Abstract
CD4+FoxP3+ regulatory T cells (Tregs) play a central role in the maintenance of immune tolerance after allogeneic hematopoietic stem cell transplantation. We recently reported that daily administration of low-dose IL-2 induces selective expansion of functional Treg and clinical improvement of chronic graft-versus-host disease (GVHD). To define the mechanisms of action of IL-2 therapy we examined the immunologic effects of this treatment on homeostasis of CD4 T cell subsets after transplant. We first demonstrate that chronic GVHD is characterized by constitutive phosphorylation of Stat5 in conventional CD4 T cells (Tcon) associated with elevated levels of IL-7 and IL-15 and relative functional deficiency of IL-2. IL-2 therapy resulted in the selective increase of Stat5 phosphorylation in Treg and decrease of pStat5 in Tcon. Over an eight-week period, IL-2 therapy induced a series of changes in Treg homeostasis, including increased proliferation, increased thymic export and enhanced resistance to apoptosis. Low-dose IL-2 had minimal effects on Tcon. These findings define the mechanisms whereby low-dose IL-2 therapy restores the homeostasis of CD4 T cell subsets and promotes the re-establishment of immune tolerance.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) can provide curative therapy for various hematologic malignancies, bone marrow failure syndromes and congenital immune deficiencies. Improvements in immune suppressive therapy and supportive care have improved patient outcomes but chronic graft-versus-host disease (GVHD) continues to be a major problem affecting long-term survivors (1–3). The clinical and laboratory manifestations of chronic GVHD resemble those of autoimmune diseases such as systemic lupus erythematous, Sjogren’s syndrome and scleroderma (4–6). Both T and B cell responses play a role in the pathogenesis of chronic GVHD (7–9) suggesting that this syndrome reflects a general loss of immune tolerance.
CD4+Foxp3+ regulatory T cells (Treg) contribute to the maintenance of peripheral tolerance, and patients with chronic GVHD have a relative deficiency of Treg (10–13). This deficiency appears to be a consequence of abnormalities in Treg homeostasis after HSCT wherein increased proliferation of Treg is not sufficient to compensate for reduced thymic output and increased susceptibility to apoptosis (14). In contrast, homeostasis of conventional CD4 T cells (Tcon) does not appear to be impaired as the reconstitution of Tcon after HSCT is maintained through increased thymic production and reduced susceptibility to apoptosis compared to Treg.
Cytokines that utilize the common γ chain play a central role in T cell homeostasis. In response to lymphopenia, IL-7 and IL-15 provide critical signals to drive T cell proliferation and survival (15–18), and administration of IL-7 or IL-15 has been shown to increase different subsets of circulating T cells (19–22). In contrast, IL-2 is a critical homeostatic cytokine for Treg (23–26). In animal models, neutralization of IL-2 results in Treg deficiency and autoimmunity, whereas administration of IL-2 can induce Treg expansion in vivo and prevent autoimmunity (25, 27–31). These findings indicate that Tcon and Treg homeostasis are regulated by distinct cytokines and manipulation of the cytokine environment may influence the balance of these subsets in the specific settings of autoimmunity and GVHD.
Although TCR activation of effector T cells leads to rapid expression of IL-2 receptors, Treg constitutively express high levels of high affinity IL-2 receptors without activation. In recent clinical trials, administration of low-doseIL-2 has been shown to result in the selective expansion of Treg and clinical improvement in symptoms of auto and allo-immunity (32, 33). At our center, daily therapy with low-dose IL-2 for 8 weeks in patients with chronic GVHD led to a rapid increase in circulating Treg, without a significant increase in CD4 Tcon or CD8 T cells (33). This was associated with clinical improvement in approximately 50% of patients and no patients experienced GVHD progression. In the present study, we examined the role of homeostatic cytokines in chronic GVHD and the effects of IL-2 administration on the homeostasis of Treg and Tcon. Patients with severe chronic GVHD had elevated levels of IL-7 and IL-15associated with higher levels of phosphorylated Stat5 (pStat5) in Tcon than Treg. This imbalance of Stat5 signaling was rapidly reversed in patients receiving low-dose IL-2, resulting in increased thymic generation and proliferation of Treg and reduced susceptibility to apoptosis. These results demonstrate that daily administration of IL-2 at physiologic doses can restore Treg homeostasis in vivo and promote immune tolerance.
Results
Differential effects of homeostatic cytokines on CD4 T cell subsets in vitro
The signals induced by IL-2, IL-7 and IL-15 interactions with their specific membrane receptors are mediated through the Jak-Stat5 pathway (34). To compare the response of human CD4 T cell subsets to these cytokines, CD4 Treg and Tcon were purified from healthy donors by cell sorting (Figure 1A). Treg isolated in this manner express high-levels of FoxP3 (Figure 1B) and effectively suppress proliferation of activated autologous Tcon (Figure 1C). Purified Treg and Tcon were stimulated by each cytokine for 15 minutes and intracellular Stat5 phosphorylation (pStat5) was evaluated by flow cytometry. Results in Figure 1D compare pStat5 levels in Treg and Tcon after stimulation with IL-2, IL-7 and IL-15 over a 7-log range of cytokine concentrations. At high IL-2 concentrations (100–1000 IU/ml), Stat5 was activated in both Treg and Tcon. However, at low IL-2 concentrations (1–10 IU/ml), Stat5 was activated only in Treg. Different patterns of Stat5 activation were observed with IL-7 and IL-15. At low concentrations, IL-7 induced similar levels of Stat5 activation in Treg and Tcon. However at high concentrations, IL-7 preferentially activated Tcon. In contrast, IL-15 induced similar levels of Stat5 activation in Treg and Tcon at all concentrations. These results indicate that human CD4 T cell subsets show distinct patterns of response to different homeostatic cytokines. The most selective effect on Treg was noted with low concentrations of IL-2 and this was confirmed in functional assays comparing proliferation of Treg and Tcon (Figure 1E) after in vitro stimulation.
Figure 1. Phenotypic and functional characterization of human Treg and Tcon after in vitro cytokine activation.
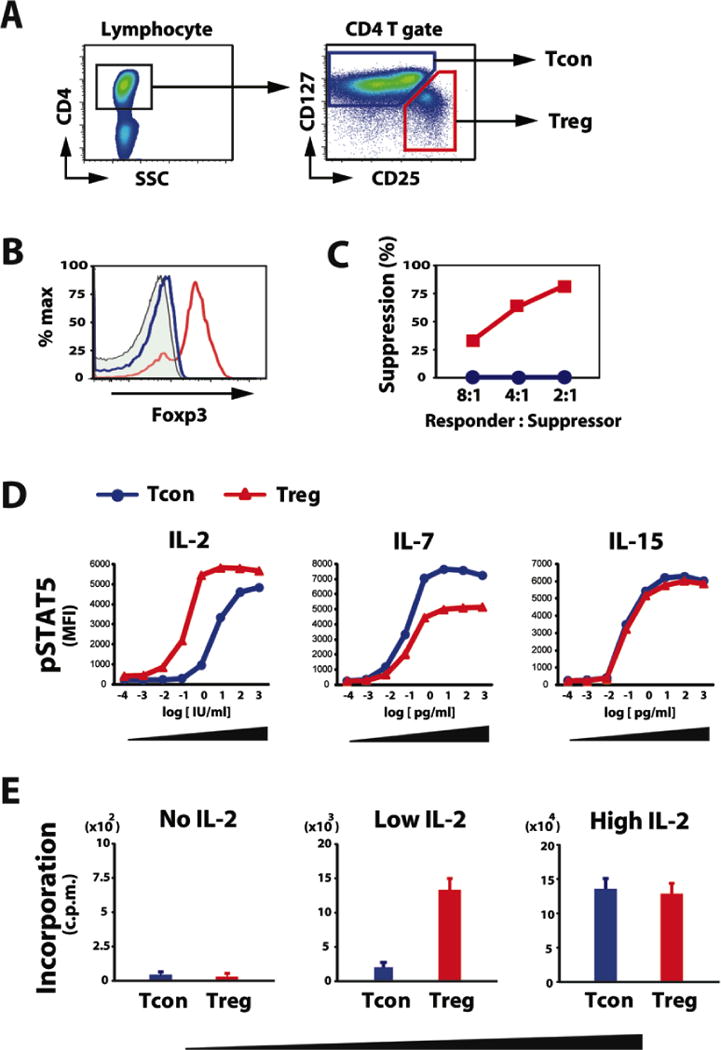
(A) Representative lymphocyte gate for identification of CD4 T cell subsets. Within the CD4 T cell gate, Treg are identified as CD25med-highCD127low and Tcon are identified as CD25neg-lowCD127med-high. (B) Gated CD4 T cell subsets were examined for intracellular Foxp3 expression. Representative data is shown. Closed histogram represents isotype control. Blue and red histograms depict Tcon and Treg, respectively. (C) Tregor Tcon isolated from peripheral blood were cultured with responder Tcon from the same donor and stimulated with irradiated allogeneic PBMCs for 5 days. Method for calculating percentage suppression of proliferation is described in Methods. Data are representative of 5 independent experiments. (D) Purified Treg and Tcon were cultured for 15 minutes in various concentrations of IL-2, IL-7 and IL-15. The level of intracellular Stat5 phosphorylation (pStat5) was determined by flow cytometry. Cytokine dose-dependent phosphorylation of Stat5 in each CD4 T cell subset is shown. Data are representative of 3 independent experiments. (E) Purified Treg and Tcon were cultured in low (10 IU/ml) or high (100 IU/ml) concentrations of IL-2 for 5 days and cell proliferation was measured by thymidine incorporation. Data are representative of 3 independent experiments.
Altered cytokine milieu in patients with severe chronic GVHD
To examine the role of homeostatic cytokines in vivo, we studied peripheral blood samples from 45 patients at a median of 3 years after transplantation (Cohort 1 – Table 1). Cohort 1 included 14 patients without chronic GVHD and 31 patients with chronic GVHD. As shown in Figure 2A and 2B, patients with chronic GVHD have fewer lymphocytes and fewer CD4 T cells than healthy donors and HSCT patients without active GVHD (p < 0.0001 and p = 0.0005, respectively, Wilcoxon-rank-sum test). Plasma IL-2 levels were not significantly elevated after HSCT and were similar to the low levels present in healthy donors (Figure 2C). Plasma IL-7 levels were significantly higher only in patients with severe chronic GVHD (p = 0.008 compared with healthy donors, Wilcoxon-rank-sum test). In contrast, IL-15 levels were higher in all HSCT patients compared with healthy donors and there was no increase associated with severity of chronic GVHD (p < 0.001 for all comparisons with healthy donors). When the absolute numbers of circulating CD4 T cells were considered, IL-2/CD4, IL-7/CD4 and IL-15/CD4 T cell ratios were significantly elevated in patients with severe chronic GVHD compared to patients without GVHD (p < 0.01, p = 0.0002 and p = 0.0006, respectively, Wilcoxon-rank-sum test), but this is the most evident for the IL-7/CD4 T cell ratio (Figure 2D).
Table 1.
Patient Characteristics
| Cohort 1 | Cohort 2 | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| no cGVHD (n = 14) | cGVHD (n = 31) | IL-2 treated cGVHD (n = 14) | |||||
|
|
|||||||
| n | % | n | % | p-value | N | % | |
| Age at SCT, median (range) | 44 (27, 52) | 48 (20, 63) | 0.31 | ||||
| M/F | 10/4 | 19/12 | 0.06 | 5/9 | |||
| Diagnosis | 0.59 | ||||||
| AML | 6 | 42.9 | 15 | 48.4 | 5 | 35.7 | |
| CLL | 1 | 7.1 | 4 | 12.9 | 5 | 35.7 | |
| CML | 2 | 14.3 | 4 | 12.9 | 1 | 7.1 | |
| HL | 0 | 0 | 1 | 3.2 | 1 | 7.1 | |
| ALL | 3 | 21.4 | 2 | 6.5 | |||
| MDS | 0 | 0 | 3 | 9.7 | 1 | 7.1 | |
| NHL | 2 | 14.3 | 2 | 6.5 | 1 | 7.1 | |
| Conditioning Regimen | 0.3 | ||||||
| Myeloablative | 14 | 100 | 26 | 83.9 | 7 | 50 | |
| Non-myeloablative | 0 | 0 | 5 | 16.1 | 7 | 50 | |
| Stem Cell Source | 1 | ||||||
| PBSC | 14 | 100 | 30 | 96.8 | 13 | 92.9 | |
| BM and PBSC | 0 | 0 | 1 | 3.2 | 1 | 7.1 | |
| Donor Type | 0.04 | ||||||
| Matched related | 12 | 85.7 | 14 | 45.2 | 4 | 28.6 | |
| Matched unrelated | 2 | 14.3 | 14 | 45.2 | 7 | 50 | |
| Mismatched unrelated | 0 | 0 | 3 | 9.7 | 3 | 21.4 | |
| Acute GVHD prophylaxis | 0.33 | ||||||
| Sirolimus-containing | 10 | 71.4 | 16 | 51.6 | 9 | 64.3 | |
| no Sirolimus-containing | 4 | 28.6 | 15 | 48.4 | 5 | 35.7 | |
| Acute GVHD | 0.02 | ||||||
| grade 2–4 | 1 | 7.1 | 14 | 45.2 | 5 | 35.7 | |
| Days to sample collection | |||||||
| median | 2168 | 1020 | 0.1 | ||||
| (range) | (440, 2952) | (412, 2807) | |||||
| Days to chronic GVHD | |||||||
| median (range) | 263 (125–927) | 269 (130, 483) | |||||
| Severity of cGVHD | |||||||
| Mild | 9 | 29 | |||||
| Moderate | 8 | 25.8 | |||||
| Severe | 14 | 45.2 | |||||
| Immune suppressive therapy# | |||||||
| Prednisone | 0.0001 | ||||||
| 0 mg | 12 | 85.7 | 6 | 19.4 | 1 | 7.1 | |
| 0 to ≦ 10mg | 2 | 14.3 | 10 | 32.3 | 2 | 14.2 | |
| > 10mg | 0 | 0.0 | 15 | 48.4 | 11 | 78.6 | |
| Sirolimus | 0.09 | ||||||
| 0 mg | 13 | 92.9 | 19 | 61.3 | 8 | 71 | |
| 0 to ≦ 1mg | 1 | 7.1 | 7 | 22.6 | 2 | 14.2 | |
| > 1mg | 0 | 0.0 | 5 | 16.1 | 4 | 28.4 | |
| Tacrolimus | 0.04 | ||||||
| 0 mg | 14 | 100.0 | 20 | 64.5 | 9 | 64.3 | |
| 0 to ≦ 1mg | 0 | 0.0 | 6 | 19.4 | 2 | 14.2 | |
| > 1mg | 0 | 0.0 | 5 | 16.1 | 3 | 21.3 | |
| MMF | 0.007 | ||||||
| 0 mg | 14 | 100.0 | 16 | 51.6 | 4 | 28.4 | |
| 0 to ≦ 500mg | 0 | 0.0 | 3 | 9.7 | 1 | 7.1 | |
| > 500mg | 0 | 0.0 | 12 | 38.7 | 9 | 64.3 | |
In Cohort 1 patients, immunosuppressive therapies when samples were collected are shown. In Cohort 2 patients, immunosuppressive therapies at the initiation of low-dose IL-2 therapy are shown.
AML, Acute Myeloid Leukemia; CLL, Chronic Lymphocytic Leukemia; CML, Chronic Myelogenous Leukemia; HL, Hodgkin Lymphoma; ALL, Acute Lymphoblastic Leukemia; MDS, Myelodyplastic Syndrome; NHL, Non-Hodgkin Lymphoma; PBSC, Peripheral Blood Stem Cells; BM, Bone Marrow; MMF, Mycophenolate mofetil
Figure 2. Altered cytokine milieu and activation of intracellular Stat5 in chronic GVHD.

(A–B)Lymphocyte counts and CD4 T cell counts in peripheral blood from healthy donors and chronic GVHD patients (Cohort 1). Box plots in each figure depict the 75th percentile, median and 25th percentile values and whiskers represent maximum and minimum values. (C) Plasma concentrations of IL-2, IL-7 and IL-15 from healthy donors and HSCT patients. (D) Ratio ofplasma cytokine concentration to CD4 T cell number for each patient group. (E) Expression of phosphorylated Stat5 (pStat5) in CD4 gated Tcon (blue) and Treg (red) from healthy donors and patients with and without chronic GVHD. Representative panels are shown. (F) Expression of pStat5 in Tcon and Treg subsets from 25 healthy donors and 45 HSCT patients. The expression of pStat5 in Tcon was significantly greater than Treg in patients with severe cGVHD (p < 0.005, Wilcoxon-signed-rank test), while pStat5 expression in other groups did not show significant differences between Treg and Tcon. (G) Ratio of Treg-pStat5 MFI to Tcon-pStat5 MFI in healthy donors and transplant patients. Treg-pStat5/Tcon-pStat5 ratio in severe chronic GVHD was significantly lower than in patients without GVHD (p < 0.001, Wilcoxon-rank-sum test).
Activation of CD4 T cell subsets in vivo was also monitored by measuring Stat5 phosphorylation (pSTAT5). In healthy donors, levels of pStat5 were equivalent in Treg and Tcon (Figure 2E, 2F). Similar results were obtained when pStat5 was measured in Treg and Tcon using an alternate gating strategy based on expression of Foxp3 and CD25 to define theseCD4 T cell subsets (Supplemental Figure 1). As shown for representative patients in Figure 2E and the entire Cohort 1 in Figure 2F, levels of pStat5 in Treg and Tcon were not different in HSCT patients with no, mild or moderate chronic GVHD. In contrast, pStat5 was selectively increased in Tcon in patients with severe chronic GVHD (p < 0.005, Wilcoxon-signed-rank test). This activation was specific for pStat5 since pStat1, pStat3, pStat4 and pStat6 were not significantly increased after HSCT (Supplemental Table 1). As shown in Figure 2G, Treg-pStat5/Tcon-pStat5 ratio steadily decreased as GVHD severity increased. We also evaluated whether pSTAT5 expression in CD4 T cell subsets was related to the time from transplantation and found no correlation between time from transplant and Tcon-pSTAT5, Treg-pSTAT5 and Treg-pSTAT5/Tcon-pSTAT5 (Spearman’s correlation coefficients are -0.23, -0.30, -0.04, respectively). Taken together, these findings suggest a dominant effect of IL-7 and IL-15 on Tcon homeostasis as well as a relative functional deficiency of IL-2 on Treg homeostasis in patients with severe chronic GVHD.
Rapid correction of signaling imbalance between Treg and Tcon during IL-2 therapy
To evaluate the effects of IL-2 on Stat5 signaling in Treg and Tcon in vivo, we examined 14 patients with refractory chronic GVHD enrolled in a Phase I clinical trial described previously (Cohort 2 – Table 1) (33). These patients received daily low-dose IL-2 for 8 weeks followed by a 4 week rest period without IL-2. One week after IL-2 therapy began, pStat5 significantly increased in Treg while pStat5 decreased in Tcon. This is shown for a representative patient in Figure 3A and for all patients examined in Figure 3B. This resulted in a significant increase in Treg-pStat5/Tcon-pStat5 ratio (p = 0.0002) one week after beginning daily low-dose IL-2 (Figure 3C, Wilcoxon-signed-rank test). However, the high level of pStat5 in Treg was not maintained with continued IL-2 treatment. After 2 weeks of daily IL-2 therapy, pStat5 levels were at their lowest levels in both Treg and Tcon (Figure 3D). Thereafter, pStat5 levels in both subsets gradually increased but the ratio of Treg-pStat5/Tcon-pStat5 was maintained in the range observed in patients without GVHD (Figure 3E). These findings indicate that exogenous low-dose IL-2 corrected the balance of Treg and Tcon activation in patients with chronic GVHD and this was maintained for the duration of therapy.
Figure 3. Selective activation of Treg in patients with chronic GVHD receiving low-dose IL-2.
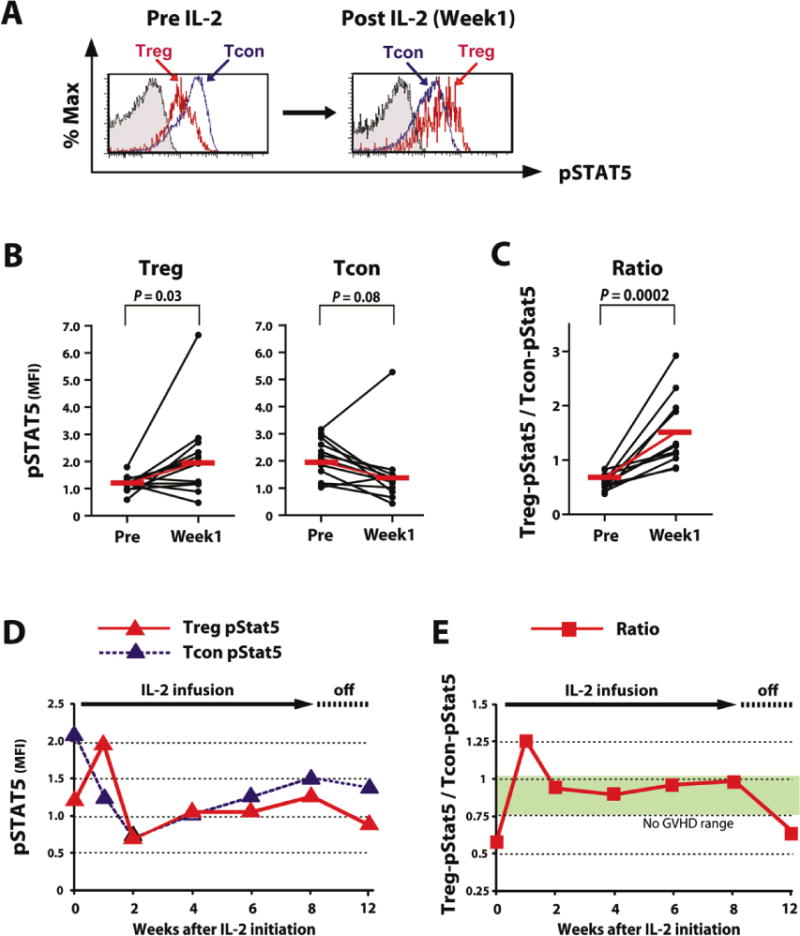
(A) Expression ofpStat5 in gated Tcon (blue) and Treg (red) from patients before and after the start of IL-2 administration. A representative panel from a single patient is shown. (B) MFI of pStat5 in Treg and Tcon were compared before and 1 week after starting IL-2 therapy. Treg pStat5 MFI is significantly elevated after IL-2. Tcon pStat5 was reduced after IL-2 but this change was not statistically significant. Median values are shown in red. (C) Ratio of Treg-pStat5 MFI / Tcon-pStat5 MFI were compared before and 1 week after starting IL-2 therapy. The ratio is significantly increased in all patients examinedafter IL-2 administration (p = 0.0002, Wilcoxon-signed-rank test). Median values are shown in red. (D) Changes of pStat5 MFI in Treg and Tcon during IL-2 therapy. Median values from 13 patients are shown. (E) Ratio of Treg-pStat5 MFI / Tcon-pStat5 MFI ratio during IL-2 therapy. Median values for MFI ratios were measured in 13 patients. Green range depicts the interquartile range of 14 patients without GVHD (Figure 2E).
Functional effects of IL-2 therapy on Treg homeostasis
We also measured plasma IL-2 levels during therapy and correlated these levels with absolute numbers of circulating Treg. As shown in Figure 4A, IL-2 levels rose sharply 1 week after starting treatment but high levels were not maintained as the number of circulating Treg increased. In contrast, IL-7 and IL-15 levels decreased after starting IL-2 (Figure 4B). Changes in expression of membrane receptors for IL-2 and IL-7 were also monitored during IL-2 therapy (Supplemental Figure 2A–B). The expression of IL-2 receptor alpha (CD25) on Treg, increased after starting IL-2 therapy and expression was maintained at a high level during the entire treatment period. In contrast, expression of CD25 did not change in Tcon and IL-7 receptor alpha (CD127) expression remained stable on both Treg and Tcon during therapy. Ki-67 expression in each T cell subset was measured as a marker of cell proliferation. Results from a representative patient are shown in Figure 4C and for the entire cohort of patients in Figure 4D. Before IL-2, Treg showed higher levels of proliferation than Tcon (14, 35). After starting IL-2, Treg proliferation increased rapidly, peaking one week after treatment began. Ki-67 also increased in Tcon during this period, but at a much lower level (Supplemental Figure 3A–B). Although patients continued to receive the same daily dose of IL-2, the initial high level of Treg proliferation rapidly returned to baseline levels as the absolute number of circulating Treg increased and IL-2 levels decreased.
Figure 4. Effects of low-dose IL-2 on Treg and Tcon in vivo.

(A) Concentrations of plasma IL-2 and absolute numbers of Treg during IL-2 therapy. Median values for 14 patients are shown. (B) Concentrations of plasma IL-7 and IL-15 during IL-2 therapy. Median values for 14 patients are shown. (C) Representative flow cytometry histograms for identification of Ki-67+ proliferating cells in Treg and Tcon subsets. Percentage of Ki-67+ cells is shown for each histogram. (D) Percentage of Ki-67+ proliferating cells in Treg and Tcon subsets during IL-2 therapy (median values). *Treg vs. Tcon (p < 0.001, Wilcoxon-signed-rank test). (E) Recent thymic emigrants (CD45RA+CD31+) within Treg and Tcon subsets during IL-2 therapy. Median fold changes are shown for each subset. *Treg vs. Tcon (p < 0.005, Wilcoxon-signed-rank test). (F) Levels of intracellular Bcl-2 in Treg and Tcon subsets during IL-2 therapy. Median % increase in mean fluorescence intensity is shown. *Treg vs. Tcon (p < 0.05, Wilcoxon-signed-rank test).
Since IL-2 also has an essential role in Treg development (36), we examined thymic generation of Treg during IL-2 therapy. Previous studies identified coexpression of CD45RA and CD31 as a marker of recent thymic emigrants (RTE) (37, 38). We confirmed this as a marker of RTE in both Treg and Tcon, by isolating CD45RA+CD31+ cells from each subset and quantified T-cell receptor excision circle (TREC) levels by real-time PCR. Only CD4 T cell subsets that expressed both CD45RAand CD31 contained high levels of TREC (Supplemental Figure 4A–B). While IL-2 treatment did not increase levels of Tcon-RTE, low-dose IL-2 significantly increased Treg-RTE (Figure 4E, Wilcoxon-signed-rank test). Treg-RTE levels peaked 4 weeks after starting IL-2 and remained elevated 4 weeks after IL-2 was stopped.
IL-2 promotes cell survival by inhibiting the mitochondrial pathway of apoptosis and anti-apoptotic proteins Bcl-2 and Mcl-1 appear to play a dominant role in this effect (39). As shown in Figure 4F, Bcl-2 expression increased during IL-2 therapy and this was significantly greater in Treg compared to Tcon. To confirm that increased Bcl-2 enhanced resistance to apoptosis, Treg and Tcon were isolated from patient samples and cultured with or without anti-Fas antibody to induce apoptosis. In the example shown in Figure 5A, Treg became more resistant to both spontaneous and Fas-induced apoptosis during IL-2 therapy. As shown in Figure 5B, Treg from healthy controls are more susceptible to both spontaneous and Fas-induced apoptosis than Tcon. Prior to starting IL-2, Treg and Tcon from HSCT patients both exhibited higher levels of apoptosis compared to healthy donors (Ctrl), but this was much more evident in Treg than Tcon. After IL-2 therapy, Treg showed significantly decreased levels of apoptosis both in the absence and presence of anti-Fas antibody. Apoptosis in Tcon did not change significantly during IL-2 therapy.
Figure 5. Increased resistance of Treg to apoptosis during low-dose IL-2.

(A) Spontaneous and Fas-induced apoptosis in Treg before and after IL-2 therapy. Treg and Tcon were isolated by cell sorting and cultured separately for 6 hours with control medium or anti-Fas antibody. Apoptosis was assessed by annexin V/7-AAD co-staining. Representative histograms for Treg are shown. (B) Spontaneous and Fas-induced apoptosis in Tcon and Treg subsets isolated from 3 healthy control donors (Ctrl) and 8 patients with chronic GVHD before and after IL-2 therapy (Pre and Post, respectively). Median values in each group are shown in red (exact Wilcoxon-rank-sum test, Wilcoxon-signed-rank test).
Suppressive function of IL-2-expanded Treg
To confirm that expanded Treg maintain suppressive activity, functional studies were carried out at various times during IL-2 treatment, including the initial proliferation phase and the later maintenance phase. As shown for a representative patient in Figure 6A, Treg suppressed the proliferation of CFSE-labeled responder Tcon from the same patient. Treg also markedly inhibited secretion of IFN-γ from activated autologous Tcon (Figure 6B). Moreover, suppression of Tcon IFN-γ secretion was dose-dependent and Treg suppression could be detected at low Treg/Tcon ratios (Figure 6C). These data indicate that IL-2-expanded Treg continue to maintain effective regulatory activity.
Figure 6. Efficient suppressive function of IL-2-expanded Treg in vitro.

(A) IL-2 expandedTreg suppress in vitro proliferation of stimulated Tcon from the same patient. Cells were harvested after 5 days and the proliferation of responder Tcon was examined by CFSE dilution. Top panel: Unstimulated Tcon maintain high level CFSE-labeling in vitro. Middle panel: Anti-CD3/CD28 stimulated Tcon exhibit extensive CFSE-dye dilution. Lower panel: Addition of Treg at 1:1 ratio inhibits proliferation of CD3/CD28 stimulated Tcon. A representative result from 6 independent experiments is shown. (B) Treg from patients receiving IL-2 were cultured with Tcon from the same patient at 1:1 ratio in the presence of anti-CD3 and anti-CD28 antibody. Suppressive activity wasassessed by the measuring the ability of Treg to reduce secretion of IFN-γ by stimulated Tcon. IFN-γ in the culture supernatants was measured by ELISA. (ND = not detected). Result of 3 independent experiments is shown (p < 0.04, exact Wilcoxon-rank-sum test).(C) Treg-mediated suppression of Tcon IFN-γ secretion was measured at various Treg/Tcon ratios. Result of 3 independent experiments is shown.* p < 0.05 vs. no Treg setting, exact Wilcoxon-rank-sum test, Savage test.
Discussion
CD4 Treg play a critical role in the maintenance of immune tolerance and relative Treg deficiency is thought to contribute to the pathogenesis of chronic GVHD. Since Treg and Tcon homeostasis are critically dependent on specific cytokines, we hypothesized that an abnormal cytokine milieu contributed to the imbalance between Treg and Tcon in patients with chronic GVHD. This was supported by our recent clinical trial demonstrating that daily administration of low-dose IL-2 results in the selective expansion of peripheral Treg and improvement of clinical manifestations of chronic GVHD (33). In the present study, we directly examined homeostatic cytokine signaling in patients with chronic GVHD and the effects of IL-2 administration on Treg and Tcon homeostasis in vivo. These studies demonstrate that severe chronic GVHD is characterized by relatively high levels of IL-7 and IL-15 with selective activation of the Stat5 signaling pathway in Tcon compared to Treg (34). Importantly, low-dose IL-2 rapidly corrected this imbalance by selectively activating Treg in vivo. This led to profound changes in Treg homeostasis, including increased proliferation of peripheral Treg, increased generation of thymic Treg and enhanced resistance to apoptosis. Taken together, low-dose IL-2 restored the homeostatic balance of Treg and Tcon and promoted the re-establishment of immune tolerance.
To examine the differential role of IL-2, IL-7 and IL-15 in the regulation of human CD4 T cells we monitored Stat5 phosphorylation in individual T cell subsets. This comparison was possible because the specific receptors for each of these cytokines utilize the common γ chain and phosphorylation of Stat5 is a shared signaling pathway that mediates their cellular effects. Although all 3 cytokines induce rapid Stat5 phosphorylation in vitro, CD4 Treg and Tcon subsets respond very differently to each homeostatic cytokine. These differences are most evident at low physiologic concentrations and, in particular, low doses of IL-2 had the most selective effect on Treg. We subsequently measured plasma cytokine levels and intracellular pStat5 in CD4 T cell subsets in patients with chronic GVHD to evaluate the response of each subset to their endogenous cytokine milieu. In healthy donors and patients without GVHD, systemic levels of these homeostatic cytokines were similar and neither Treg nor Tcon were found to have high levels of constitutive pStat5. In contrast, the cytokine environment in patients with severe GVHD was characterized by elevated levels of IL-7 and IL-15, which primarily support Tcon homeostasis, and a relative functional deficiency of IL-2. This was confirmed by demonstrating increased levels of pStat5 in Tcon in these patients and the ratio of Treg-pStat5/Tcon-pStat5 was inversely correlated with the severity of chronic GVHD. Since all patients with severe chronic GVHD also received immune suppressive agents, it is possible that these therapies also influenced the constitutive activation on Stat5 in these patients. Nevertheless, these findings suggest that the altered endogenous cytokine environment contributes directly to the abnormal balance of Treg and Tcon in chronic GVHD.
There are several possible causes for the altered cytokine milieu after HSCT. Patients often experience profound and prolonged periods of lymphopenia after HSCT, which stimulates production of IL-7 and IL-15 by stromal cells to promote lymphocyte recovery (15, 16, 18). Inflammation and tissue damage associated with active GVHD also promotes production of IL-7 (15–17, 40). Immune suppressive agents also contribute to the altered cytokine environment after transplant. In contrast to IL-7 and IL-15, IL-2 is only produced by activated T cells. Calcineurin inhibitors selectively inhibit IL-2 production by activated T cells, and all patients in our study received immunosuppressive agents for GVHD prophylaxis after HSCT. This effect is amplified in patients who develop GVHD and continue to receive intensive immunosuppressive therapy for prolonged periods.
Although CD4 Treg do not secrete IL-2, these cells constitutively express high levels of high affinity receptors for IL-2 and are therefore primed to respond to low concentrations of exogenous cytokine (41, 42). CD4 Tcon constitutively express intermediate affinity receptors (IL-2Rβ and IL-2Rγ) and therefore require higher concentrations of IL-2 for activation in the absence of TCR engagement (43–45). The predicted selectivity of low concentrations of IL-2 on CD4 T cell subsets was confirmed in patients with chronic GVHD receiving IL-2. In these patients, low-dose IL-2 was sufficient to induce rapid and selective up-regulation of pStat5 in Treg as well as the down-regulation of pStat5 in Tcon. After the balance of pStat5 activation in Treg and Tcon was normalized, this balance was maintained for the duration of IL-2 therapy. Although there was no statistical correlation between pSTAT5 expression and clinical response to IL-2 therapy in this small number of patients, changes in levels of pSTAT5 in Treg and Tcon may be useful as a functional biomarker to assess the effect of IL-2 therapy in future studies. Although the activation of pStat5 in Treg is presumed to be a direct effect of IL-2, the mechanism responsible for suppression of pStat5 in Tcon is likely to be more complex. IL-2 activated Treg may directly suppress activation of Tcon. Alternatively, lower levels of pSTAT5 in Tcon may simply reflect reduced levels of IL-7 and IL-15. As expanded Treg ameliorate inflammation in damaged tissues, this may result in the reduced production of IL-7. Resolution of lymphopenia after IL-2 therapy may also lead to the lower levels of IL-7 and IL-15.
Selective activation of Treg by administration of low-dose IL-2 had profound effects on Treg homeostasis in vivo. Interestingly, these effects varied with the duration of IL-2 therapy. The initial effect was primarily on Treg proliferation, which peaked 1 week after starting IL-2. Treg proliferation appeared to be related to systemic levels of IL-2, which also peaked at this time and subsequently declined as the number of circulating Treg rapidly increased. The high affinity receptor expressed by Treg rapidly binds exogenous IL-2 and increasing numbers of Treg likely limit the levels of free ligand in vivo. Moreover, exogenous IL-2 has a short half-life in vivo that may limit the ability to maintain high systemic cytokine levels during continued treatment. As systemic concentrations of IL-2 decreased and Treg proliferation returned to baseline levels, the number of peripheral Treg stabilized and gradually decreased despite continued administration of IL-2. Since we were only able to monitor circulating levels of Treg, we do not know whether tissue Treg also declined or whether the decrease in circulating Treg might actually reflect increased trafficking of Treg to sites of inflammation.
IL-2-induced Treg proliferation was followed by a 6–7 fold increase in thymic-derived Treg. Thymic generation of Treg peaked 4–6 weeks after starting IL-2 and this effect also waned by week 8. The reasons for this transient effect are unknown. Thymic Treg development may also be IL-2 concentration dependent and thus this may simply reflect changes in systemic levels of IL-2 during prolonged therapy. It is also possible that the inability to sustain high levels of Treg thymic maturation may reflect intrinsic factors related to thymic function in adults. These might include limited numbers of T cell progenitors, limited functional capacity of involuted adult thymus, effects of other immune suppressive therapies on thymic function and auto-regulatory pathways within the thymus that may limit the generation of excessive numbers of Treg.
In patients with chronic GVHD, circulating Treg have lower levels of Bcl-2 and are more susceptible to apoptosis compared to Tcon (14). Low-dose IL-2 induced increased Bcl-2 expression in Treg, which peaked 8 weeks after starting therapy. Functional studies confirmed that this resulted in increased resistance to apoptosis in vitro. Notably, all of these cellular effects were highly selective for Treg and in vitro experiments confirmed the functional suppressive function of Treg that had been expanded in vivo during IL-2 therapy. Although low-dose IL-2 had slight effects on Tcon homeostasis, the numbers of Tcon did not change during therapy, and importantly, this did not result in chronic GVHD progression in any patient.
Although the immunologic effects of low dose IL-2 therapy were very consistent, our study was limited to the analysis of T cells in serial blood samples. We were not able to examine tissues affected by chronic GVHD and further studies are needed to define the effects of IL-2 on chronic allo-reactivity at sites of clinical disease. Indeed, expansion of Treg in peripheral blood and modification of pStat5 levels occurred in all patients but only 50% demonstrated clinical improvement. Further modifications of this approach will be needed to enhance the clinical efficacy of low dose IL-2 therapy. These might include earlier intervention in the course of chronic GVHD, before extensive tissue damage and fibrosis have developed. Adoptive therapy with highly purified donor Treg can also be combined with low dose IL-2 therapy. In this setting, low dose IL-2 may be used to selective expand infused Treg in vivo and this may reduce the need to expand large numbers of Treg in vitro for adoptive therapy. Low-dose IL-2 may also be more effective if combined with immune suppressive agents such as sirolimus (rapamycin) that would selectively suppress effector T cell function while sparing Treg. It is also evident that most of the immunologic effects of IL-2 therapy only persisted as long as IL-2 treatment was continued. It is likely that long-term IL-2 treatment will be needed to maintain peripheral tolerance and additional toxicities may be identified as further studies extend the duration of therapy.
The present study focused on allogeneic stem cell transplantation as a model for studying human CD4 Treg homeostasis. Autoimmune diseases have also been associated with elevated levels of IL-7 or IL-15 without increased IL-2 and improvement of disease manifestations have been reported following blockade of these cytokines (46, 47). These observations suggest that homeostatic imbalance of CD4 T subsets may also be involved in the pathogenesis of autoimmune diseases. Considering the restoration of normal Treg homeostasis during IL-2 therapy in patients with chronic GVHD, low-dose IL-2 may provide a new strategy for restoration of immune tolerance in other clinical settings characterized by Treg deficiency.
Materials and Methods
Patient characteristics
Laboratory studies were undertaken in 59 adult patients who underwent allogeneic HSCT at the Dana-Farber Cancer Institute and Brigham and Women’s Hospital, Boston. All patients were enrolled in clinical research protocols approved by the Human Subjects Protection Committee of the Dana-Farber/Harvard Cancer Center. Written informed consent was obtained fromeach patient prior to sample collection, in accordance with the Declaration of Helsinki. Clinical characteristics of these patients are summarized in Table 1. All patients received peripheral blood stem cells (PBSC) with standard immunosuppressive regimens for GVHD prophylaxis. No patients received T cell depleted stem cell products or prophylactic therapy with anti-thymocyte immunoglobulin. Chronic GVHD status was categorized according to documented clinical exam and laboratory studies using NIH chronic GVHD consensus criteria.
Patient Cohort 1
Plasma cytokines and pStat5 expression in CD4 T cell subsets were tested in 45 patients. Blood samples were collected at relatively late times after transplantation (median 3 years). Chronic GVHD status shown in Table 1 was determined at the time of sample collection. We also studied 25 age-matched healthy adults.
Patient Cohort 2
Detailed analysis of CD4 T cell homeostasis was undertaken in 14 patients with chronic GVHD during low-dose IL-2 therapy. Recombinant IL-2 was administrated subcutaneously once daily and all patients completed 8 weeks of treatment. 7 patients received 3.0 x 105 IU/m2/day and 7 received 1.0–1.5 × 106 IU/m2/day of IL-2. Blood samples were obtained before and at 1, 2, 4, 6, 8, and 12 weeks after starting IL-2.
Flow cytometry
Peripheral blood mononuclear cells (PBMC) were isolated from blood samples by density gradient centrifugation (Ficoll-Hypaque; GE Healthcare) and cryopreserved in 10% DMSO before being analyzed. After thawing, PBMC were first incubated with the following directly conjugated monoclonal antibodies for 20 minutes at 4°C: anti-CD4-Pacific Blue (clone RPA-T4; BD Biosciences), anti-CD25-PC7 (clone M-A251; BD Biosciences), anti-CD45RA-FITC (clone M-A251; Beckman Coulter), anti-CD31-APC (clone WM59; eBioscience) and anti-CD127-APC-Alexa Fluor® 750 (clone eBioRDR5; eBioscience). To detect intracellular Ki-67 and Bcl-2, surface-stained PBMC were processed using Cytofix/Cytoperm buffer (BD Biosciences) and incubated with PE-conjugated anti-Ki-67 antibody (clone B56; BD Biosciences) or PE-conjugated anti-BCL-2 antibody (clone Bcl-2/100; BD Biosciences) for 30 minutes at room temperature. To detect intracellular phosphorylated Stat proteins and Foxp3, PBMC were first incubated with anti-CD4-Pacific Blue, anti-CD25-PC7, anti-CD45RA-FITC and anti-CD127-APC-Alexa Fluor® 750. Surface-stained PBMC were processed using Fixation buffer and Fix/Perm buffer (BD Biosciences) or Fixation buffer and Permeabilization buffer (eBioscience), and incubated with following APC-conjugated antibodies from BD Biosciences:pStat1 (clone pY701), pStat3 (clone pS727), pStat4 (clone pY693), pStat5 (clone pY694) and pStat6 (clone pY641) or Foxp3 (clone PCH101; eBioscience). CD4 Tcon are defined as CD4+CD25neg-lowCD127med-high and CD4 Treg are defined as CD4+CD25med-highCD127low. Expression of Foxp3, pStat proteins, CD45RA, CD31, Ki-67 and BCL-2 was determined for each T cell subset. Cell debris and doublets were excluded on the basis of side versus forward scatter. All cells were analyzed on a FACSCanto II (BD Biosciences) using FACSDiva (BD Biosciences) and FlowJo software (Tree Star).
Cell sorting
For in vitro functional assays, specific cell populations were isolated by cell sorting using FACSAria (BD Biosciences). CD4 Treg and Tcon were sorted by the expression pattern of CD25 and CD127 (Figure 1A). Sorted cell populations were confirmed to be more than 95% pure.
TREC analysis
Measurement of signal-joint TCR excision circle (sjTREC) DNA was performed according to a previously described protocol (11). Briefly, DNA was isolated from each Treg and Tcon subset using the QIAamp DNA Blood Mini Kit (QIAGEN). Measurement of sjTREC DNA was performed by Taqman real-time polymerase chain reaction using ABI 7300/7500 Real-Time PCR (Applied Biosystems).
In vitro suppression assays
In vitro suppression was evaluated using CFSE dilution to measure Tcon proliferation or ELISA to measure interferon-γ secretion. In CFSE dilution assay, freshly sorted Tcon were labeled with CFSE (Invitrogen) according to manufacturer’s directions. Briefly, cells were incubated at 37°C for 10 min with 5μM CFSE. Staining was stopped by adding RPMI 1640 containing 10% FBS at 4°C, followed by 1 wash in PBS. CFSE-labeled responder Tcon were cultured with Treg sorted from the same donor in the presence of 0.1ug/mL anti-CD3 antibody (clone OKT3; eBioscience), 1ug/mL anti-CD28 antibody (clone L293; BD Biosciences) in 96-well round-bottom plates at a 1:1 ratio (1 × 104 each T cell subset/well). After 5 days, cells were harvested and incubated with anti-CD4-Pacific Blue (clone RPA-T4; BD Biosciences). Cell division analysis of CD4 Tcon was performed on a FACSCanto II (BD Biosciences). IFN-γ concentration in culture supernatant in each well was also measured by ELISA according to the manufacturer’s instructions (Pierce Biotechnology).
Fas-induced apoptosis assay
Treg and Tcon were purified from thawed PBMC by cell sorting and cultured separately with 5ug/mL purified mouse anti-human CD95 antibody (clone EOS9.1; BD Biosciences) or with control medium in 96-well round-bottom plates, at a concentration of 1 × 104 T cells per well. Apoptosis induction was measured 6 hours after addition of anti-CD95. Cell death was assessed by Annexin V / 7-Amino-actinomycin D (7-AAD) costaining and forward to side scatter profiles.
Plasma cytokine measurement
Plasma IL-2, IL-7 and IL-15 concentrations were measured by ELISA according to the manufacturer’s instructions (Pierce Biotechnology; R&D Systems). Samples were obtained from patients after HSCT and cryopreserved in aliquots before being analyzed. We also studied plasma samples from 18 healthy adults.
Statistical analysis
Descriptive statistics was used for patient and transplant-related characteristics. Fisher’s exact test or a chi-square test was used for group comparisons for categorical variables in Table 1. The Wilcoxon-rank-sum test or the Kruskal-Wallis test was performed for 2 or more group comparisons for continuous variables. Non-parametric one-way analysis of variance (Savage test) was performed for analysis of functional suppression of Tcon IFN-γ secretion. The Wilcoxon signed-rank test was used for difference of paired samples for continuous variables. Spearman’s rank test was used to describe the correlation between time from transplant and pStat5 expression. All tests were 2-sided at the significance level of 0.05 and multiple comparisons were not adjusted.
Supplementary Material
Acknowledgments
We thank John Daley, Suzan Lazo-Kallanian and Robert Smith for excellent assistance with flow cytometric studies; Doreen Hearsey, Gorka Murga and Britt Selland for help obtaining clinical samples.
Funding:
This work was supported by NIH grants AI29530, CA142106, the Jock and Bunny Adams Research and Education Endowment, and the Ted and Eileen Pasquarello Research Fund. Dr. Koreth is supported in part by a Dana–Farber Dunkin’ Donuts Rising Star award and an American Society of Blood and Marrow Transplantation/Pharmion New Investigator award.
Footnotes
Author contributions:
KM designed and performed experiments and wrote the paper. JK designed and supervised the clinical trial and clinical data collection and edited the paper. HTK designed the clinical trial, performed statistical analysis and edited the paper. OGB, SM, KM and YK performed experiments and edited the paper. CC, VTH, EPA, PA, JHA and RJS designed and carried out the clinical trial, analyzed data and edited the paper. BRB reviewed data and edited the paper. JR designed the clinical trial, supervised the laboratory studies and edited the paper.
Competing interests:
The authors have declared that no conflicts of interest exist.
References
- 1.Bhatia S, Francisco L, Carter A, Sun CL, Baker KS, Gurney JG, McGlave PB, Nademanee A, O’Donnell M, Ramsay NK, Robison LL, Snyder D, Stein A, Forman SJ, Weisdorf DJ. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, Martin PJ, Sandmaier BM, Marr KA, Appelbaum FR, Storb R, McDonald GB. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood. 2009;114:7–19. doi: 10.1182/blood-2008-10-182592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ratanatharathorn V, Ayash L, Lazarus HM, Fu J, Uberti JP. Chronic graft-versus-host disease: clinical manifestation and therapy. Bone Marrow Transplant. 2001;28:121–129. doi: 10.1038/sj.bmt.1703111. [DOI] [PubMed] [Google Scholar]
- 5.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 6.Arai S, Jagasia M, Storer B, Chai X, Pidala J, Cutler C, Arora M, Weisdorf DJ, Flowers ME, Martin PJ, Palmer J, Jacobsohn D, Pavletic SZ, Vogelsang GB, Lee SJ. Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH Consensus Criteria. Blood. 2011;118:4242–4249. doi: 10.1182/blood-2011-03-344390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, Hochberg EP, Wu CJ, Alyea EP, Cutler C, Ho V, Soiffer RJ, Antin JH, Ritz J. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973–2978. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarantopoulos S, Stevenson KE, Kim HT, Cutler CS, Bhuiya NS, Schowalter M, Ho VT, Alyea EP, Koreth J, Blazar BR, Soiffer RJ, Antin JH, Ritz J. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113:3865–3874. doi: 10.1182/blood-2008-09-177840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srinivasan M, Flynn R, Price A, Ranger A, Browning JL, Taylor PA, Ritz J, Antin JH, Murphy WJ, Luznik L, Shlomchik MJ, Panoskaltsis-Mortari A, Blazar BR. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2012;119:1570–1580. doi: 10.1182/blood-2011-07-364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 11.Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S, Bellucci R, Alyea EP, Antin JH, Soiffer RJ, Ritz J. Reduced frequency of FOXP3 + CD4 + CD25 + regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106:2903–2911. doi: 10.1182/blood-2005-03-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Q, Bluestone JA. The Foxp3 + regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rieger K, Loddenkemper C, Maul J, Fietz T, Wolff D, Terpe H, Steiner B, Berg E, Miehlke S, Bornhauser M, Schneider T, Zeitz M, Stein H, Thiel E, Duchmann R, Uharek L. Mucosal FOXP3 + regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107:1717–1723. doi: 10.1182/blood-2005-06-2529. [DOI] [PubMed] [Google Scholar]
- 14.Matsuoka K, Kim HT, McDonough S, Bascug G, Warshauer B, Koreth J, Cutler C, Ho VT, Alyea EP, Antin JH, Soiffer RJ, Ritz J. Altered regulatory T cell homeostasis in patients with CD4 + lymphopenia following allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2010;120:1479–1493. doi: 10.1172/JCI41072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiant S, Yakoub-Agha I, Magro L, Trauet J, Coiteux V, Jouet JP, Dessaint JP, Labalette M. Plasma levels of IL-7 and IL-15 in the first month after myeloablative BMT are predictive biomarkers of both acute GVHD and relapse. Bone Marrow Transplant. 2010;45:1546–1552. doi: 10.1038/bmt.2010.13. [DOI] [PubMed] [Google Scholar]
- 16.Thiant S, Labalette M, Trauet J, Coiteux V, de Berranger E, Dessaint JP, Yakoub-Agha I. Plasma levels of IL-7 and IL-15 after reduced intensity conditioned allo-SCT and relationship to acute GVHD. Bone Marrow Transplant. 2011;46:1374–1381. doi: 10.1038/bmt.2010.300. [DOI] [PubMed] [Google Scholar]
- 17.Dean RM, Fry T, Mackall C, Steinberg SM, Hakim F, Fowler D, Odom J, Foley J, Gress R, Bishop MR. Association of serum interleukin-7 levels with the development of acute graft-versus-host disease. J Clin Oncol. 2008;26:5735–5741. doi: 10.1200/JCO.2008.17.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 19.Sportes C, Babb RR, Krumlauf MC, Hakim FT, Steinberg SM, Chow CK, Brown MR, Fleisher TA, Noel P, Maric I, Stetler-Stevenson M, Engel J, Buffet R, Morre M, Amato RJ, Pecora A, Mackall CL, Gress RE. Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clin Cancer Res. 2010;16:727–735. doi: 10.1158/1078-0432.CCR-09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK, Fry TJ, Engels J, Buffet R, Morre M, Amato RJ, Venzon DJ, Korngold R, Pecora A, Gress RE, Mackall CL. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sneller MC, Kopp WC, Engelke KJ, Yovandich JL, Creekmore SP, Waldmann TA, Lane HC. IL-15 administered by continuous infusion to rhesus macaques induces massive expansion of CD8 + T effector memory population in peripheral blood. Blood. 2011;118:6845–6848. doi: 10.1182/blood-2011-09-377804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perales MA, Goldberg JD, Yuan J, Koehne G, Lechner L, Papadopoulos EB, Young JW, Jakubowski AA, Zaidi B, Gallardo H, Liu C, Rasalan T, Wolchok JD, Croughs T, Morre M, Devlin SM, van den Brink MR. Recombinant human interleukin-7 (CYT107) promotes T-cell recovery after allogeneic stem cell transplantation. Blood. 2012;120:4882–4891. doi: 10.1182/blood-2012-06-437236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 24.Yu A, Malek TR. Selective availability of IL-2 is a major determinant controlling the production of CD4 + CD25 + Foxp3 + T regulatory cells. J Immunol. 2006;177:5115–5121. doi: 10.4049/jimmunol.177.8.5115. [DOI] [PubMed] [Google Scholar]
- 25.Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O’Gorman WE, Abbas AK. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J Immunol. 2010;185:6426–6430. doi: 10.4049/jimmunol.0903940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popmihajlov Z, Smith KA. Negative feedback regulation of T cells via interleukin-2 and FOXP3 reciprocity. PloS one. 2008;3:e1581. doi: 10.1371/journal.pone.0001581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humrich JY, Morbach H, Undeutsch R, Enghard P, Rosenberger S, Weigert O, Kloke L, Heimann J, Gaber T, Brandenburg S, Scheffold A, Huehn J, Radbruch A, Burmester GR, Riemekasten G. Homeostatic imbalance of regulatory and effector T cells due to IL-2 deprivation amplifies murine lupus. Proc Natl Acad Sci U S A. 2010;107:204–209. doi: 10.1073/pnas.0903158107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, Bellucci R, Raderschall E, Canning C, Soiffer RJ, Frank DA, Ritz J. IL-2 regulates FOXP3 expression in human CD4 + CD25 + regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, Sene D, Cacoub P, Klatzmann D. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med. 2011;365:2067–2077. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- 33.Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP, 3rd, Armand P, Cutler C, Ho VT, Treister NS, Bienfang DC, Prasad S, Tzachanis D, Joyce RM, Avigan DE, Antin JH, Ritz J, Soiffer RJ. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365:2055–2066. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Junge S, Kloeckener-Gruissem B, Zufferey R, Keisker A, Salgo B, Fauchere JC, Scherer F, Shalaby T, Grotzer M, Siler U, Seger R, Gungor T. Correlation between recent thymic emigrants and CD31 + (PECAM-1) CD4 + T cells in normal individuals during aging and in lymphopenic children. Eur J Immunol. 2007;37:3270–3280. doi: 10.1002/eji.200636976. [DOI] [PubMed] [Google Scholar]
- 38.Kohler S, Thiel A. Life after the thymus: CD31 + and CD31- human naive CD4 + T-cell subsets. Blood. 2009;113:769–774. doi: 10.1182/blood-2008-02-139154. [DOI] [PubMed] [Google Scholar]
- 39.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19:320–326. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 40.Sawa Y, Arima Y, Ogura H, Kitabayashi C, Jiang JJ, Fukushima T, Kamimura D, Hirano T, Murakami M. Hepatic interleukin-7 expression regulates T cell responses. Immunity. 2009;30:447–457. doi: 10.1016/j.immuni.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Stauber DJ, Debler EW, Horton PA, Smith KA, Wilson IA. Crystal structure of the IL-2 signaling complex: paradigm for a heterotrimeric cytokine receptor. Proc Natl Acad Sci U S A. 2006;103:2788–2793. doi: 10.1073/pnas.0511161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Rickert M, Garcia KC. Structure of the quaternary complex of interleukin-2 with its alpha, beta, and gammac receptors. Science. 2005;310:1159–1163. doi: 10.1126/science.1117893. [DOI] [PubMed] [Google Scholar]
- 43.Nakarai T, Robertson MJ, Streuli M, Wu Z, Ciardelli TL, Smith KA, Ritz J. Interleukin 2 receptor gamma chain expression on resting and activated lymphoid cells. J Exp Med. 1994;180:241–251. doi: 10.1084/jem.180.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grant AJ, Roessler E, Ju G, Tsudo M, Sugamura K, Waldmann TA. The interleukin 2 receptor (IL-2R): the IL-2R alpha subunit alters the function of the IL-2R beta subunit to enhance IL-2 binding and signaling by mechanisms that do not require binding of IL-2 to IL-2R alpha subunit. Proc Natl Acad Sci U S A. 1992;89:2165–2169. doi: 10.1073/pnas.89.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsudo M, Kitamura F, Miyasaka M. Characterization of the interleukin 2 receptor beta chain using three distinct monoclonal antibodies. Proc Natl Acad Sci U S A. 1989;86:1982–1986. doi: 10.1073/pnas.86.6.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yokoyama S, Watanabe N, Sato N, Perera PY, Filkoski L, Tanaka T, Miyasaka M, Waldmann TA, Hiroi T, Perera LP. Antibody-mediated blockade of IL-15 reverses the autoimmune intestinal damage in transgenic mice that overexpress IL-15 in enterocytes. Proc Natl Acad Sci U S A. 2009;106:15849–15854. doi: 10.1073/pnas.0908834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartgring SA, Willis CR, Alcorn D, Nelson LJ, Bijlsma JW, Lafeber FP, van Roon JA. Blockade of the interleukin-7 receptor inhibits collagen-induced arthritis and is associated with reduction of T cell activity and proinflammatory mediators. Arthritis Rheum. 2010;62:2716–2725. doi: 10.1002/art.27578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


