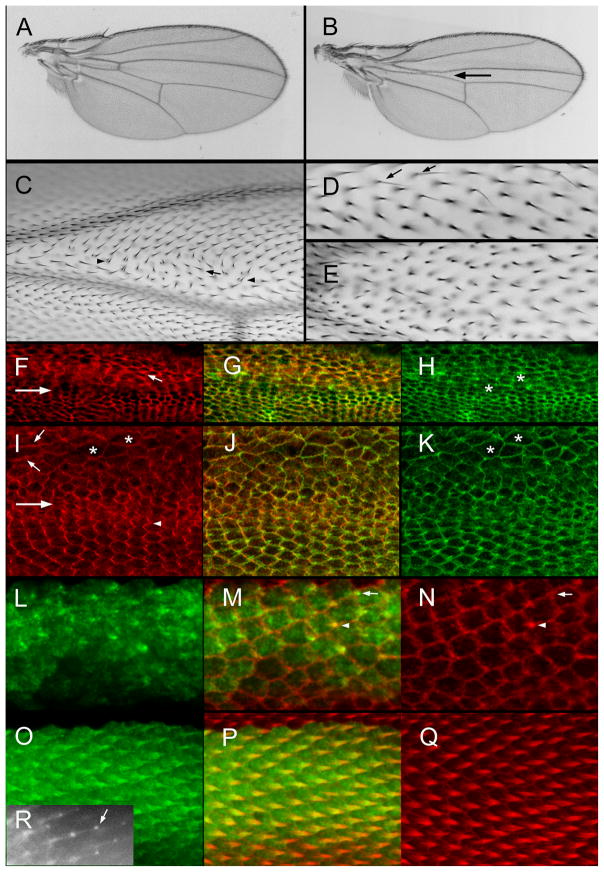
When animals were grown at 29.5°C (restrictive condition) most died prior to pupation although a small number of pupae were formed after 8–9 days of culture. None of these exceptional pupae eclosed as adults. A few made it to the pharate adult stage and these showed a variety of defects including legs that were truncated distally and/or proximally (Fig S6AB). When flies were grown at the permissive condition (21°C) no mutant phenotype was detected on the wing (Fig 5A, Table S1). In contrast, when they were grown at a semi-restrictive condition (27.5°C) several phenotypes were seen and these were made more severe when the animals were shifted to 29.5°C at white prepupae (wpp). Consistent with Rab11 functioning in growth, larvae grown at 27.5°C had a reduction in the size of the ptc domain in the center of the wing (Fig 5B). Along with this phenotype was a reduction or loss of the anterior cross vein. This “growth” function of Rab11 needs to be antagonized for a substantial period of time prior to 20 hr awp for the phenotype to be manifested as it was suppressed when animals were shifted from 27.5°C to 21°C at wpp (Table S1). Further, flies raised at 21°C and then shifted to 29°C at wpp did not show a reduction in the size of the ptc domain. A second growth related phenotype was the appearance of occasional polypoid cells. Several other phenotypes were commonly seen in wings of flies raised at 27.5°C. The most interesting was a PCP phenotype that resembled a loss of function in one or more of the genes of the fz/stan pathway (Fig 5C). This was seen in most wings of flies grown at 27.5°C. We immunolocalized two members of the fz/stan pathway in such pupal wings. We noticed that cells where DN-Rab11 was expressed most strongly were irregular in shape and had a larger cross section compared to the normal hexagonal cells (Fig 5H,K). We detected an increase in Stan staining and a degradation of the normal zig zag pattern (Fig 5F) (Usui et al., 1999). We also immunolocalized the downstream planar cell polarity effector protein Inturned (Adler et al., 2004). The normal zig zag accumulation of this protein along the proximal side of wing cells was similarly disrupted (Fig 5I).
Fig. 5.
Rab11 and wing development. (A) A ptcGal4 Gal80ts/UAS-DN-Rab11 wing from a fly grown at 21°C. (B) A ptcGal4 Gal80ts/UAS-DN-Rab11 wing from a fly grown at 27.5°C. The arrow points to the reduced size of the ptc domain. (C) A micrograph of a ptcGal4 Gal80ts/UAS-DN-Rab11 wing from a fly grown at 27.5°C. Just distal to the anterior cross vein (ACV) is a group of cells showing abnormal hair polarity (arrows) and multiple hair cells (arrowheads). (D). A micrograph of a ptcGal4 Gal80ts/UAS-DN-Rab11 wing from a fly grown at 21°C until wpp and then shifted to 27.5°C. Note the presence of several thin split hairs (arrows). (E). A micrograph of a ptcGal4 Gal80ts/UAS-DN-Rab11 wing from a fly grown at 21°C until wpp and then shifted to 27.5°C. Note the lack of precise alignment of neighboring hairs. (F). A micrograph of a ptcGal4 Gal80ts/UAS-DN-Rab11 pupal wing from a fly grown at 27.5°C. The large arrow shows the boundary of the ptc domain (above inside). This image shows immunolocalization of Stan. The small arrow shows a cell with an increased level of improperly localized Stan. (G). A merge of F and H. (H). The same wing shown in F but showing F-actin staining in green. The asterisks are on abnormally shaped cells. (I). A micrograph of a ptcGal4 Gal80ts/UAS-DN-Rab11 pupal wing from a fly grown at 27.5°C. The large arrow shows the boundary of the ptc domain (above inside). This image shows immunolocalization of In. Note the zigzag accumulation pattern for In outside of the ptc domain and the aberrant cell shape and In localization inside the ptc domain. (J). A merge of I and K. (K). The same wing as in I but showing F-actin staining. The asterisks are on abnormally shaped cells. (L). A 32 hr ptc-Gal/UAS-GFP-Rab11 pupal wing. (M) A merge of L and N. The arrow points to a cell where GFP-Rab11 is showing distal accumulation prior to F-actin accumulation. This shows that GFP-Rab11 is an earlier marker of hair outgrowth than F-actin. The arrowhead points to a cell where the hair is also marked by F-actin accumulation. (O). A 34 hr ptc-Gal/UAS-GFP-Rab11 pupal wing stained for GFP. (P) A merge of O and Q. (Q). A 34 hr ptc-Gal/UAS-GFP-Rab11 pupal wing stained for F-actin. (R) A 35 hr ap-Gal/UAS-GFP-Rab11 pupal wing imaged in vivo. The arrow points to the “blob” of GFP-Rab11 at the tip of the growing hair.
We also detected hair abnormalities that resembled those seen in dyl. These included thin, split, multipled, and very short hairs and hairs that were not well aligned with their neighbors (Fig 5DE). We also detected cuticle abnormalities in some wings. All of these phenotypes were seen in a small number of cells but in a substantial fraction of wings (Table S1). These were also seen when animals were shifted from 21°C to 27.5 or 29.5°C 20–24 hrs awp and thus represent a “late hair” phenotype. In other experiments we found that a reduction in Rab11 gene dose (1 vs 2 functional copies) appeared to enhance the phenotypes seen with the expression of DN-Rab11. This observation supports the hypothesis that the effects of the DN-Rab11 were specific for normal Rab11 functions. The presence of these dyl like phenotypes suggests that Dyl may be a Rab11 effector in hairs as well as bristles. We were unable to get compelling data on this point as we did not detect an alteration in Dyl localization after the expression of DN-Rab11 (data not shown). This seems likely to be due to the very weak nature of the DN-Rab11 dyl like phenotype. Our results on the effects of expressing DN-RAb11 differ somewhat from those reported by (Gault et al., 2012) who reported short, missing and deformed hairs in pupal wings. The experiments were done differently and this may have shifted the predominant phenotypes.
We also carried out experiments where we expressed constitutively active Rab11. As was reported recently (Gault et al., 2012; Purvanov et al., 2010) this led to distally pointing multiple hair cells (data not shown). We also detected polyploid cells after the expression of CA-Rab11, consistent with Rab11 functioning in cytokinesis in wing cells (Giansanti et al., 2007; Xu et al., 2011).
Rab11 is a good hair reporter
We examined the subcellular distribution of Rab11 in pupal wing cells using a GFP-Rab11 reporter shown to be functional in bristle cells by a rescue assay (Nagaraj and Adler, 2012). Rab11 accumulated in growing hairs from the time of initiation (Fig 5L–Q). Indeed it appeared to accumulate there earlier than F-actin, although that may simply be a reflection on the sensitivity of detection. In fixed cells we did not see evidence of enrichment at the distal tip of growing hairs as we had seen in growing bristles and laterals. However, in vivo imaging experiments showed preferential accumulation at the tip in what appeared as a small blob (Fig 5R). It seems likely that the “blob” is not stable to fixation/dissection. At late stages in hair outgrowth (e.g. >38hr awp) it was clear that Rab11 was less abundant in the hair shaft and was concentrated around the base (Fig S7 BC). That Rab11 accumulated in growing hairs was reported recently (Gault et al., 2012). During the process of hair elongation we observed an intracellular gradient of Rab11 across the apical region of the cell that deceased as one moved back from the base of the hair (Fig S8). This could be an indication of there being an overall bias in intracellular trafficking needed to build the hair. We also examined Rab11 accumulation in wing cells mutant for the mwh gene. Such mutations result in each wing cell forming multiple hairs of abnormal polarity. Rab11 appeared to accumulate over a larger region of the cell at hair initiation and it accumulated in all of the multiple hairs produced in the mutant (Figure S7EF). The latter of these results were recently reported by Gault and colleagues (Gault et al., 2012).
The dyl split hair phenotype is not due to a lack of chitin
As reported above a lack of dyl function results in abnormal chitin deposition. This was also seen in developing bristles (Nagaraj and Adler, 2012) and is also likely in developing denticles (Fernandes et al., 2010). To determine if a lack of chitin could be responsible for the dyl hair phenotypes we examined clones of cells that were homozygous for a null allele in kkv (kkv1), which encodes the chitin synthase responsible for synthesizing exoskeleton cuticle chitin (Gagou et al., 2002; Moussian et al., 2005; Ostrowski et al., 2002). kkv null hairs were almost invisible on first inspection in the light microscope, however they were visible with careful examination (Fig 6C). This is due to very limited pigmentation and to the hairs being flacid and lying close to the wing blade cuticle. We did not observe any evidence of hair branching as seen with a lack of dyl function. We previously reported similar results with a hypomorphic kkv allele (Ren et al., 2005). We also examined kkv clone bearing wings in the SEM. These images confirmed that the kkv hairs were flacid and established they had an irregular surface (Fig 6AB). They also confirmed that the kkv mutant hairs were neither shorter nor thinner than wild type hairs nor were the mutant hairs branched. Indeed, they often appeared slightly longer than wild type neighbors. We also did not see any evidence for the hair “cup” associated with the dyl kd. We conclude that the presence of chitin is not required for the maintenance of hair integrity and that the branching of dyl mutant hairs requires an alternative explanation.
Fig. 6.
kkv and hair morphogenesis. (A) An SEM of an adult wing that contains a pair of small kkv1 clones. These are outlined in white. (B) A higher mag image of part of A. The arrow points to a kkv1 hair and the arrowhead to a neighboring wild type hair. Note how the kkv1 is flaccid and fainter. However, it is not branched and is neither thinner nor shorter than wild type. (C). Shown is a bright field image of a region of an adult wing bearing two small kkv1 clones. The arrow points to a faint kkv1 hair and the arrowhead to a location where the faint mutant hair cannot be seen due to being out of the plane of focus. (D) A bright field micrograph of a knk clone (arrow). Note the similarity to the kkv1 clone. (E). A region of a 34 hr pupal wing stained for F-actin (red) that contains a kkv1 clone. (F). The image from E showing the location of the clone as marked by the loss of GFP. (G). A region of a 44 hr pupal wing stained for chitin (red) that contains a kkv1 clone. (H) The image from G showing the location of the clone as marked by the loss of GFP. Note at this late stage GFP staining quality is lower than in younger wings. Note the bright spot of chitin staining at the base of the hairs. Note that chitin staining is lost in the clone cells showing the specificity of the chitin staining. (I) A region of a 34 hr pupal wing stained for Dyl (red) that contains a kkv1 clone. (J) The image from I showing the location of the clone as marked by the loss of GFP. Note that the hairs inside the clone do not show altered Dyl staining. (K) A kkv1 clone inside of the ptc domain where dyl has been knocked down by RNAi. The arrow points to a faint and flaccid hair (hence one that is mutant for kkv) that shows dramatic branching. (L). A different focal plane from the same region of the same wing shown in K. The asterisk marks the location of the kkv clone cell. No hair is seen due to it laying on the wing blade surface.
Since the deposition of chitin is abnormal in dyl kd cells it remained possible that abnormal chitin and not a lack of chitin was involved in hair branching. To eliminate this possibility we generated kkv null clones in wings where dyl function was knocked down in the central but not peripheral regions of the wing (ptc-GA4>dyl RNAi). kkv clones outside of the ptc domain had the same morphology seen previously (Fig 6C). Clones within the ptc domain showed hairs that had the branching phenotype of dyl and the flacid unpigmented phenotype of kkv (Fig 6KL) (i.e the phenotypes were additive). Thus, chitin appears to be unrelated to the dyl hair branching phenotype.
We also examined marked kkv clones in the pupal wing. We first examined clones in older wings (>40 hr apf) by chitin staining. As expected chitin staining was lost in the clone cells (Fig 6GH). This also served as a control for the specificity of our chitin staining protocol. Further, we did not see any effect on hair morphology as revealed by F-actin staining during outgrowth (Fig 6EF).
We next asked if the localization of Dyl to the hair requires the presence of chitin. To do this we immunolocalized Dyl in wings that contained marked kkv1 clones. We did not detect any alteration in Dyl in clone cells (Fig 6IJ), thus Dyl localization is independent of the presence of chitin.
A screen for additional genes that function along with dyl and kkv in hair morphogenesis
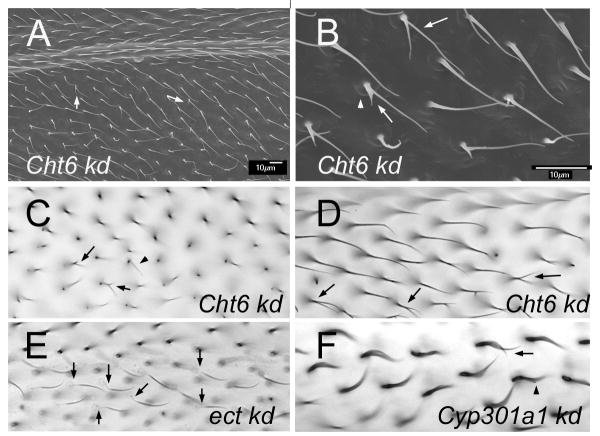
The dyl and kkv genes are notable in that their expression is highly modulated during wing development. We previously characterized the mRNA populations at 3 times in pupal wing development (Ren et al., 2005). At 24 hrs awp, around the time when cell division ceases, at 32 hr awp the time of hair initiation and at 40 hr around the time wing expansion and chitin deposition start. We found that several of the genes identified in this way had dramatic wing hair phenotypes. These included kkv, knk and sha. The results described above allow us to add dyl to this group. To try to identify other genes that worked with dyl and kkv in hair morphogenesis we carried out a small directed RNAi screen. We screened 107 genes whose wing expression increased at 32 and/or 40 hrs, or that were identified on FlyBase (McQuilton et al., 2012) as having an overall gene expression profile that was similar to dyl or kkv. A number of genes fit more than one criterion. Included are several genes that fit these criteria and were known prior to the screen to have a hair phenotype (e.g pawn (Arruda and Dolph, 2003) and shaven baby (Delon et al., 2003). In the screen we used a directed kd of the gene using transgene induced RNAi. Knockdowns of forty-seven of the genes produced a mutant phenotype that was reminiscent of one or more of the phenotypes seen with dyl or kkv (Table 1). In addition to dyl we found 9 genes that gave split hairs, 17 that resulted in thin hairs, 16 that resulted in misaligned neighboring hairs and 2 that produced hair cups. In addition to kkv and knk we identified 3 additional genes where a kd resulted in faint hairs. In our screen we noticed a new phenotype that was similar in some ways to the kkv and dyl phenotypes but distinct from both. We first clearly identified it in knockdowns of the ectodermal (ect) gene. It was characterized by a somewhat faint and usually curved hair with a very faint and flacid base (Fig 7E). In addition to ect, a kd of 7 other genes gave this phenotype.
Table 1.
Genes that showed a hair phenotype
| gene | RNAi line or mutant |
Split hairs (dyl-like) |
Thin hairs (dyl-like) |
Faint hairs (kkv-like) |
ect like hairs | Hair cup (dyl-like) | Misaligned hairs |
Cuticle defects |
Reason for screening* |
Comment |
|---|---|---|---|---|---|---|---|---|---|---|
| dyl (CG15013) | V102166 V39022 V39023 dylMI |
+++ | +++ | ++ | +++ | + | 32, 40, d, l, m | ZP domain | ||
| Cht6 (CG43374) | V107916 V38886 |
+ | ++ | + | ++ | +/− | 40, l | Chitinase | ||
| ect (CG6611) | V104650 | + | ++ | + | + | 32, 40, d, m | ||||
| CG15020 | V101086 | +/− | + | 32, d | ZP domain | |||||
| Cyp301a1 (CG8587) | V109771 V26989 |
+/− | 40, d | Curved hairs | ||||||
| kkv (CG2666) |
kkv1 V100327 |
+++ | + | 40, k, l | Chitin synthase1 | |||||
| knk (CG6217) |
knkf01902 V106302 |
+++ | + | 40, k, l | Chitin stability | |||||
| CG5873 | V14374 | + | + | 40, k | Haem peroxidase | |||||
| mtg (CG7549) | V108951 | + | 32, d, l, m | Chitin binding | ||||||
| CG15211 | V104929 | + | + | + | 32, 40 | MARVEL domain | ||||
| cyr (CG15335) | V28555 | +/− | 32, 40, m | ZP domain | ||||||
| uif (CG9138) | V101153 | + | 32, 40 | Lectin, EGF domain | ||||||
| mey (CG12063) | V106568 | +/− | 32, m | ZP domain | ||||||
| CG12206 | V14558 | +/− | +/− | 32 | ||||||
| ed (CG12676) | V104279 V27044 |
+ | + | 32 | Cell - cell adhesion | |||||
| CG30463 | V4923 | +/− | 32 | Glycosyltransfe rase Ricin B lectin |
||||||
| Rab23 (CG2108) | T28025 | + | 32, l | Multiple hair cells | ||||||
| sha (CG13209) | V36512 Several alleles |
+ | ++ | 32, l, m | Localizes to growing hairs | |||||
| CG10933 | V104282 | + | 32 | Src homology 3 domain | ||||||
| svb (CG6824) | V41584 | +++ | ++ | + | + | + | 32, l | Transcription factor | ||
| CG3036 | V108500 | + | 40 | MFS transporter family | ||||||
| CG16953 | V109873 | ++ | 40 | |||||||
| CG14770 | V106550 | +/− | 40 | |||||||
| Cpr97Eb (CG15884) | V19545 | +/− | +/− | 40 | Cuticle protein | |||||
| Cpr49Ah (CG8515) | V23183 | +/− | +/− | 40 | Cuticle protein | |||||
| Eip71CD (CG7266) | V26009 | +/− | 40 | methionine-(S)- S-oxide reductase |
||||||
| CG13917 | V32082 | +/− | + | ++ | 40 | BTB/POZ | ||||
| karst (CG12008) | V37074 | +/− | 40 | βH-spectrin | ||||||
| UGP (CG4347) | V109632 | + b | + | +/− | + | 40 | UTP:glucose-1- phosphate uridylyltransfer ase |
|||
| pwn (CG11101) | V101282 pwn1 |
+/− b | +++ | d, l, m | EGF domains | |||||
| dy (CG9355) | V102255 dy1 |
+++ | d, l | ZP domain | ||||||
| CG15740 | V107087 | +/− | + | d, m | ||||||
| CG42331 | V104585 | +/− | + | d | peroxidase | |||||
| m (CG9369) | V8036 m1, mD |
++ | + | + | +++ | d, m | ZP domain | |||
| CG9095 | V104608 | + tip | + | d, m | C type lectin | |||||
| CG43366 | V101186 | +/− | d | Endopeptidase inhibitor |
||||||
| CG14107 | V102232 | +/− | + | d, m | ||||||
| CG6347 | V101253 | + | d | peptidase | ||||||
| CG15080 | V106073 | + | d | |||||||
| CG15017 | V103326 | +/− | d | |||||||
| CG31559 | V33967 | + | +/− | +/− | d, m | |||||
| CG13188 | V102370 | + | k | Smad domain | ||||||
| CG10232 | V100033 | + | + | d | peptidase | |||||
| CG1869 | V104445 | +/− | +/− | k | Chitinase 7 | |||||
| Syn1 (CG7152) | V104992 | +/− | +/− | k | ||||||
| TwdlE (CG14534) | V107483 | +/− | k |
32 – Increased expression at 32 hr awp from Ren et. al., 2005
40 – Increased expression at 40 hr awp from Ren et. al., 2005
d – Expression similar to dyl FlyBase McQuilton et. al., 2012
k – Expression similar to kkv Flybase McQuilton et. al., 2012
m – mE2 34 mRNA expression cluster 07 ModEncode - FlyBase
l – literature
Fig. 7.
(A) An SEM of an adult ptc-Gal4 UAS-Cht6 RNAi wing. Arrows point to branched hairs. Note the poor alignment of neighboring hairs. (B). A higher magnification image of a region of A. The arro points to a split hair. The arrowhead points to a “cup” at the base of the hair. (C). A brightfield micrograph of an adult ptc-Gal4 UAS-Cht6 RNAi wing. The arrows point to branched and thin hairs. (D). A brightfield micrograph of an adult ptc-Gal4 UAS-Cht6 RNAi wing. The arrows point to branched hairs. (E). A brightfield micrograph of an adult ptc-Gal4 UAS-ect RNAi wing. The arrows point to hairs that show the “ect” phenotype of hairs with a faint and wimpy proximal region. (F). A brightfield micrograph of an adult ptc-Gal4 UAS-Cyp301a RNAi wing. The arrows point to split hairs. Note the curved shape of all hairs in this wing region.
One gene that stood out was Chitinase6 (Cht6), as the kd resulted in a weak version of all of the dyl phenotypes (Fig 7A–D). This makes it a strong candidate for a gene that works along with dyl in hair morphogenesis. That the Cht6 phenotypes were so much weaker could be due to the kd being less effective for Cht6, to Cht6 being relatively less important for hair morphogeneis or to Cht6 being partially redundant with one or more unknown genes (e.g. other chitinases). The relative weakness of the Cht-6 vs dyl kd complicated efforts to examine the relationship between the two genes. For example, we did not see a clear alteration in dyl immunostaining in the Cht6 kd but the significance of that is not clear as the vast majority of hairs are completely normal in such a wing. We identified several genotypes and wing regions where there was a weak dyl kd phenotype and we then assessed the phenotype in the dyl, Cht6 double kd. The clearest results were seen by examining the dorsal surface of the wing just distal to the posterior cross vein (from the vein to 5 cells distal). About 1/2 of vg-Gal4/UAS-dyl-RNAi wings did not show a phenotype in this region, while the others showed a small number of hairs with the thin and or split phenotype characteristic of dyl. On average we found 1.6 (sd 2.1) such hairs per wing region. No phenotype was seen in vg-Gal4/UAS-Cht6-RNAi wings in the same location. In contrast in the double kd (UAS-dyl-RNAi vg-Gal4/UAS-Cht6-RNAi) all wings showed a phenotype with a mean of 10.5 (sd 4.6) dyl like hairs per wing region. This difference was highly significant (p=5×10−5 (t test comparing UAS-dyl-RNAi vg-Gal4 vs UAS-dyl-RNAi vg-Gal4/UAS-Cht6-RNAi). Thus, in this sensitized situation we saw an interaction that indicated the two genes functioned in an additive way. We also examined UAS-dicer2; ptc-Gal4/Cht6-RNAi pupal wings by Dyl immunostaining but we did not detect any changes in the accumulation of Dyl. This could be due to the weakness of the Cht6 kd or to Dyl accumulation not being sensitive to Cht6 function. A way to obtain a stronger Cht6 loss of function will be needed to resolve this issue.
Several of the other genes identified are worth noting. The shaven baby transcription factor was previously reported to be important for hair morphogenesis and we confirmed those results (Delon et al., 2003). A kd of a second likely transcription factor, CG13188, which encodes a Smad protein gave a faint hair phenotype that appeared to be a weak version of the kkv phenotype. Knockdowns of other ZP domain proteins gave a range of hair and wing blade phenotypes consistent with the results seen for this family of proteins in embryonic denticle morphogenesis and cuticle deposition (Fernandes et al., 2010). None of these were as severe as the dyl kd with regard to hair morphology. Several genes that encode cuticle proteins or cuticle binding proteins also gave hair phenotypes. These included Cht-6, kkv, knk, mtg, Cpr97Eb, Cpr49Ah and Cht-7. One of the most interesting and surprising phenotypes was seen with kd of Cyp301a1. This member of the cytochrome P450 family was screened because its expression increased at 40 hr and it’s overall expression pattern was similar to dyl. It is listed in Table 1 due to it causing split hairs but its most prominent phenotype was a curvy hair (Fig 7F). This was also seen in bristles (data not shown). We do not consider the failure to see a wing hair phenotype in the other 60 genes screened (Table S2) to be particularly informative. Our results could be due to the genes not functioning in hair morphogenesis, due to the genes being functionally redundant or to the knock downs not being effective enough to see a phenotype.
Discussion
Dyl and actin
Previous studies have implicated Dyl and other ZP domain proteins in linking the cuticle to the plasma membrane and in the patterned deposition of chitin (Fernandes et al., 2010; Nagaraj and Adler, 2012). Other studies suggested that it could regulate the cytoskeleton (Bokel et al., 2005; Brodu et al., 2010; Roch et al., 2003). Our finding premature hair initiation in dyl kd wing cells establishes that dyl regulates the cytoskeleton and suggests the possibility that Dyl negatively regulates the actin cytoskeleton prior to hair initiation. At later stages in hair morphogenesis the situation is more complicated. In older kd cells we observed an increase in the accumulation of F-actin in the basal cup but a decrease in hair F-actin. The decrease in the hair could be due to a role for Dyl in promoting F-actin accumulation there and the increase in the basal disc could be due to a displacement of F-actin from the hair. The evidence for possibly increased hair F-actin with oe dyl is consistent with this possibility. Alternatively, the increased F-actin in the basal disc could be due to Dyl normally negatively regulating actin polymerization there. Regardless of the mechanism involved the effects on the actin cytoskeleton are stage and site specific arguing that Dyl does not act as a general actin regulator. Other observations were consistent with that hypothesis. For, example we did not see any alteration in F-actin staining associated with a kd of dyl in wing discs or young pupal wings (data not shown). In contrast, mutations in genes that encode proteins that are key general components in the regulation of F-actin such as cofilin (tsr) and AIP1 (flr) lead to enormous increases in F-actin levels and increased F-actin stability (Gunsalus et al., 1995; Ren et al., 2007) in a variety of cell types at all stages of development. Further evidence against Dyl functioning as a general regulator of the actin cytoskeleton is the nature of the dyl hair phenotype. For example, mutations in the F-actin bundling protein genes forked and singed (Cant et al., 1994; Petersen et al., 1994; Tilney et al., 1995), in non-muscle myosin encoding genes such as crinkled (Kiehart et al., 2004) or zipper (Franke et al., 2010), in regulators such as Rho kinase (Winter et al., 2001) and in F-actin depolymerizing protein encoding genes such as twin star (Gunsalus et al., 1995) and flare (Ren et al., 2007) all result in hair phenotypes. The phenotypes associated with these classes of genes are distinct from one another and from that of dyl. Thus, it seems unlikely the dyl mutant phenotype is mediated by effects on actin bundling, polymerization state or myosin related functions. We suggest that Dyl and perhaps other ZP domain proteins regulate the actin cytoskeleton by assembling and locally concentrating a mix of actin regulators.
The vast majority of the Dyl protein is predicted to be extracellular with only the C terminal 15 amino acids predicted to be cytoplasmic (Plaza et al., 2010). This region of the protein could function directly in the regulation of the actin cytoskeleton, however the sequence does not contain similarity to known actin regulators. This segment of the protein is very highly conserved in other Drosophila species, as is the entire Dyl protein, suggesting it is of functional importance, although in comparisons to Dyl homologs in more distant insects it is not the most highly conserved region. This segment is positively charged (5 positively charged residues compared to a single negatively charged one) thus it might interact with the negatively charged phosphate groups of membrane lipids and in this way indirectly signal to the actin cytoskeleon (Shewan et al., 2011; Zhao et al., 2010). An alternative explanation is that the extracellular domain interacts with one or more proteins in the extracellular space that then signal back to the cytoplasm to regulate the actin cytoskeleton. This hypothesis could explain the non-autonomous hair defects associated with dyl oe.
The Dyl protein contains a potential consensus furin cleavage site (RRRR aa 499–502) (Roch et al., 2003) located in its large putative extracellular domain. Hence it is possible that most of the Dyl protein is completely extracellular rather than being part of an integral membrane protein (Plaza et al., 2010). Given the possible cleavage site it is a bit surprising that we found strict cell autonomy in dyl kd flip out clones. Consistent with Dyl being cleaved we observed oe Dyl beyond the borders of flip out clones. To explain this set of observations we suggest that under normal expression conditions Dyl is either protected from cleavage by a binding partner or that the cleaved protein remains connected to the synthesizing cell. This could be a consequence of binding to the extracellular domain of another transmembrane protein. In either case the Dyl binding partner could share the highly modulated dyl expression profile. When dyl is expressed constitutively in the flip out clone cells the binding partner would not be present. Hence, Dyl would be cleaved and able to migrate to produce the non-autonomous accumulation observed
The over expressed Dyl protein appeared fibrous consistent with previous observations that ZP domain proteins polymerize and can form fibrous structures (Plaza et al., 2010). Our observation may be useful in establishing an in vivo assay for fiber assembly by ZP domain proteins. It will be interesting to see if other ZP domain proteins will form such fibrous structures when expressed in the pupal wing or if there are gene specific factors that are needed. Why there appeared to be a distal bias to the non-autonomous accumulation is unclear. Further experimentation will be required to determine if, when and how Dyl is cleaved.
The dyl PCP phenotype is unique
The abnormal polarity of wing hairs in dyl mutants represents a novel planar cell polarity (PCP) phenotype. It differs in many ways from those seen in fz/stan and ds/ft in that it does not alter polarity at early stages of hair outgrowth; rather it results from a failure to maintain normal PCP. Further, it does not result in extensive swirling of mutant hairs as in dyl neighboring hairs are often not well aligned with one another (Adler, 2002; Adler, 2012; Goodrich and Strutt, 2011; Lawrence et al., 2007; Wu and Mlodzik, 2009). Those same general characteristics have been seen with mutations in septate junction components (Moyer and Jacobs, 2008; Venema et al., 2004) but in those mutations the abnormal polarity is associated with the hair base/pedestal being tilted with respect to the surface of the wing blade. That is not the case for dyl. Hence, dyl and likely Cht6 appear to be openings into a new mechanism for maintaining hair polarity on the fly wing. We suggest that this involves the cuticle acting as a “glove” to maintain the orientation of hairs as wing cells go through their later steps in wing morphogenesis (Mitchell et al., 1990) (Roch et al., 2003). These steps, which have not been intensively studied in recent years include cell flattening, the formation of the hair pedestal and the movement of the hair to the central region on the apical surface of the cell.
Rab11 and Dyl in hairs
During bristle morphogenesis Rab11 is required for the plasma membrane localization of Dyl (Nagaraj and Adler, 2012). Mutants for either of these genes give rise to a bristle “stub” phenotype due to collapse of the bristle that appears to be a consequence of a failure to secrete a patterned and organized cuticle. Although we observed an analogous phenotype for dyl in wing hairs we did not detect any wing hair phenotypes in Rab11 null mutant clones or after a Rab11 kd. However, we found Rab11 to accumulate at the site of hair outgrowth, in growing hairs and preferentially in blobs at the tips of growing hairs. These localization patterns are reminiscent of that in bristles and arista laterals where Rab11 is functionally important (Nagaraj and Adler, 2012) and suggest the lack of a mutant phenotype in hairs is due to redundancy or to other cell essential functions being more sensitive to a loss of Rab11. Consistent with these possibilities the expression of either dominant negative or constitutively active Rab11 leads to a range of hair phenotypes including mimics of the dyl hair phenotype. Another was a planar cell polarity phenotype typical of mutations in fz/stan pathway genes. We found this was associated with both abnormal cell shape and a disruption in the normal zig zag accumulation of the fz/stan pathway proteins Stan and In at the distal/proximal sides of pupal wing cells (Adler et al., 2004; Usui et al., 1999). This result was unexpected as previous results from Strutt and Strutt (Strutt and Strutt, 2008) implicated Rab4 and not Rab11 in the endosomal trafficking of Stan and Fz. They found that Stan co-localized with Rab4 and Rab5 but not with Rab11. However, the authors could not rule out Rab11 taking over in the absence of Rab4 function. They found the expression of DN-Rab11 to be toxic (as we have) but did not report any effects on PCP. It is unclear if DN-Rab11 directly interfered with the intracellular trafficking involved in the formation of the proximal and distal protein complexes that contain fz/stan pathway components. The effects could be indirect, for example, due to the abnormal cell shape or to defects in the trafficking of other components.
We suspect that bristle cells are more sensitive than hairs to a loss of Rab11 function and that this is due to their much greater length. An observation consistent with this hypothesis is that a Rab11 hypomorph produces a bristle morphology phenotype that is obvious in the largest bristles (thoracic macrochaetae) but not in smaller bristles or in hairs. The largest bristles were also far more sensitive to a Rab11 kd than shorter bristles (Nagaraj and Adler, 2012). This could be due to Rab11 being important for long-range intracellular transport while other pathways might suffice for shorter distances.
Chitin is not the only target of dyl
Drosophila ZP domain proteins as a group have been implicated in cuticle assembly (see for example (Fernandes et al., 2010; Nagaraj and Adler, 2012; Roch et al., 2003)). Here we established that while dyl is required for normal chitin deposition, chitin is not required for normal Dyl accumulation. Thus, Dyl appears to function upstream of chitin in cuticle assembly. We suggest this will be true for other ZP domain proteins.
It is striking that both the dyl bristle and hair mutant phenotypes are associated with defects after outgrowth. We found defects in chitin deposition in dyl mutant hairs much as we previously found in bristles (Nagaraj and Adler, 2012). The range of bristle phenotypes seen in kkv mutants overlapped but was weaker on average than that seen in dyl mutants, which lead us to argue that Dyl had functions other than organizing chitin deposition (Nagaraj and Adler, 2012). More compelling data was obtained studying wing hairs where the mutant phenotypes are distinctly different and additive.Thus a defect in chitin deposition cannot explain the dyl mutant phenotype. It seems likely that other targets of Dyl are important for maintaining hair structure. Obvious candidates include cuticle proteins, cuticle interacting proteins and the actin cytoskeleton. Due to the large number of putative cuticle proteins encoded in the Drosophila genome it is not obvious which ones might be important in hairs. Further the large number of such genes suggests the possibility that they will be redundant making it difficult to obtain convincing functional data. Nonetheless it is interesting that knockdowns of several cuticle proteins produced hair phenotypes, albeit weak ones. These are certainly good candidates for being possible Dyl targets. Our finding that a knock down of Cht6 could mimic all of the hair morphology phenotypes seen in adult dyl hairs is strong support for the idea that the adult hair phenotypes are a result of a defect in cuticle formation and it could be a Dyl target.
As noted above, Dyl appears to regulate the actin cytoskeleton in hair forming cells. It is possible that the late hair morphology abnormalities associated with a lack of dyl function are due to effects on the cytoskeleton and not to effects on cuticle deposition. Such an explanation is not tenable for the similar bristle collapse phenotype as no defects in F-actin organization were seen in that cell type. Thus, by analogy we think it unlikely that most or all of the dyl mutant hair phenotype is due to effects on the cytoskeleton. Further, many mutations are known that alter the function of the actin cytoskeleton and none have been found to produce a mutant hair or bristle phenotype due to collapse.
The function of Cyp301a1
Published data suggested that Cyp301a1 may function in the metabolism of ecdysone and in the formation of cuticle (Sztal et al., 2012). The increase in expression of Cyp301a1 around the time of the start of wing cuticle deposition is consistent with that suggestion. However, the wing hair phenotype we found fits better with an alternative mechanism. The curved hair and bristle phenotypes are reminiscent of phenotypes associated with mutations in genes that regulate the actin cytoskeleton such as singed and forked that bundle F-actin (Tilney et al., 1995). We suggest that Cyp301a1 may function in the regulation of the cytoskeleton. There is little evidence in the literature for P450s regulating the cytoskeleton so it is difficult to guess as to a possible mechanism. We note that recently the Mical protein, which contains redox activity, is a potent regulator of actin dynamics in flies (Hung et al., 2010). Hence, novel mechanisms for regulating the cytoskeleton continue to be discovered.
Supplementary Material
Highlights.
The ZP domain protein Dyl is required for the morphogenesis of wing hairs.
Dyl is required for maintaining planar cell polarity in the fly wing.
dyl is essential for normal chitin deposition.
Effects on Chitin cannot explain the dyl mutant phenotype.
Chitinase 6 appears to function along with dyl in hair morphogenesis.
Acknowledgments
This work was supported by a grant from the NIGMS to pna and an ARRA supplement to that grant. We thank our colleagues in the fly community for generously sharing reagents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler PN. Planar signaling and morphogenesis in Drosophila. Dev Cell. 2002;2:525–535. doi: 10.1016/s1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- Adler PN. The frizzled/stan pathway and planar cell polarity in the Drosophila wing. Curr Top Dev Biol. 2012;101:1–31. doi: 10.1016/B978-0-12-394592-1.00001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler PN, Charlton J, Liu J. Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development. 1998;125:959–968. doi: 10.1242/dev.125.5.959. [DOI] [PubMed] [Google Scholar]
- Adler PN, Zhu C, Stone D. Inturned Localizes to the Proximal Side of Wing Cells under the Instruction of Upstream Planar Polarity Proteins. Curr Biol. 2004;14:2046–2051. doi: 10.1016/j.cub.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, Tomlin CJ. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science. 2005;307:423–426. doi: 10.1126/science.1105471. [DOI] [PubMed] [Google Scholar]
- Arruda SE, Dolph PJ. Molecular cloning of the pawn locus from Drosophila melanogaster. Gene. 2003;310:169–173. doi: 10.1016/s0378-1119(03)00548-1. [DOI] [PubMed] [Google Scholar]
- Bogard N, Lan L, Xu J, Cohen RS. Rab11 maintains connections between germline stem cells and niche cells in the Drosophila ovary. Development. 2007;134:3413–3418. doi: 10.1242/dev.008466. [DOI] [PubMed] [Google Scholar]
- Bokel C, Prokop A, Brown NH. Papillote and Piopio: Drosophila ZP-domain proteins required for cell adhesion to the apical extracellular matrix and microtubule organization. Journal of cell science. 2005;118:633–642. doi: 10.1242/jcs.01619. [DOI] [PubMed] [Google Scholar]
- Brodu V, Baffet AD, Le Droguen PM, Casanova J, Guichet A. A developmentally regulated two-step process generates a noncentrosomal microtubule network in Drosophila tracheal cells. Developmental cell. 2010;18:790–801. doi: 10.1016/j.devcel.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Cant K, Knowles BA, Mooseker MS, Cooley L. Drosophila singed, a fascin homolog, is required for actin bundle formation during oogenesis and bristle extension. J Cell Biol. 1994;125:369–380. doi: 10.1083/jcb.125.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delon I, Chanut-Delalande H, Payre F. The Ovo/Shavenbaby transcription factor specifies actin remodelling during epidermal differentiation in Drosophila. Mechanisms of development. 2003;120:747–758. doi: 10.1016/s0925-4773(03)00081-9. [DOI] [PubMed] [Google Scholar]
- Devine WP, Lubarsky B, Shaw K, Luschnig S, Messina L, Krasnow MA. Requirement for chitin biosynthesis in epithelial tube morphogenesis. Proc Natl Acad Sci U S A. 2005;102:17014–17019. doi: 10.1073/pnas.0506676102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollar G, Struckhoff E, Michaud J, Cohen RS. Rab11 polarization of the Drosophila oocyte: a novel link between membrane trafficking, microtubule organization, and oskar mRNA localization and translation. Development. 2002;129:517–526. doi: 10.1242/dev.129.2.517. [DOI] [PubMed] [Google Scholar]
- Emery G, Hutterer A, Berdnik D, Mayer B, Wirtz-Peitz F, Gaitan MG, Knoblich JA. Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell. 2005;122:763–773. doi: 10.1016/j.cell.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Fernandes I, Chanut-Delalande H, Ferrer P, Latapie Y, Waltzer L, Affolter M, Payre F, Plaza S. Zona pellucida domain proteins remodel the apical compartment for localized cell shape changes. Dev Cell. 2010;18:64–76. doi: 10.1016/j.devcel.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Franke JD, Montague RA, Kiehart DP. Nonmuscle myosin II is required for cell proliferation, cell sheet adhesion and wing hair morphology during wing morphogenesis. Dev Biol. 2010;345:117–132. doi: 10.1016/j.ydbio.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagou ME, Kapsetaki M, Turberg A, Kafetzopoulos D. Stage-specific expression of the chitin synthase DmeChSA and DmeChSB genes during the onset of Drosophila metamorphosis. Insect Biochem Mol Biol. 2002;32:141–146. doi: 10.1016/s0965-1748(01)00101-1. [DOI] [PubMed] [Google Scholar]
- Gangishetti U, Breitenbach S, Zander M, Saheb SK, Muller U, Schwarz H, Moussian B. Effects of benzoylphenylurea on chitin synthesis and orientation in the cuticle of the Drosophila larva. Eur J Cell Biol. 2009;88:167–180. doi: 10.1016/j.ejcb.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Gault WJ, Olguin P, Weber U, Mlodzik M. Drosophila CK1-gamma, gilgamesh, controls PCP-mediated morphogenesis through regulation of vesicle trafficking. J Cell Biol. 2012;196:605–621. doi: 10.1083/jcb.201107137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng W, He B, Wang M, Adler PN. The tricornered gene, which is required for the integrity of epidermal cell extensions, encodes the Drosophila nuclear DBF2-related kinase. Genetics. 2000;156:1817–1828. doi: 10.1093/genetics/156.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giansanti MG, Belloni G, Gatti M. Rab11 is required for membrane trafficking and actomyosin ring constriction in meiotic cytokinesis of Drosophila males. Mol Biol Cell. 2007;18:5034–5047. doi: 10.1091/mbc.E07-05-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus KC, Bonaccorsi S, Williams E, Verni F, Gatti M, Goldberg ML. Mutations in twinstar, a Drosophila gene encoding a cofilin/ADF homologue, result in defects in centrosome migration and cytokinesis. J Cell Biol. 1995;131:1243–1259. doi: 10.1083/jcb.131.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Adler PN. Cellular mechanisms in the development of the Drosophila arista. Mech Dev. 2001;104:69–78. doi: 10.1016/s0925-4773(01)00368-9. [DOI] [PubMed] [Google Scholar]
- He Y, Fang X, Emoto K, Jan YN, Adler PN. The tricornered Ser/Thr protein kinase is regulated by phosphorylation and interacts with furry during Drosophila wing hair development. Mol Biol Cell. 2005;16:689–700. doi: 10.1091/mbc.E04-09-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung RJ, Yazdani U, Yoon J, Wu H, Yang T, Gupta N, Huang Z, van Berkel WJ, Terman JR. Mical links semaphorins to F-actin disassembly. Nature. 2010;463:823–827. doi: 10.1038/nature08724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafar-Nejad H, Andrews HK, Acar M, Bayat V, Wirtz-Peitz F, Mehta SQ, Knoblich JA, Bellen HJ. Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev Cell. 2005;9:351–363. doi: 10.1016/j.devcel.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Kiehart DP, Franke JD, Chee MK, Montague RA, Chen TL, Roote J, Ashburner M. Drosophila crinkled, mutations of which disrupt morphogenesis and cause lethality, encodes fly myosin VIIA. Genetics. 2004;168:1337–1352. doi: 10.1534/genetics.104.026369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G, Casal J. Planar cell polarity: one or two pathways? Nat Rev Genet. 2007;8:555–563. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuilton P, St Pierre S, Thurmond J, Consortium F. FlyBase 101- the basics of navigating FlyBase. Nucleic Acids Res. 2012;40:D706–714. doi: 10.1093/nar/gkr1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell HK, Edens J, Petersen NS. Stages of cell hair construction in Drosophila. Dev Genet. 1990;11:133–140. doi: 10.1002/dvg.1020110203. [DOI] [PubMed] [Google Scholar]
- Moussian B, Schwarz H, Bartoszewski S, Nusslein-Volhard C. Involvement of chitin in exoskeleton morphogenesis in Drosophila melanogaster. J Morphol. 2005;264:117–130. doi: 10.1002/jmor.10324. [DOI] [PubMed] [Google Scholar]
- Moyer KE, Jacobs JR. Varicose: a MAGUK required for the maturation and function of Drosophila septate junctions. BMC Dev Biol. 2008;8:99. doi: 10.1186/1471-213X-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj R, Adler PN. Dusky-like functions as a Rab11 effector for the deposition of cuticle during Drosophila bristle development. Development. 2012;139:906–916. doi: 10.1242/dev.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski S, Dierick HA, Bejsovec A. Genetic control of cuticle formation during embryonic development of Drosophila melanogaster. Genetics. 2002;161:171–182. doi: 10.1093/genetics/161.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen NS, Lankenau DH, Mitchell HK, Young P, Corces VG. forked proteins are components of fiber bundles present in developing bristles of Drosophila melanogaster. Genetics. 1994;136:173–182. doi: 10.1093/genetics/136.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza S, Chanut-Delalande H, Fernandes I, Wassarman PM, Payre F. From A to Z: apical structures and zona pellucida-domain proteins. Trends Cell Biol. 2010;20:524–532. doi: 10.1016/j.tcb.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Price MH, Roberts DM, McCartney BM, Jezuit E, Peifer M. Cytoskeletal dynamics and cell signaling during planar polarity establishment in the Drosophila embryonic denticle. J Cell Sci. 2006;119:403–415. doi: 10.1242/jcs.02761. [DOI] [PubMed] [Google Scholar]
- Purvanov V, Koval A, Katanaev VL. A direct and functional interaction between Go and Rab5 during G protein-coupled receptor signaling. Sci Signal. 2010;3:ra65. doi: 10.1126/scisignal.2000877. [DOI] [PubMed] [Google Scholar]
- Ren N, Charlton J, Adler PN. The flare gene, which encodes the AIP1 protein of Drosophila, functions to regulate F-actin disassembly in pupal epidermal cells. Genetics. 2007;176:2223–2234. doi: 10.1534/genetics.107.072959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren N, Zhu C, Lee H, Adler PN. Gene Expression During Drosophila Wing Morphogenesis and Differentiation. Genetics. 2005;171:625–638. doi: 10.1534/genetics.105.043687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roch F, Alonso CR, Akam M. Drosophila miniature and dusky encode ZP proteins required for cytoskeletal reorganisation during wing morphogenesis. J Cell Sci. 2003;116:1199–1207. doi: 10.1242/jcs.00298. [DOI] [PubMed] [Google Scholar]
- Shewan A, Eastburn DJ, Mostov K. Phosphoinositides in cell architecture. Cold Spring Harb Perspect Biol. 2011;3:a004796. doi: 10.1101/cshperspect.a004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G, Basler K. Organizing acitivity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Strutt D. The asymmetric subcellular localisation of components of the planar polarity pathway. Seminars in Cell and Developmental Biology. 2002;13:225–231. doi: 10.1016/s1084-9521(02)00041-1. [DOI] [PubMed] [Google Scholar]
- Strutt H, Strutt D. Differential stability of flamingo protein complexes underlies the establishment of planar polarity. Current biology: CB. 2008;18:1555–1564. doi: 10.1016/j.cub.2008.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztal T, Chung H, Berger S, Currie PD, Batterham P, Daborn PJ. A cytochrome p450 conserved in insects is involved in cuticle formation. PloS one. 2012;7:e36544. doi: 10.1371/journal.pone.0036544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Connelly PS, Vranich KA, Shaw MK, Guild GM. Actin filaments and microtubules play different roles during bristle elongation in Drosophila. J Cell Sci. 2000;113 (Pt 7):1255–1265. doi: 10.1242/jcs.113.7.1255. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS, Guild GM. F actin bundles in Drosophila bristles. I. Two filament cross-links are involved in bundling. J Cell Biol. 1995;130:629–638. doi: 10.1083/jcb.130.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CM, Adler PN. Distinct roles for the actin and microtubule cytoskeletons in the morphogenesis of epidermal hairs during wing development in Drosophila. Mech Dev. 1998;70:181–192. doi: 10.1016/s0925-4773(97)00194-9. [DOI] [PubMed] [Google Scholar]
- Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, Takeichi M, Uemura T. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- Venema DR, Zeev-Ben-Mordehai T, Auld VJ. Transient apical polarization of Gliotactin and Coracle is required for parallel alignment of wing hairs in Drosophila. Dev Biol. 2004;275:301–314. doi: 10.1016/j.ydbio.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Winter CG, Wang B, Ballew A, Royou A, Karess R, Axelrod JD, Luo L. Drosophila Rho-Associated Kinase (Drok) Links Frizzled-Mediated Planar Cell Polarity Signaling to the Actin Cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- Wong LL, Adler PN. Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. J Cell Biol. 1993;123:209–221. doi: 10.1083/jcb.123.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Mlodzik M. A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol. 2009;19:295–305. doi: 10.1016/j.tcb.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lan L, Bogard N, Mattione C, Cohen RS. Rab11 is required for epithelial cell viability, terminal differentiation, and suppression of tumor-like growth in the Drosophila egg chamber. PLoS One. 2011;6:e20180. doi: 10.1371/journal.pone.0020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Zhao H, Hakala M, Lappalainen P. ADF/cofilin binds phosphoinositides in a multivalent manner to act as a PIP(2)-density sensor. Biophys J. 2010;98:2327–2336. doi: 10.1016/j.bpj.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.