Mouse hair development and subsequent cycling follow a carefully choreographed rhythm (Muller-Rover et al., 2001; Paus et al., 1999). Cycling behavior is controlled by multiple genes and pathways. Although regulatory processes can be overridden by compensatory mechanisms, losing the function of key regulators will produce irreversible and irreparable defects during cycling. New progress has been made on how key regulators monitor hair stem cell behavior and maintain normal hair cycling. Among them, β-catenin signaling is considered to be the earliest and most important factor (Huelsken et al., 2001). In hair follicles, β-catenin activity is activated via Wnt signaling and suppressed by BMP signaling (Plikus et al., 2011). Wnt signaling has been found in both the epithelium and mesenchyme but it is unknown which initiates the Wnt signaling cascade that is essential for ectodermal organ production.
It was previously shown that epidermal Wnt signaling was sufficient to induce a dermal Wnt signaling response during hair follicle development (Chen et al., 2012). To further explore this issue, Wntless(Wls also called Gpr177), a gene regulating Wnt protein sorting and secretion, was genetically deleted from either epithelium or mesenchyme (Fu et al., 2011). Deleting epithelial Wls expression using a Krt5 driven Cre excision inhibited the canonical Wnt signaling molecules Dvl2, GSK3 and nuclear β-catenin levels as well as the epithelial placode markers Eda, BMP2, BMP4, and SHH. It prevented hair placodes from forming at E14.5. The phenotype was partially rescued by restoring expression of a stabilized form of β-catenin to the epidermis. Edar and Shh positive epithelial placodes could form but underlying dermal condensations and dermal papillae could not. These data imply that canonical Wnt signaling in the epithelium is sufficient to induce epithelial hair formation but cannot activate a dermal response.
They then tested a dermis-specific deletion of Wls using a Dermo1 driven Cre excision. Several assays did not show a reduction of β-catenin levels in vivo but an in vitro TOPFLASH assay showed a significant reduction. Although Edar and Shh expression was reduced in the skin, epithelial placodes could still form. The dermis beneath the placodes was loosely arranged and dermal cell proliferation was greatly reduced. Dermal cells preferentially differentiated to become adipocytes. This finding supports the earlier report that co-culturing dermal papilla cells with cells secreting Wnt3a could activate β-catenin and thereby maintain their hair inducing properties (Kishimoto et al., 2000). It is also consistent with the finding that epidermal Wnts can activate a dermal β-catenin response (Chen and Atit, 2012). However, knocking out Wls directly in the dermis did not prevent hair placode formation which suggests that it may not be essential for epidermal hair follicle activation. Perhaps there are sufficient levels of epidermal Wnts present to stimulate the pathway.
Interestingly, of the 19 mammalian Wnts regulating organ development and growth, deletion of Wls blocked Wnts2, 7a, 7b, 10a and10b expression. Of these, Wnt 2, 7b, 10a, and 10b were expressed in the hair placode suggesting some of these may be more critical for epithelial hair placode initiation. Wnt3, 4, 6 and 16 do not require Wls to be secreted but they are not sufficient to induce hair placodes.
Wnts play important roles in hair regeneration during adult life (Collins et al., 2011). Wls was used to identify the cellular source of Wnt ligands during anagen induction in adult hair follicle cycling (Myung et al., 2012). They found that Wls is normally expressed in cells with nuclear β-catenin expression. To determine whether epidermal Wnt ligands are required for hair cycling, the authors genetically deleted Wls(WlsK14cKO) in the basal layer of epidermis and hair follicles. They showed that most hair follicles from P35-37 in a WlsK14cKO mice did not uniformly enter anagen, but remained arrested in telogen and anagen I phase with a few in anagen II and III phase. This is a rare phenotype. Most gene deletion induced hair cycle abnormalities cause hair follicles to be blocked at one single stage. However, Wnts do not always serve as activators of hair development and regeneration. Wnt5a has been found to both activate and repress canonical Wnt signaling depending on the receptors and cellular context in which it is expressed (van Amerongen et al., 2012). Inhibition of repression could enable some hair follicles to reenter anagen phase. Even if stem cells could not be activated they still maintained many of their molecular properties. There were normal numbers of K15+, CD34+, TCF3+ epithelial progenitor cells and alkaline phosphatase+, LEF-1+ dermal papilla cells. Similar findings were observed when they specifically targeted hair follicle progenitor cells with a WlsK15cKO mouse. These cells did not show increased numbers of nuclear β-catenin nor increased proliferation.
After a big wound, new hair buds can form de novo from the center of wounds if the full thickness wound opening is greater than 1 cm2 (Ito et al., 2007). Wnt7a signaling was shown to enhance wound induced follicle neogenesis (Ito et al., 2007). However, it is not clear whether epithelial Wnt signals are essential to induce hair neogenesis. To test this, the ability of WlsK14cKO mice to undergo hair neogenesis in a big open wound was examined. These mice could not form new hair follicles within the wound bed. This study showed the importance of epidermal Wnts in wound induced follicle neogenesis.
If Wnts are important, can the delivery of ectopic Wnts alter hair regenerative cycling? The role of Wnt10b in hair follicle regeneration was tested in this capacity (Li et al., 2012). Wnt10b is expressed during anagen but not during catagen or telogen phases of the hair cycle. Using adenovirus mediated ectopic expression, they showed β-catenin was translocated to the nucleus and induced hair follicles to enter anagen earlier than controls. The regenerating hair follicles expressed normal follicle markers including AE15, and Sox9. To further demonstrate the essential role of Wnt10b in hair regeneration, they interfered with Wnt10b expression by intradermal injection of siRNA (AdSim10b). Anagen was delayed in the siRNA treated area. Furthermore, siRNA suppression of β-catenin inhibited hair follicle regeneration even when Wnt10b was over-expressed, suggesting β-catenin activation is downstream to Wnt10b stimulation. This paper demonstrates that Wnt10b can activate anagen reentry of the telogen hair follicle through a canonical signaling pathway, although other Wnts may play a similar role and a noncanonical Wnt signaling pathway may also be involved.
While hair stem cell activity within single hair follicles is regulated via intra-follicular Wnt/BMP signaling (Kobielak et al., 2007), the extra-follicular dermal macroenvironment also plays an important role in regulating the coordinated regeneration of hair follicle populations. BMP from subcutaneous adipose tissue was shown to inhibit regeneration of the hair populations by maintaining them in a refractory telogen phase, unable to respond to Wnt signaling (Plikus et al., 2011). Once BMP expression was turned off, hairs entered a competent telogen phase where they could respond to Wnt and enter anagen. Hence the ratio of activators/inhibitors is critical in regulating hair cycle progression. The extrafollicular macroenvironment in the adult mouse contains inhibitors including the Wnt antagonists, Dkk1 and Sfrp4, which coordinate hair cycling behavior. Furthermore, the expression of these regulatory molecules can be controlled by extrinsic environmental signals (ie., temperature, day length, etc) to modulate hair regeneration. This adaptability to extrinsic environmental signals enables mammals to live in a wide range of environments. Thus Wnt activity serves as an integrator of activators/inhibitors derived from different levels of environmental inputs (Chen and Chuong, 2012)
Abnormal hair development and regeneration has been implicated in diseases of the skin (ie., hirsutism, alopecia, etc) or in open wounds when hair follicles are completely eliminated. To manage these clinical conditions, it is important to understand molecular pathways which regulate the number, size, growth and regeneration of hair follicles. Wnt signaling plays a fundamental role in this process. We need a deeper understanding so we can reliably adjust Wnt levels in existing follicles. This studies reviewed here have future translational value for skin regeneration following severe wound injuries or in the context of tissue engineering. Tuning the levels of Wnt ligands can directly modulate the number and growth of hairs. Using this new knowledge, we now know that Wnt activity can be modulated by adjusting the secretion of Wnt ligands, altering binding of ligands to receptors, inhibiting β-catenin tanslocation, or by regulating extra-follicular dermal Wnt and Wnt inhibitors.
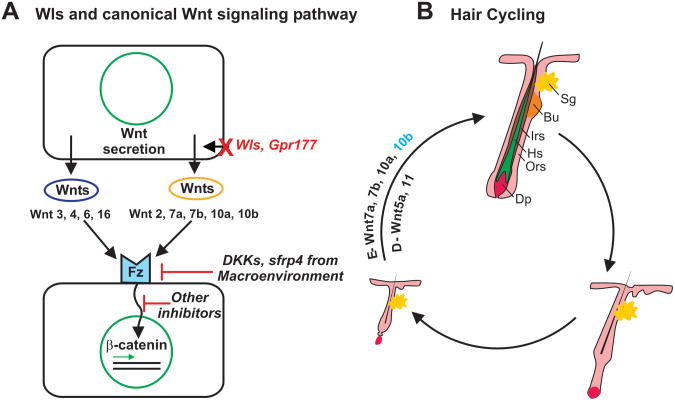
Figure 1.
(a) Model depicting the role of Wls/Gpr177 in regulating the Wnt signaling pathway. (b) Involvement of Wls-regulated Wnts in the hair cycle. E, epidermis; D, dermis; Sg, sebaceous gland; Bu, bulge; Irs, inner root sheath; Hs, hair shaft; Ors, outer root sheath; Dp, dermal papillae.
Acknowledgments
This work is supported by US NIH grants AR42177, AR47364, and AR 060306 (C.M.C.)
Footnotes
Conflict of Interest: The authors state no conflict of interest.
References
- Chen CC, Chuong CM. Multi-layered environmental regulation on the homeostasis of stem cells: the saga of hair growth and alopecia. J Dermatol Sci. 2012;66:3–11. doi: 10.1016/j.jdermsci.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Jarrell A, Guo C, Lang R, Atit R. Dermal beta-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development. 2012;139:1522–33. doi: 10.1242/dev.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Kretzschmar K, Watt FM. Reprogramming adult dermis to a neonatal state through epidermal activation of beta-catenin. Development. 2011;138:5189–99. doi: 10.1242/dev.064592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Ivy Yu HM, Maruyama T, Mirando AJ, Hsu W. Gpr177/mouse Wntless is essential for Wnt-mediated craniofacial and brain development. Dev Dyn. 2011;240:365–71. doi: 10.1002/dvdy.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–45. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–20. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000;14:1181–5. [PMC free article] [PubMed] [Google Scholar]
- Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci U S A. 2007;104:10063–8. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YH, Zhang K, Yang K, Ye JX, Xing YZ, Guo HY, et al. Adenovirus-Mediated Wnt10b Overexpression Induces Hair Follicle Regeneration. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.235. [DOI] [PubMed] [Google Scholar]
- Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- Myung PS, Takeo M, Ito M, Atit RP. Epithelial Wnt Ligand Secretion Is Required for Adult Hair Follicle Growth and Regeneration. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus R, Muller-Rover S, Van Der Veen C, Maurer M, Eichmuller S, Ling G, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113:523–32. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- Plikus MV, Baker RE, Chen CC, Fare C, de la Cruz D, Andl T, et al. Self-organizing and stochastic behaviors during the regeneration of hair stem cells. Science. 2011;332:586–9. doi: 10.1126/science.1201647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R, Fuerer C, Mizutani M, Nusse R. Wnt5a can both activate and repress Wnt/beta-catenin signaling during mouse embryonic development. Dev Biol. 2012;369:101–14. doi: 10.1016/j.ydbio.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]