Abstract
A facile N2 flow-accelerated N-carboxyanhydride ring opening polymerization (NCA ROP) is demonstrated, herein, with rigorous kinetic studies to evaluate the methodology in detail. By using n-hexylamine as initiator and γ-benzyl-L-glutamate N-carboxyanhydride (BLG-NCA) as monomer, the NCA ROP via a normal amine mechanism (NAM) reached 90% conversion in 2 h under N2 flow at room temperature in a fume hood, much shorter than the time required for the same polymerization conducted in a glove box (14 h). The efficient removal of CO2 from the reaction by N2 flow drove the carbamic acid-amine equilibrium toward the formation of active nucleophilic amino termini and promoted polymerization. The detailed kinetic studies of the polymerization with different feed ratios and N2 flow rates were conducted, demonstrating the living feature of the NCA ROP and the tuning of the polymerization rate by simply changing the flow rate of N2. Maintenance of the reactivity of the amino ω-chain terminus and control during a subsequent polymerization were confirmed by performing chain extension reactions. The N2 flow method provides a new straightforward strategy to synthesize well-defined polypeptides with predictable molecular weights and narrow molecular weight distributions (PDI < 1.19).
Keywords: NCA, ring opening polymerization, Nitrogen flow
Introduction
Synthetic polypeptides consisting of α-amino acids linked covalently have significant similarities to natural materials, which have facilitated their utilization extensively in biomedical areas, such as serving as scaffolds for tissue engineering, matrices for drug and gene delivery, and responsive materials for biosensors, and also in technological applications, due to their liquid crystalline characteristics and other properties.1-3 Moreover their origination from renewable feedstocks and potential biodegradability make polypeptides important materials for environmental purposes. There has been increasing interest, therefore, in developing efficient routes to synthesize well-defined polypeptides. Although Leuchs and coworkers synthesized the first examples of α-amino acid N-carboxyanhydrides (NCAs) in 1906,4 significant advances in the concepts of polymer structures and analytical techniques for their characterization were needed before NCA ring-opening polymerization (ROP) emerged as a synthetically straightforward methodology to construct polypeptide materials.5-8 Over the past century,9 significant advances have been made toward NCA ROPs, with the greatest achievements being realized over only the past ca. two decades, to synthesize polypeptides with controlled structures and varied architectures.8,10-12 However, much remains to be understood about the reaction conditions to achieve rapid and reproducible production of well-defined polypeptide materials of controlled degrees of polymerization and sequence.
In the past decade, several groups have developed strategies to achieve well-defined polypeptides with high molecular weights (MW) and narrow molecular weight distributions (MWD) by using NCA ROP. For instance, Deming and coworkers developed transition metal complexes as active species to control the addition of NCA monomers and eliminate side reactions in NCA polymerization.13 Schlaad and coworkers reported primary amine hydrochloride initiated NCA polymerizations, which avoided the formation of NCA anions and inhibited the activated monomer mechanism (AMM) of polymerization.14 In 2004, the Hadjichristidis group applied high vacuum techniques (HVT) to purify NCA monomers and conduct polymerizations with intermittent removal of CO2 generated during the reaction, and effectively promote polymerization via the normal amine mechanism (NAM).15 The controlled living polymerization under high vacuum was also confirmed by Messman and coworkers.16 Recently, Cheng’s group reported organosilicon reagent-mediated living NCA polymerization, which allows for the synthesis of homo, block and brush polypeptides with predictable MWs and narrow MWDs.17 However, there is still plenty of opportunity to develop improved methods to achieve efficient and convenient preparation of polypeptides by NCA ROP.
In this communication, we report a straightforward method to enhance the polymerization rate while maintaining the living features of the polymerization by simply using N2 flow during the NCA ROP. Although the influence of CO2 in NCA ROP was studied fifty years ago, when it was observed that the immediate removal of CO2 affected the kinetics of polymerization,18 the “livingness” of the NCA ROP with removal of CO2 from the reaction was not demonstrated. With the great impact that polypeptides have been experiencing recently, we chose to re-investigate the influence of CO2 removal, confirm the advantages of N2 flow methods, and confirm the living characteristics for NCA ROPs by employing γ-benzyl L-glutamate (BLG NCA), as a well-studied monomer, and n-hexylamine as initiator. Compared to the methods for the preparation of polypeptides from NCAs using primary amines as initiators without catalyst activation, our method has several advantages: 1) promoted polymerization rates, to allow NCA conversions to reach >95% in a matter of hours rather than the multiple day time period that is required typically; 2) glove box-free operation in a normal fume hood, to increase the convenience, decrease the time and allow for greater variation of the reaction conditions, for instance the temperature; 3) control over the polymerization rate by altering the flow rate of nitrogen; 4) maintenance of the living features of NCA ROP even at high conversions and high monomer : initiator feed ratios.
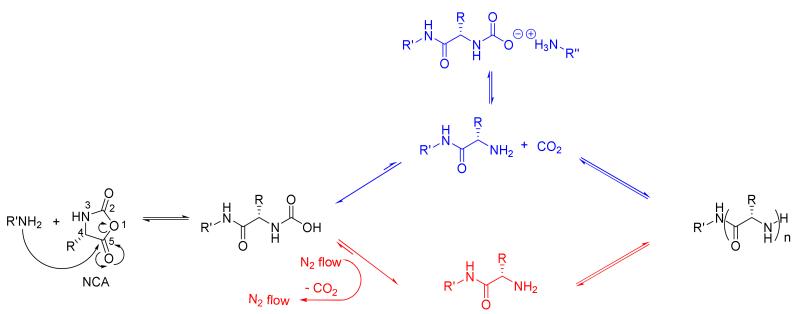
The primary amine-initiated NCA ROP without catalyst is one of the most studied strategies to synthesize polypeptides. By using n-hexylamine initiator, the NCA ROP follows the NAM (Scheme 1). The primary amine attacks the 5-C=O of the NCA monomer, leading to opening of the ring and proton transfer to form a carbamic acid intermediate. Decarboxylation of the carbamic acid generates a free amino group, which continues to serve as a nucleophile for chain propagation. The loss of CO2 from the carbamic acid is the rate determining step in NAM, therefore, with the efficient removal of CO2 from the reaction mixture, the decarboxylation of the carbamic acid continuously shifts the equilibrium to generate active amine for further chain propagation and, thus, first order kinetics are observed.19,20 However, without removal of CO2, the carbamic acid dominates and the formation of carbamic acid salt is favored (as shown in Scheme 1), which reduces the nucleophilicity of the active polymer chain end and presents different polymerization kinetics.18,21,22 The polymerization usually requires days to reach high conversion, and in order to minimize potential side reactions induced by water and other impurities, glove box techniques have been used widely during the process of polymerization to achieve well defined polypeptides with narrow MWDs. If only freeze-pump-thaw operation is performed to remove water and air and the polymerization is conducted under sealed conditions, very long reaction times (even one week) are needed and polymers are obtained with relatively broad MWDs.23 High vacuum techniques can promote the polymerization by removal of the CO2 during the reaction and produce polypeptides with predictable MWs and narrow MWDs. However, the polymerizations must be conducted in specially designed equipment, to avoid loss of monomer, initiator, and solvent, which may experience difficulties with scale up.
Scheme 1.
NCA ROP under N2 flow via normal amine mechanism.
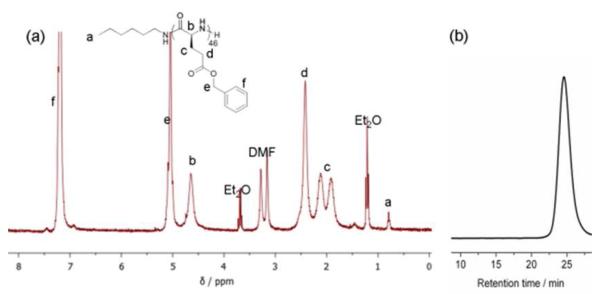
Fortuitously, it was observed that N2 flow during the BLG-NCA ROP greatly promoted the polymerization rate, presumably by facilitating the removal of CO2, so that this effect was explored further. Amino acids were recently reported to behave as efficient CO2 capture materials via the formation of carbamic acids with 1 : 1 stoichiometry of amino acid : CO2. The highly efficient binding of CO2 to a variety of amino acids to form stable carbamic acids was confirmed by FT-IR, 1H NMR and 13C NMR spectroscopies.24 It was also reported that the carbamic acids could be efficiently decarboxylated by simply bubbling N2 through the solution at 40 °C.24 We, therefore, investigated in detail the effects of N2 flow during the BLG-NCA ROP, by introducing various rates of N2 flow during the polymerization to alter the efficiency of CO2 removal from the carbamic acid intermediate and further shift the equilibrium to form active amines on the propagating polymer chain ends. In a general experiment, the polymerization of BLG-NCA monomer (recrystallized 5 times from ethyl acetate/hexane, 250 mg, 50 eq) was initiated by n-hexylamine in dry DMF and allowed to proceed, using standard Schlenk techniques, in which the Schlenk flask was capped with a rubber stopper with a needle outlet connected to a tube filled with drying agent. During an initial polymerization attempt, monitoring of the reaction by attenuated total reflectance (ATR) FT-IR indicated that the conversion was complete within a few hours, although we had expected much slower progression of the polymerization. Therefore, after only 4 h, the reaction mixture was precipitated into 80 mL dry Et2O and a white solid product of poly(γ-benzyl-L-glutamate) (PBLG) was obtained, following centrifuga tion and drying in vacuo. Gel permeation chromatography (GPC) characterization indicated that the PBLG was well-defined, having a PDI = 1.10 and Mn = 12.0 kDa (Figure 1). The degree of polymerization determined by 1H NMR spectroscopy, by comparing the integration values of the terminal methyl protons of the n-hexylamido α-chain terminus (0.79 ppm) and the benzyl methylene protons of the repeat units (5.1 ppm) was determined to be 46, which is in agreement with the GPC result. Based upon this finding of relatively rapid polymerization to obtain uniform PBLG material, further experiments to probe the polymerization kinetics and the effects of N2 flow were performed.
Figure 1.
(a) 1H NMR spectrum of PBLG46. (b) The GPC trace of PBLG46.
In order to understand how the N2 flow affected the polymerization rate, the kinetics of NCA-ROP were investigated. The typical kinetic study of n-hexylamine-initiated BLG-NCA ROP was conducted in a 25 mL flame-dried Schlenk flask, the feed ratio of BLG-NCA : initiator(I) was fixed at 100 : 1. The initial monomer concentration was 50 mg/mL (0.19 M) with 500 mg BLG-NCA dissolved in 10 mL dry DMF. The polymerization rate was obtained by plotting the natural logarithm of monomer concentration versus time and fitting of the data using equation 1.
| (1) |
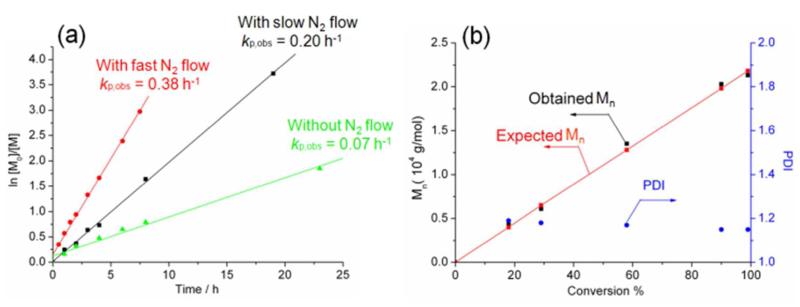
The conversion was determined by ATR-FTIR using the intensity of BLG-NCA anhydride absorption band at 1788 cm−1. The linear fitting of the plot gave kp.obs, which reflected the rate of polymerization. With increasing N2 flow speed (from 0 to 250 mL/min), the kp.obs increased respectively (Figure 2), which indicated that the polymerizations were promoted by N2 flow. The accelerated rate of polymerization could be attributed to the efficient removal of CO2 in the reaction system and forcing of the equilibrium to form nucleophilic amine to promote chain propagation. The kinetic studies of a different feed ratio (50 : 1) were conducted and those results also indicated that the N2 flow promoted polymerization (Figure S1). This approach of N2 flow method provided an easy way to control the polymerization rate by simply tuning the N2 flow rate. With higher N2 flow rate (250 mL/min), a higher kp.obs (kp.obs = 0.38 h−1) was observed than that with low flow rate (100 mL/min, kp.obs = 0.20 h−1). Interestingly, the half-life time (t½ = ln2/kp.obs) was 0.91 h for the polymerization with feed ratio of 50 : 1, which is close to the value (t½ = 0.71 h) obtained by Hadjichristidis using HVT.15 The “living” feature of the NCA ROP under N2 flow was also supported by the following results: (i) over 95 % monomer conversion was observed in 8 h and the obtained PBLG had Mn = 20.1 kDa, as determined by 1H NMR spectroscopy, which agreed well with the expected PBLG MW (Mn = 20.9 kDa) calculated based upon conversion; (ii) first order kinetic characteristics were observed in BLG-NCA ROP; (iii) the obtained polymers had narrow molecular weight distributions.
Figure 2.
(a). The kinetics studies of n-hexylamine initiated BLG-NCA ROP at room temperature with BLG-NCA/I ratio of 100:1. The initial BLG-NCA concentration was 50 mg/mL (0.19 M). The N2 flow rates were 0 mL/min (green line), 100 mL/min (black line) and 250 mL/min (red line). (b). NCA ROP terminated at selected monomer conversion with flow rate of 100 mL/min.
Chain extension experiments to confirm the livingness of the ω-chain terminus were also performed, by introducing additional aliquots of BLG-NCA monomer via syringe, sequentially, into the reaction mixture. With the feed ratio of BLG-NCA : initiator = 50 : 1, the monomer conversion reached > 99 % after 6 h, monitored by ATR-FTIR. The obtained PBLG53 had Mn = 11.7 kDa from1H NMR and retention time = 24.50 min and PDI = 1.26, as determined by GPC. Another supply of BLG-NCA was added into the reaction mixture with the feed ratio of BLG-NCA : initiator = 25 : 1. The achieved PBLG74, precipitated into diethyl ether after 18 h, was characterized to have a 1H NMR-determined Mn = 16.3 kDa, retention time = 22.49 min and PDI = 1.26.
To further investigate the best conditions to obtain well-defined polypeptides with minimum polymerization time by using the N2 flow methods, parameters such as feed ratio, flow rate of N2 and sparging were screened (Table 1). With different feed ratios, accelerated polymerizations were observed in all entries using this N2 flow method. However, with a moderate flow rate (100 mL/min), the obtained polymers had the narrowest molecular weight distributions (Entry 2 and Entry 5). When polymerizations were conducted with the venting needle in place and without N2 flow, the BLG-NCA ROP required longer reaction time and had more side reactions, resulting in ill-defined polymers with broad PDIs (Entry 3 and Entry 6). Especially, when attempts were made to synthesize polymers with high DPn under these N2-flow-free conditions, impurities and moisture in the air caused the polymerization lose the living features and polymers with broader PDI were produced (Entry 6). When the feed ratio was increased to 200 : 1, the N2 flow methods continued to afford well-defined PBLG with narrow PDIs at high conversion (Figure S2), while the absence of N2 flow resulted in dead polymer chains even at low conversion (results not shown). When sparging N2 into the reaction mixture, the polymerization rate increased compared to the flow methods (Entry 9). However at high feed ratios, termination of the polymerizations was observed (Entries 10 and 11).
Table 1.
n-hexylamine initiated BLG-NCA ROP with different feed ratio and N2 flow rate.
| Entry | Feed ratio [M0] / [I0] |
Conc. (M) |
N2 flow rate (mL/min) |
Time (h) |
Conv. (%) |
Obtained Mn (kDa)d |
Expected Mn (kDa) |
PDI |
|---|---|---|---|---|---|---|---|---|
| 1 | 50 / 1 | 0.19 | 250 | 2 | 90 | 9.6 | 10.0 | 1.15 |
| 2 | 50 / 1 | 0.19 | 100 | 4 | 96 | 10.6 | 10.6 | 1.11 |
| 3 | 50 / 1 | 0.19 | 0 | 19 | 96 | 13.2 | 10.6 | 1.38 |
| 4 | 100 / 1 | 0.19 | 250 | 7.5 | 95 | 20.1 | 20.9 | 1.18 |
| 5 | 100 / 1 | 0.19 | 100 | 19 | 99 | 21.3 | 21.8 | 1.12 |
| 6 | 100 / 1 | 0.19 | 0 | 28 | 81c | 22.1 | 17.8 | 2.19 |
| 7 | 200 / 1 | 0.19 | 100 | 28 | 75 | 29.5 | 33.4 | 1.14 |
| 8 | 200 / 1 | 0.19 | 0a | 48 | 75 | 34.7 | 33.4 | 1.18 |
| 9 | 50 / 1 | 0.19 | 100b | 2 | 99 | 10.6 | 11.1 | 1.19 |
| 10 | 100 / 1 | 0.19 | 100b | 2 | 45c | 13.3 | 10.0 | 1.23 |
| 11 | 200 / 1 | 0.19 | 100b | 3 | 37c | 20.1 | 16.3 | 1.48 |
The reaction was conducted in a glove box.
N2 was sparged into the reaction mixture.
After a certain conversion, the chain propagation halted.
Determined by 1H NMR spectroscopy.
Conclusions
A N2 flow accelerated NCA ROP via NAM has been explored in detail. This easy operational method offers a new approach for the synthesis of polypeptides with controllable polymerization rates, predictable molecular weights and narrow molecular weight distributions. This approach is attractive because most of the initiators are commercially available and there is no need for removal of catalyst after polymerization. Since no metal catalyst was used, this polymerization strategy diminishes potential toxicity issues that may arise in the use of synthetic polypeptide materials for biomedical applications. The N2 flow can be easily tuned to balance the polymerization kinetics and structural control in NCA ROP. The significantly accelerated polymerization decreases side reactions that may be introduced by moisture and solvent.25 In addition to the BLG-NCA monomer, the nitrogen flow method can also be applied in NCA ROP with varieties of monomers, detailed results will be reported in future works. For instance, expansion of this economic, easy operational and straightforward method is being explored to install chain end functionality, perform block polypeptide synthesis and exert structural control for materials comprising combinations of different NCA monomers.
Supplementary Material
ACKNOWLEDGMENT
We gratefully acknowledge financial support from the National Heart Lung and Blood Institute of the National Institutes of Health as a Program of Excellence in Nanotechnology (HHSN268201000046C) and the National Science Foundation under grant number DMR-1105304. The Welch Foundation is gratefully acknowledged for support through the W. T. Doherty-Welch Chair in Chemistry, Grant No. A-0001.
Footnotes
Detailed experimental section, GPC traces and kinetics study. This material is free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Deming TJ. Nat. Mater. 2010;9:535. doi: 10.1038/nmat2789. [DOI] [PubMed] [Google Scholar]
- 2.Lalatsa A, Schatzlein AG, Mazza M, Thi BHL, Uchegbu IF. J. Control. Release. 2012;161:523. doi: 10.1016/j.jconrel.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 3.Matson JB, Stupp SI. Chem. Commun. 2012;48:26. doi: 10.1039/c1cc15551b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leuchs H. Ber. Detsch. Chem. Ges. 1906;39:857. [Google Scholar]
- 5.Habraken GJM, Heise A, Thornton PD. Macromol. Rapid. Comm. 2012;33:272. doi: 10.1002/marc.201100730. [DOI] [PubMed] [Google Scholar]
- 6.Kricheldorf HR. Angew. Chem. Int. Ed. 2006;45:5752. doi: 10.1002/anie.200600693. [DOI] [PubMed] [Google Scholar]
- 7.Hadjichristidis N, Iatrou H, Pitsikalis M, Sakellariou G. Chem. Rev. 2009;109:5528. doi: 10.1021/cr900049t. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez JR, Klok HA. J. Polym. Sci. Pol. Chem. 2003;41:1167. [Google Scholar]
- 9.Curtius T, Sieber W. Ber. Detsch. Chem. Ges. 1921;54:1430. [Google Scholar]
- 10.Habraken GJM, Wilsens KHRM, Koning CE, Heise A. Polym. Chem. 2011;2:1322. [Google Scholar]
- 11.Lu H, Cheng JJ. J. Am. Chem. Soc. 2007;129:14114. doi: 10.1021/ja074961q. [DOI] [PubMed] [Google Scholar]
- 12.Kramer JR, Deming TJ. Biomacromolecules. 2010;11:3668. doi: 10.1021/bm101123k. [DOI] [PubMed] [Google Scholar]
- 13.Deming TJ. Nature. 1997;390:386. doi: 10.1038/37084. [DOI] [PubMed] [Google Scholar]
- 14.Dimitrov I, Schlaad H. Chem. Commun. 2003:2944. doi: 10.1039/b308990h. [DOI] [PubMed] [Google Scholar]
- 15.Aliferis T, Iatrou H, Hadjichristidis N. Biomacromolecules. 2004;5:1653. doi: 10.1021/bm0497217. [DOI] [PubMed] [Google Scholar]
- 16.Pickel DL, Politakos N, Avgeropoulos A, Messman JM. Macromolecules. 2009;42:7781. [Google Scholar]
- 17.Lu H, Cheng JJ. J. Am. Chem. Soc. 2008;130:12562. doi: 10.1021/ja803304x. [DOI] [PubMed] [Google Scholar]
- 18.Thunig D, Semen J, Elias HG. Makromol. Chem. 1977;178:603. [Google Scholar]
- 19.Goodman M, Hutchiso J. J. Am. Chem. Soc. 1965;87:3524. [Google Scholar]
- 20.Rinaudo M, Domard A. Biopolymers. 1976;15:2185. doi: 10.1002/bip.1976.360151107. [DOI] [PubMed] [Google Scholar]
- 21.Ballard DGH, Bamford CH, Weymouth FJ. Proc. R. Soc. Lon. Ser-A. 1955;227:155. [Google Scholar]
- 22.Ballard DGH, Bamford CH. Proc. R. Soc. Lon. Ser-A. 1954;223:495. [Google Scholar]
- 23.Zhang XQ, Li JG, Li W, Zhang A. Biomacromolecules. 2007;8:3557. doi: 10.1021/bm700729t. [DOI] [PubMed] [Google Scholar]
- 24.Liu AH, Ma R, Song C, Yang ZZ, Yu A, Cai Y, He LN, Zhao YN, Yu B, Song QW. Angew. Chem. Int. Ed. 2012;51:11306. doi: 10.1002/anie.201205362. [DOI] [PubMed] [Google Scholar]
- 25.Muzart J. Tetrahedron. 2009;65:8313. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.