Abstract
Purpose
Recent research on Head-shake Posturography has demonstrated a modest increase in sensitivity to identifying peripheral vestibular system asymmetry when horizontal head-movements were added to portions of the standard Sensory Organization (SOT) test battery. However, limitations with respect to the head-shake protocol were outlined and usable data for assessing performance could not be established. The purpose of this study was to test a change in protocol for use of head-shake SOT to address the noted limitations.
Method
Forty subjects ranging in age from 20-79 years with no history of dizziness completed conditions 2 and 5 of the SOT portion of Computerized Dynamic Posturography on EquiTest ™ equipment while maintaining head still, as well as four horizontal head movement velocity tasks.
Results
Slope of a linear regression fit to six performance points was used to characterize each subject. Spearman’s ranked correlation (r) indicated a significant relationship between the slope of the line representing a decline in performance with age (r = −0.52; p = 0.0006).
Conclusions
The head-shake modification shows a trend in increasing the separation of normal individuals across age and eliminated the limitations addressed in earlier research. Future research will investigate the head-shake modification for identifying vestibular peripheral system asymmetries.
Keywords: Head-shake, SOT = Sensory Organization Test, CDP = Computerized Dynamic Posturography, Dizziness
Introduction
Computerized Dynamic Posturography (CDP) is an assessment of an individual’s ability to maintain static and dynamic stance. The assessment is composed of a battery of tests that provide an analogy to everyday conditions associated with daily living. There are four primary components to CDP that are used clinically: Sensory Organization Test (SOT), Motor Control Test (MCT) and Adaptation testing and Postural Evoked Response Test (PER). The Sensory Organization Test (SOT) of CDP is intended to help determine how well an individual can use the visual and proprioceptive systems with the vestibular system or the vestibular system alone. Furthermore, it is the most frequently used component of CDP. SOT is made up of six conditions, which assess the individual’s balance performance during a sequence of six, increasingly difficult, subtests. The subtests include combinations of eyes open, eyes closed, and conditions with a moving sway reference (i.e. movement of the visual background and/or movement of the forceplate) (see Figure 1). In particular, subjects are asked to stand on a forceplate and maintain stable stance while the magnitude of sway in the anterior / posterior dimension is quantified (Shepard, Schulz, Alexander, Gu, and Boismier, 1993).
Figure 1.
Schematic of the Sensory Organization Test (SOT) portion of Computerized Dynamic Posturography (CDP). (Reprinted with permission from NeuroCom International, Inc., EquiTest® operator’s manual, Version 3.0)
The information obtained from the various components of CDP is highly valuable when the goal of the assessment is to investigate the functional status of compensation or rehabilitative needs and/or progress (Nashner, 1993; El-Kashlan, Shepard, Asher, Smith-Wheelock, and Telian, 1998). However CDP (with SOT as the most commonly used protocol) is limited as a tool to screen for site-of-lesion for possible peripheral vestibular system unilateral paresis (Shepard et al., 1993; Nashner, 1993; Chandra and Shepard, 1996; El-Kashlan et al., 1998; Allum and Shepard, 1999).
Prior work by Mishra, Davis, Speers, and Shepard (2009) demonstrated increased sensitivity to the identification of peripheral vestibular system asymmetry (site-of-lesion diagnostics) when dynamic head movements (head-shake) were added to portions of the SOT battery (SOT condition 2 and SOT condition 5). For clarification, during condition 2 of the SOT protocol, the forceplate is stationary while the individual is instructed to stand quietly with eyes closed for twenty seconds. During condition 5 of the SOT protocol, the forceplate rotates forwards or backwards in the sagittal plane proportionally to the amount of sway by the individual. The individual is again instructed to stand quietly with eyes closed while maintaining upright stance on the forceplate.
The head-shake modification to the standard SOT conditions 2 and 5 proposed by Mishra et al. (2009) incorporated horizontal head movements with a peak head velocity of 60°/sec. Their work revealed a limitation to the performance of the head-shake SOT protocol for the diagnostic site-of-lesion purpose using the head-shake modification to SOT conditions 2 and 5. The limitation was that the two conditions (head-shake condition 2, eyes closed while standing on a stable support surface) at 60°/sec and head-shake condition 5 (eyes closed while standing on a sway-referenced support surface) at 60°/sec used to assess postural control performance during head movement, proved to be restricted in their range. The more difficult of the two (head-shake condition 5 at 60°/sec) was too difficult for a number of subjects (i.e. yielding fall reactions on the trials) giving a floor effect and not allowing for data useable for assessing performance. The easier of the two conditions (head-shake condition 2 at 60°/sec), was not challenging enough and did not differentiate patients in an adequate manner based on head movement sensitivity. Therefore, the current project was proposed in order to first, broaden the range of testing to allow for increased challenge in head-shake condition 2 (the easier of the two test conditions) and for less of a challenge in head-shake condition 5 (the more difficult of the two test conditions) with the intent that this information will be used in a future study to improve identification of unilateral peripheral vestibular hypofunction and determine its sensitivity and specificity. Second, investigate the use of a single outcome variable that incorporated all of the data points acquired during the testing.
Methods
Normal subjects were recruited for participation from community sources in Omaha, Nebraska and Rochester, Minnesota. Volunteers signed an Institutional Review Board approved written consent form prior to data collection. Forty subjects (17 males and 23 females) were included in the statistical analysis. Based on age, each subject was placed into the appropriate age group as follows: Group 1 consisted of ten subjects ranging in age from 20 – 39 yrs; Group 2 included ten subjects ranging in age from 40-59 yrs; Group 3 was composed of ten subjects ranging in age from 60-69 yrs; and Group 4 consisted of ten subjects ranging in age from 70-79 yrs. The determination of the age grouping was based on prior work investigating the effects of age on postural control as assessed by EquiTest ™ (Shepard et al., 1993; Nashner, 1993). All subjects were considered normal based on the following criteria obtained through an interview process: 1.) Negative history of any form of dizziness (including complaints of lightheadedness, vertigo, unsteadiness) lasting longer than 1 hour or recurring for greater than 1 day; 2.) Negative history of any current otologic disease actively involving the middle ear; 3.) Negative history of perceived, progressive unilateral hearing loss; 4.) Negative history of current or past neuromuscular disorder; 5.) Negative history of any disorder interfering with mobility, stance or neck range-of-motion; 6.) Currently not taking any medications from the following drug groups: anti-anxiety, anti-seizure, or narcotic-based pain medications; 7.) No use of alcohol within 24 hours of participation in the study.
Study Protocol
After inclusion criteria were met, participants were asked to remove their shoes prior to testing. During all standing conditions subjects wore a safety harness that was fastened to a roll bar on the EquiTest ™ equipment. The standard SOT using three trials of conditions 2 and 5 was performed on all subjects prior to the head-shake modification protocol. Analyses of the results were obtained by taking the average equilibrium score from each of the three trials of the condition. The equilibrium score is calculated by the EquiTest™ based on the maximum excursions of sway in the anterior/posterior plane of 12.5° during the 20 seconds of recording for each trial within a specific condition (Allum and Shepard, 1999).
Once the SOT trials 2 and 5 were completed in the traditional manner (as also executed by Mishra et al., 2009), all subjects then performed the modified SOT protocol of conditions 2 and 5 while performing horizontal head movements with a peak head velocity of 60°/sec as proposed by Mishra et al. (2009). Subjects also completed two additional head-shake conditions that consisted of peak head velocity of 120°/sec for SOT condition 2 and for SOT condition 5 a peak head velocity of 15°/sec. All horizontal head movements were executed with an excursion of 15 degrees to both sides of center, giving a total excursion of 30 degrees and the head velocities as noted. Therefore, the frequency of horizontal head rotation was 0.16 Hz with a velocity of 15°/sec; 0.64 Hz with velocity of 60°/sec; and 1.28 Hz with a velocity of 120°/sec. These head-shake conditions were randomized as were the different head-shake velocity trials within SOT conditions 2 and 5. Three trials of each velocity condition were also performed for the head-shake protocol. Thus, subjects fell into one of eight groups based on order of the four head-movement conditions (see Table 1):
Table 1.
Eight Groups Based on Order of the Four Head-Movement Conditions
| Group | Order of Head-Movement Conditions |
|---|---|
| Group 1 | Condition 2 three trials of head-shake (HS) 60 °/sec (HS 2- 60°/s) and three trials of 120 °/sec (HS 2 - 120°/s) , Condition 5 three trials of 15 °/sec (HS 5 - 15°/s) and three trials of 60 °/sec (HS 5 - 60°/s). |
| Group 2 | Condition 2 three trials of 60 °/sec (HS 2- 60°/s) and three trials of 120 °/sec (HS 2 - 120°/s), Condition 5 three trials of 60 °/sec (HS 5 - 60°/s) and three trials of 15 °/sec (HS 5 - 15°/s). |
| Group 3 | Condition 2 three trials of 120 °/sec (HS 2 - 120°/s) and three trials of 60 °/sec (HS 2- 60°/s), Condition 5 three trials of 15 °/sec (HS 5 - 15°/s) and three trials of 60 °/sec (HS 5 - 60°/s). |
| Group 4 | Condition 2 three trials of 120 °/sec (HS 2 - 120°/s) and three trials of 60 °/sec (HS 2- 60°/s), Condition 5 three trials of 60 °/sec (HS 5 - 60°/s) and three trials of 15 °/sec (HS 5 - 15°/s). |
| Group 5 | Condition 5 three trials of 15 °/sec (HS 5 - 15°/s) and three trials of 60 °/sec (HS 5 - 60°/s), Condition 2 three trials of 60 °/sec (HS 2- 60°/s) and three trials of 120 °/sec (HS 2 - 120°/s). |
| Group 6 | Condition 5 three trials of 15 °/sec (HS 5 - 15°/s) and three trials of 60 °/sec (HS 5 - 60°/s), Condition 2 three trials of 120 °/sec (HS 2 - 120°/s) and three trials of 60 °/sec (HS 2- 60°/s). |
| Group 7 | Condition 5 three trials of 60 °/sec (HS 5 - 60°/s) and three trials of 15 °/sec (HS 5 - 15°/s), Condition 2 three trials of 60 °/sec (HS 2- 60°/s) and three trials of 120 °/sec (HS 2 - 120°/s). |
| Group 8 | Condition 5 three trials of 60 °/sec (HS 5 - 60°/s) and three trials of 15 °/sec (HS 5 - 15°/s), Condition 2 three trials of 120 °/sec (HS 2 - 120°/s) and three trials of 60 °/sec (HS 2- 60°/s). |
During each of the head-shake conditions, a three-dimensional rate sensor accelerometer was worn on the subject’s head via a comfortable headband. This provided the speed and the excursion of the head movement for the examiner to monitor during the head-shake SOT trials. Subjects were allowed to practice the task and were cued by an audible signal from a metronome as well as verbal feedback provided by the examiner during the head-shake conditions. All subjects were instructed to maintain the head-shaking task for 20 seconds during each trial. In order to eliminate fatigue, subjects were given a one minute sitting break in-between each of the head-shake conditions. Additional sitting breaks were permitted if indicated by the subject.
Statistical Analysis
Mean and standard deviation for each condition within each age group was calculated. In addition, mean, standard deviation and range values were calculated to determine how well each subject was able to match the desired head-shake condition velocities. Each subject’s change in performance with increasing difficulty of the task was characterized by the slope of a linear regression line fit between condition difficulty rank (condition 1 = SOT 2, condition 2 = HS 2- 60°/s, condition 3= HS 2- 120°/s, condition 4 = SOT 5, condition 5 = HS 5- 15°/s, condition 6 = HS 5-60°/s) and equilibrium score; in addition, the scatterplot and corresponding coefficient of correlation were assessed for each individual subject to ensure that the slope was an appropriate summary measure.
The association between individual slopes and age was assessed using Spearman’s ranked correlation (r). The mean slopes were compared between age groups using Wilcoxon Rank – sum tests. Mean equilibrium scores were also compared between conditions using Wilcoxon Signed-rank tests for the overall age groups. P-values < 0.05 were considered statistically significant. All statistical calculations were performed using JMP (Version 7.0.1, SAS Institute Inc., Cary, NC).
Results
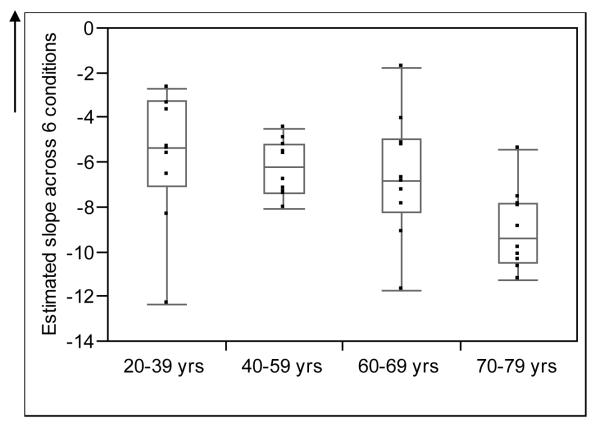
Average equilibrium scores decreased as condition difficulty increased within each age group as demonstrated by the means and negative average slope in each age group (see Table 2). The relationship between equilibrium score and condition ranking demonstrated a good correlation for each individual subject with correlation coefficients ranging from −0.70 to −0.97 and a median of −0.89. Thus, we used the individual subject slopes to estimate change across conditions. The average mean slope was highest (i.e. lowest decline in performance across the 6 conditions) for Group 1 followed by Group 2, Group 3 and then Group 4. However, it should be noted that differences between Groups 1, 2 and 3 were not significantly different (Group 1 vs Group 2, p = 0.31; Group 1 vs Group 3, p = 0.39, Group 2 vs Group 3, p = 0.91) while Group 4 demonstrated a significantly higher rate of decline across the 6 conditions when compared to each of the other 3 Groups (Group 4 vs Group 1, p = 0.01; Group 4 vs Group 2, p = 0.002; Group 4 vs Group 3, p = 0.02) (Figure 2). In addition there was a significant correlation between slope and age as a continuous variable (r = −0.52; p = 0.0006). Table 3 provides the mean, standard deviation and range of the four head-shake conditions velocity values by age. Overall, all subjects were able to maintain the desired velocity task during the four head-shake conditions within a small variance. Of importance the older groups performed equally as well as the younger subjects in the accuracy with which they maintained the task velocity.
Table 2.
Mean, Standard Deviation and Average Slope Values for Modified Head-Shake Conditions by Age
| Condition |
Slope |
||||||
|---|---|---|---|---|---|---|---|
| Age Group (years) |
SOT 2 |
HS 2- 60 |
HS 2- 120 |
SOT 5 |
HS 5- 15 |
HS 5- 60 |
|
| 20-39 | |||||||
| M | 91.63 | 91.77 | 91.00 | 73.10 | 68.87 | 69.40 | −5.6507 |
| SD | 2.76 | 4.61 | 4.79 | 6.40 | 13.34 | 11.50 | 3.05 |
| 40-59 | |||||||
| M | 91.47 | 91.07 | 89.83 | 68.50 | 67.53 | 65.77 | −6.2983 |
| SD | 3.54 | 3.17 | 2.32 | 5.25 | 5.91 | 4.90 | 1.28 |
| 60-69 | |||||||
| M | 89.27 | 89.63 | 87.30 | 66.17 | 64.43 | 62.23 | −6.6257 |
| SD | 2.92 | 4.12 | 5.45 | 11.42 | 10.9 | 12.04 | 2.64 |
| 70-79 | |||||||
| M | 90.67 | 90.87 | 89.60 | 61.70 | 54.90 | 54.50 | −9.051 |
| SD | 2.48 | 2.94 | 2.59 | 10.35 | 9.00 | 8.69 | 1.87 |
Note. The slope for each individual subject was estimated across the 6 conditions in order of difficulty; individual subject slopes were then averaged for subjects within each age group.
Figure 2.
Box plots showing the distribution of estimated slope across 6 conditions for the four age groups. The line in the middle of the box represents the median, while the ends of the box represent the 25th and 75th percentiles. The lines extending from the box represent the minimum and maximum observed values.
Table 3.
Mean, Standard Deviation and Range Values for Velocity Presentations During The Four Head-Shake Conditions by Age
| Condition |
||||
|---|---|---|---|---|
| Age Group (years) |
HS 2- 60 |
HS 2- 120 |
HS 5- 15 |
HS 5- 60 |
| 20-39 | ||||
| M | 63.6 | 117.2 | 22.1 | 64.7 |
| SD | 5.89 | 4.05 | 3.6 | 5.18 |
| Range | 56-73 | 112-123 | 17-27 | 58-77 |
| 40-59 | ||||
| M | 63 | 122.7 | 20.5 | 60.6 |
| SD | 5.4 | 13.78 | 4.77 | 4.7 |
| Range | 54-69 | 101-156 | 14-26 | 54-67 |
| 60-69 | ||||
| M | 61.6 | 116.8 | 21.6 | 61.7 |
| SD | 4.06 | 6.78 | 4.55 | 5.95 |
| Range | 55-66 | 111-125 | 16-31 | 50-67 |
| 70-79 | ||||
| M | 65.3 | 124.6 | 19.9 | 63.3 |
| SD | 2.63 | 3.92 | 3.67 | 9.68 |
| Range | 62-69 | 116-130 | 16-25 | 47-82 |
|
| ||||
| Overall 20-79 |
||||
| M | 63.38 | 120.32 | 21.03 | 62.57 |
| SD | 4.68 | 8.57 | 4.11 | 6.6 |
| Range | 54-73 | 101-156 | 14-31 | 47-82 |
Note: Velocity averages in deg/s for the three trials in each head-shake condition.
Mean equilibrium scores were compared between conditions using the Wilcoxon Signed-rank tests with all age groups collapsed to evaluate the benefit of the new head-shake modifications (i.e. HS 2-120°/s and HS 5 −15°/s) in eliminating the ceiling and floor effect noted by Mishra et al. (2009) and to justify the need for all 6 parameters in future research with patient populations. Specifically, performance on HS 2 −120°/s was compared to the standard condition SOT 2 and HS 2- 60°/s was compared to standard condition SOT 2 whereas HS 5- 15°/s was compared to the standard condition SOT 5 and HS 5- 60°/s was compared to standard condition SOT 5.
There was a significant difference in performance between HS 2 −120°/s and SOT 2 (p = 0.0237); however there was not a significant difference in performance between condition HS 2 - 60°/s and SOT 2 (p = 0.8640). There was also a significant difference in performance between HS 2 −120°/s and HS 2- 60°/s conditions (p = 0.01). These findings suggest that HS 2 −120°/s was a more challenging condition than HS 2 −60°/s for the normal subjects to perform than the standard SOT 2 condition, thus eliminating the ceiling effect noted by Mishra et al. (2009). Based on these findings, it may not be necessary to include HS 2 - 60°/s in a future study with patients.
When comparing the head-shake modifications to the standard condition SOT 5, there was a significant difference in performance between HS 5- 15°/s and SOT 5 conditions (p = 0.018) and between HS 5 – 60°/s and SOT 5 (p = 0.001). No statistical difference in performance was noted for HS 5 −60°/s or HS 5 – 15°/s conditions (p = 0.41). It should also be noted that only 2 subjects (both from Group 6) presented with fall reactions to the first trial on HS 5- 60°/s; no fall reactions were noted for HS 5 - 15°/s. From a qualitative standpoint, this finding differs from that reported by Mishra et al. (2009) where a majority of their patients had fall reactions on HS 5 - 60°/s, thus causing the floor effect. Our results suggest that the single outcome variable including HS 5 – 15°/s eliminated the floor effect reported by Mishra et al. (2009).
Discussion
The addition of active head movements to a postural task causes degradation of postural control (Paloski et al., 2006). The advantage of the head-shake condition is that it allows for simultaneous stimulation of the peripheral vestibular system while performing a postural control task.. The brain must discriminate body sway and head-shake stimuli in order to maintain balance during head-shake posturography (Peters, 2007). Prior work by Mishra et al. (2009) stated that modification to conditions 2 and 5 of SOT that stemmed from previous work by Hain et al. (1987) and Walker & Zee (2000) incorporates the concept of post head-shake nystagmus. The head-shake test produces a build up of neural activity in the velocity storage integrator resulting in induced nystagmus in individuals with unilateral peripheral vestibular system hypofunction (Panosian & Paige, 1995). While our head-shake procedure is not specifically looking at post-velocity storage integration (post head-shake nystagmus), it presents an analogous protocol. The addition of a head-shake task to standard SOT testing disrupts an individual’s stance and we hypothesize that this disruption is likely a combination of the mechanics of moving the head while attempting to maintain quiet stance in addition to the stimulation of the peripheral vestibular system providing additional sensory cues that need to be integrated into the task of standing. It is also hypothesized that the stimulation of the peripheral vestibular system will cause increased postural control disruption for individuals with asymmetrical peripheral vestibular system functioning as modestly suggested by Mishra et al. (2009). Of importance, our modifications to the head-shake protocol allowing for use of 6 points and a single outcome parameter (slope of the linear fit line to the six conditions) no longer showed the ceiling and floor effects reported from Mishra et al. (2009).
Our results have shown that deterioration in performance on SOT with head-shake was appreciated during later decades of life given the statistically significant difference across age. While our results should not be considered normative data, they are consistent with normative values for standard SOT testing (Nashner, 1993) showing a decrease in equilibrium scores with advancing age. However, one may argue that this increased deterioration with age may be the result of older subjects performing a harder dual task (i.e. head movements with eyes closed on a fixed forceplate or one that rotates in relation to body sway) and not necessarily implying age related changes to the stimulated vestibular system. Lundin-Olsson, Nyberg, and Gustafson (1997) have reported that older individuals have difficulty managing attention to simultaneous dual tasks. The addition of the head-shake condition places an additional task demand on the subject and decline in the ability to perform this dual task may be affected by aging (Peters, 2007).
In conclusion, our results suggest that the use of a head-shake modification to SOT conditions 2 and 5 shows a trend in increasing the separation of normal individuals across age. The addition of the head-shake conditions that consisted of peak head velocity of 120°/sec for condition 2 and 15°/sec for condition 5 eliminated the ceiling and floor effects reported by Mishra et al. (2009). Future research on the head-shake modification needs to be performed in the patient population to retest the hypothesis addressed by Mishra et al. (2009) for the identification of peripheral vestibular system asymmetry. This new study will specifically test sensitivity and specificity at predicting patient’s with unilateral peripheral vestibular system involvement vs. gold standard caloric irrigation testing. The need for all six parameters to be included as part of the single outcome variable to determine sensitivity and specificity for identifying unilateral peripheral vestibular hypofunction will also be addressed in the next phase of study.
While we do not anticipate that knowledge on the sensitivity of the head-shake modification protocol will take the place of gold standard procedures such as caloric irrigations as a tool for identifying peripheral vestibular system hypofunction, it may be used in combination with other clinical tests (e.g. electronystagmography, rotational chair) in the assessment of vestibular pathologies. It may also serve to alert physical therapist that routinely use posturography assessment and often see balance disorder patients first that an index of suspicion regarding hypofunction is high and that the patient needs to be referred for more definitive testing. It is also possible that the addition of the headshake protocol with increased sensitivity to peripheral asymmetry may serve as a better indicator of changes over time in the central compensation process since it attempts to link active peripheral vestibular stimulation with postural control.
Acknowledgements
Subject recruitment partially funded by: Human Subjects Research Core Grant P30DC004662, Michael Gorga, Ph.D. PI, Boys Town National Research Hospital; Small Grant Department of Otorhinolaryngology, Mayo Clinic – Rochester.
Footnotes
A portion of this paper was presented as a poster presentation at the American Academy of Audiology’s annual conference on April 2-5, 2008 in Charlotte, North Carolina.
References
- Allum JHJ, Shepard NT. An overview of the clinical use of dynamic posturography in the differential diagnosis of balance disorders. Journal of Vestibular Research. 1999;9:223–252. [PubMed] [Google Scholar]
- Chandra NS, Shepard NT. Clinical utility of lateral head tilt Posturography. American Journal of Otology. 1996;17(2):271–277. [PubMed] [Google Scholar]
- El-Kashlan HK, Shepard NT, Asher AM, Smith-Wheelock M, Telian SA. Evaluation of clinical measures of equilibrium. The Laryngoscope. 1998;108:311–319. doi: 10.1097/00005537-199803000-00002. [DOI] [PubMed] [Google Scholar]
- Hain TC, Fetter M, Zee DS. Head-shaking nystagmus in patients with unilateral peripheral vestibular lesions. American Journal of Otolaryngology. 1987;8:36–47. doi: 10.1016/s0196-0709(87)80017-0. [DOI] [PubMed] [Google Scholar]
- Lundin-Olsson L, Nyberg L, Gustafson Y. “Stops waking when talking” as a predictor of falls in elderly people. The Lancet. 1997;349:617. doi: 10.1016/S0140-6736(97)24009-2. [DOI] [PubMed] [Google Scholar]
- Mishra A, Davis S, Speers R, Shepard NT. Head-shake computerized dynamic posturography in peripheral vestibular lesions. American Journal of Audiology. 2009 doi: 10.1044/1059-0889(2009/06-0024). in press. [DOI] [PubMed] [Google Scholar]
- Nashner LM. Computerized dynamic Posturography. In: Jacobson GP, Newman CW, Kartush JM, editors. Handbook of Balance Function Testing. Singular Publishing Group; San Diego: 1993. pp. 280–334. [Google Scholar]
- Paloski WH, Wood SJ, Feiveson AH, Black FO, Hwany EY, Reschke MF. Destabilization of human balance control by static and dynamic head tilts. Gait & Posture. 2006;23(3):315–323. doi: 10.1016/j.gaitpost.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Panosian MS, Paige GD. Nystagmus and postural instability after headshake in patients with vestibular dysfunction. Otolaryngology-Head and Neck Surgery. 1995;112(3):399–404. doi: 10.1016/S0194-59989570273-3. [DOI] [PubMed] [Google Scholar]
- Peters JF. Computerized dynamic posturography (CDP) and the assessment of balance with active head movements. Journal of the Korean Balance Society. 2007;6(2):243–247. [Google Scholar]
- Shepard NT, Schulz A, Alexander NB, Gu MJ, Boismier T. Postural control in young and elderly adults when stance is challenged: Clinical versus laboratorymeasurements. Annals Otology, Rhinology & Laryngology. 1993;102(7):508–517. doi: 10.1177/000348949310200704. [DOI] [PubMed] [Google Scholar]
- Shepard NT, Telian SA. Practical management of the balance disorder patient. Singular Publishing Group; San Diego: 1996. [Google Scholar]
- Walker MF, Zee DS. Bedside vestibular examination. The Otolaryngologic Clinics of North America. 2000;33(3):495–506. doi: 10.1016/s0030-6665(05)70223-1. [DOI] [PubMed] [Google Scholar]